Summary
The degree of T cell self-reactivity considered dangerous by the immune system, thereby requiring thymic selection processes to prevent autoimmunity, is unknown. Here, we analyzed a panel of T cell receptors (TCRs) with a broad range of reactivity to ovalbumin (OVA323-339) in the rat insulin promoter (RIP)-mOVA self-antigen model for their ability to trigger thymic self-tolerance mechanisms. Thymic regulatory T (Treg) cell generation in vivo was directly correlated with in vitro TCR reactivity to OVA-peptide in a broad ~1,000-fold range. Interestingly, higher TCR affinity was associated with a larger Treg cell developmental “niche” size, even though the amount of antigen should remain constant. The TCR-reactivity threshold to elicit thymic negative selection and peripheral T cell responses was ~100 fold higher than that of Treg cell differentiation. Thus, these data suggest that the broad range of self-reactivity that elicits thymic Treg cell generation is tuned to secure peripheral tolerance to self.
Introduction
The adaptive immune system generates a diverse array of antigen receptors to allow recognition of a variety of pathogens. However, a consequence of this diversity is that some receptors will recognize self with the potential to cause autoimmunity. For T cells, the issue of self-reactivity is mitigated as development occurs in a specialized organ, the thymus, where the immature T cell population is educated to the self-antigenic repertoire prior to their release into the periphery as mature T cells with the ability to cause autoimmunity. One important mechanism of education is the deletion of selfreactive T cells, also known as negative selection (McCaughtry and Hogquist, 2008; Palmer and Naeher, 2009). However, not all self-reactive thymocytes are eliminated, and some escape the thymus as effector cells with the potential to cause autoimmunity (Bouneaud et al., 2000; Zehn and Bevan, 2006). A second mechanism of education to self-antigens is now recognized to be the differentiation of self-reactive thymocytes to become regulatory T (Treg) cells that suppress, rather than induce, inflammatory responses.
T cell receptor (TCR) specificity was initially thought to be important for thymic Treg cell development as it was observed that TCR transgenic mice on a Rag-deficient background do not have Treg cells (Apostolou et al., 2002; Itoh et al., 1999), implying that only certain TCRs can facilitate Treg cell differentiation. Subsequent studies of TCR and antigen double transgenic mice suggested that recognition of self-antigen is the pertinent requirement for thymic Treg cell induction (Apostolou et al., 2002; Jordan et al., 2001). Other reports using fetal thymic organ cultures (FTOC) and in vivo peptide injection support this model (Atibalentja et al., 2009; Feuerer et al., 2007). Taken together with other studies(Bautista et al., 2009; Hsieh et al., 2004; Leung et al., 2009), the preponderance of the data suggests that self-recognition is an important requirement for thymic Treg cell selection.
While these studies provide proof of principle that self-reactivity is required to trigger thymic education via Treg cell differentiation and negative selection, a number of questions remain. First, many studies modeled interactions with ubiquitous antigens via transgenic expression or peptide administration in vivo or in vitro (Atibalentja et al., 2009; Feuerer et al., 2007). However, recent data suggests that thymic Treg cell development utilizes a limited antigenic niche (Bautista et al., 2009; Leung et al., 2009), implying that ubiquitous antigen presentation may not be an appropriate model. Thus, the threshold of self-reactivity that elicits thymic education mechanisms for CD4+ T cells to tissue-specific antigens is unknown.
Second, the study of TCR transgenic cells at high clonal frequencies may not represent what happens during normal thymic development, which occurs at very low clonal frequencies. For example, high clonal frequencies have recently been shown to markedly decrease the efficiency of thymic Treg cell development, presumably due to intraclonal competition (Bautista et al., 2009; Leung et al., 2009). Thus, it may be possible that Treg cell development or negative selection may be different at high versus low clonal frequencies.
Finally, while there have been studies of major histocompatibility complex (MHC) class I restricted T cells regarding thresholds of negative selection (Daniels et al., 2006; Zehn and Bevan, 2006), the range of TCR self-reactivities that elicit thymic tolerance mechanisms in CD4+ T cells in vivo has not been examined. In some studies, the threshold of self-reactivity required to trigger thymic Treg cell differentiation has been suggested to be quite high (Hinterberger et al., 2010; Jordan et al., 2001), being at or near the threshold of self-reactivity that induces negative selection. Moreover, thymic Treg cell differentiation may be dependent on a certain threshold of TCR affinity for antigen, as Treg cell development is not observed with low affinity interactions, even if antigen expression is high enough to induce negative selection (Cozzo Picca et al., 2011). On the other hand, it was reported that there is great overlap between the Treg and non-Treg TCR repertoires (Pacholczyk et al., 2007), suggesting that a broad range of self-reactivity, perhaps including TCRs that recognize self at the level of positive selection, is sufficient for Treg cell development. Also, direct demonstration of natural Treg cell TCR recognition of self-antigens presented on splenic antigen presenting cells (APCs) has not been successful (Pacholczyk et al., 2007), suggesting that the antigen is rare or that the TCR affinity for antigen is low. Determining the self-reactivity thresholds for thymic Treg cell induction and negative selection would be important for understanding how the immune system perceives the problem of self-recognition and utilizes thymic education to control it. Thus, the quantitative threshold of self-reactivity that triggers thymic education mechanisms, and how that relates to the threshold of self-reactivity required for peripheral immune responses, is not known.
To address this question, we decided to fix the amount of self-antigen and vary the efficiency of TCR recognition. Since natural Treg cell ligands are currently unknown, we utilized as a well-characterized model of a tissue-specific antigen, the rat insulin promoter-membrane ovalbumin (RIP-mOVA) transgenic line, in which the rat insulin promoter drives the expression of membrane bound ovalbumin (OVA) (Kurts et al., 1996). The developmental effects of thymic encounter with a relatively rare “self-antigen” can then be assessed at low clonal frequencies of T cells expressing a panel of naturally occurring TCRs with varying reactivity to OVA323-339 identified from DO11β TCR transgenic mice. Remarkably, we observed a direct correlation between the degree of in vitro antigen-reactivity and in vivo thymic Treg cell generation over a broad ~1,000-fold range. Negative selection was apparent with the more self-reactive TCRs. Finally, peripheral responses as measured by proliferation of naive T cells after transfer into irradiated RIP-mOVA mice required a degree of OVA-reactivity that induces both thymic negative selection and Treg cell development. Thus, these data demonstrate that the extent of self-reactivity plays a crucial instructive role in thymic Treg cell differentiation, which is tuned to be substantially below the threshold for peripheral immune responses to self-antigen.
Results
Identification of naturally occurring OVA323-339 peptide-specific TCRs
The degree of self-reactivity that triggers thymic Treg cell differentiation of CD4+ T cells is unknown, with studies favoring high (Cozzo Picca et al., 2011) versus low (Pacholczyk et al., 2007) thresholds of self-reactivity. Different thresholds for thymic Treg cell development may be predicted to generate different models regarding how self-reactive Treg and effector cells participate in immune responses to self- and foreign antigens in the periphery (Figure S1A). To define this threshold, we identified a panel of naturally rearranged TCRs with a broad range of reactivity to OVA peptide. Our strategy for obtaining these TCRs is diagrammed in Figure S1B. Briefly, we utilized a TCRβ transgenic line from an OVA-specific TCR, DO11 (Ise et al., 2010) (Figure S1C), to increase the precursor frequency based on observations from the MHC class I restricted OT-Iβ transgenic line (Dillon et al., 1994), and to provide a similar physical framework for recognition of OVA peptide:MHC molecules using different TCRα chains. To enrich for OVA-specific TCRs, we expanded CD4+ T cells in vitro with OVA peptide prior to sequencing the TRAV14 (Vα2) repertoire (Figure S1D). We obtained 3,785 sequences from the various proliferated T cell subsets (Figure S1E). Consistent with the proliferation observed in the OVA unstimulated wells (Figure S1D), there was substantial overlap between the OVA stimulated and un-stimulated TCR repertoires (Table S1). In particular, the 9 most frequent Foxp3− TCRs on T cells from RIP-mOVA+ mice were found at comparable frequencies irrespective of OVA-peptide stimulation, suggesting that the precursor frequency of OVA-specific cells is much lower in these mice due to negative selection. To confirm OVA-peptide specificity, we selected the most frequent TCRs found in the OVA but not control cultures (highlighted, Table S1) and expressed them on DO11β T cell hybridoma lines that contain green fluorescent protein (GFP) driven by nuclear factor of activated T cells (NFAT) as a reporter for TCR stimulation (Ise et al., 2010). However, only a subset of these TCRs showed reactivity to OVA peptide (Table S1, Figure S1F), and appeared to have either higher affinity for OVA (R1, R2, R4), or exist at higher precursor frequency (P1, Figure S1G).
In these screening experiments, we noted that the only TCRs which exhibited OVA-reactivity, regardless of T cell subset or RIP-mOVA status, utilized TRAJ21 (Jα21), which happens to be the same J-region utilized by the TRAV5D (Vα13) DO11α chain. This suggests that Jα21 provides structural features that facilitate recognition of OVA-peptide in the context of DO11β. We therefore tested all naturally rearranged Jα21 containing TCRs in our data set (Figure S2A–C), from which we identified 4 more TCRs with either lower affinity to OVA or are present at lower precursor frequency. Interestingly, only TCRs with a CDR3 length of 10 showed reactivity to OVA. In summary, we identified a panel of 8 naturally arising OVA-reactive TCRs that all use Jα21 and the same CDR3 length (Figure S2D), suggesting that these TCRs recognize in a very similar manner OVA323-339 bound to I-Ad in a single register.
The panel of TCRs exhibit a broad range of reactivity to OVA
To calculate the efficiency of OVA recognition, we determined the effective concentration of OVA peptide that elicited half-maximal responses (EC50) for each TCR using non-linear regression analysis (Figure 1A). Since the EC50 is dependent on the functional characteristics of the assay, we decided to use DO11αβ as a reference TCR for an agonist level response to foreign antigen. Normalizing TCR responses to that observed for DO11 also provided an inter-experiment control. Thus, the relative efficiency by which a TCR recognizes OVA peptide in comparison with DO11 is denoted as ΔLog(EC50) = Log(EC50 DO11) − Log(EC50 TCR).
Figure 1. In vitro and in vivo analysis of OVA-reactive TCRs.
(A) In vitro assessment of TCR sensitivity to OVA peptide. NFAT-GFP reporter hybridomas expressing each of 9 TCRs, including DO11 (red) were tested against OVA peptide presented by Flt3L induced dendritic cells. Data shown are the frequency of NFAT-GFP+ cells normalized to the maximum value seen with DO11 in that experiment. Data are representative of three independent experiments. (B,C) In vivo assessment of thymic Treg cell development in WT mice. Double-negative (DN) thymocytes from Foxp3gfpRag1−/− mice were retrovirally transduced with each TCR and transferred into the thymuses of WT mice, and analyzed for Treg cell generation 14-days post transfer. Representative flow cytometry plots are shown in (B) and summarized in (C) of 3–6 mice for each TCR from at least 2 independent experiments. Each dot represents data from an individual recipient.
Using the NFAT-GFP reporter hybridoma cells described above, we observed that the TCRs spanned over 3 logs in relative EC50 to the reference TCR DO11, with TCRs N7 and N9 within 1 log of DO11, R1 and R4 within the 2nd log, and R2, N12, N13 and P1 with even lower reactivity to OVA (Figure 1A, S2D). Note that changes in one or two amino acids greatly affected the recognition of OVA, such that DO11.10 exhibits greater than a thousand fold-higher sensitivity to peptide antigen than P1, although there may also be effects in the CDR1 or CDR2 regions for R2 and N9 (Figure S2D). Thus, we have identified a panel of 8 TCRs that recognize OVA with a broad range of efficiencies.
Thymic Treg cell selection of TCRs with foreign antigen-reactivity
We first wanted to establish that our panel of OVA-reactive TCRs did not facilitate thymic Treg cell development in the absence of OVA, which would preclude straightforward interpretation of OVA-dependent Treg cell generation. We utilized retroviral transduction of CD4−CD8− double negative (DN) thymocytes followed by intrathymic transfer into congenically marked wild-type (WT) hosts (Haxhinasto et al., 2008; Lathrop et al., 2011) to assess whether these TCRs show OVA-independent development of Foxp3+ cells 2 weeks after transfer. We did not observe Treg cell development in T cells expressing the DO11 TCR, as well as five OVA-reactive TCRs (Figure 1B,C). Interestingly, there were three TCRs which facilitated thymic Treg cell development in the absence of OVA, suggesting that these TCRs may recognize unknown self-antigens for Treg cell selection. This is further supported by the observation of R1, R2, and N9, in the Treg cell TCR repertoires from wild-type mice (Figure S1G). It appears that both TRAV14 subtype and CDR3 sequence affected this presumed self-reactivity, as R2 and N9 use the same TRAV14 subtype but different CDR3 sequence, whereas R1 and R2 utilize the same CDR3 sequence, but have different TRAV regions. Thus, these data demonstrate that it is possible to generate Treg cells in the thymus that also recognize foreign antigens, presumably due to the promiscuity of TCR recognition.
A direct role of self-reactivity for thymic Treg cell development
To avoid the complication of OVA-independent Treg cell generation, we decided to focus on the 6 TCRs, including DO11, which did not facilitate thymic Treg cell development in wild-type mice. The differences between the 5 TCRs are only in the CDR3 amino acid sequence, as all of these TCRs use the same TRAV14-3*01 subtype. Because measurement of OVA reactivity so far was based on a single readout using the NFAT-GFP reporter, we sought to expand the analysis of functional responses to TCR stimulation. We utilized another marker, NF-κB-GFP, which was introduced into the same parent hybridoma line used by NFAT-GFP (Ise et al., 2010). Since this parent hybridoma line produced little interleukin-2 (IL-2), we tested IL-2 production after re-expressing these TCRs in a different TCR-deficient hybridoma line, 5KC-73.8.20 (Scott-Browne et al., 2009). Although the range of measured EC50s varied from ~100–6,000 fold depending on the assay, the ordering of TCRs in terms of efficiency of OVA recognition remained mostly constant (Figure 2A, Table 1).
Figure 2. Defining efficiency of OVA recognition using hybridoma and primary T cells.
(A) Hybridoma assays. Hybridoma cells were assessed for NF-κB-GFP expression (left) and IL-2 production (right) in response to OVA peptide presented by TA3 APCs. (B) Assays of TCR reactivity using primary cells. T cells were obtained from retroviral bone marrow chimeras using WT hosts. CD4SP thymocytes were assessed for CD25 upregulation (left), and peripheral CD4+ T cells were tested for in vitro proliferation (right), in response to OVA-peptide presented by Flt3L DCs. Calculated ΔEC50 is summarized in Table 1. Graphs shown are representative of three independent experiments. (C) Binding of OVA:I-Ad tetramer to hybridoma cells expressing OVA-reactive TCRs. Representative plots are shown on the left, and summarized on the right (mean ± s.d., n=3 independent experiments). As the lines were generated via retroviral transduction of TCRs, the cells shown are gated on TCR+ cells. MFI was calculated from all TCR+ cells. 3K:I-Ab tetramer was used as a control.
Table 1.
Summary of assessments of TCR reactivity
| Clone | CDR3 a.a Sequence | Hybridoma† | T cells† | Tetramer binding‡ | Thymic selection | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| NFAT activation | NFκB activation | IL-2 production | CD25 on CD4SP | CD4 T cell proliferation | ||||
| DO |
|
Neg.+Treg | ||||||
|
| ||||||||
| N7 |
|
−0.2±0.3 | −0.2±0.1 | −0.1±0.2 | −0.8±0.6 | −0.7±0.3 | −0.30±0.03 | Neg.+Treg |
| R4 |
|
−1.2±0.6 | −0.4±0.1 | −0.6±0.1 | −1.5±0.6 | −1.2±0.4 | −0.84±0.04 | Treg |
| N12 |
|
−2.6±0.3 | −1.2±0.5 | −1.5±0.2 | −2.3±0.6 | −2.1±0.5 | ≤−1.30±0.05 | Treg |
| N13 |
|
−3.5±0.1 | −1.8±0.5 | −2.3±0.3 | −3.0±0.8 | −3.6±0.2 | ≤−1.35±0.04 | Treg |
| P1 |
|
−3.8±0.3 | −2.1±0.8 | −2.7±0.3 | −2.2±0.5 | −3.1±0.4 | ≤−1.34±0.05 | Tconv |
The relative sensitivity of OVA recognition is shown as the difference of Log (EC50) from that of DO11.
The relative affinities of TCRs are shown as the difference of Log (MFI) from that of DO11.
Data shown are mean ± s.e.m. (n=3).
Thymic selection is based on in vivo assays presented throughout the paper. Neg., negative selection. Treg, Treg cell selection.
Since TCR signaling may differ between hybridoma cells and primary T cells, we also assessed peptide reactivity using thymocytes and peripheral T cells. T cells expressing individual OVA peptide-reactive TCRs were generated using retroviral bone marrow chimeras using Rag1−/− donors. The EC50 for each TCR was determined in vitro by assessing CD25 upregulation on CD4+CD8− (CD4 single positive, CD4SP) thymocytes and proliferation of peripheral CD4+ T cells (Figure 2B). Taken together, the data from 5 different hybridoma and primary T cell assays are consistent with the notion that these 6 TCRs exhibit a broad range of sensitivity for OVA recognition (Table 1).
To estimate the TCR affinity for peptide:MHC, we measured the mean fluorescence intensity (MFI) of equilibrium tetramer binding, which provides a relative assessment of monomeric TCR:pMHC affinity (Crawford et al., 1998; Huseby et al., 2006; Savage et al., 1999). Consistent with the functional assessment of OVA-reactivity, tetramer binding classified N7 and R4 as higher affinity TCRs (Figure 2C) in comparison with N12, N13, and P1, which showed little to no binding. Although the latter 3 TCRs can elicit in vitro responses to OVA-peptide, these TCRs are below the affinity range that can be quantified using this tetramer binding assay. Thus, these tetramer data suggest that this panel of TCRs recognize OVA peptide:MHC with a broad range of affinities.
We next assessed the ability of thymic OVA expression via RIP-mOVA to induce thymic Treg cell development using retroviral transduction of TCRs into Rag1−/− thymocytes followed by intrathymic transfer as described above. As expected, DO11 facilitated thymic Treg cell development in the presence of RIP-mOVA (Walker et al., 2003). Interestingly, all of the other TCRs except for P1, the lowest-affinity TCR, induced Treg cells to a degree that varied with the efficiency of OVA recognition, both at the level of Foxp3+ cell frequency and number (Figure 3A,B). Treg cell generation could even be detected in cells expressing the low affinity TCRs N12 and N13, which could not be reliably measured by equilibrium tetramer binding, and require ~1670 fold (average response in 5 assays between P1 and DO11 in Table 1) more antigen to achieve the same response in comparison to DO11. However, we were unable to observe Treg cells generated by P1, which was very close to N13 in its sensitivity to OVA. Although stochastic expression of RIP-mOVA in thymic mTEC cells may contribute to mouse to mouse variability (Derbinski et al., 2008), differences between TCRs were readily observed. Thus, these data show that TCRs which are on average 1% to 0.1% percent as sensitive as DO11 for OVA recognition in vitro can generate thymic Treg cells, demonstrating that Treg cell differentiation can occur within a broad range of self-reactivity.
Figure 3. Thymic Treg cell generation is instructed by the extent of TCR reactivity to self-antigen.
(A) Treg cell generation by OVA-reactive TCRs in the presence of RIP-mOVA. Retrovirally transduced Rag1−/− DN cells were transferred into the thymuses of RIP-mOVA mice, and analyzed at 2 weeks as in Figure 1B. (B) Data in (A) are summarized, with each dot representing the frequency (left) and absolute number (right) of Foxp3+ cells from an individual recipient. The plot in the middle shows the mean Foxp3+ percentage (± S.E.M.). The middle and right plots are correlated with the ΔLog(EC50) of the TCR for NFAT-GFP activation as compared with DO11. P1 was not plotted on the right because no Foxp3+ cells were observed. (C) Correlation of Treg cell generation with in vitro sensitivity to OVA. To determine whether TCR affinity is directly correlated with the efficiency of Treg cell selection in vivo, we plotted the absolute number of Treg cells versus the ΔLog(EC50) of the TCR as compared with DO11, and analyzed it by linear regression. For tetramer binding, we used the Log(MFI) as compared with DO11. Each symbol represents an individual TCR indicated in the legend. (D) The frequency of Foxp3−CD25hi CD4SP cells in the experiments described in (A) are shown (mean ± s.d., 5 independent experiments). (E) Correlation of Foxp3−CD25hi CD4SP cells with sensitivity of TCR to OVA peptide. Frequencies of Foxp3−CD25hi cells shown in (D) are plotted as per (C).
Linear regression analyses for all assays revealed that the ability of TCRs to facilitate Treg cell generation was directly proportional to their affinity for OVA peptide as provided by RIP-mOVA (Figure 3C, S3). The efficiency of self-recognition was also directly correlated with the generation of Foxp3− CD25+ cells (Figure 3D,E), consistent with the proposal that the process of thymic Treg cell generation occurs via TCR-dependent generation of Foxp3− Treg precursor cells (Lio and Hsieh, 2008). Thus, these data demonstrate that the degree of self-recognition is the primary determinant for Treg cell differentiation in the thymus.
Treg cell selection “niche” is dependent on TCR affinity for self-antigen
Thymic Treg cell selection utilizes a small “niche” based on the observation of intraclonal competition in studies of transgenic mice using naturally arising Treg cell TCRs (Bautista et al., 2009; Leung et al., 2009). Since the natural ligands for these Treg cell TCRs are unknown, the factors that affect this developmental niche are unclear. We therefore asked whether affinity to self-antigen affects the size of the Treg cell developmental niche. As the intrathymic transfer of retrovirally transduced thymocytes does not assess Treg cell generation at steady state, we decided to analyze chimeras with retrovirally-transduced bone marrow. Because generating chimeras at varying clonal frequencies is more difficult than the intrathymic transfer experiment described above, we examined the two highest affinity TCRs along with DO11. Various ratios of congenically marked wild-type bone marrow were also added to achieve varying clonal frequencies. We confirmed that these OVA-reactive TCRs did not facilitate Treg cell generation in wild-type mice, even at low clonal frequencies (Figure 4A. S4). Consistent with previous reports (Bautista et al., 2009; Leung et al., 2009), we observed that Treg cell development with all 3 TCRs was inversely correlated with clonal frequency in the presence of OVA peptide presentation (Figure 4A). Moreover, the efficiency of Treg cell generation was directly correlated with affinity, as evidenced by the ability of higher affinity TCRs to generate a higher frequency of Treg cells at a given clonal frequency (Figure 4B). The affinity of the TCR also correlated with the absolute number of Treg cells that are generated in the thymus, implying that higher affinity TCRs have a larger niche size for thymic Treg cell development (Figure 4C,D). Since the amount of antigen generated by the RIP-mOVA transgene should be equal between different bone marrow chimeras, we hypothesize that niche size in this case represents the number of APCs that present sufficient antigen to trigger Treg cell selection for a given TCR affinity.
Figure 4. A role for TCR affinity in the thymic Treg cell selection “niche”.
(A) Inverse relationship between clonal frequency and Treg cell development. Thymic Treg cell development was assessed in mixed bone marrow chimeras with varying ratios of WT to retrovirally transduced bone marrow. Data shown are the percentage of Foxp3+CD4SP cells versus the clonal frequency in the CD4SP subset for the indicated TCR. Each symbol represents data from an individual recipient from 3–5 independent experiments for each TCR. Data points in the dashed red boxes fall outside of the previously described inverse relationship. (B) Data from the experiment shown in (A) are plotted log-log to illustrate the similar slopes, with differences in the intercept. Note that the points in the red boxes are not shown in this plot. (C) Absolute number of Treg cells from the data shown in (B) are plotted versus clonal frequency. (D) Correlation of Treg cell selection niche size to TCR affinity. The number of Foxp3+ cells was analyzed by linear regression with OVA-reactivity measured by NFAT activation (upper) and tetramer binding (lower) as per Figure 3. Each symbol represents an individual TCR as indicated in the legend.
Threshold for negative selection
Some studies using TCR transgenic models have reported negative selection to be coincident with Treg cell generation (Jordan et al., 2001; Van Santen et al., 2004), whereas other studies have suggested that Treg cell selection may occur without obvious negative selection (Bautista et al., 2009). Thus, the threshold of self-reactivity required to elicit Treg cell differentiation versus negative selection remains poorly defined.
An interesting observation from our studies of clonal frequency and Treg cell development was that the frequency of Treg cells using the higher affinity TCRs, DO11 and N7, actually went down again at clonal frequencies below 0.1% (red box, Figure 4A). This was not apparent for the intermediate affinity TCR R4, nor for other TCRs tested in previous reports (Bautista et al., 2009), although it remains possible that this may be observed if lower clonal frequencies could be achieved experimentally. One potential explanation for this observation is that TCRs with high affinity to antigen can induce negative selection rather than Treg cell differentation at low clonal frequencies. In this case, the remaining Foxp3− CD4SP cells have presumably have not yet encountered antigen.
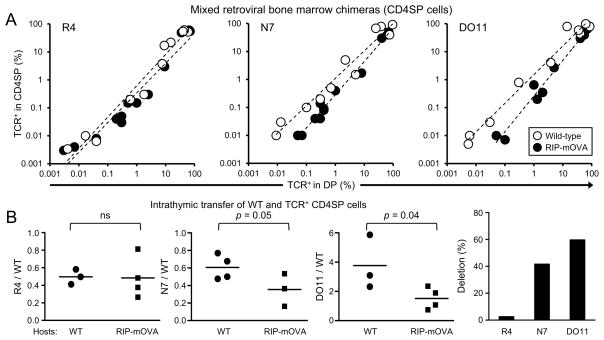
To test the hypothesis that negative selection to RIP-mOVA occurs at low clonal frequencies with the N7 and DO11, but not R4 TCRs, we asked whether the frequency of TCR-expressing cells in the CD4SP relative to the DP subset was affected by the presence of RIP-mOVA (Figure 5A). We observed a decrease in the CD4SP frequency in N7 and DO11, but not R4, consistent with negative selection. Interestingly, deletion appeared to be enhanced with decreasing clonal frequency, analogous to the demonstration of intraclonal competition during Treg cell development (Bautista et al., 2009; Leung et al., 2009). However, these data do not fully support the hypothesis that negative selection occurs only at very low clonal frequencies to prevent Treg cell selection (Figure 4A), as negative selection appears to occur at higher clonal frequenices (Figure 5A). One speculation is that the reduction in intraclonal competition with very low clonal frequency may increase the strength of TCR signaling such that self-antigen encounter will always result in negative selection (Hinterberger et al., 2010).
Figure 5. Treg cell development coincident with negative selection by high affinity TCRs.
(A) Analysis of mixed bone marrow chimeras. Data from Figure 4 are plotted to compare the frequency of TCR+ cells (Thy1.1+CD45.1+CD45.2−) amongst total DP (x-axis) and CD4SP (y-axis) cells in WT versus RIP-mOVA recipients. Each dot represents data from an individual recipient. (B) Negative selection of CD4SP thymocytes. TCR expressing CD4SP thymocytes and Cell-Trace Violet labeled WT cells were intrathymically injected into WT and RIP-mOVA mice. Flow cytometry was performed 3 days later. Negative selection was assessed by the ratio of OVA-specific CD4SP cells to WT cells added as an injection control. Each dot represents an individual recipient, with 2 independent experiments per TCR. Statistical differences were accessed by unpaired t test (left). The percent difference in the mean values from WT compared with RIP-mOVA hosts is shown for each TCR (right).
To confirm that negative selection occurred at the CD4SP stage, we assessed whether purified CD4SP thymocytes from chimeras of retrovirally-transduced bone marrow in wild-type hosts would be deleted after intrathymic transfer into RIP-mOVA+ recipients. The transferred cells were mostly immature based on high heat stable antigen (HSA, CD24) expression at time of transfer (Figure S5A,B). At day 3 post-transfer, we observed that the relative ratio of thymocytes expressing the higher affinity TCRs DO11 and N7, but not R4, to the co-injected polyclonal thymocytes were decreased in RIP-mOVA hosts, indicative of negative selection (Figure 5B; S4C). Consistent with previous reports (Cozzo Picca et al., 2011), these data suggest that the propensity to induce Treg cell differentiation does not always rescue the cells from negative selection. Taken together, these data suggest that that detectable negative selection occurs at an affinity above R4, which is ~7% as sensitive as DO11 at recognizing OVA (average of 5 assays, Table 1). As Treg selection is observed at affinities above P1 (~0.06% as sensitive as DO11), these data suggest that detectable negative selection requires a substantially higher affinity interaction with self than does Treg cell selection.
Differential affinity thresholds for thymic Treg cell development and peripheral responses to self
Our studies in the thymus suggest that a broad range of TCR affinity can elicit Treg cell development, albeit at varying degrees. As the affinity for self-antigen decreases, the percentage of Foxp3− conventional T cells emigrating from the thymus increased. Thus, understanding the relationship between the range of affinities that facilitate thymic Treg cell generation versus peripheral responses is important for clarifying how self-reactive Treg cells may mediate self-tolerance in the periphery.
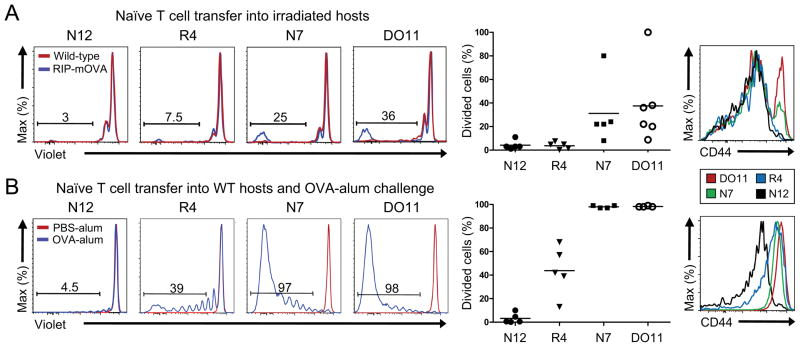
To address this question, we adoptively transferred peripheral Foxp3− CD4 T cells bearing OVA-reactive TCRs into sub-lethally irradiated RIP-mOVA recipients, as very little proliferative responses were observed in non-irradiated mice (Figure S6A,B). Intriguingly, the intermediate affinity TCR R4 or low affinity TCR N12 did not respond to OVA in vivo under these conditions (Figure 6A). By contrast, the higher affinity TCRs DO11 and N7 induced peripheral expansion of Foxp3− cells as well as a small amount of Treg cell differentiation. Since endogenous presentation of OVA from RIP-mOVA may represent a low amount of peripheral self-antigen expression, we tested whether abundant amounts of OVA protein in alum adjuvant would stimulate in vivo proliferation in TCR expressing cells transferred into WT hosts. We observed substantially higher proliferation overall, and were able to visualize proliferation of R4, but not N12, in this case (Figure 6B). In these assays, we observed relatively little peripheral conversion, presumably due to environmental effects of irradiation or immunization (Figure S6C,D). As N12 and N13 facilitated thymic Treg cell development (Figure 3), but not peripheral T cell responses even under abundant antigen, these data suggest a model where thymic Treg cell differentiation is tuned such that T cells with sufficient self-reactivity to elicit peripheral immune responses cannot wholly escape the thymus as effector cells (Figure S5E). Thus, these data suggest that T cells with sufficient reactivity to react with self-antigens in the periphery will likely be accompanied by Treg cells generated via thymic selection triggered by the same self-antigen, thereby preventing the development of autoimmunity.
Figure 6. High affinity TCR recognition of peripheral self-antigen is required to elicit peripheral naive T cell responses.
(A) Peripheral T cells responses to RIP-mOVA. Naive peripheral Foxp3−CD4+ T cells were intravenously transferred into sublethally irradiated RIP-mOVA recipients. Representative flow cytometry plots are shown of the transferred splenic T cells after 14 days to determine proliferation via dilution of Cell-Trace Violet dye (left). Frequencies of proliferated cells are summarized for each TCR (middle), and CD44 expression is shown (right). Each dot represents data from a single recipient, with 2 independent experiments per TCR. (B) Peripheral T cell responses to abundant antigen. Naive peripheral Foxp3−CD4+ T cells were intravenously transferred into normal WT recipients immunized with OVA protein-Alum, and proliferation of the transferred T cells in the spleen was analyzed after 7 days. Representative flow cytometric plots of proliferation are shown on the left and summarized in the middle graph. Each dot represents an individual recipient, with 2–3 independent experiments per TCR. Representative CD44 expression is shown on the right.
Discussion
Although previous studies provided proof of principle that self-reactivity is an important factor for thymic Treg cell differentiation and negative selection, there was little sense of how much self-reactivity is required at low clonal frequencies. Quantification of the relationship between self-reactivity and thymic Treg cell selection is important for understanding how thymic Treg cells may provide tolerance in the periphery. A high threshold of self-reactivity for Treg cell differentiation (Cozzo Picca et al., 2011) would predict that many low-affinity self-reactive T cells would escape the thymus as effector cells, relying primarily on the presence of high affinity self-reactive Treg cells to provide tolerance. By contrast, a low threshold of self-reactivity for Treg cell differentiation would broaden the Treg cell repertoire such that it may almost approximate that of the effector cell repertoire (Pacholczyk et al., 2007), increasing the likelihood that thymic Treg cells will participate in not only immune responses to self, but also non-self antigens. Thus, understanding the relationship between self-reactivity and thymic education mechanisms would be useful to understand the process of self:non-self discrimination.
We addressed this question by analyzing a panel of TCRs with different affinities for OVA peptide:MHC molecules for their in vivo response to the RIP-mOVA model antigen. While using a single antigen transgenic line has technical advantages as it should fix the amount and distribution of the antigen, another important consideration is that it models a tissue specific, rather than ubiquitous, antigen. Whereas T cell autoimmunity to ubiquitous antigens is not well described, tissue specific antigens are likely targets in a number of human autoimmune diseases such as Hashimoto’s thyroiditis and type 1 diabetes. Thus, understanding tolerance to a model tissue specific antigen may be clinically relevant.
Our data suggests that tolerance mechanisms to tissue specific antigens expressed in the thymus are broad and robust. We were surprised to observe that Treg cell development to this model tissue-specific self-antigen still occurs with TCRs which are ~0.1% as sensitive to OVA peptide in comparison with the reference TCR DO11, which represents an agonist interaction with foreign antigen. Although we did not perform a direct measurement of affinity, the steady state tetramer binding studies in conjunction with the broad range of functional assessments of TCR reactivity provide a clear estimate of relative affinity for this panel of TCRs compared with the DO11 TCR. It is interesting to contrast these results from analysis of negative selection of a CD8+ TCR, in which a narrow affinity range was observed (Palmer and Naeher, 2009). Whether this is explained by differences in the assays used, or whether this reflects a fundamental difference between T cells at different stages of development or MHC restriction, will need to be clarified by future studies. None-the-less, in contrast with CD8+ T cell negative selection, the range of affinity that the immune system considers of sufficient self-reactivity to warrant thymic Treg cell generation is extremely broad.
We also observed that the extent of self-reactivity is directly correlated with the efficiency of thymic Treg cell generation. In fact, thymic Treg cell development appeared indistinguishable from any other in vitro or in vivo TCR-dependent process. It is interesting to note that increasing TCR affinity has also be reported to facilitate peripheral Treg cell induction, although the optimum amount of antigen is inversely correlated with TCR affinity (Gottschalk et al., 2010). Presumably, this results from T cell expansion at higher amounts of antigen in the periphery, whereas negative selection would occur in the thymus. These data therefore support the notion that one of the primary purposes of TCR activation at this particular stage in development is Treg cell generation, which appears to wane during T cell maturation (Schallenberg et al., 2010; Wirnsberger et al., 2009). Thus, the TCR’s autoimmune potential dictates its ability to generate thymic Treg cells.
The relationship between TCR affinity and the efficiency of thymic Treg cell generation is also manifest in the apparent “niche” size, as represented by the number of Treg cells generated over a broad range of clonal frequencies. One interesting observation is that TCR affinity changes the intercept, but not slope, of the line representing the relationship between clonal frequency and Foxp3+ percentage. Such differences have been observed previously between TCRs with vastly different efficiencies for Treg cell generation (Bautista et al., 2009), and was hypothesized to represent the number of APCs expressing the Treg cell selecting ligand. However, the amount of antigen should remain constant in this case because only one antigen transgenic line is used. A recent study of thymic TCR activation using a Nur77-GFP reporter suggested that clonal frequency impacts the number of T cells receiving strong TCR signaling due to intraclonal competition (Moran et al., 2011). Taken together, one possible explanation is that the requirement for TCR affinity reflects the number of APCs that are capable of signaling T cells above the threshold for Treg cell generation.
Although the affinity of the TCR for self-antigen and the Treg cell niche size is directly correlated, this may only be true at higher clonal frequencies for TCRs with agonist level affinity for self-antigen. It appears that negative selection likely perturbs this linear relationship at lower clonal frequencies, as T cells encountering self-antigen are often deleted instead of becoming Treg cells. While it has been difficult to quantify the role of intraclonal competition for negative selection, this may be expected based on studies of Treg cell development (Bautista et al., 2009; Leung et al., 2009) and positive selection (Huesmann et al., 1991). A possible mechanism leading to increased deletion at low clonal frequencies is that minimal competition for antigen bearing APCs allows the antigen specific developing thymocyte to make a long conjugate and trigger apoptotic negative selection. A decrease in the TCR affinity or an increase in clonal frequency would be predicted to shorten the thymocyte:APC conjugation time resulting in decreased deletion and increased thymic Treg cell generation.
The threshold of observable negative selection appears to be substantially higher than that of Treg cell differentiation. However, it must be noted that the sensitivity of detecting Foxp3 induction as determined by the induction of Foxp3gfp is excellent, whereas measuring the loss of cells is comparatively insensitive. It may be possible that negative selection occurs at lower affinity, but cannot be measured. However, a small fraction of cells undergoing negative selection is unlikely to have any impact on immune tolerance, as essentially the same numbers of self-reactive T cells escape the thymus, whereas a small amount of Treg cell development may have a substantial impact due to the importance of Treg cells in dominant tolerance.
Thymic Treg cell generation may be considered a window into how the immune system perceives the need to combat self-reactivity during development prior to the export of cells into the periphery. In comparison with thymic responses, we found that the TCR affinities required for peripheral T cell responses were much higher than that required for thymic Treg cell selection. While these data will need to be validated for other model self-antigens, and if possible, true self-antigens if they can be genetically changed without affecting function, this suggests that the range of self-reactivity required for Treg cell selection is substantially below that required for peripheral T cell responses.
In summary, our data using a model tissue specific antigen suggest that the immune system utilizes straighforward rules for determining the thymic Treg cell population. Self-reactivity within a broad range, but above that for positive selection, selects for Treg cells in a graded manner depending on the extent of self-reactivity. Cognate, or agonist levels of self-reactivity also result in negative selection. In this manner, the immune system selects for a Treg cell population which is highly tuned towards high affinity self-antigen recognition while including some low affinity TCRs. Together with deletion of high affinity TCRs, these rules imprint the self-antigen repertoire on the Treg cell population. Interestingly, we found that incidental self-reactivity can also clearly generate Treg cells to foreign antigens, which is consistent with studies showing that a small fraction of cells binding to tetramers of foreign antigens are Treg cells (Moon et al., 2011). Thus, self:non-self discrimination likely revolves around the quantitative bias of the TCRs amongst the Treg and naive T cell population.
Our data clarify how self-reactivity in the CD4+ T cell subset is constrained by the immune system. Based on these data, we would predict that the bulk of the TCRs that cause autoimmune disease would be of higher affinity than R4, which is ~7% as sensitive as DO11 for OVA. These TCRs are low enough affinity to avoid marked negative selection and incompletely drive thymic Treg cell selection, permiting the escape of self-reactive naive T cells (Zehn and Bevan, 2006). Under normal circumstances, it is difficult to imagine that these escaped self-reactive T cells could overcome the suppression by the thymic Treg cell population concomitantly generated in response to a broad range of self-reactivity. It seems that pro-inflammatory conditions that obviate or block Treg cell suppression would be required to release these potential autoimmune effector cells. Such an inciting event is often not obvious in spontaneous human autoimmunity. One interesting future question is whether the B6 genotype studied here has an unusually low threshold for thymic Treg cell generation, as it is known to be highly resistant to spontaneous autoimmunity. Perhaps other autoimmune prone backgrounds will show a higher threshold of self-reactivity for thymic Treg cell generation. Alternatively, these robust thymic mechanisms may hint at the possibility that spontaneous autoimmunity may not result from a failure of central tolerance, but rather occurs to antigens that are poorly expressed in the thymus. For example, we found no indication in mixed bone marrow chimeras that the self-reactive TCR 2D2 induces thymic Treg cell generation even at low clonal frequencies (not shown), suggesting that the MOG-epitope is either not presented in the thymus or the affinity of the 2D2 TCR for MHC-MOG peptide complex is too low relative to the amount of thymic MOG-peptide. Future studies will be required to test the hypothesis that spontaneous autoimmunity is primarily a failure of peripheral tolerance.
Experimental Procedures
Mice
Foxp3gfp mice (Fontenot et al., 2005) were a gift from A. Rudensky (MSKCC). DO11.10 TCRβ transgenic mice (Ise et al., 2010) were provided by K. Murphy (Wash. U.). RIP-mOVA (Kurts et al., 1996), CD45.1, and B6.C mice were obtained from Jackson Labs. All mice were backcrossed to B6.C (B6 background congenic at the MHC locus with BALB/c) to facilitate breeding to mutant mice of B6 background. Mice were housed in a specific pathogen–free animal facility and were used according to protocols approved by the Institutional Animal Care and Use Committee at Wash. U.
TCR sequencing
Spleen and lymph node cells from DO11.10 TCRβ Foxp3gfp Tcra+/− RIP-mOVA− or + mice were labeled with Cell Trace DDAO (Invitrogen) and cultured with or without 1μM of OVA323-339 for 5 days. DDAO low-Vβ8+ CD4 T cells were purified from Foxp3− and Foxp3+ subsets with a FACSAria (Becton Dickenson). Sequencing and analysis of TRAV14 TCRα (Vα2) chains was performed as described (Hsieh et al., 2006).
Chimeras with retrovirally-transduced bone marrow
Retrovirally transduced Foxp3gfpRag1−/− B6.C bone marrow mixed with CD45.1 Foxp3gfp bone marrow at ratios of 1:0, 1:1 or 1:4 were injected (5×106 total cells) into lethally irradiated (1,000 rads) CD45.1 WT or RIP-mOVA hosts (Bautista et al., 2009). At 6 weeks, the mice were analyzed by flow cytometry. Non-mixed (1:0) chimeras in WT hosts were used as a source of T cells for in vitro assays and in vivo transfer experiments.
In vitro assays of TCR reactivity to OVA peptide
For the NFAT activation assay, T cell hybridomas expressing DO11 TCRβ, mCD4, and an NFAT-GFP reporter (Ise et al., 2010) provided by Ken Murphy (Wash. U.), were retrovirally transduced with TCRα chains. FACS sorted mCD4+Vβ8+Vα2+ cells were cultured with flt3 ligand-activated CD11c+ APCs and OVA-peptide. For the NF-κB activation assay, an NF-κB-GFP construct (Bredemeyer et al., 2008) provided by B. Sleckman (Wash. U.) was transduced into DO11 TCRβ-mCD4 hybridomas, then transduced with TCRα chains prior to stimulation with OVA-peptide and TA3 B cell hybridoma APCs, which decreased the background. GFP expression was measured by flow cytometry 40 hrs post stimulation.
To measure IL-2 production, 5KC T cell hybridomas expressing DO11β were transduced with TCRα chains and cultured with TA3 APCs and OVA-peptide. IL-2 in the supernatant was assessed using a CTLL-2 bioassay with Alamar blue (Arcus biologicals).
Bone marrow chimeras were used as a source of T cells. CD4SP thymocytes were enriched by depletion of CD8-expressing cells with biotinylated anti-CD8 antibody and anti-biotin microbeads (AutoMACS, Miltenyi Biotec), and cultured with irradiated splenic APCs pulsed with OVA323-339 to measure CD25 induction at 24 hours by flow cytometry. Spleen and lymph node cells were labeled with CFSE and cultured with OVA-peptide to assess cell division of retrovirally transduced cells (CD45.2+CD45.1−TCRβ+Vα2+ CD4+) by flow cytometry at 72 hours.
To generate the dose response curve of each TCR to OVA323-339 peptide, the functional readout of TCR activation in vitro at each peptide concentration was normalized to the maximum value seen with DO11, and EC50 was determined by nonlinear regression using GraphPad software.
Tetramer binding
T cell hybridomas (2×105) expressing individual TCRs were stained with 20μg/ml of PE labeled tetramers of I-Ad protein with linked DO11 OVA epitope (Scott-Browne et al., 2011) at 37°C for 90 min, and were further incubated with 2μg/ml of anti-TCRβ antibody at 4°C for 30 min. 3K:I-Ab tetramer was used as a control. Tetramer binding to TCRs was measured using flow cytometry.
Retroviral transduction of TCRs into CD4−CD8− thymocytes
Thymocytes from Foxp3gfpRag1−/− mice were transduced with MigR1-derived retroviruses expressing TCRα-P2A-DO11 TCRβ IRES-Thy1.1 as described (Haxhinasto et al., 2008; Lathrop et al., 2011). One million cells in 10μl PBS were intrathymically injected into sublethally irradiated (600 rads) WT or RIP-mOVA recipients. Development of Foxp3+ thymocytes was analyzed by flow cytometry 2 weeks later.
Assessment of negative selection by intrathymic injection
CD4SP thymocytes expressing individual TCRs were sorted from bone marrow chimeras, mixed with Cell Trace Violet (Invitrogen)-labeled polyclonal thymocytes at a 1:1 ratio, injected into the thymuses of either CD45.1 WT or RIP-mOVA mice, and analyzed by flow cytometry 3 days later.
In vivo peripheral T cell assays
Peripheral CD4+ T cells expressing individual TCRs were obtained from bone marrow chimeras, and labeled with Cell-Trace Violet to follow cell division. Foxp3− CD4+ T cells (105) were intravenously transferred into irradiated (500 rads) CD45.1 RIP-mOVA recipients. Splenocytes were analyzed for proliferation and Treg cell conversion 14 days later by flow cytometry. In another set of experiments, cells were intravenously transferred into CD45.1 WT recipients and intraperitoneally injected with OVA protein (100 μg/mouse) dissolved in Imject Alum Adjuvant (Thermo Fisher Scientific). Splenocytes were analyzed 7 days later by flow cytometry.
Supplementary Material
Highlights.
Treg cell development is correlated with self-reactivity over a broad range
Foreign antigen reactive Treg cells may be generated by cross-reactivity to self
TCR affinity is correlated with the size of the Treg cell developmental “niche”
Peripheral responses need greater self-reactivity than thymic Treg cell selection
Acknowledgments
We thank N. Santacruz and J. Hunn for technical assistance, W. Ise, K. Murphy, B. Helmink, and B. Sleckman for reagents; W. McCoy, D. Fremont, D. Donermeyer, and P.M. Allen, for technical assistance and helpful discussions; and K. Murphy, T. Egawa, P.M. Allen, E. Unanue, C.W. Lio (all Wash. U), Y. Zheng (Salk), L.F. Lu (UCSD) for helpful discussion and critical reading of the manuscript. Supported by the NIH (C.-S.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nature Immunology. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- Atibalentja DF, Byersdorfer CA, Unanue ER. Thymus-blood protein interactions are highly effective in negative selection and regulatory T cell induction. J Immunol. 2009;183:7909–7918. doi: 10.4049/jimmunol.0902632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, Hsieh CS. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009;10:610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–840. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- Bredemeyer AL, Helmink BA, Innes CL, Calderon B, McGinnis LM, Mahowald GK, Gapud EJ, Walker LM, Collins JB, Weaver BK, et al. DNA double-strand breaks activate a multifunctional genetic program in developing lymphocytes. Nature. 2008;456:819–823. doi: 10.1038/nature07392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzo Picca C, Simons DM, Oh S, Aitken M, Perng OA, Mergenthaler C, Kropf E, Erikson J, Caton AJ. CD4+CD25+Foxp3+ regulatory T cell formation requires more specific recognition of a self-peptide than thymocyte deletion. Proc Natl Acad Sci U S A. 2011;108:14890–14895. doi: 10.1073/pnas.1103810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NR, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- Derbinski J, Pinto S, Rosch S, Hexel K, Kyewski B. Promiscuous gene expression patterns in single medullary thymic epithelial cells argue for a stochastic mechanism. Proc Natl Acad Sci U S A. 2008;105:657–662. doi: 10.1073/pnas.0707486105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SR, Jameson SC, Fink PJ. V beta 5+ T cell receptors skew toward OVA+H-2Kb recognition. J Immunol. 1994;152:1790–1801. [PubMed] [Google Scholar]
- Feuerer M, Jiang W, Holler PD, Satpathy A, Campbell C, Bogue M, Mathis D, Benoist C. Enhanced thymic selection of FoxP3+ regulatory T cells in the NOD mouse model of autoimmune diabetes. Proc Natl Acad Sci U S A. 2007;104:18181–18186. doi: 10.1073/pnas.0708899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J Exp Med. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterberger M, Aichinger M, da Costa OP, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol. 2010;11:512–519. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- Hsieh C-S, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- Huesmann M, Scott B, Kisielow P, von Boehmer H. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell. 1991;66:533–540. doi: 10.1016/0092-8674(81)90016-7. [DOI] [PubMed] [Google Scholar]
- Huseby ES, Crawford F, White J, Marrack P, Kappler JW. Interface-disrupting amino acids establish specificity between T cell receptors and complexes of major histocompatibility complex and peptide. Nat Immunol. 2006;7:1191–1199. doi: 10.1038/ni1401. [DOI] [PubMed] [Google Scholar]
- Ise W, Kohyama M, Nutsch KM, Lee HM, Suri A, Unanue ER, Murphy TL, Murphy KM. CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nat Immunol. 2010;11:129–135. doi: 10.1038/ni.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: Production of CD25+ CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. Journal of Immunology. 1999;162:5317–5326. [PubMed] [Google Scholar]
- Jordan MS, Boesteanu A, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+ CD25+ regulatory T cells induced by an agonist self-peptide. Nature Immunology. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung MW, Shen S, Lafaille JJ. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. J Exp Med. 2009;206:2121–2130. doi: 10.1084/jem.20091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaughtry TM, Hogquist KA. Central tolerance: what have we learned from mice? Semin Immunopathol. 2008;30:399–409. doi: 10.1007/s00281-008-0137-0. [DOI] [PubMed] [Google Scholar]
- Moon JJ, Dash P, Oguin TH, 3rd, McClaren JL, Chu HH, Thomas PG, Jenkins MK. Quantitative impact of thymic selection on Foxp3+ and Foxp3− subsets of self-peptide/MHC class II-specific CD4+ T cells. Proc Natl Acad Sci U S A. 2011;108:14602–14607. doi: 10.1073/pnas.1109806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011 doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholczyk R, Kern J, Singh N, Iwashima M, Kraj P, Ignatowicz L. Nonself-antigens are the cognate specificities of foxp3(+) regulatory T cells. Immunity. 2007;27:493–504. doi: 10.1016/j.immuni.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E, Naeher D. Affinity threshold for thymic selection through a T-cell receptor-co-receptor zipper. Nat Rev Immunol. 2009;9:207–213. doi: 10.1038/nri2469. [DOI] [PubMed] [Google Scholar]
- Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- Schallenberg S, Tsai PY, Riewaldt J, Kretschmer K. Identification of an immediate Foxp3(−) precursor to Foxp3(+) regulatory T cells in peripheral lymphoid organs of nonmanipulated mice. J Exp Med. 2010;207:1393–1407. doi: 10.1084/jem.20100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Browne JP, Crawford F, Young MH, Kappler JW, Marrack P, Gapin L. Evolutionarily Conserved Features Contribute to alphabeta T Cell Receptor Specificity. Immunity. 2011 doi: 10.1016/j.immuni.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Browne JP, White J, Kappler JW, Gapin L, Marrack P. Germline-encoded amino acids in the alphabeta T-cell receptor control thymic selection. Nature. 2009;458:1043–1046. doi: 10.1038/nature07812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Santen H-M, Benoist C, Mathis D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. Journal of Experimental Medicine. 2004;200:1221–1230. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. Journal of Experimental Medicine. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirnsberger G, Mair F, Klein L. Regulatory T cell differentiation of thymocytes does not require a dedicated antigen-presenting cell but is under T cell-intrinsic developmental control. Proc Natl Acad Sci U S A. 2009;106:10278–10283. doi: 10.1073/pnas.0901877106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D, Bevan MJ. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 2006;25:261–270. doi: 10.1016/j.immuni.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.