Abstract
Objective
Some studies suggest that HIV infection progresses slowly in patients with sickle cell disease (SCD). The authors aimed to determine the relationships between SCD and HIV infection.
Methods
National Hospital Discharge Survey data from adult Africane–Americans in the period of 1997–2009 were analysed. The comorbidities of SCD with HIV infections in hospital discharges were analysed. Multiple logistic regression was used to test the association between SCD and HIV. For comparative purposes, the relationships of SCD with hepatitis B virus (HBV) and hepatitis C virus (HCV) were also assessed.
Results
423 431 records were divided into two time periods 1997–2003 (53% of records) and 2004–2009 (47% of records). The frequency of HIV diagnosis was lower in patients with SCD (1.5% vs 3.3% in patients without SCD). In logistic regression, SCD diagnosis was associated with an OR of 0.24 (95% CI 0.18 to 0.32) for HIV diagnosis in the first period and with an OR of 0.31 (95% CI 0.22 to 0.42) in the second period. In contrast, SCD was associated with higher risk of HCV (OR=2.01, 95% CI 1.56 to 2.59 in the first period and OR=2.12, 95% CI 1.71 to 2.63 in the second period). SCD was also associated with a higher risk of HBV (OR=1.15, 95% CI 0.72 to 1.83 in the first period and OR=1.82, 95% CI 1.24 to 2.68 in the second period).
Conclusions
The lower risk of HIV comorbidity, but not HCV and HBV, with SCD is consistent with the possibility that SCD has a unique effect in altering the risk of HIV infection or progression. Investigation of how the haemolytic and immunological changes of SCD influence HIV might lead to new therapeutic or preventive approaches.
INTRODUCTION
The Centers for Disease Control estimated that 1.1 million adults and adolescents were living with HIV infection in the USA in 2006 and that 46% of them were Africane–American. In 2008, the prevalence of HIV infection for the population aged ≥13 years was estimated to be 469 per 100 000 overall and 1819 per 100 000 in Africane–Americans. The HIV prevalence for Africane–American men was six times the rate for white men and the rate for Africane–American women was 18 times the rate for white women.1 Sickle cell disease (SCD) is the most common inherited blood disorder in the USA, affecting approximately 90 000 people, 90% of them Africane–American.2
Several studies suggest that SCD may influence the course of HIV infection and progression. The prevalence of HIV seropositivity in patients with SCD who were transfused in the 1970s and 1980s was reported to be lower than in haemophiliacs receiving transfusions,3 although this difference could plausibly be due to exposure to blood products from more donors in the haemophiliacs. A cohort of SCD patients infected with HIV from five centres in the USA had slower HIV progression based on viral load and CD4+ cell count than matched HIV-positive patients without SCD when followed for a median of 12 years (range 2–13 years).4 In another study in the USA, none of 686 HIV patients with SCD died during hospitalisation compared with 3.1% of 62 004 HIV patients without SCD.5 A recent report from the Republic of Congo6 indicated that the frequency of HIV among 127 patients with SCD was lower than among 3390 blood donors7 (0.9% vs 5.4%; p=0.022). In contrast, the prevalence of HIV among patients with SCD (0.8%, 95% CI 0.4% to 1.4%) in Brazil8 was not lower than that in the general adult population as reported in a separate source (0.6%, 95% CI 0.5% to 0.8%).9
The purpose of the present study was to determine whether SCD diagnosis is associated with lower rate of concurrent HIV diagnosis in Africane–American adults who are hospitalised, based on data collected in the USA. This is the first population-based study with adequate sample size that tests if SCD could protect from HIV. A small study suggested that SCD may be associated with protection from HIV protection, but this is by no means established.3 SCD is associated with upregulated inflammatory and haemolytic pathways; if these responses provide protection from HIV, it is very important to do further research and identify the mechanism. This could open the door to new therapeutic or preventive strategies for HIV. The relationship of SCD with hepatitis C virus (HCV) infection and hepatitis B virus (HBV) infection was also examined for comparative purposed.
METHODS
The National Center for Health Statistics at Centers for Disease Control has conducted the National Hospital Discharge Survey since 1965. This survey recruits discharge records from random samples of non-institutional hospitals, excluding Federal, military and Veterans Administration hospitals, located in the 50 States and the DC. Only short-stay hospitals (hospitals with an average length of stay for all patients of <30 days) or those whose specialty is general (medical, surgical or paediatric) are included in the survey. For each discharge record, up to seven medical diagnostic codes were recorded. The International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) was used for coding the diagnoses.10
This study used National Hospital Discharge Survey data from 1997 to 2009. Analysis was restricted to adult (≥20 years) Africane–American individuals. Different comorbidity categories were classified according to the ICD-9-CM codes (see table 1 in online appendix). Each patient was assigned to these categories if any of the seven diagnoses had the corresponding ICD-9 code, so each patient could have more than a single diagnosis.
Statistical analysis
The year 1997 was chosen for the start of this study as this approximately coincides with the introduction of Highly Active Antiretroviral Therapy (HAART) for HIV11 and Food and Drug Administration (FDA) approval of hydroxyurea12 for SCD. Data were divided into two periods to determine whether observed relationships could be confirmed in consecutive time periods. Each period covered approximately 50% of the samples. Logistic regression analysis was applied to assess the relationship of SCD diagnosis at discharge with HIV, HCV and HBV diagnoses at discharge. Since exploratory analysis indicated that the risks of HIV infection and SCD varied according to certain demographic variables, adjustment was made for the effects of these variables including age (categorised in decades) in analyses. HIV diagnosis was different according to some of the general diagnostic categories, so a model was built to adjust for demographic variables as well as general category of disease as potential confounders. Applying survey weight did not change the results, so unweighted results are presented in the Results section.
RESULTS
From 1997 to 2009, 423 431 records from adult Africane–American patients were available. We divided these records into two periods, an early period (1997–2003) including 53% of the records and a later period (2004–2009) including 47% of the records (table 1).
Table 1.
Characteristics of patients by study period
| Characteristic | Study period |
|
|---|---|---|
| 1997–2003 | 2004–2009 | |
| Total number of records | 22 5819 | 197 612 |
| Age group (years), N (%) | ||
| 20–29 | 35 007 (15) | 27 355 (14) |
| 30–39 | 37 396 (17) | 27 447 (14) |
| 40–49 | 39 595 (18) | 35 562 (18) |
| 50–59 | 32 771 (14) | 34 774 (18) |
| 60–69 | 30 130 (13) | 27 920 (14) |
| 70–79 | 28 829 (13) | 24 291 (12) |
| 80+ | 22 091 (10) | 20 263 (10) |
| Median (IQR) age, years | 50 (35–68) | 52 (38–68) |
| Male, N (%) | 84 764 (38) | 76 704 (39) |
| Sickle cell diagnosis, N (%) | 3370 (1.5) | 3147 (1.6) |
| HIV diagnosis, N (%) | 7277 (3.2) | 6073 (3.1) |
| HCV diagnosis, N (%) | 3010 (1.3) | 4645 (2.4) |
| HBV diagnosis, N (%) | 870 (0.4) | 882 (0.5) |
| Other infectious and parasitic diseases, N (%) | 23 387 (10) | 21 316 (11) |
| Neoplasm, N (%) | 24 280 (11) | 20 607 (10) |
| Endocrine, nutritional and metabolic diseases, N (%) |
90 246 (40) | 92 884 (47) |
| Other haematological diseases, N (%) | 35 494 (16) | 32 444 (16) |
| Mental disorders, N (%) | 53 008 (23) | 54 546 (28) |
| Nervous system disease, N (%) | 20 727 (9) | 22 651 (11) |
| Cardiovascular diseases, N (%) | 116 107 (51) | 114 718 (58) |
| Respiratory diseases, N (%) | 49 961 (22) | 48 898 (25) |
| Digestive diseases, N (%) | 43 835 (19) | 42 811 (22) |
| Genitourinary diseases, N (%) | 44 198 (20) | 53 065 (27) |
| Skin diseases, N (%) | 15 271 (7) | 15 541 (8) |
| Musculoskeletal and connective tissue diseases, N (%) |
24 212 (11) | 25 842 (13) |
| Injury and poisoning, N (%) | 26 311 (12) | 25 609 (13) |
HBV, hepatitis B virus; HCV; hepatitis C virus.
The frequency of SCD diagnosis was stable, approximately 1.5%. The frequency of HIV diagnosis had two increases, one from 1997 to 1999 and the second from 2006 to 2008. In 2009, the frequency of HIV diagnosis decreased from 3.5% to 2.1%. HCV frequency continuously increased from 1997 to 2004 and then declined from 2007. HBV diagnosis was stable during the study periods, approximately 0.5% (figure 1).
Figure 1.
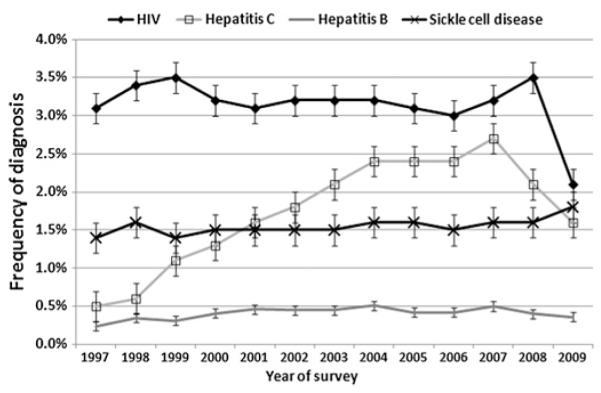
The frequency of different diagnoses in hospital discharges. Bars indicate the CI of frequencies calculated from Poisson distribution.
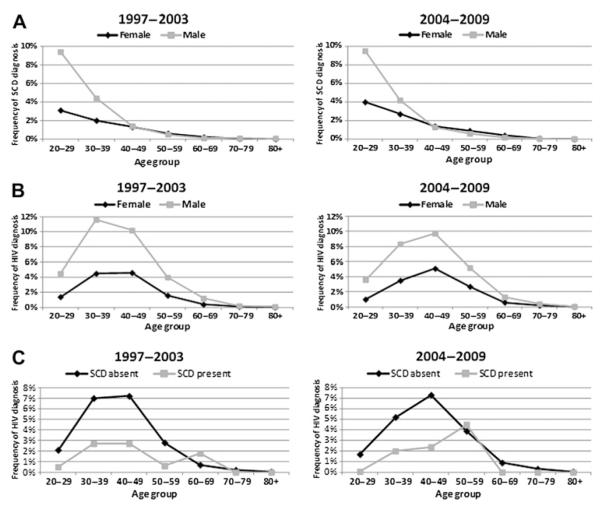
In both study periods, patients with SCD were of younger age (figure 2A). The median (IQR) age was 31 (25–40) years in patients with SCD versus 51 (37–68) years in other diagnoses (p<0.0001). SCD frequency was higher among men than women aged <40 years. SCD frequency was not different by gender over 40 years of age (figure 2A).
Figure 2.
(A) Frequency of sickle cell disease (SCD) diagnosis by age group and gender. Denominator for each frequency is total number of hospital discharges for the same age group and gender. (B) Frequency of HIV diagnosis by age group and gender. Denominator for each frequency is total number of hospital discharges for the same age group and gender. (C) Frequency of HIV diagnosis by age group and sickle cell diagnosis. Denominator for each frequency is total number of hospital discharges for the same age group and sickle cell status.
SCD and HIV comorbidity
In the earlier period, the frequency of HIV diagnosis peaked in the 30–39 years of age group in both genders. In the later period, this peak happened in the 40–49 years of age group in both genders (figure 2B). In the earlier study period, the frequency of HIV diagnosis was lower among patients with concurrent SCD up to 60 years of age. In the later period, HIV diagnosis was lower among patients with concurrent SCD up to 50 years of age (figure 2C).
In both study periods, the overall frequency of HIV diagnosis was lower among patients who had concurrent SCD diagnosis (approximately 1.5%) than in patients without SCD diagnosis (approximately 3.3%). Sickle cell diagnosis was associated with an OR of 0.46 (95% CI 0.35 to 0.60) for concurrent HIV diagnosis in the earlier period and with an OR of 0.43 (95% CI 0.32 to 0.59) in the later period.
The frequency of HIV diagnosis differed according to the presence or absence of other comorbidities in both study periods. HIV frequency was higher in patients with a diagnosis of other infectious disease as well as other haematological, mental, respiratory and skin diseases (see figure 1 in online appendix). Therefore, we adjusted for other comorbidities in our analyses. In both study periods, the prevalence of HIV differed according to geographic region (higher in the northeast and south areas) and marital status (higher in single and divorced or separated individuals). Therefore, we also performed an analysis that adjusted for these variables in addition to the broad diagnostic categories.
In multiple logistic regression and adjusting for age, gender, geographic location, marital status and other comorbidities (including infectious, neoplasm, endocrine, haematological, mental, nervous, cardiovascular, respiratory, digestive, genitourinary, skin, musculoskeletal disease and injuries), SCD was associated with an OR of 0.24 (95% CI 0.18 to 0.32) for concurrent HIV diagnosis in the earlier period and with an OR of 0.31 (95% CI 0.22 to 0.42) in the later period (table 2).
Table 2.
Relationship between sickle cell disease diagnosis and HIV comorbidity by study period
| HIV diagnosis with no sickle cell disease diagnosis |
HIV diagnosis with sickle cell disease diagnosis |
Unadjusted |
Adjusted* |
Full model† |
||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |||
| 1997–2003 | 7226/222 449 (3.3%) | 51/3370 (1.5%) | 0.46 (0.35 to 0.60) | <0.0001 | 0.27 (0.21 to 0.36) | <0.0001 | 0.24 (0.18 to 0.32) | <0.0001 |
| 2004–2009 | 6030/194 465 (3.1%) | 43/3147 (1.4%) | 0.43 (0.32 to 0.59) | <0.0001 | 0.33 (0.24 to 0.45) | <0.0001 | 0.31 (0.22 to 0.42) | <0.0001 |
Adjusted for age, gender and geographic area.
Adjusted for age, gender, geographic area, marital status and other comorbidities (including infectious, neoplasm, endocrine, haematological, mental, nervous, cardiovascular, respiratory, digestive, genitourinary, skin, musculoskeletal disease and injuries).
SCD and comorbidity with HCV and HBV
To determine if the observed decreased association of SCD and HIV was a general observation for viral infections or unique to HIV, we also examined the comorbidities of SCD and HCV and of SCD and HBV.
In the earlier study period, the frequency of HCV diagnosis was higher among patients with concurrent SCD up to 50 years of age. In the later period, HCV diagnosis was more frequent in patients with concurrent SCD up to 60 years of age (figure 2A in online appendix). In both study periods, the overall frequency of HCV diagnosis was higher among patients who had concurrent SCD diagnosis (2.0% vs 1.3% in patients without SCD diagnosis in the early period and 3.1% vs 2.3% in the later period). In bivariate analysis, SCD diagnosis was associated with an OR of 1.49 (95% CI 1.16 to 1.91) for concurrent HCV diagnosis in the earlier period and with an OR of 1.32 (1.08 to 1.63) in the later period. In multiple logistic regression after adjusting for age, gender, geographic location, marital status and other comorbidities, SCD was associated with an OR of 2.01 (95% CI 1.56 to 2.59) for concurrent HCV diagnosis in the earlier period and with an OR of 2.12 (95% CI 1.71 to 2.63) in the later period (table 3, a).
Table 3.
Relationship between sickle cell disease, HCV and HBV comorbidity by study period
| Hepatitis diagnosis with no sickle cell disease diagnosis |
Hepatitis diagnosis with sickle cell disease diagnosis |
Unadjusted |
Adjusted* |
Full model* |
||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |||
| a) HCV | ||||||||
| 1997–2003 | 2944/222 449 (1.3%) | 66/3370 (2.0%) | 1.49 (1.16 to 1.91) | 0.002 | 1.77 (1.38 to 2.27) | <0.0001 | 2.01 (1.56 to 2.59) | <0.0001 |
| 2004–2009 | 4548/194 465 (2.3%) | 97/3147 (3.1%) | 1.32 (1.08 to 1.63) | 0.006 | 1.97 (1.60 to 2.44) | <0.0001 | 2.12 (1.71 to 2.63) | <0.0001 |
| b) HBV | ||||||||
| 1997–2003 | 851/222 449 (0.4%) | 19/3370 (0.6%) | 1.48 (0.94 to 2.33) | 0.09 | 1.12 (0.71 to 1.77) | 0.6 | 1.15 (0.72 to 1.83) | 0.6 |
| 2004–2009 | 854/194 465 (0.4%) | 28/3147 (0.9%) | 2.04 (1.39 to 2.97) | 0.0002 | 1.82 (1.24 to 2.67) | 0.002 | 1.82 (1.24 to 2.68) | 0.002 |
Adjusted for age, gender and geographic area.
Adjusted for age, gender, geographic area, marital status and other comorbidities (including infectious, neoplasm, endocrine, haematological, mental, nervous, cardiovascular, respiratory, digestive, genitourinary, skin, musculoskeletal disease and injuries).
HBV, hepatitis B virus; HCV; hepatitis C virus.
In the earlier period, the frequency of HBV diagnosis was higher among patients with SCD up to age of 40 years, and then, HBV was more frequent in patients without the diagnosis of SCD. In the later period, the diagnosis of HBV was more frequent in patients with concurrent SCD up to age of 50 years (figure 2B in online appendix). In both study periods, the overall frequency of HBV diagnosis was higher among patients who had SCD (6.0% vs 0.4% in patients without SCD for the earlier period and 0.9% vs 0.4% in the later period). In the earlier period, SCD was not associated with significantly higher frequency of HBV. In the later period, and after adjustment for age, gender, geographic location, marital status and other comorbidities, SCD was associated with an OR of 1.82 (95% CI 1.24 to 2.68) for concurrent HBV diagnosis (table 3, b).
DISCUSSION
While Africane–Americans represent approximately 13% of the US population,13 they account for almost half of people living with HIV in the USA. In 2009, in adult Africane–American men, the main route of transmission was male-to-male sexual contact, followed by heterosexual contact and injection drug use; in women, heterosexual contact and injection drug use were the most important transmission routes.14
In this study, we found that SCD among Africane–American hospital discharges was associated with lower rate of HIV comorbidity. This effect was consistent for two periods of study, one of 7 years duration and the other of 6 years duration. The younger age of patients with SCD was a potential major confounder, so adjustment for age was necessary in our analyses. The observed effect of SCD on HIV comorbidity was even more significant after adjustment for confounders. In striking contrast, SCD was associated with a higher risk of HCV and HBV comorbidities in this database. This is consistent with a unique effect of the immunological or haemolytic changes of SCD on HIV versus other viral infections.
It is also conceivable that a lower chance of exposure to HIV through sexual activity or intravenous drug abuse could explain the lower risk of HIV comorbidity in patients with SCD. SCD is associated with a delay in sexual maturation15,16 and lower sexual activity.17,18 However, adjusting for marital status and the diagnosis of drug abuse did not affect the inverse relationship between SCD and HIV in the present study. We did not have information about sexual activity. Marital status is rather an inaccurate measure of sexual activity. It is therefore possible that the strength of the observed association has been overestimated due to residual confounding.
We could not rule out the effect of other potential biases in this study. Any factor that could change the chance of hospital admission could affect our interpretation. HIV, HBV or HCV comorbidities could increase the severity of disease and risk of hospital admission in patients with SCD.5,8 This could partially explain the effect of SCD on higher HBV and HCV rate of comorbidities but could not predict the lower rate of HIV diagnosis in patients with SCD. We were not able to identify repeat admissions. SCD,19 HBV and HCV infections20 are associated with higher rate of hospital readmission, which could overestimate the association between SCD diagnosis and these infections in hospital records. The observed frequency of HIV diagnosis in hospital discharges is 3%, which is higher than 1.8% expected from the Africane–American (aged ≥13 years) statistics. We have studied adult patients who could partially explain this higher prevalence. HIV diagnosis is also associated with increased risk of hospital readmission,21 which will increase the estimated prevalence of HIV infection in hospital discharge records and may bias the observed association.
Interindividual variability has been reported in the response to HIV infection. This includes susceptibility to infection by HIV, transmission of HIV and HIV disease progression.22 While more than 90% of infected people progress to AIDS if untreated, long-term non-progressors, who have <2000 copies of virus per millilitre without antiretroviral therapy, and elite controllers, who have <50 copies per millilitre without therapy, remain naturally resistant to the disease. Natural human susceptibility could potentially be determined by a number of factors, including chemokines, cytokines, HIV co-receptors, immune responses and innate antiviral resistance.23
Several mechanisms might explain an ameliorating effect of SCD on the course of HIV disease. (1) Chronic haemolysis upregulates haeme oxygenase-1.24-26 Upregulation of haeme oxygenase-1 was recently shown to block HIV-1 infection in macrophages and T cells treated with haemin.27,28 Haemin administration also significantly reduced viral load in HIV-infected humanised mice.27 Suppression of HIV-1 by haemin was reversed by protoporphyrin, an HO-1 inhibitor.28 (2) Hypoxia related to anaemia and vaso-occlusive episodes may contribute to inhibition of HIV.29-31 Our previous molecular studies showed that HIV-1 transcription is inhibited in cells cultured under lower oxygen conditions.30 (3) Higher expression of inflammatory cytokines32 may be associated with enhanced innate immunity and protection from HIV infection.33 For example, IL-10, a TH-2 cell cytokine that is increased in SCD,32,34 inhibits HIV-1 replication in macrophages.35 (4) HIV transcription is inhibited when cellular iron is reduced with iron chelators36,37 or when the iron export protein ferroportin is overexpressed.38 Iron chelators deregulate cellular activities of CDK2 and CDK9, which are critical for HIV-1 transcription.36,37 HIV-1 is also induced in primary cells treated with hepcidin. Thus, reduced hepcidin production and lower intracellular labile iron levels in SCD39 might lead to the inhibition of HIV replication.36,37 (5) Duffy Antigen Receptor for Chemokines-negative status40 and CCR5 blockage23 could slow HIV progression. Lower expression of Duffy Antigen Receptor for Chemokines41 and CCL5 (also known as RANTES) in patients with SCD42 could also explain lower HIV progression in these patients. (6) Hydroxyurea, widely used in patients with SCD to reduce vaso-occlusive episodes, is a virostatic drug against HIV.43
SCD was associated with increased risk of HBV, a condition associated with injection drug use and risky sexual behaviour, and HCV, which is transmitted mainly by injection drug use.44 The frequency of both infections has been reported to be higher in patients with SCD.45,46 HIV, HCV and HBV infection also could be transmitted by contaminated blood, even though there is minimal risk in recent years. The higher frequency of HCV in patients with SCD may be related to transfusion of blood products prior to the availability of blood screening. Despite the common transmission routes, the higher rate of HCV and HBV in SCD diagnosis is in contrast to the lower rate of HIV in our analysis. This could indicate a specific protection against HIV infection and progression or different routes of exposure between these infections among patients with SCD. In our data, higher risk of HCV and HBV comorbidities among patients with SCD are limited to younger age groups. Higher frequency of HBV and HCV diagnosis among younger age could reflect the importance of other admission diagnosis in older ages. These comorbidities increase the severity of disease in sickle cell anaemia8 so they may decreases the chance of survival in patients with SCD. The apparent increase in HCV diagnosis during the study period could reflect the effect of introduction of serological test for HCV and also increasing inpatient care and treatment indication for HCV.
Causal inferences in a cross-sectional study are limited by many potential biases, including selection and information bias and lack of temporality. Ideally, a cohort study would collect data about the incidence of HIV infection in Africane–Americans with and without SCD, together with data about possible confounding factors and immunological status. Also our results may not be applicable to other populations with different prevalence of SCD and HIV, or populations where patients tend to present very late in the course of their illness, such as is typically the case in most developing countries.
In conclusion, our results show that SCD diagnosis is associated with a lower rate of HIV comorbidity among adult Africane–Americans discharged from hospital in the USA. A higher rate of HCV and HBV diagnoses in these patients could indicate that, rather than a lower exposure rate, an enhanced immune or innate antiviral defence that is effective against HIV but not other viruses may ameliorate HIV infection in patients with SCD. If this observation is confirmed in molecular studies, it could potentially provide insights into protective and therapeutic measures for HIV infection.
Supplementary Material
Key messages.
-
▶
SCD is associated with lower risk of HIV comorbidity.
-
▶
SCD increased the risk of HBV and HCV comorbidities.
-
▶
The protection against HIV infection or progression could be related to enhanced immune defence in SCD against HIV.
Acknowledgments
Funding This study was supported by Howard University Research Scientist Award (UH1 HL03679), Pulmonary Hypertension and Hypoxic Response grant (R01 HL079912), walk-PHaSST (treatment of Pulmonary Hypertension and Sickle cell disease with Sildenafil Therapy) Study (HHSN268200617182C) and Research Centers in Minority Institutions (RCMI) grant (RCMI-NIH 2G12RR003048) from the Division of Research Infrastructure, National Center for Research Resources, NIH, NIH SCORE Grant (SC1GM082325) and NHLBI Research Center at Howard University grant (1P30HL107253).
Footnotes
Contributors MN extracted the data, did the analysis and participated in writing the paper. VRG participated in data analysis and writing the paper. SN participated in data analysis and writing the paper.
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
Additional appendices are published online only. To view these files please visit the journal online (http://sti.bmj.com/content/early/recent).
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC) HIV surveillanced United States, 1981-2008. MMWR Morb Mortal Wkly Rep. 2011;60:689–93. [PubMed] [Google Scholar]
- 2.Brousseau DC, Panepinto JA, Nimmer M, et al. The number of people with sickle-cell disease in the United States: national and state estimates. Am J Hematol. 2010;85:77–8. doi: 10.1002/ajh.21570. [DOI] [PubMed] [Google Scholar]
- 3.Castro O, Saxinger C, Barnes S, et al. Prevalence of antibodies to human immunodeficiency virus and to human T cell leukemia virus type I in transfused sickle cell disease patients. J Infect Dis. 1990;162:743–5. doi: 10.1093/infdis/162.3.743. [DOI] [PubMed] [Google Scholar]
- 4.Bagasra O, Steiner RM, Ballas SK, et al. Viral burden and disease progression in HIV-1-infected patients with sickle cell anemia. Am J Hematol. 1998;59:199–207. doi: 10.1002/(sici)1096-8652(199811)59:3<199::aid-ajh4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Kourtis AP, Bansil P, Johnson C, et al. Children with sickle cell disease and human immunodeficiency virus-1 infection: use of inpatient care services in the United States. Pediatr Infect Dis J. 2007;26:406–10. doi: 10.1097/01.inf.0000259953.79654.d0. [DOI] [PubMed] [Google Scholar]
- 6.Batina Agasa S, Dupont E, Kayembe T, et al. Multiple transfusions for sickle cell disease in the Democratic Republic of Congo: the importance of the hepatitis C virus. Transfus Clin Biol. 2010;17:254–9. doi: 10.1016/j.tracli.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Batina A, Kabemba S, Malengela R. Rev Med Brux. 2007;28:145–9. Infectious markers among blood donors in Democratic Republic of Congo (DRC) [PubMed] [Google Scholar]
- 8.Neto JP, Lyra IM, Reis MG, et al. The association of infection and clinical severity in sickle cell anaemia patients. Trans R Soc Trop Med Hyg. 2011;105:121–6. doi: 10.1016/j.trstmh.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 9.USAID Brazil HIV/AIDS Health profile. 2010
- 10.Hall MJ, DeFrances CJ, Williams SN, et al. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010:1–20. 24. [PubMed] [Google Scholar]
- 11.Volberding PA, Deeks SG. Antiretroviral therapy for HIV infection: promises and problems. JAMA. 1998;279:1343–4. doi: 10.1001/jama.279.17.1343. [DOI] [PubMed] [Google Scholar]
- 12.Lanzkron S, Haywood C, Jr, Hassell KL, et al. Provider barriers to hydroxyurea use in adults with sickle cell disease: a survey of the Sickle Cell Disease Adult Provider Network. J Natl Med Assoc. 2008;100:968–73. [PubMed] [Google Scholar]
- 13.U.S. Census Bureau 2010 Census Data. 2011 [Google Scholar]
- 14.HIV Surveillance by Race/Ethnicity (through 2009) Centers for Disease Control and Prevention; Atlanta: 2011. [Google Scholar]
- 15.Zemel BS, Kawchak DA, Ohene-Frempong K, et al. Effects of delayed pubertal development, nutritional status, and disease severity on longitudinal patterns of growth failure in children with sickle cell disease. Pediatr Res. 2007;61:607–13. doi: 10.1203/pdr.0b013e318045bdca. [DOI] [PubMed] [Google Scholar]
- 16.Zago MA, Kerbauy J, Souza HM, et al. Growth and sexual maturation of Brazilian patients with sickle cell diseases. Trop Geogr Med. 1992;44:317–21. [PubMed] [Google Scholar]
- 17.Samuels-Reid JH, Scott RB, Brown WE. Contraceptive practices and reproductive patterns in sickle cell disease. J Natl Med Assoc. 1984;76:879–83. [PMC free article] [PubMed] [Google Scholar]
- 18.Alleyne SI, Rauseo RD, Serjeant GR. Sexual development and fertility of Jamaican female patients with homozygous sickle cell disease. Arch Intern Med. 1981;141:1295–7. [PubMed] [Google Scholar]
- 19.Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol. 2005;79:17–25. doi: 10.1002/ajh.20336. [DOI] [PubMed] [Google Scholar]
- 20.Steinke DT, Weston TL, Morris AD, et al. Epidemiology and economic burden of viral hepatitis: an observational population based study. Gut. 2002;50:100–5. doi: 10.1136/gut.50.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh YH, Rothman RE, Bartlett JG, et al. HIV seropositivity predicts longer duration of stay and rehospitalization among nonbacteremic febrile injection drug users with skin and soft tissue infections. J Acquir Immune Defic Syndr. 2008;49:398–405. doi: 10.1097/qai.0b013e318183ac84. [DOI] [PubMed] [Google Scholar]
- 22.Telenti A, Goldstein DB. Genomics meets HIV-1. Nat Rev Microbiol. 2006;4:865–73. doi: 10.1038/nrmicro1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur G, Mehra N. Genetic determinants of HIV-1 infection and progression to AIDS: susceptibility to HIV infection. Tissue Antigens. 2009;73:289–301. doi: 10.1111/j.1399-0039.2009.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jison ML, Munson PJ, Barb JJ, et al. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood. 2004;104:270–80. doi: 10.1182/blood-2003-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bains SK, Foresti R, Howard J, et al. Human sickle cell blood modulates endothelial heme oxygenase activity: effects on vascular adhesion and reactivity. Arterioscler Thromb Vasc Biol. 2010;30:305–12. doi: 10.1161/ATVBAHA.109.196360. [DOI] [PubMed] [Google Scholar]
- 26.Belcher JD, Mahaseth H, Welch TE, et al. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest. 2006;116:808–16. doi: 10.1172/JCI26857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devadas K, Dhawan S. Hemin activation ameliorates HIV-1 infection via heme oxygenase-1 induction. J Immunol. 2006;176:4252–7. doi: 10.4049/jimmunol.176.7.4252. [DOI] [PubMed] [Google Scholar]
- 28.Devadas K, Hewlett IK, Dhawan S. Lipopolysaccharide suppresses HIV-1 replication in human monocytes by protein kinase C-dependent heme oxygenase-1 induction. J Leukoc Biol. 2010;87:915–24. doi: 10.1189/jlb.0307172. [DOI] [PubMed] [Google Scholar]
- 29.Ammosova T, Washington K, Debebe Z, et al. Dephosphorylation of CDK9 by protein phosphatase 2A and protein phosphatase-1 in Tat-activated HIV-1 transcription. Retrovirology. 2005;2:47. doi: 10.1186/1742-4690-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charles S, Ammosova T, Cardenas J, et al. Regulation of HIV-1 transcription at 3% versus 21% oxygen concentration. J Cell Physiol. 2009;221:469–79. doi: 10.1002/jcp.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell A, Minniti CP, Nouraie M, et al. Prospective evaluation of haemoglobin oxygen saturation at rest and after exercise in paediatric sickle cell disease patients. Br J Haematol. 2009;147:352–9. doi: 10.1111/j.1365-2141.2009.07854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niu X, Nouraie M, Campbell A, et al. Angiogenic and inflammatory markers of cardiopulmonary changes in children and adolescents with sickle cell disease. PLoS One. 2009;4:e7956. doi: 10.1371/journal.pone.0007956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naicker DD, Werner L, Kormuth E, et al. Interleukin-10 promoter polymorphisms influence HIV-1 susceptibility and primary HIV-1 pathogenesis. J Infect Dis. 2009;200:448–52. doi: 10.1086/600072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walter PB, Fung EB, Killilea DW, et al. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol. 2006;135:254–63. doi: 10.1111/j.1365-2141.2006.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrington M, Nelson G, O’Brien SJ. Considering genetic profiles in functional studies of immune responsiveness to HIV-1. Immunol Lett. 2001;79:131–40. doi: 10.1016/s0165-2478(01)00275-9. [DOI] [PubMed] [Google Scholar]
- 36.Debebe Z, Ammosova T, Breuer D, et al. Iron chelators of the di-2-pyridylketone thiosemicarbazone and 2-benzoylpyridine thiosemicarbazone series inhibit HIV-1 transcription: identification of novel cellular targetse–iron, cyclin-dependent kinase (CDK) 2, and CDK9. Mol Pharmacol. 2011;79:185–96. doi: 10.1124/mol.110.069062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Debebe Z, Ammosova T, Jerebtsova M, et al. Iron chelators ICL670 and 311 inhibit HIV-1 transcription. Virology. 2007;367:324–33. doi: 10.1016/j.virol.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu M, Kashanchi F, Foster A, et al. Hepcidin induces HIV-1 transcription inhibited by ferroportin. Retrovirology. 2010;7:104. doi: 10.1186/1742-4690-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroot JJ, Laarakkers CM, Kemna EH, et al. Regulation of serum hepcidin levels in sickle cell disease. Haematologica. 2009;94:885–7. doi: 10.3324/haematol.2008.003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He W, Neil S, Kulkarni H, et al. Duffy antigen receptor for chemokines mediates trans-infection of HIV-1 from red blood cells to target cells and affects HIV-AIDS susceptibility. Cell Host Microbe. 2008;4:52–62. doi: 10.1016/j.chom.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nebor D, Durpes MC, Mougenel D, et al. Association between Duffy antigen receptor for chemokines expression and levels of inflammation markers in sickle cell anemia patients. Clin Immunol. 2010;136:116–22. doi: 10.1016/j.clim.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 42.Rivera A, Jarolim P, Brugnara C. Modulation of Gardos channel activity by cytokines in sickle erythrocytes. Blood. 2002;99:357–603. doi: 10.1182/blood.v99.1.357. [DOI] [PubMed] [Google Scholar]
- 43.Romanelli F, Hoven AD. Use of virostatics as a means of targeting human immunodeficiency virus infection. Curr Pharm Des. 2006;12:1121–7. doi: 10.2174/138161206776055868. [DOI] [PubMed] [Google Scholar]
- 44.Daniels D, Grytdal S, Wasley A. Surveillance for acute viral hepatitisd United States, 2007. MMWR Surveill Summ. 2009;58:1–27. [PubMed] [Google Scholar]
- 45.Hassan M, Hasan S, Giday S, et al. Hepatitis C virus in sickle cell disease. J Natl Med Assoc. 2003;95:939–42. [PMC free article] [PubMed] [Google Scholar]
- 46.Ocak S, Kaya H, Cetin M, et al. Seroprevalence of hepatitis B and hepatitis C in patients with thalassemia and sickle cell anemia in a long-term follow-up. Arch Med Res. 2006;37:895–8. doi: 10.1016/j.arcmed.2006.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.