Abstract
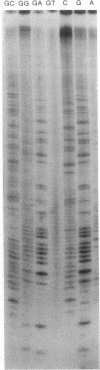
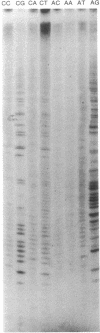
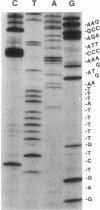
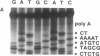
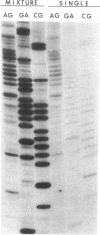
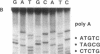
The poly(A) site of bovine prolactin (bPRL) mRNA was examined by phased priming of cDNA synthesis with oligodeoxynucleotides of the general sequence d(pT8-N-N'). The existence of multiple poly(A)-adjacent sequences in bPRL mRNA was indicated by the production of specific chain-termination fragments with at least three d(pT8-N-N') sequences. Comparison of the sequence bands produced by initiation of cDNA synthesis on the bPRL mRNA template with d(pT8-A-G), d(pT8-G-A), and d(pT8-C-G) revealed a shifted pattern of identical fragments. The shift in position of related sequence bands on the gel suggested that the difference in length of the three major bPRL mRNA species occurred within a span of 12 nucleotides. Sequence analysis conducted with the three d(pT8-N-N') primers gave identical nucleotide sequences for the 3' noncoding region of the bPRL mRNA species and suggested that the mRNA molecules were heterogeneous in length. The existence of multiple poly(A) sites was confirmed by determination of the nucleotide sequence of bPRL cDNA clones containing two of the major poly(A)-adjacent sequences predicted by the oligodeoxynucleotide primers. The mRNA molecules containing these multiple poly(A)-addition sites were shown to be present in the bPRL mRNA obtained from a single pituitary gland. The variation in the poly(A) junction of bPRL mRNA may be a reflection of the processing events at the 3' terminus of mRNAs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Darnell J. E., Jr Transcription units for mRNA production in eukaryotic cells and their DNA viruses. Prog Nucleic Acid Res Mol Biol. 1979;22:327–353. doi: 10.1016/s0079-6603(08)60803-x. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Ford J. P., Hsu M. T. Transcription pattern of in vivo-labeled late simian virus 40 RNA: equimolar transcription beyond the mRNA 3' terminus. J Virol. 1978 Dec;28(3):795–801. doi: 10.1128/jvi.28.3.795-801.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillam S., Smith M. Use of E. coli polynucleotide phosphorylase for the synthesis of oligodeoxyribonucleotides of defined sequence. Methods Enzymol. 1980;65(1):687–701. doi: 10.1016/s0076-6879(80)65067-8. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Dhar R., Khoury G. Mapping the spliced and unspliced late lytic SV40 RNAs. Cell. 1978 Aug;14(4):971–982. doi: 10.1016/0092-8674(78)90351-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Blanchard J. M., Darnell J. E., Jr Transcription units of adenovirus type 2. Termination of transcription beyond the poly(A) addition site in early regions 2 and 4. J Mol Biol. 1980 Dec 15;144(3):377–386. doi: 10.1016/0022-2836(80)90096-0. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E., Jr Steps in the processing of Ad2 mRNA: poly(A)+ nuclear sequences are conserved and poly(A) addition precedes splicing. Cell. 1978 Dec;15(4):1477–1493. doi: 10.1016/0092-8674(78)90071-5. [DOI] [PubMed] [Google Scholar]
- Nilson J. H., Barringer K. J., Convey E. M., Friderici K., Rottman F. M. Ontogeny of pituitary hormone mRNAs in the bovine fetus. Quantitation of pre-prolactin mRNA as a function of gestation. J Biol Chem. 1980 Jun 25;255(12):5871–5878. [PubMed] [Google Scholar]
- Nilson J. H., Convey E. M., Rottman F. M. Purification of pre-prolactin mRNA from bovine anterior pituitary glands. J Biol Chem. 1979 Mar 10;254(5):1516–1520. [PubMed] [Google Scholar]
- Nilson J. H., Thomason A. R., Horowitz S., Sasavage N. L., Blenis J., Albers R., Salser W., Rottman F. M. Construction and characterization of a cDNA clone containing a portion of the bovine prolactin sequence. Nucleic Acids Res. 1980 Apr 11;8(7):1561–1573. doi: 10.1093/nar/8.7.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasavage N. L., Smith M., Gillam S., Astell C., Nilson J. H., Rottman F. Use of oligodeoxynucleotide primers to determine poly(adenylic acid) adjacent sequences in messenger ribonucleic acid. 3'-Terminal noncoding sequence of bovine growth hormone messenger ribonucleic acid. Biochemistry. 1980 Apr 29;19(9):1737–1743. doi: 10.1021/bi00550a003. [DOI] [PubMed] [Google Scholar]
- Smith A. J. The use of exonuclease III for preparing single stranded DNA for use as a template in the chain terminator sequencing method. Nucleic Acids Res. 1979 Mar;6(3):831–848. doi: 10.1093/nar/6.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]
- Zain B. S., Roberts R. J. Sequences from the beginning of the fiber messenger RNA of adenovirus-2. J Mol Biol. 1979 Jun 25;131(2):341–352. doi: 10.1016/0022-2836(79)90080-9. [DOI] [PubMed] [Google Scholar]