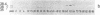Abstract
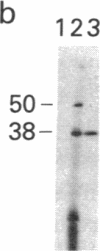
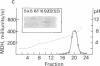
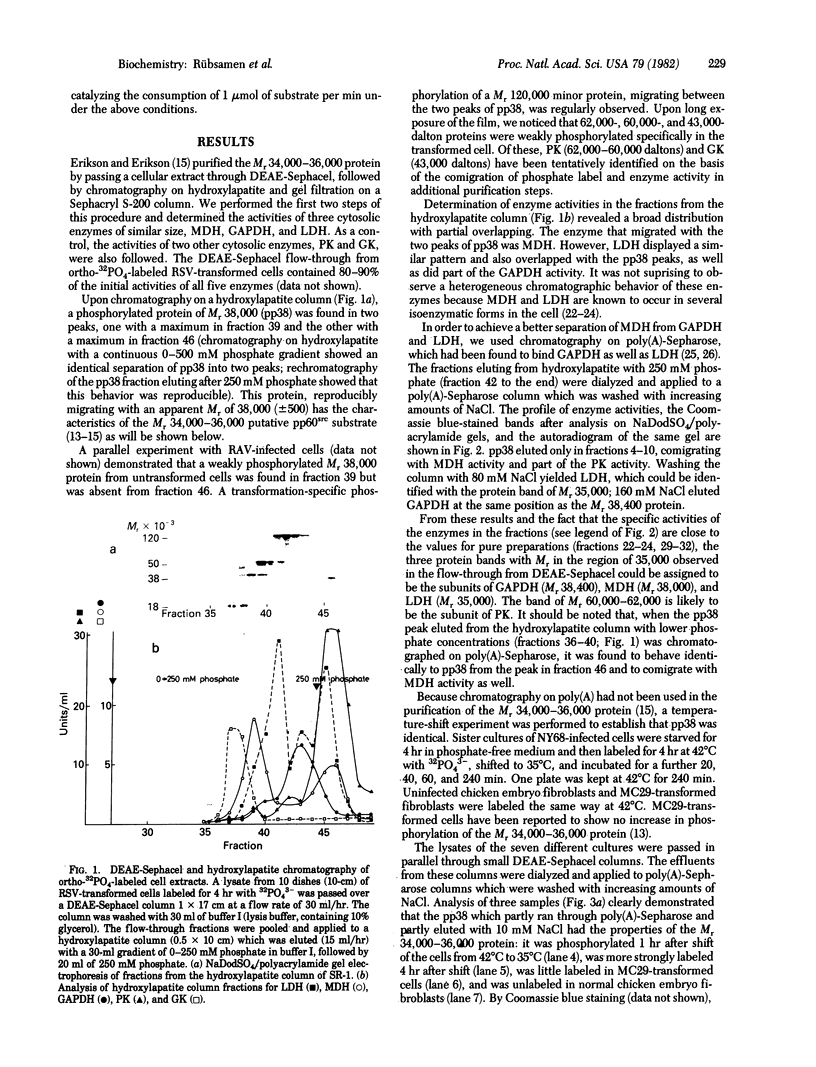
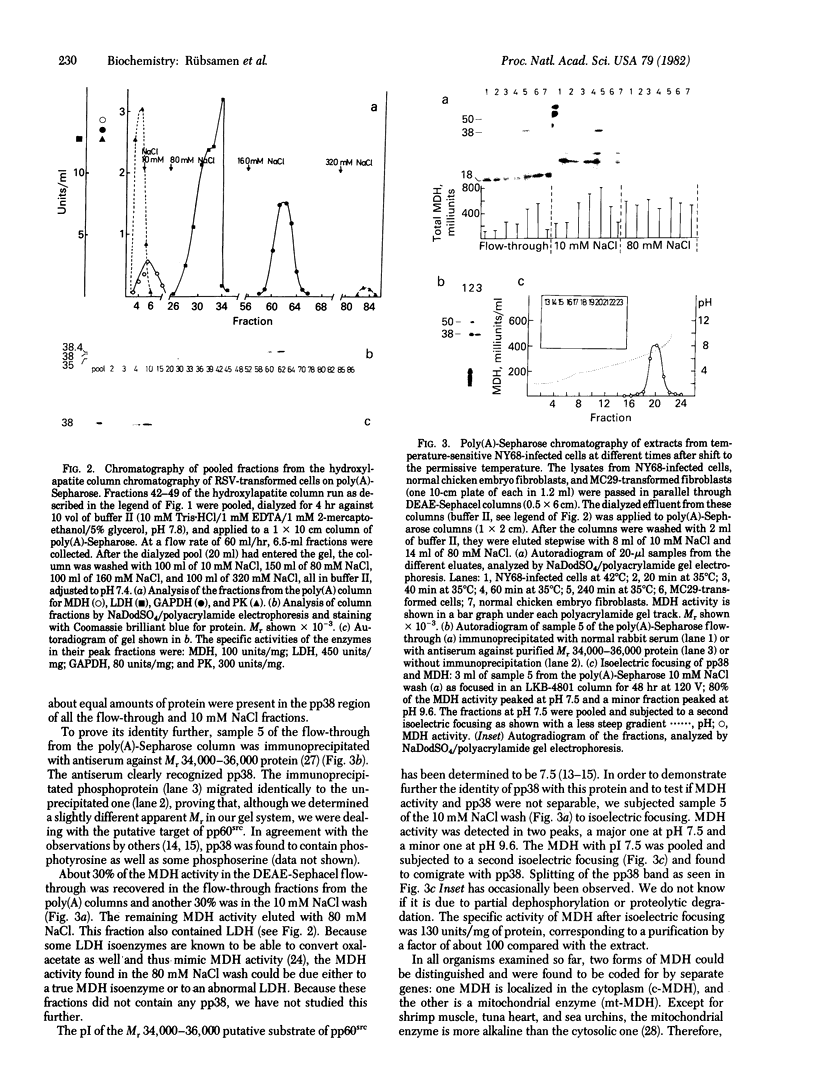
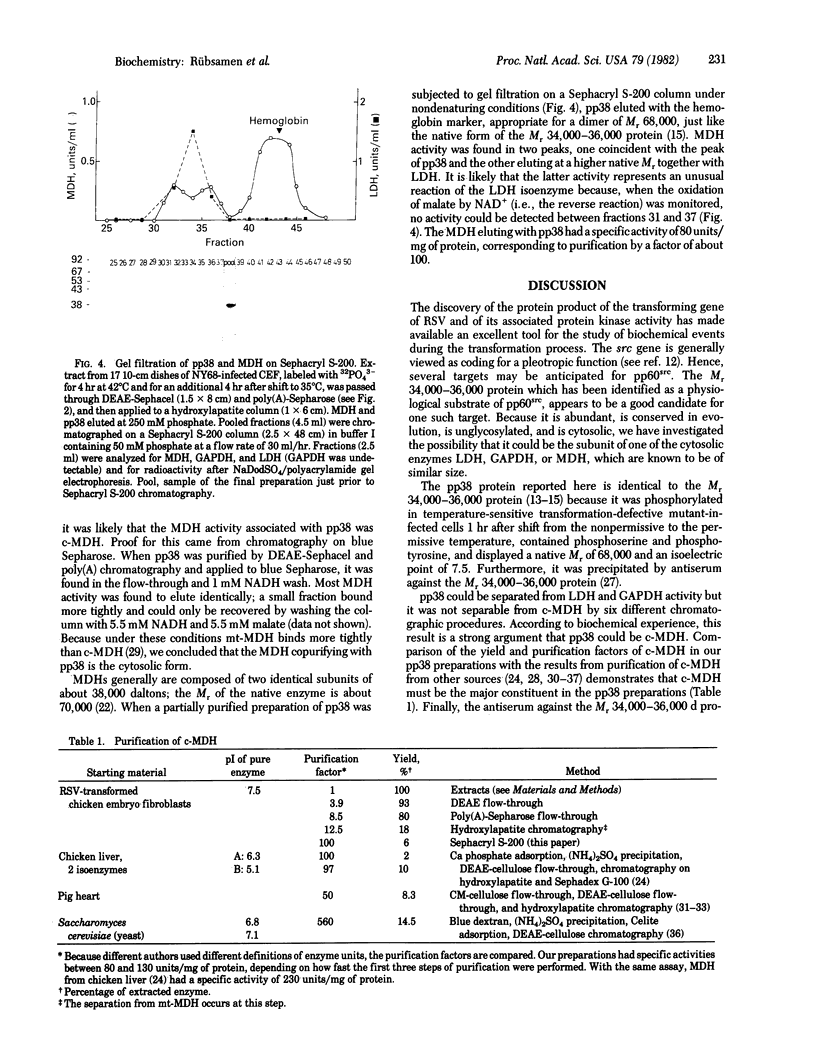
A cellular protein of apparent Mr 34,000--36,000 was suggested as a possible physiological substrate for the protein kinase (EC 2.7.1.37) activity associated with the transforming gene product of Rous sarcoma virus. We find this protein to migrate with an apparent Mr of 38,000 in NaDodSO4/polyacrylamide gels. It was not separable from cytosolic malic dehydrogenase activity when purified by chromatography on DEAE-Sephacel, hydroxylapatite, poly(A)-Sepharose, and blue Sepharose, by gel filtration, and by isoelectric focusing. The Mr 38,000 protein as well as cytosolic malic dehydrogenase activity focused with a pI of 7.5. In gel filtration experiments, both displayed an apparent native Mr of 68,000. The male dehydrogenase activity contained in homogeneous preparations of the Mr 38,000 protein had a specific activity of up to 130 units/mg of protein. The recovery of the enzyme was 5--10% of the activity in the extract. Antiserum against the Mr 38,000 protein inactivated the malic dehydrogenase activity associated with the Mr 38,000 protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Brugge J., Erikson E., Collett M. S., Erikson R. I. Peptide analysis of the transformation-specific antigen from avian sarcoma virus-transformed cells. J Virol. 1978 Jun;26(3):773–782. doi: 10.1128/jvi.26.3.773-782.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk D., Woods M., Hunter J. On the significance of glucolysis for cancer growth, with special reference to Morris rat hepatomas. J Natl Cancer Inst. 1967 Jun;38(6):839–863. [PubMed] [Google Scholar]
- Busquets M., Baró J., Cortés A., Bozal J. Separation and properties of the two forms of chicken liver (Gallus domesticus) cytoplasmic malate dehydrogenase. Int J Biochem. 1979;10(10):823–835. doi: 10.1016/0020-711x(79)90056-9. [DOI] [PubMed] [Google Scholar]
- Cassman M., Vetterlein D. Allosteric and nonallosteric interactions with reduced nicotinamide adenine dinucleotide in two forms of cytoplasmic malic dehydrogenase. Biochemistry. 1974 Feb 12;13(4):684–689. doi: 10.1021/bi00701a008. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Cook R., Miller G. J., Erikson R. L. The same normal cell protein is phosphorylated after transformation by avian sarcoma viruses with unrelated transforming genes. Mol Cell Biol. 1981 Jan;1(1):43–50. doi: 10.1128/mcb.1.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Erikson R. L., Collett M. S., Erikson E., Purchio A. F. Evidence that the avian sarcoma virus transforming gene product is a cyclic AMP-independent protein kinase. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6260–6264. doi: 10.1073/pnas.76.12.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodge D. W., Rubin H. Stimulation of lactic acid production in chick embryo fibroblasts by serum and high pH in the absence of external glucose. J Cell Physiol. 1975 Dec;86(3 Pt 1):453–457. doi: 10.1002/jcp.1040860303. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Temperature-sensitive mutants of avian RNA tumor viruses: a review. Curr Top Microbiol Immunol. 1978;79:261–293. doi: 10.1007/978-3-642-66853-1_6. [DOI] [PubMed] [Google Scholar]
- Gerding R. K., Wolfe R. G. Malic dehydrogenase. 8. Large scale purification and properties of supernatant pig heart enzyme. J Biol Chem. 1969 Mar 10;244(5):1164–1171. [PubMed] [Google Scholar]
- Goldberg E. Amino acid composition and properties of crystalline lactate dehydrogenase X from mouse testes. J Biol Chem. 1972 Apr 10;247(7):2044–2048. [PubMed] [Google Scholar]
- Guha A., Englard S., Listowsky I. Beef heart malic dehydrogenases. VII. Reactivity of sulfhydryl groups and conformation of the supernatant enzyme. J Biol Chem. 1968 Feb 10;243(3):609–615. [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägele E., Neeff J., Mecke D. The malate dehydrogenase isoenzymes of Saccharomyces cerevisiae. Purification, characterisation and studies on their regulation. Eur J Biochem. 1978 Feb 1;83(1):67–76. doi: 10.1111/j.1432-1033.1978.tb12069.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. E., Rupley J. A. Binding of reduced and oxidized nicotinamide adenine dinucleotide to pig heart supernatant malate dehydrogenase. Biochemistry. 1979 Aug 7;18(16):3611–3616. doi: 10.1021/bi00583a027. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. The Pasteur effect and the relations between respiration and fermentation. Essays Biochem. 1972;8:1–34. [PubMed] [Google Scholar]
- Kuan K. N., Jones G. L., Vestling C. S. Rapid preparation of mitochondrial malate dehydrogenase from rat liver and heart. Biochemistry. 1979 Oct 2;18(20):4366–4373. doi: 10.1021/bi00587a016. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L., McKeehan K. A. Oxocarboxylic acids, pyridine nucleotide-linked oxidoreductases and serum factors in regulation of cell proliferation. J Cell Physiol. 1979 Oct;101(1):9–16. doi: 10.1002/jcp.1041010103. [DOI] [PubMed] [Google Scholar]
- Milhaud P., Blanchard J. M., Jeanteur P. Hela cytosolic protein isolated by poly (A)-sepharose chromatography is glyceraldehyde-3-P-dehydrogenase. Biochimie. 1978;60(11-12):1343–1346. doi: 10.1016/s0300-9084(79)80454-x. [DOI] [PubMed] [Google Scholar]
- Narise S. Biochemical differences between cytoplasmic malate dehydrogenase allozymes of Drosophila virilis. Biochem Genet. 1979 Jun;17(5-6):433–444. doi: 10.1007/BF00498881. [DOI] [PubMed] [Google Scholar]
- Owada M., Donner P., Dittmar K. E., Moelling K. Comparison of protein kinase activities in normal cells and cells transformed by a temperature-sensitive mutant of avian sarcoma virus to those of cell-free viral translational products. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):959–965. doi: 10.1101/sqb.1980.044.01.103. [DOI] [PubMed] [Google Scholar]
- Pedersen P. L. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- Perucho M., Salas J., Salas M. L. Study of the interaction of glyceraldehyde-3-phosphate dehydrogenase with DNA. Biochim Biophys Acta. 1980 Feb 29;606(2):181–195. doi: 10.1016/0005-2787(80)90028-3. [DOI] [PubMed] [Google Scholar]
- Presek P., Glossmann H., Eigenbrodt E., Schoner W., Rübsamen H., Friis R. R., Bauer H. Similarities between a phosphoprotein (pp60src)-associated protein kinase of Rous sarcoma virus and a cyclic adenosine 3':5'-monophosphate-independent protein kinase that phosphorylates pyruvate kinase type M2. Cancer Res. 1980 May;40(5):1733–1741. [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Erikson R. L. Translation of 35S and of subgenomic regions of avian sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4661–4665. doi: 10.1073/pnas.74.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke K., Gilmore T., Martin G. S. Transformation by Rous sarcoma virus: a cellular substrate for transformation-specific protein phosphorylation contains phosphotyrosine. Cell. 1980 Oct;21(3):821–828. doi: 10.1016/0092-8674(80)90445-6. [DOI] [PubMed] [Google Scholar]
- Radke K., Martin G. S. Transformation by Rous sarcoma virus: effects of src gene expression on the synthesis and phosphorylation of cellular polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5212–5216. doi: 10.1073/pnas.76.10.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzer L. J., Wice B. M., Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979 Apr 25;254(8):2669–2676. [PubMed] [Google Scholar]
- Reitzer L. J., Wice B. M., Kennell D. The pentose cycle. Control and essential function in HeLa cell nucleic acid synthesis. J Biol Chem. 1980 Jun 25;255(12):5616–5626. [PubMed] [Google Scholar]
- Rotmans J. P. Schistosoma mansoni: purification and characterization of malate dehydrogenases. Exp Parasitol. 1978 Nov;46(1):31–48. doi: 10.1016/0014-4894(78)90154-6. [DOI] [PubMed] [Google Scholar]
- Rübsamen H., Friis R. R., Bauer H. Src Gene product from different strains of avian sarcoma virus: Kinetics and possible mechanism of heat inactivation of protein kinase activity from cells infected by transformation-defective, temperature-sensitive mutant and wild-type virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):967–971. doi: 10.1073/pnas.76.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. P., Passonneau J. V., Johnson G. S., Pastan I. The effect of growth conditions on NAD+ and NADH concentrations and the NAD+:NADH ratio in normal and transformed fibroblasts. J Biol Chem. 1974 Jul 10;249(13):4138–4143. [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Snyder T. P., Chambers G. K., Ayala F. J. Isolation of the cytoplasmic form of malate dehydrogenase from honey bee (Apis mellifera) larvae. Biochem Biophys Res Commun. 1979 May 28;88(2):668–675. doi: 10.1016/0006-291x(79)92100-4. [DOI] [PubMed] [Google Scholar]