Abstract
Myosin has an intrinsic ability to organize into ordered thick filaments that mediate muscle contraction. Here, we use surface plasmon resonance and light scattering analysis to further characterize the molecular determinants that guide myosin filament assembly. Both assays identify a cluster of lysine and arginine residues as important for myosin polymerization in vitro. Moreover, in cardiomyocytes, replacement of these charged residues by alanine severely affects the incorporation of myosin into the distal ends of the sarcomere. Our findings show that a novel assembly element with a distinct charge profile is present at the C-terminus of sarcomeric myosins.
Keywords: myosin, assembly, SPR, light scattering
Introduction
The ability of myosin II to move along actin filaments is central to the function of striated muscles. The motion driven by the enzymatic activity of the myosin head is dependent on the ability of the coiled coil structure of the myosin rod to assemble into thick filaments [1]. This process is driven by charge interactions occurring along the rod [2], and by sequence determinants located in the C-terminal portion of the molecule also known as light meromyosin (LMM) [3]. Myosin and LMM are soluble in high salt but insoluble in physiological salt solutions. Under these conditions, LMM forms periodic paracrystalline assemblies. These arrays are generally accepted as surrogates for myosin thick filaments because they show characteristic repeats at 43 and 14.3 nm [4]. Using a combination of differential solubility and paracrystal assays, a conserved 29- residue region named Assembly Competence Domain (ACD) was identified as a critical modulator of LMM assembly in vitro [3]. Moreover, detailed sequence analysis revealed that the ACD is part of a longer conserved C-terminal region characterized by an unusual distribution of apolar amino acids and a unique charge profile [5]. We have sought to broaden these earlier observations by monitoring LMM assembly with surface plasmon resonance (SPR), light scattering (LS) and cellular assays. This integrated approach has allowed identification of a cluster of four positive residues not previously identified in paracrystal assays that is important for myosin polymerization in vitro and incorporation into sarcomeres in cultured cardiac cells. The data presented add new valuable information to the molecular processes that promote myosin assembly.
Materials and Methods
Expression vectors and proteins
The WT-LMM sequence corresponding to residues 1231–1938 of rat α-cardiac myosin, was initially amplified from pMT21 α plasmid with Pfu DNA polymerase using forward and reverse primers carrying the XhoI and SpeI restriction sites respectively. The resulting blunt end PCR product that introduces 2 extra amino acids at the beginning and at the end of LMM (L-E and T-S respectively), was then cloned into pUC18 SmaI site for mutagenesis. To avoid aggregation through oxidation, the 3 cysteine residues present along the protein (C1341, C1412, and C1749) were replaced by serines. These modifications, as well as the truncations and point mutations introduced in the LMM analyte constructs were carried out by inverse PCR according to standard procedures. All mutations were confirmed by sequencing. Constructs were double digested with XhoI and SpeI, and the resulting LMM fragments were then inserted into a modified pET3a expression vector. This cloning added N-terminal T7 and 6X-His tags and a C-terminal Cys residue to the LMM proteins. Finally, for the SPR assay, the amino acid sequence MAGC was added to the N- terminus of the ligand to allow a unique point of attachment to the chip. EGFP and mCherry myosin tagged constructs were generated by fusing each fluorescent reporter gene at amino acid 841 of the rat α-cardiac myosin gene as previously described [6].
Recombinant proteins used in SPR and LS assays were expressed in E.coli BL21 (DE3) as previously described [7]. Protein purity was assessed by polyacrylamide gel electrophoresis, and relative concentrations were determined by BCA assay (Pierce) using BSA as a standard. Proteins were stored as small aliquots at pH 7.3 in 0.5 M NaCl at −80° C.
NRVM preparation and transfection
Neonatal rat ventricular myocytes (NRVMs) were prepared from Sprague-Dawley neonatal rat hearts as previously described [8]. Cells were electroporated using the Rat Cardiomyocyte-Neonatal Nucleofector Kit (Lonza) according to the manufacturer’s protocol and plated onto 1% gelatin coated glass coverslips. Following overnight recovery, cells were washed and then treated with 15 mM L-Phenylephrine. After 48 hrs, cells on the coverslips were fixed with 4% paraformaldehyde, mounted with Vectashield (Vector Laboratories) and analyzed by confocal microscopy with a Nikon Eclipse TE 2000-U microscope. Imaging was performed with the 100X Nikon Plan Apo VC oil objective. MetaMorph software was used for image acquisition and cell image analysis.
Surface Plasmon Resonance (SPR)
All experiments were made in a Biacore model X (GE). LMM ligand was attached to a sensor C1 chip (GE) by first derivatizing both Biacore flow cells with ethylenediamine according to the manufacturer’s directions, and then reacting the free amino group with a solution of 250 µg N-gamma-maleimidobutyryloxy succinimidyl ester (Pierce) in 20 µl 0.1 M sodium bicarbonate pH 8.8 to produce flow cells with N-alkyl maleimide groups. Unreacted amino groups were blocked by reaction with sulfo-NHS acetate (ThermoScientific) in 0.1 M sodium bicarbonate (pH 8.8). 50 µl of 0.1 µM LMM ligand in 0.5 M NaCl, 0.5 mM Tris (2-carboxyethyl)phosphine at pH 7.3 was run over flow cell 2 (Fc2) at 20 µl per minute; the amount of protein attached varied from 200 to 300 RU, where 1 RU (Resonance Units) corresponds to about 1.2 pg of protein. A solution of 50 mM β-mercaptoethanol was used to block unreacted maleimides in Fc 1 and Fc 2 cells. For binding assays, the chip was first washed with 6M guanidinium chloride and then equilibrated for 2 minutes with 10 mM TES pH 7.3, 150 mM NaCl, and 3.5 mM EDTA. Immediately after dilution from 300 to 150 mM NaCl, 70 µl of analyte solution was flowed over the two cells at 20 µl per minute; the sensorgram ordinate (RU) represents the difference between binding of analyte to Fc 2 and Fc 1.
Light Scattering (LS)
Light scattering at 320 nm was measured as previously described, in a PTI QM-2000–6SE fluorescence spectrometer (Photon Technology International) at 25 °C with a 2 and 8 nm slit width for excitation and emission respectively [7]. To show that the changes observed were reversible, 5 M NaCl was added at the end of each assembly reaction (carried out at 150 mM NaCl) to bring the final salt concentration to 300 mM.
Results and Discussion
Surface Plasmon Resonance (SPR) assay for LMM assembly
To better define the sequence determinants and the earliest events of LMM assembly, we have developed a novel approach based on SPR, a technique used to study a wide range of biomolecular interactions [9]. In our assay, we measure the non-covalent binding of LMM analyte in solution to the LMM ligand immobilized on the biosensor chip. As the ligand and analyte interact, the formation of a myosin polymer on the chip increases the refractive index of the solution proportionally to the mass of analyte bound. Thus, a real-time quantification of the early steps of assembly of the WT and mutant LMM can be carried out.
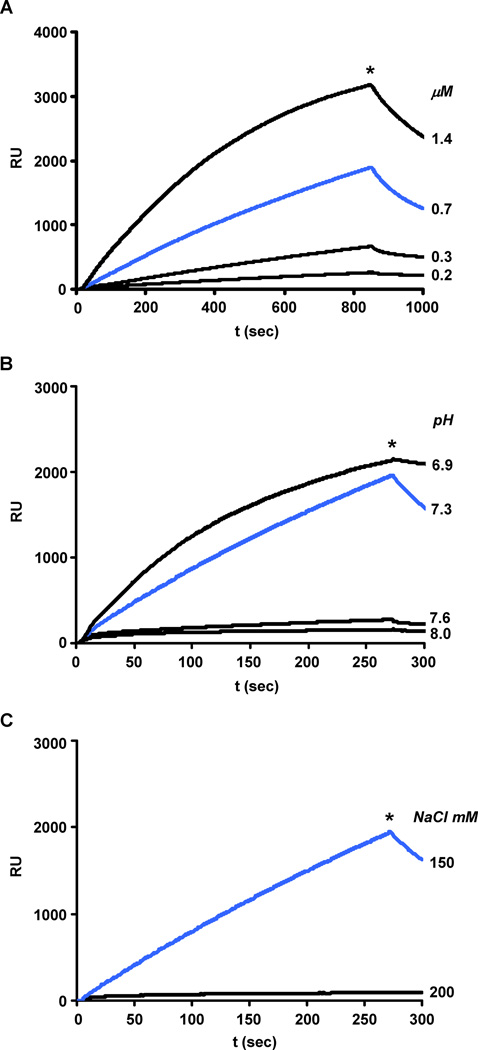
Figure 1A shows the results of a typical experiment in which WT-LMM ligand (residues 1231–1938), covalently attached to the chip through a Cys at its N-terminus, was exposed to solutions of WT-LMM analyte in 150 mM NaCl at pH 7.3. At analyte concentrations greater than 0.2 μM the amount of LMM bound increases with time with a rate that is approximately first order in protein concentration. Since the amount of noncovalently bound LMM analyte quickly exceeds stoichiometric equivalence with the covalently bound LMM ligand (see Materials and Methods) the LMM analyte is either polymerizing on the LMM ligand, or is polymerizing in solution and then binding to the LMM ligand. The complex formed under these conditions dissociates rapidly when highsalt buffer replaces the analyte (asterisks in each panels). The kinetics of dissociation are complex and do not lend themselves to a simple analysis of this process. Polymerization of LMM on the ligand is very sensitive to the pH and salt concentration of the solution of analyte. At pH 8.0, the amount of LMM analyte bound to the LMM ligand is less than 5% of that bound at pH 7.3 (Figure 1B). A similar reduction in binding is seen when the salt concentration is raised to 200 from 150mM (Figure 1C). These effects of pH and ionic strength on assembly are consistent with an assembly process that is driven by ionic interactions between LMM molecules as observed for myosin [10, 11].
Fig. 1.
Binding of WT-LMM analyte to WT-LMM ligand depends on protein concentration, pH and salt concentration. The ligand was equilibrated in 10 mM TES at the pH and NaCl concentrations indicated below. Binding was initiated by flowing a solution of analyte over the ligand at protein concentration, pH, and NaCl molarity shown on the right side of each sensorgram in Panel A, B and C respectively. At the time indicated by the asterisk, the analyte solution was replaced by 10 mM TES/300 mM NaCl. The SPR sensorgram shown in Panel A was obtained at pH 7.3, NaCl 150 mM and analyte concentrations as indicated. The analyte concentration in the sensorgrams shown in Panels B and C was 0.45 µM: the reactions were carried out in NaCl 150 mM at the pH values indicated (panel B) and at pH 7.3 at the NaCl concentration indicated (panel C).
A novel assembly determinant in the C-terminus of LMM
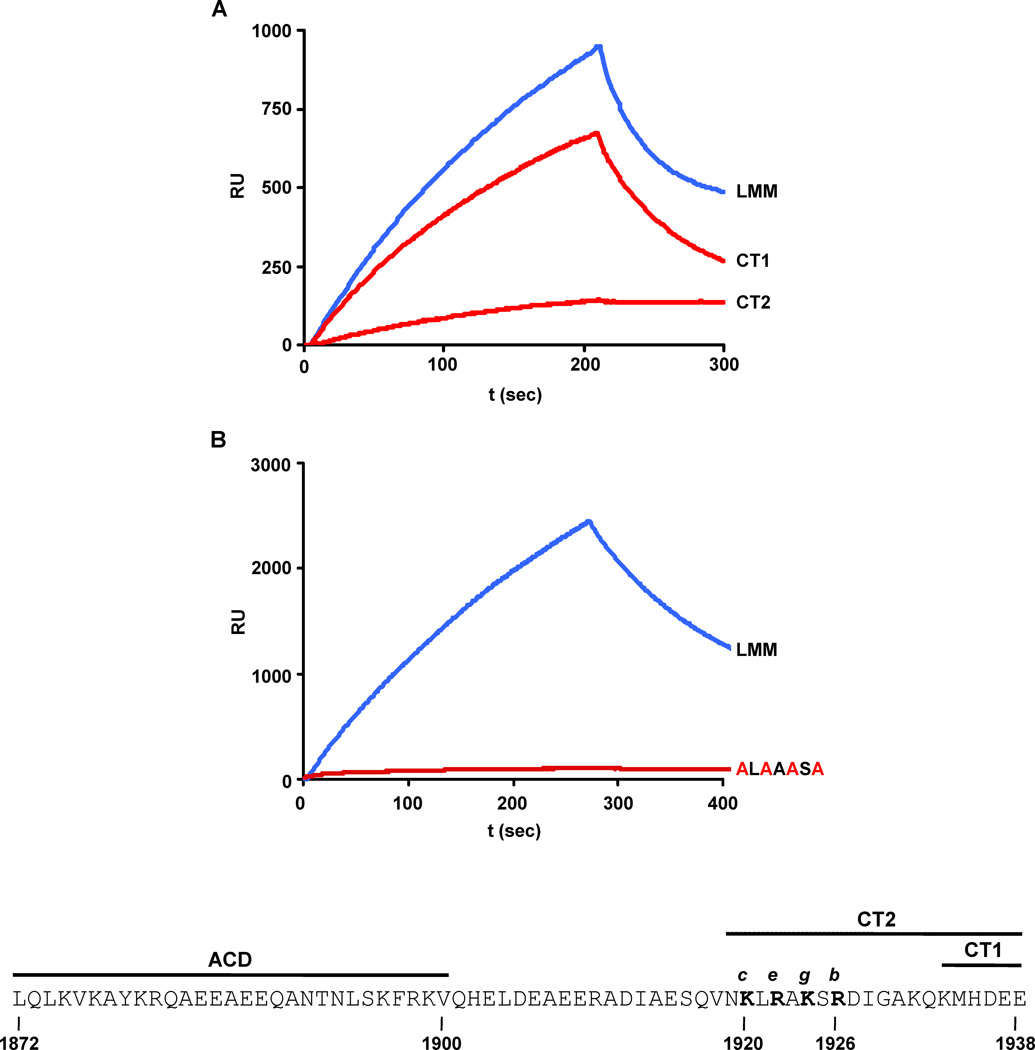
Earlier work has suggested that interactions involving the C-terminus of LMM are important for assembly into paracrystals [3, 12]. To further define those amino acid residues responsible for assembly we have investigated the properties of a number of C-terminally truncated LMM analytes in the SPR assay (Figure 2A). While binding was hardly affected by truncation of the C-terminal 6 amino acids (CT1), it was severely reduced by truncation of the C-terminal 20 residues of LMM (CT2). Little further loss of ability to assemble was detected when the analyte was further shortened by 28 amino acids (data not shown).
Fig. 2.
LMM analyte truncations and amino acid substitutions affect the binding to WT-LMM ligand. Truncation analysis (A): LMM corresponds to the WT-LMM; CT1 and CT2 correspond to truncations of 6 and 20 amino acids respectively at the carboxyl-terminus of LMM. Amino acid substitution analysis (B): the alanine replacements in the KLRAKSR sequence are shown in red. Binding reactions in Panels A, and B were carried out in 150 mM NaCl, pH 7.3 and analyte concentrations of 0.5, and 0.6 µM respectively. The bottom of the figure shows the sequence of the last 67 amino acids of the rat α-myosin, containing the assembly competence domain (ACD), the cluster of positive charges identified in this study (bold), their relative heptad repeat position, and the CT1 and CT2 deletions shown in Panel A.
To further localize the residues in the C-terminal region identified by the truncation analysis as being important for the polymerization reaction, we closely examined the sequence contained in the CT2 deletion and focused on a cluster of four charged amino acids, (KLRAKSRresidues 1920–1926) which we postulated could be involved in ionic interactions between adjacent coiled-coils since present in the exposed positions of the heptad repeat (Figure 2, bottom). In accord with our hypothesis, simultaneous replacement of all four positively charged residues with alanine reduced the LMM analyte’s ability to bind to the LMM ligand (Figure 2B). Interestingly, the alanine replacement mutagenesis has a greater impact on polymerization than the CT2 truncation. We believe that this difference could be ascribable to the different charge profile of the two mutant rods: while alanine substitutions leave the end of the rod negatively charged (4 negative residues are in fact present downstream of the KLRAKSR sequence) the truncation neutralizes this effect. Although alanine substitution of the first two positive charges (K1920 and R1922) or the last two (K1924 and R1926) also resulted in a reduction of the protein’s ability to polymerize on the LMM ligand, single residue replacements tested in each position of the positive cluster did not affect the reaction (data not shown).
Light scattering (LS) assay for LMM assembly
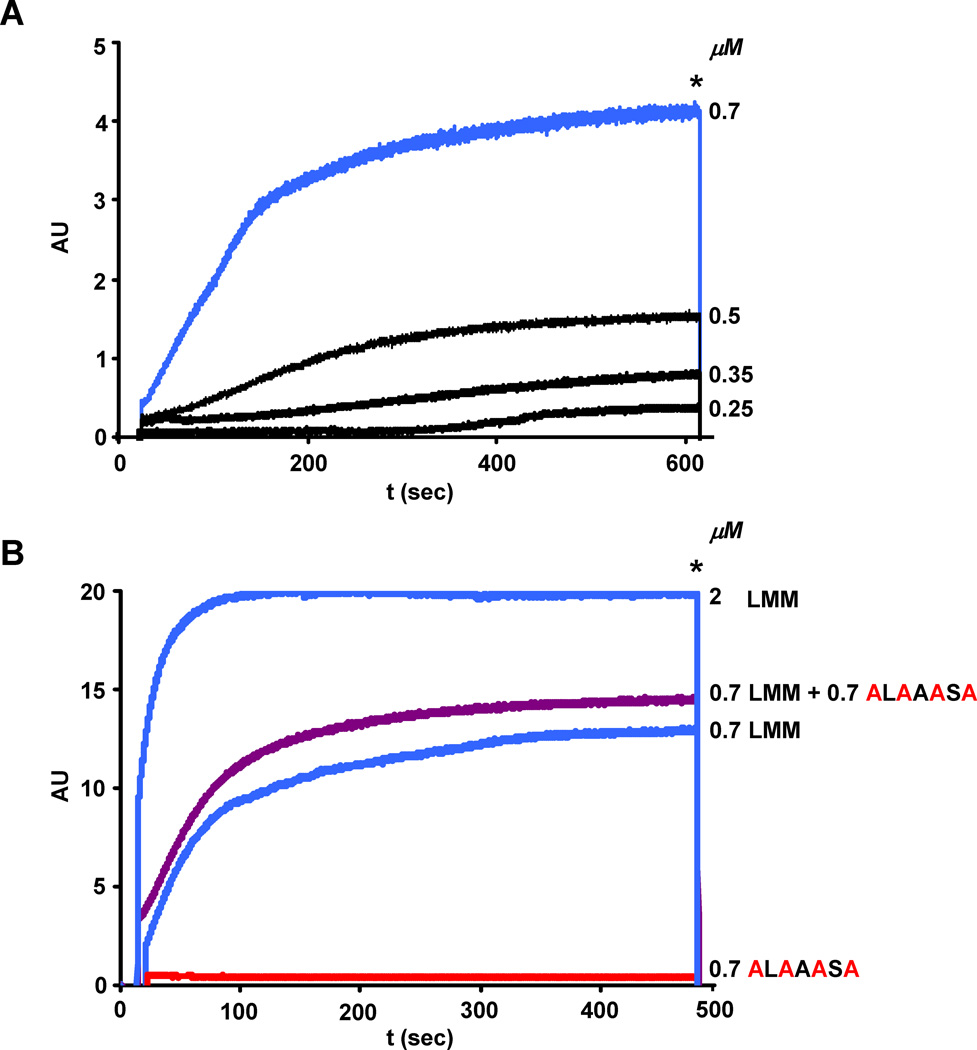
A decrease in SPR signal could result from impaired binding of the mutant analyte to the WT ligand, or impaired self-polymerization of the mutant. To discriminate between these two alternatives, we examined LMM assembly using the LS assay. Previously established for studying the formation of myosin-II minifilaments in Acanthamoeba [13], the LS assay has been recently used in our lab to study the effects of pathogenic myosin mutations on myosin assembly [7]. Since molecules scatter light according to their molecular weight, the extent of LMM polymerization can be determined by this assay. As for SPR, the LS assay shows that LMM polymerization is dependent on its concentration (Figure 3A) and on the ionic strength of the solution (data not shown). At LMM concentrations between 0.25 and 0.5 µM, there appears to be a lag in the formation of light scattering complex, indicating that under these conditions, formation of a small intermediate(s) may be rate-determining for the overall assembly process. At the concentrations used in this study, the final level of light scattering does not decrease on extended incubation, indicating that the complex formed does not precipitate from solution. The absence of any measurable increase in light scattering observed when the C-terminal KLRAKSR→ALAAASA mutant was assayed indicates that the loss of the four positive residues severely affects the ability of LMM to self-polymerize (Figure 3B, red curve). This result also suggests that the dramatic signal reduction observed in the SPR assay with the same mutant is probably due to lack of analyte self-polymerization. Moreover, mixing equimolar amounts of the ALAAASA mutant with wild-type LMM only slightly enhanced the extent of light scattering (Figure 3B, purple curve) indicating that the mutant cannot efficiently copolymerize with, or inhibit the polymerization of the wild-type protein.
Fig. 3.
The C-terminal ALAAASA mutant cannot self-polymerize or efficiently copolymerize with the WT-LMM. (A) WT-LMM self-polymerization depends on protein concentration. At the time indicated by the asterisk, the NaCl concentration was increased from 150 to 300 mM to show the reversibility of the assembly reaction. The concentration of WT-LMM tested are shown on the right side of each curve; the intensity of the light scattering is plotted in arbitrary units (AU) versus time. (B) WT-LMM and ALAAASA mutant self-polymerization reactions were carried out at 0.7 µM; for copolymerization analysis the final total protein concentration was 1.4 µM. The linear response of the assay at higher protein concentration was verified by monitoring the WT-LMM self-polymerization reaction at 2µM.
The KLRAKSR sequence is important for assembly in myocytes
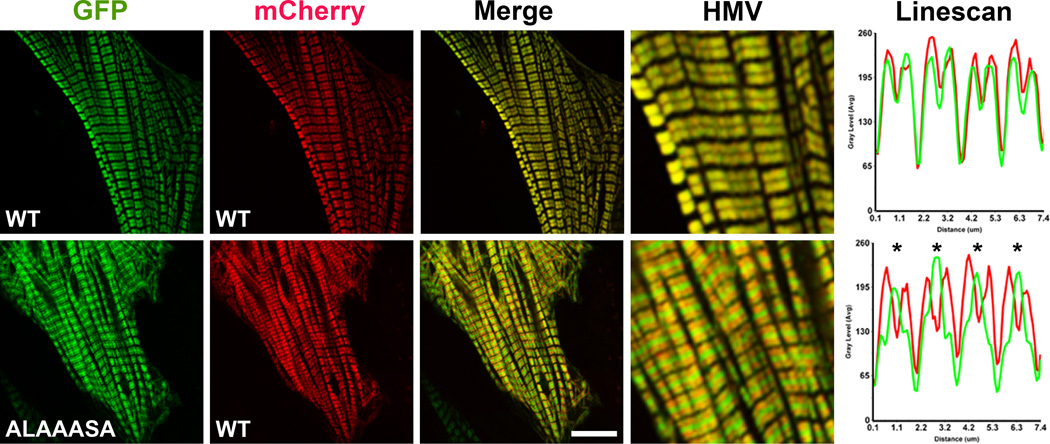
To extend our in vitro results into the biological context of a muscle cell background, we next investigated whether the alanine mutations in the KLRAKSR affect assembly of myosin into the sarcomere. Neonatal rat cardiac myocytes (NRVMs) were co-transfected with a plasmid expressing the mutant rat α-myosin rod N-terminally fused to GFP and a plasmid expressing the WT rat α-myosin fused to mCherry, and subsequently imaged by confocal microscopy. As shown in Figure 4, the myosin mutant was incorporated into the sarcomere but its presence was mainly restricted to the bare zone, where the myosin molecules organize in an antiparallel arrangement, and was only barely detectable along the A band (HMV and Linescan). However, the presence of the mutant does not affect efficient incorporation of the WT mCherry myosin along the sarcomere. The intensity of the fluorescence signal spiking at the center of the bare zone suggests that the mutant is incorporated into the sarcomere but may assemble incorrectly; loss of interactions between the mutant and the myomasp/LRRC39 protein that interacts with the C-terminal region of myosin, could in fact result in a change of rod localization in the M-band [14]. Interestingly, a similar phenotype has been previously described for a rod mutation causing Laing distal myopathy [6]. The reduction in the fraction of mutant localized in the sarcomere arms indicates that the positively charged side chains could primarily be involved in the interactions occurring between parallel coiled-coils. These data also concur with the result obtained with the LS assay: lack of dominant negative effects on WT mCherry myosin incorporation and limited assembly in the sarcomere bare zone match the mixing experiment presented in Figure 3 (purple curve).
Fig. 4.
The ALAAASA mutant is incorporated into sarcomeres but accumulates in the bare zone. Neonatal rat ventricular myocytes were co-transfected with GFP- and mCherry-WT myosin rods (Top Panel), or with GFP-mutant myosin (ALAAASA) and mCherry-WT myosin rods (Bottom Panel). Representative images from the mCherry and GFP channels are shown together with the merge. HMV: high magnification view of merged images. Linescan: analysis of the relative intensity of wt-mCherry myosin and GFP-myosin mutant along four sarcomeres. Asterisks indicate accumulation of the ALAAASA mutant in the bare zone. The bar represents 10 µm.
The cationic cyclical pattern of the KLRAKSR sequence is unique
Using a combined biochemical and cellular approach we have found that four positive charges located in the KLRAKSR sequence (AA 1920–1926 of the α-cardiac myosin) are important for thick filament formation. These positive residues are highly conserved among all muscle myosin isoforms as well as across different species (for a detailed analysis see the myosin-weighted consensus sequence available at http://bmf2.colorado.edu/myomapr/ [15]). Their identification is of special significance since the last two K and R amino acids were previously identified as important for sarcomere assembly in C. elegans [16]. The importance of these residues for myosin assembly in cardiac myocytes, and nematodes indicates that there are significant similarities between the biochemically and biologically accessible vertebrate systems and the genetically accessible nematode system. This suggests that hypotheses generated in either of these systems can be usefully investigated in the other in a way that will benefit our understanding of both.
The KLRAKSR region lies in the extended ACD region that lacks the periodicity of positive and negative charges that characterize the rest of the myosin rod, and shows a distinctive distribution of charges and a high proportion of large apolar residues in surface positions [5]. Remarkably, the topology of the lysine and arginine residues is unique: four alternating positively charged residues (clustering in c, e, g and b positions of the heptad repeat). We have considered the possibility that the alanine substitutions could hinder the formation of the rod coiled-coil structure by disrupting stabilizing ion pairs between the two α-helices. However, the first R (R1922) and the second K (K1924) are predicted to form ionic pairs with residues 1917 and 1929 of the partner helix [17, 18]. The finding that in rat α-myosin these positions are occupied by Q and G residues respectively, strongly suggests that inter-helical electrostatic interactions do not occur in this region. Thus, we believe that the four positive residues contained in the KLRAKSR sequence are involved in higher-level assembly of the coiled-coils. In particular, based on the in vivo experiments that show a preferential exclusion of the mutant from the arms of the sarcomere, we propose that the positive character of the KLRAKSR sequence mainly promotes parallel assembly of adjacent coiled-coils. Interestingly, one-dimensional modeling of electrostatic interactions between the 4 positive residues K-R-K-R and anionic partners located at the predicted rod staggers of 43/14.3 nm [2], identifies the sequences EGDLNEME (AA 1620–1628; negative charges located in e, g, c, and e positions of the heptad repeat) and RELENELE (AA 1821–1828; negative charges located in c, e, g, and b positions of the heptad repeat) as potential candidates for ionic pairing.
By defining new sequences involved in thick filament formation, our data may be useful to better understand the links between mutations occurring in the myosin assembly domain and a growing class of myopathies [19].
Highlights.
Surface Plasmon Resonance was used to survey myosin assembly.
Four positive residues guiding myosin polymerization in vitro are defined.
Their topology in the myosin coiled-coil rod is unique.
Their mutagenesis affects myosin incorporation in the sarcomere in vivo.
Acknowledgements
We thank Ann Robinson for preparing NRVMs. This research was supported by National Institutes of Health grants R01GM29090.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Craig R, Woodhead JL. Structure and function of myosin filaments. Curr Opin Struct Biol. 2006;6:204–212. doi: 10.1016/j.sbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 2.McLachlan AD, Karn J. Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature. 1982;99:226–231. doi: 10.1038/299226a0. [DOI] [PubMed] [Google Scholar]
- 3.Sohn RL, Vikstrom KL, Strauss M, Cohen C, Szent-Gyorgyi AG, Leinwand LA. A 9 residue region of the sarcomeric myosin rod is necessary for filament formation. J Mol Biol. 1997;66:317–330. doi: 10.1006/jmbi.1996.0790. [DOI] [PubMed] [Google Scholar]
- 4.Bennett PM. The structure of spindle-shaped paracrystals of light meromyosin. J Mol Biol. 1981;46:201–221. doi: 10.1016/0022-2836(81)90432-0. [DOI] [PubMed] [Google Scholar]
- 5.Cohen C, Parry DA. A conserved C-terminal assembly region in paramyosin and myosin rods. J Struct Biol. 1998;22:180–187. doi: 10.1006/jsbi.1998.3983. [DOI] [PubMed] [Google Scholar]
- 6.Buvoli M, Buvoli A, Leinwand LA. Effects of pathogenic proline mutations on Myosin assembly. J Mol Biol. 2012;15:807–818. doi: 10.1016/j.jmb.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armel TZ, Leinwand LA. Mutations in the beta-myosin rod cause myosin storage myopathy via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;06:6291–6296. doi: 10.1073/pnas.0900107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maass AH, Buvoli M. Cardiomyocyte preparation, culture, and gene transfer. Methods Mol Biol. 2007;66:321–330. doi: 10.1007/978-1-59745-030-0_18. [DOI] [PubMed] [Google Scholar]
- 9.Boozer C, Kim G, Cong S, Guan H, Londergan T. Looking towards label-free biomolecular interaction analysis in a high-throughput format: a review of new surface plasmon resonance technologies. Curr Opin Biotechnol. 2006;7:400–405. doi: 10.1016/j.copbio.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Josephs R, Harrington WF. Studies on the formation and physical chemical properties of synthetic myosin filaments. Biochemistry. 1966:3474–3487. doi: 10.1021/bi00875a013. [DOI] [PubMed] [Google Scholar]
- 11.Reisler E, Cheung P, Borochov N, Lake JA. Monomers, dimers, and minifilaments of vertebrate skeletal myosin in the presence of sodium pyrophosphate. Biochemistry. 1986;5:326–332. doi: 10.1021/bi00350a007. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson SJ, Stewart M. Expression in Escherichia coli of fragments of the coiled-coil rod domain of rabbit myosin: influence of different regions of the molecule on aggregation and paracrystal formation. J Cell Sci. 1991;9(Pt 4):823–836. doi: 10.1242/jcs.99.4.823. [DOI] [PubMed] [Google Scholar]
- 13.Sinard JH, Pollard TD. The effect of heavy chain phosphorylation and solution conditions on the assembly of Acanthamoeba myosin-II. J Cell Biol. 1989;09:1529–1535. doi: 10.1083/jcb.109.4.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Will RD, et al. Myomasp/LRRC39, a heart- and muscle-specific protein, is a novel component of the sarcomeric M-band and is involved in stretch sensing. Circ Res. 2010;07:1253–1264. doi: 10.1161/CIRCRESAHA.110.222372. [DOI] [PubMed] [Google Scholar]
- 15.Buvoli M, Hamady M, Leinwand LA, Knight R. Bioinformatics assessment of beta-myosin mutations reveals myosin's high sensitivity to mutations. Trends Cardiovasc Med. 2008;8:141–149. doi: 10.1016/j.tcm.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoppe PE, Andrews RC, Parikh PD. Differential requirement for the nonhelical tailpiece and the C terminus of the myosin rod in Caenorhabditis elegans muscle. Mol Biol Cell. 2003;4:1677–1690. doi: 10.1091/mbc.E02-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLachlan AD, Stewart M. Tropomyosin coiled-coil interactions: evidence for an unstaggered structure. J Mol Biol. 1975;8:293–304. doi: 10.1016/s0022-2836(75)80119-7. [DOI] [PubMed] [Google Scholar]
- 18.O'Shea EK, Klemm JD, Kim PS, Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991;54:539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- 19.Oldfors A, Lamont PJ. Thick filament diseases. Adv Exp Med Biol. 2008;42:78–91. doi: 10.1007/978-0-387-84847-1_7. [DOI] [PubMed] [Google Scholar]