Abstract
Rats exposed to the odor of a predator or to the elevated plus maze express fear behaviors without a prior exposure to either stimulus. The expression of innate fear provides for an ideal model of anxiety which can aid in the elucidation of brain circuits involved in anxiety-related behaviors. The current experiments compared activation of neuropeptide-containing neuronal populations in the amygdala of rats exposed to either the elevated plus maze (EPM; 5 minutes) versus home cage controls, or predator ferret odor versus butyric acid, or no odor (30 minutes). Sections of the brains were prepared for dual-labeled immunohistochemistry and counts of c-Fos co-localized with somatostatin (SOM) or neuropeptide Y (NPY) were made in the basolateral (BLA), central (CEA), medial (MEA) nucleus of the amygdala. Ferret odor and butyric acid exposure significantly decreased the percentage of SOM–positive neurons also immunoreactive for c-Fos in the anterior BLA compared to controls, whereas EPM exposure yielded a significant increase in the activation of SOM-positive neurons versus home cage controls. In the CEA, ferret odor and butyric exposure significantly decreased the percentage of SOM-positive neurons also immunoreactive for c-Fos compared to no-odor controls whereas EPM exposure yielded no change versus controls. In the MEA, both ferret odor exposure and EPM exposure resulted in increased SOM co-localized with c-Fos compared to control groups whereas NPY co-localized with c-Fos occurred following ferret odor exposure, but not EPM exposure. These results indicate that phenotypically distinct neuronal populations of the amygdala are differentially activated following exposure to different anxiogenic stimuli. These studies further elucidate the fundamental neurocircuitry of anxiety and could possibly explain the differential behavioral effects of predator versus novelty-induced stress.
1. Introduction
The amygdala links sensory perception with emotive and behavioral responses and thus the elucidation of the functional neurocircuitry of the amygdala during anxious states is valuable for the fundamental understanding of anxiety or fear. Dysregulation of the amygdala has also been associated with anxiety disorders such as post-traumatic stress disorder (for review see Jovanovic and Ressler, 2010). However, the amygdala contains several subregions and phenotypically-divergent populations of neurons which may play stimulus- or behavior-specific roles in fear responses (Haubensak et al., 2010; Herry et al., 2008; Herry et al., 2010; McDonald et al., 2002; McDonald et al., 1999). Thus, increasing our knowledge of the phenotypic characteristics of the neuronal populations involved in anxiety can lead to specific targeting of the neuronal populations through pharmacological tools or genetic manipulation (Haubensak et al., 2010).
The amygdala is involved in the behavioral response to numerous unconditioned tests of anxiety (Davis et al., 1997a; Davis et al., 1997b; for review see Rosen and Donley, 2006). Exposure of rats to the odor of a predator elicits a fear response even if the rat has never before encountered the predator. Numerous studies have demonstrated that the amygdala is involved in the innate fear processing following exposure to predators or predator cues (Butler et al., 2011; Choi and Kim, 2010; Martinez et al., 2011). The innate fear behavior response of rodents to predator odor represents an important survival response and is often utilized as a model of anxiety. We have previously shown that rat exposure to predator ferret odor elicits defensive behaviors such as burying of the source of the odor with bedding (defensive burying) and that subregions of the amygdala are involved in the expression of this behavior (Butler et al., 2011; Wilson and Junor, 2008). Another popular method to initiate an innate fear response is to expose rodents to an elevated plus maze (Handley and Mithani, 1984). Amygdala lesions ameliorate fear processing following EPM exposure (File et al., 1998) – a similar effect displayed upon exposure to predator scent (Blanchard and Blanchard, 1972; Blanchard et al., 2005). We have shown that drug challenges in the amygdala also modulate behaviors in these two tasks (Wilson and Junor, 2008). However, the behavioral outputs of the two tests, predator odor exposure and EPM, are drastically different. Rodents exposed to predator odor demonstrate increased motor activity such as defensive burying and escape attempts (Butler et al., 2011). Fear behaviors elicited by EPM exposure, however, are characterized by thigmotaxis, a rodent aversion to open spaces and are measured by the percent time and the number of entries into the open arm of the maze (for reviews see Carobrez and Bertoglio, 2005; Rodgers and Dalvi, 1997; Stock et al., 2001). The role of specific neuronal subtypes, however, in the amygdala as they relate to different behavioral outputs to different anxiogenic stimuli has not been well characterized.
The amygdala can be subdivided into three major subnuclei – medial (MEA), central (CEA), and basolateral/lateral (BLA) – each with distinct roles in the expression of anxiety. InButler et al. (2011), we showed that Ca2+/Calmodulin-Dependent Protein Kinase II (CAMKII)-positive BLA neurons display increased expression of c-Fos following exposure to ferret odor. In contrast, BLA neurons which contain the calcium-binding protein calbindin (CB) showed decreased expression of c-Fos following exposure to ferret odor. CAMKII-positive neurons of the BLA represent the major glutamatergic projection population (McDonald et al., 2002) whereas somatostatin (SOM)- and neuropeptide Y (NPY)-positive neurons in the BLA are interneuronal GABAergic populations (McDonald and Pearson, 1989) of smaller density than CB populations (McDonald and Betette, 2001). In the current study, we wish to further elucidate the functional neurocircuitry of the BLA by examining the activation of SOM- or NPY-positive interneurons following exposure to ferret odor and elevated plus maze.
The CEA functions as the primary output region for the amygdala (for review see Sah et al., 2003). In addition, studies have shown that ~95% of all neurons in the CEA are GABAergic (for review see Cassell et al., 1999). Despite the homogeneity of GABA throughout the CEA, different populations of CEA neurons have distinct functional roles. For example, neuronal populations in the CEA which contain SOM or NPY project outside the CEA (Gray and Magnuson, 1992) and receive projections from an inhibitory CEA interneuronal population which contains the neuropeptide enkephalin (Chieng and Christie, 2010; Chieng et al., 2006). In addition, a prior study has suggested that pharmacological attenuation of anxiety induced by the EPM exposure is, at least in part, due to the activation of the lateral subdivision of the CEA (Linden et al., 2004). The second aim of this study is to compare the percentage of CEA neurons with NPY or SOM which express c-Fos following exposure to ferret odor and the EPM test.
Our lab and others have previously shown that the MEA displays increased expression of c-Fos following exposure to predator odor (Baisley et al., 2011; Butler et al., 2011; Dielenberg et al., 2001; Martinez et al., 2011). In the current study we further examine the phenotypic characteristics of the activated neurons in the MEA following exposure to predator ferret odor and the EPM test. Specifically, we explored our hypothesis that SOM and NPY neurons of the MEA show increased c-Fos co-localization following exposure to ferret odor or the EPM.
2. Experimental Procedures
2.1 Animals
Male Long-Evans rats (Harlan Laboratories, Indianapolis, IN), weighing approximately 250–300g were single-housed in an environmentally controlled animal facility on a 12:12 h light: dark cycle with lights on at 0700 hours. Purina rat chow and water were available ad libitum. All experiments were conducted during the light phase, beginning at least 2 hours after light phase onset and concluding at least 2 hours before transition to the dark phase. Animals were housed in an animal facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care. Animal care and use procedures were carried out in accordance with protocols written under the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of South Carolina.
2.2 Predator Odor Exposure
The defensive burying test was performed as described in Wilson and Junor (2008) with the addition of a noxious odor control group of rats which were exposed to butyric acid. A Plexiglas chamber (45 × 30 × 44 cm) filled up to a depth of 5 cm with fresh pine bedding with either a piece of untreated gauze (control), gauze with 100 µL butyric acid (based on Hebb et al., 2004), or a piece of ferret-scented towel (5 cm2) placed 2 cm above bedding, was used for this analysis (towels a generous donation from Dr. John Hines from Yale University). Rats were habituated to the novel arena for 2 consecutive days prior to testing. The habituation phase lasted for 30 minutes and was recorded onto videotape. On the test day, rats were exposed to either a piece of untreated gauze, gauze treated with butyric acid, or the ferret-scented towel and behavior was recorded onto videotape for a duration of 30 minutes. Immediately after the 30 minute odor exposure trial, rats were placed back into the home cage.
2.3 Elevated Plus Maze
The elevated plus maze (EPM) was comprised of two open (56 × 10 × 1 cm) and two closed arms (56 × 10 × 40 cm) made of black Plexiglas. The maze was elevated 50 cm above the floor. One week after arrival in the colony, animals receiving EPM exposure (N=8 in the first test group and N=6 in the second test group) were individually transported into the testing room. Rats were placed immediately into the center of the EPM facing an open arm and their behavior recorded for five minutes. Immediately after testing, rats were returned to their home cages. Controls remained in their home cages without testing. The maze was cleaned with a 7% ammonium hydroxide solution between subjects. Analysis of time spent in open and closed arms, entries into open and closed arms, and overall distance traveled was conducted using the Ethovision software program (Noldus Information Technology, The Netherlands). Zones for each arm were drawn such that all four paws must enter the arm in order for the rat to be considered in an open or closed arm of the maze. Percent open arm entries was calculated as the number of open arm entries divided by total entries (open arm entries plus closed arm entries). Percent open arm time was calculated as the amount of time spent in open arms divided by the total amount of time spent in both open and closed arms. Distance traveled (cm) was used as the measure of activity.
2.4 Perfusion and immunohistochemistry
The brain tissue for predator odor part of this study was collected from rats which have previously been shown to display 1) increased defensive burying and decreased latency to bury upon exposure to ferret odor- or butyric acid-exposed rats compared to controls and 2) increased c-Fos expression in the posterior MEA in ferret exposed rats compared to butyric acid- and nonodor- exposed controls (Butler et al., 2011).
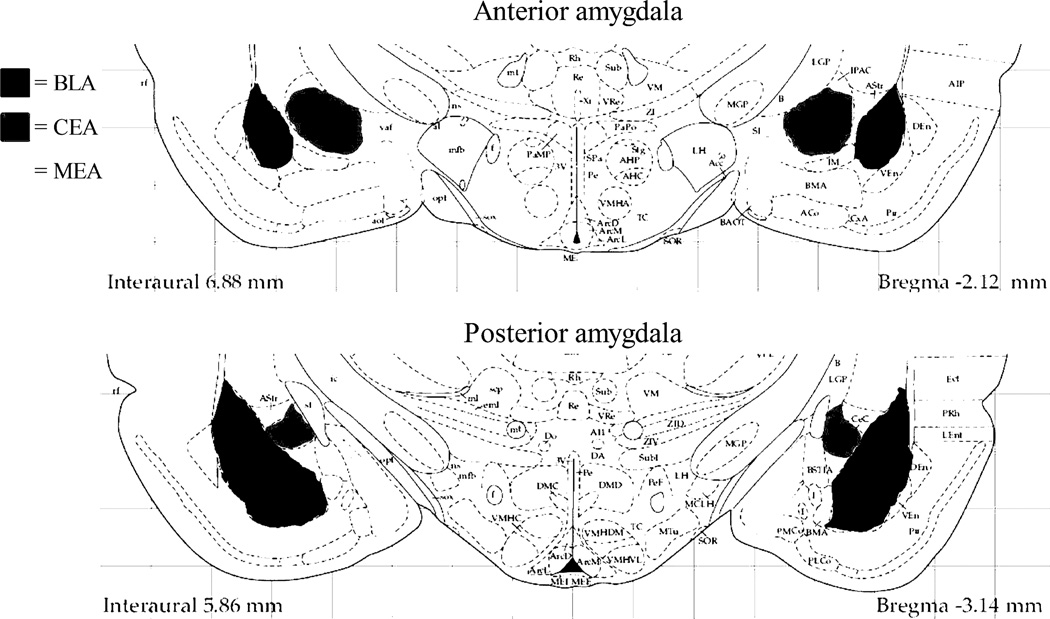
Two hours from the beginning of the odor exposure and EPM tests, rats were placed under deep isoflurane anesthesia and transcardially perfused with 0.1M phosphate buffered saline followed by 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4). The two hour timepoint was chosen because that is within the 1–3 hour post-exposure time frame in which c-Fos is maximally expressed (Nestler et al., 2001) and to maintain consistency with our previous studies (Butler et al., 2011). The brains were removed, post-fixed overnight and transferred to 30% sucrose solution in 0.1 M phosphate buffer for cryoprotection. Serial coronal sections were cut on a microtome and stored at −20°C in anti-freezing solution (30% sucrose and 30% ethylene glycol in 0.1M phosphate buffer) until they were processed for immunohistochemistry; brains from the first set of EPM-exposed rats were cut at 50 microns and brains for predator odor exposed rats were sectioned at 45 microns. An initial experiment was conducted to determine the pattern of single-label Fos expression in the amygdala and related limbic regions following EPM exposure. The following limbic brain regions were examined for single-labeled Fos expression due to their involvement in anxiety-related responses including behaviors in the elevated plus maze: lateral septum (LaSep), claustrum (Cl), dorsal endopiriform nucleus (DEN), ventral endopiriform nucleus (VEN), rostral and caudal interstitial nucleus of the posterior limb of the anterior commissure (IPAC), hippocampal subregions (CA1, CA3, and dentate gyrus [DG]), bed nucleus of the stria terminalis (BNST), and basolateral (BLA), anterodorsal medial (MeAD), and central (CeA) nuclei of the amygdala. Dual-labeled immunohistochemistry (c-Fos and NPY or c-Fos and SOM) was performed on random sections in both the anterior (Bregma: −1.80 mm to −2.30 mm) and posterior (from Bregma: −2.56 mm to −3.30 mm) portions of the amygdala (Figure 1). For each marker, immunohistochemistry from amygdalar sections from the groups of control and EPM-exposed rats or the control, butyric acid-exposed and ferret odor-exposed rats were processed at the same time. Briefly, all sections were initially incubated with goat (1:500 or 1:1000) anti-c-Fos (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) antibody in Tris-buffered saline (TBS) with 4% normal horse serum and 0.2% Triton X-100 for 48 h at 4°C. This was followed by incubation with a biotinylated donkey anti-goat secondary antibody (1:1000) for 1.5 h at room temperature (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) and then horseradish peroxidase-conjugated streptavidin for 1 h at room temperature (1:1600; Jackson ImmunoResearch Laboratories Inc.). c-Fos immunoreactivity was visualized by developing the sections in a nickel-cobalt intensified diaminobenzidine solution with 0.3 % hydrogen peroxide, yielding a blue-black reaction product confined to the nucleus of c-Fos-positive cells. The immunolabeling continued for a second set of sections to determine co-localization of c-Fos and NPY or c-Fos and SOM. After c-Fos staining, sections were incubated in mouse primary antisera directed against SOM (1:500; see antibody details below) or rabbit primary antisera directed against NPY (1:1000 or 1:4000; Peninsula Laboratories, LLC, San Carlos, CA) at 4°C for 48 h, followed by incubation with unlabeled donkey anti-mouse or rabbit secondary antibodies (1:100; Jackson ImmunoResearch Laboratories Inc.) for 2 hours at room temperature and finally with mouse or rabbit peroxidase-anti-peroxidase (1:250; Covance, Florida). The mouse monoclonal antibody to somatostatin (SOMA-8 antibody; a generous gift from Dr. Alison M.J. Buchan from the University of British Columbia) was raised against somatostatin-14 conjugated to keyhole limpet hemocyanin. This antibody has been well-characterized; ELISA studies indicate that it recognizes somatostatin-28, as well as both cyclic and linear forms of somatostatin-14 (Buchan et al., 1985; Muller et al., 2007b). Immunoreactivity for SOM and NPY was visualized by developing the sections in plain diaminobenzidine solution with 3.0 % hydrogen peroxide, yielding a brown reaction product confined to the cytoplasm of immunoreative cells with these markers. After standard mounting, dehydration and coverslipping procedures, total numbers of neurons labeled with c-Fos, NPY, or SOM were counted, as well as numbers of dual-labeled neurons showing c-Fos positive nuclei and cytoplasm stained with NPY or SOM using a Nikon E600 microscope at 20× magnification and counts were obtained using the Neurolucida software program (Version 7, MBF Bioscience, 2006)‥ Subregions of the amygdala (BLA, CEA, and MEA) in the left and right hemispheres were counted from a random section taken from both the anterior (from Bregma: −1.80 mm to −2.30 mm) and posterior (from Bregma: −2.56 mm to −3.30 mm) portions of the amygdala (Figure 1). This allowed analysis of hemispheric and rostro-caudal differences in activation (see analysis description below). No hemispheric differences were seen, so data from left and right regions were combined to enhance consistency and numbers of neurons counted per region. The boundaries of the entire basolateral complex (minus the basomedial), the entire CEA (centromedial and centrolateral), and the entire MEA (mediodorsal and medioventral) were included in the respective counts. Neuronal counts were done by investigators blinded to the treatment condition of the sections. The total number of neurons from single and double-stained tissue was divided by the area of the subregion to determine the density of the immunoreactive cells per mm2. Photomicrographic images were captured with a Photometrics CoolSnap digital camera (Roper Scientific, Trenton, NJ, USA) linked to a computer equipped with IPLab software using a Nikon E600 microscope. Images were collected and imported into Adobe Photoshop where minor adjustments for contrast and brightness were made.
Figure 1.
Depiction of the anatomical levels of the amygdala analyzed in this study. Separate counts were made in the anterior (−1.80 to −2.30 mm from bregma) and posterior (−2.56 to −3.30 mm from bregma) portions of the basolateral/lateral (BLA), central (CEA), and medial (MEA) nuclei of the amygdala (Paxinos G. and Watson C, 1998).
2.5 Data analysis
Immunohistochemistry data were expressed both as a percentage of c-Fos-positive neurons with SOM or NPY [(total number of double-labeled neurons / total number of c-Fos-labeled neurons) * 100); tables 1 and 2] and the percentage of SOM- or NPY-labeled neurons with c-Fos-positive nuclei [(total number of double-labeled neurons / total number of SOM or NPY-labeled neurons) * 100); figures 3–8] in each subregion of the amygdala. Average cell counts (density) for single-labeled and double-labeled neurons with each marker were also compared (Tables 1 and 2). For single-labeled c-Fos, cell counts were performed on one random section in the regions of interest (Figure 2). For double-labeled immunohistochemistry, cell counts were performed in left and right hemispheres at both anterior and posterior levels to assess the possibility of hemispheric and rostro-caudal differences in neuronal activation. To determine total anterior or posterior labeling, counts from the left and right were added together; similarly, to determine total left and right labeling, counts from the anterior and posterior portions of each amygdala subregion were added together; finally, to determine total labeling for a specific subregion, total counts for the left, right, anterior, and posterior portions were added together. If any of the left/right, anterior/posterior portions of amygdala were damaged the counts from that subject were not included in the final data set; if only one subregion was uncountable the other subregions of amygdala were included. The three odor exposure groups were compared using a one-way analysis of variance (ANOVA) for immunohistochemistry parameters in each region. The source of significant (P<0.05) main effects was determined by post-hoc Student-Newman-Keuls (SNK) comparisons. EPM immunohistochemistry data was analyzed using Student’s unpaired t-test. Data were analyzed using GraphPad Prism software (v. 5.03; La Jolla, CA) with significance level set at alpha = 0.05.
Table 1.
c-Fos, SOM, NPY density and percentage c-Fos with phenotype in amygdalar subregions of odor-exposed rats.
| Region | Treatment | c-Fos densitya |
SOM density |
SOM+c- Fos density |
% c- Fos with SOM |
c-Fos densityb |
NPY density |
NPY+c- Fos density |
% c- Fos with NPY |
|---|---|---|---|---|---|---|---|---|---|
| BLA | control | 17±2 (5) | 18±1 (5) | 2±0 (5) | 13±1 (5) | 23±2 (5) | 18±1 (5) | 1±0 (5) | 3±1 (5) |
| butyric acid | 15±2 (7) | 25±2 (7) | 2±0 (7) | 13±3 (7) | 16±1 (7) | 20±2 (7) | 1±0 (7) | 5±1 (7) | |
| ferret | 30±4 (5) ** | 27±5 (5) | 2±1 (5) | 8±2 (5) | 27±4 (5) | 22±4 (5) | 2±1 (5) | 7±3 (5) | |
| CEA | control | 22±2 (5) | 32±3 (5) | 3±0 (5) | 14±3 (5) | 17±3 (5) | 17±3 (5) | 2±1 (5) | 8±4 (5) |
| butyric acid | 18±3 (7) | 48±4 (7) | 3±1 (7) | 16±5 (7) | 16±2 (7) | 19±1 (7) | 0±0 (7) | 3±1 (7) | |
| ferret | 25±4 (5) | 47±9 (5) | 3±1 (5) | 14±6 (5) | 17±4 (5) | 18±2 (5) | 1±1 (5) | 7±3 (5) | |
| MEA | control | 91±8 (6) | 103±9 (6) | 6±1 (6) | 7±1 (6) | 64±7 (6) | 78±10 (6) | 6±1 (6) | 9±1 (6) |
| butyric acid | 80±13 (7) | 128±9 (7) | 7±2 (7) | 9±1 (7) | 57±7 (7) | 69±6 (7) | 4±1 (7) | 7±1 (7) | |
| ferret | 172±21 (6) ** | 132±7 (6) | 20±4 (6) ** | 12±2 (6) | 133±9 (6) *** | 95±7 (6) | 10±2 (4)* | 8±1 (4) |
Density of neurons in subregions of the amygdala which are immunoreactive for c-Fos, somatostatin (SOM) or neuropeptide Y (NPY) following exposure to control odor, butyric acid, or ferret odor. The data is presented as the density of labeled neurons in the subregion (per mm2) and is a summation of the bilateral counts from the anterior and posterior portions. The numbers in parentheses are the N for each group.
From tissue slices double-labeled for c-Fos and SOM.
From tissue slices double labeled for c-Fos and NPY. The data represent cumulative counts from anterior and posterior sections. Percent c-Fos with SOM or NPY neurons represent: (the total number of double-labeled neurons / total c-Fos-labeled neurons) * 100.
P<0.001,
P<0.01,
P<0.05 vs. butyric acid and control.
Table 2.
c-Fos, SOM, NPY density and percentage c-Fos with phenotype in amygdalar subregions of home cage and elevated plus maze-exposed rats.
| Region | Treatment | c-Fos densitya |
SOM density |
SOM+c- Fos density |
% c- Fos with SOM |
c-Fos densityb |
NPY density |
NPY+c- Fos density |
% c-Fos with NPY |
|---|---|---|---|---|---|---|---|---|---|
| BLA | home | 29±6 (5) | 80±39 (5) | 6±2 (4) | 21±5 (4) | 19±6 (8) | 8±2 (8) | 1±0 (8) | 1.0±0.6 (6) |
| EPM | 63±5 (5) ** | 70±6 (5) | 18±2 (5) ** | 29±3 (5) | 74±16 (8) ** | 10±3 (8) | 1±0 (8) | 1.1±0.7 (8) | |
| CEA | home | 22±4 (5) | 49±12 (5) | 8±2 (5) | 35±4 (5) | 56±9 (8) | 9±2 (8) | 1±0 (8) | 1.3±0.9 (8) |
| EPM | 20±5 (5) | 39±5 (5) | 7±1 (5) | 39±5 (5) | 93±12 (8) * | 7±1 (8) | 0±0 (8) | 0.1±0.1 (8) | |
| MEA | home | 27±6 (5) | 92±41 (5) | 10±3 (5) | 34±5 (5) | 36±7 (8) | 27±7 (8) | 1±0 (8) | 0.8±0.5 (8) |
| EPM | 106±12 (5) *** | 76±11 (5) | 23±2 (5) ** | 22±2 (5) | 236±28 (8) *** | 36±9 (8) | 1±0 (8) | 0.1±0.1 (8) |
Density of neurons in subregions of the amygdala which are immunoreactive for c-Fos, somatostatin (SOM) or neuropeptide Y (NPY) following exposure to home cage or elevated plus maze (EPM). The data is presented as the density of labeled neurons in the subregion (per mm2) and is a summation of the bilateral counts from the anterior and posterior portions. The numbers in parentheses are the N for each group.
From tissue slices double-labeled for c-Fos and SOM.
From tissue slices double labeled for c-Fos and NPY. The data represent cumulative counts from anterior and posterior sections. Percent c-Fos with SOM or NPY neurons represent: (the total number of double-labeled neurons / total c-Fos-labeled neurons) * 100.
P<0.001,
P<0.01,
P<0.05 vs. home cage control.
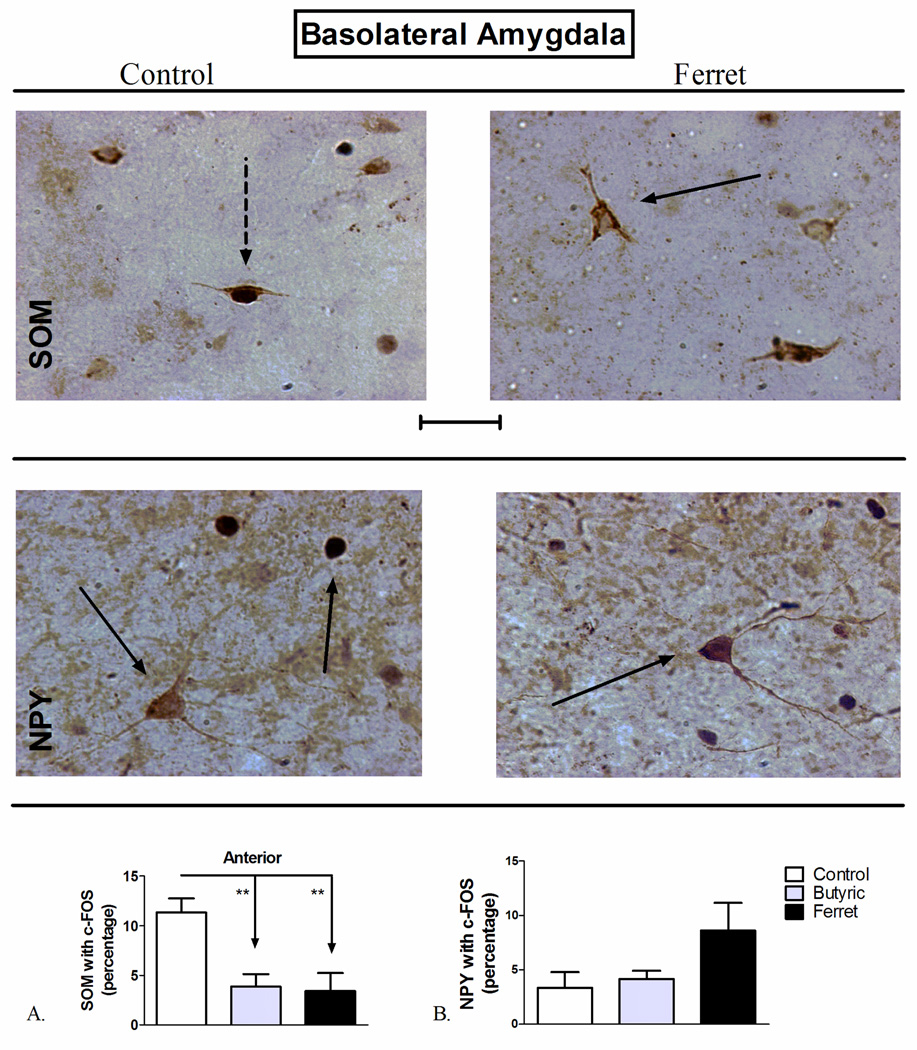
Figure 3.
Odor exposure-induced changes in c-Fos expression of A) SOM+ and B) NPY+ neurons of the basolateral amygdala (BLA). (Top) Representative photomicrograph with SOM (brown) and c-Fos (blue/black) labeling in control (left) and ferret odor-exposed (right) rats. (Middle) Representative photomicrograph with NPY (brown) and c-Fos (blue/black) labeling in control (left) and ferret odor-exposed (right) rats. Exposure to butyric acid or ferret odor decreased the percentage of SOM-positive neurons with c-Fos in the BLA (Bottom, A) compared to control odor. **P<0.01, (N=5–7). The data represent counts from the anterior sections (A) and cumulative counts from anterior and posterior sections (B). Percent SOM or NPY with c-Fos neurons represent: (the total number of double-labeled neurons / total NPY or SOM-labeled neurons) * 100. Pictures are at 40× magnification. Scale bar represents approximately 50 microns. The solid arrow points to a single-labeled neuron (blue/black = c-Fos and brown = SOM or NPY), and the dotted arrow points to a double-labeled SOM/c-Fos neuron.
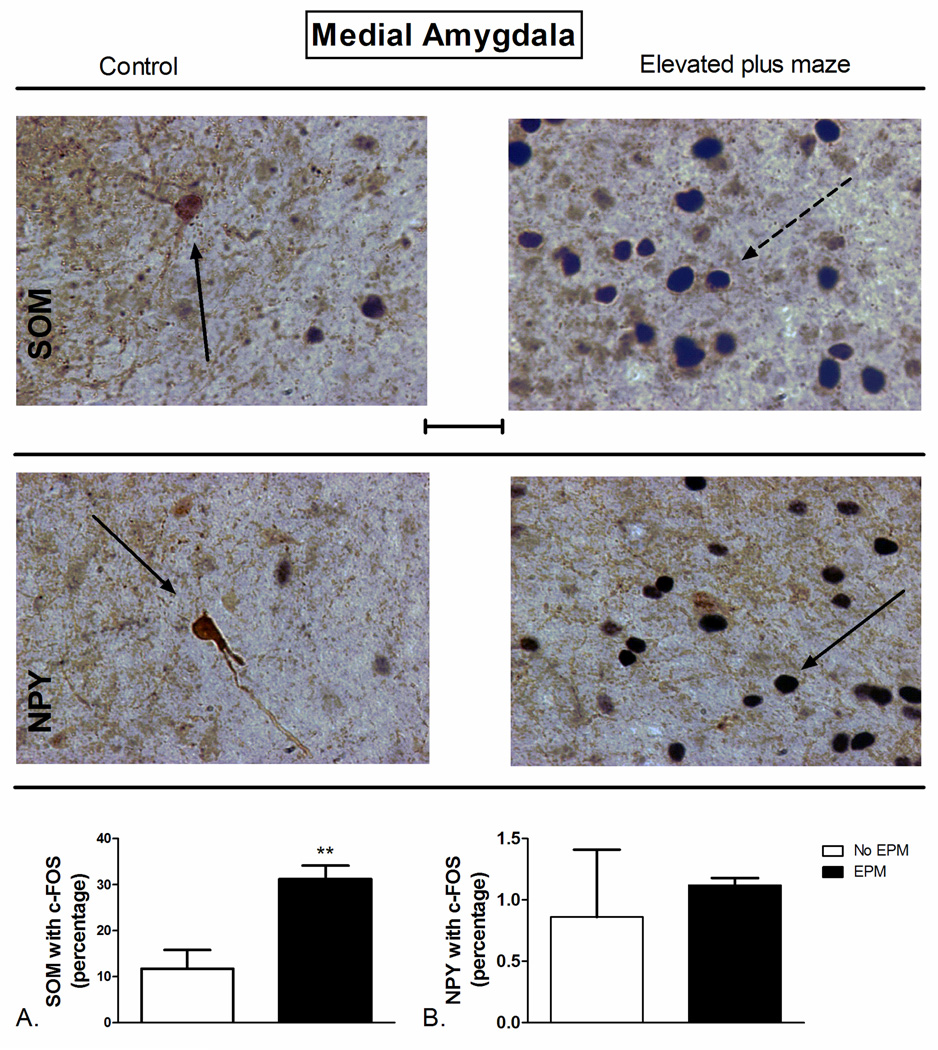
Figure 8.
Elevated plus maze (EPM) exposure-induced changes in c-Fos expression of A) SOM+ and B) NPY+ neurons of the medial amygdala (MEA). (Top) Representative photomicrograph with SOM (brown) and c-Fos (blue/black) labeling in control (left) and EPM-exposed (right) rats. (Middle) Representative photomicrograph with NPY (brown) and c-Fos (blue/black) labeling in control (left) and EPM-exposed (right) rats. Exposure to the EPM increased the percentage of SOM-positive neurons with c-Fos in the MEA (Bottom, A) compared to controls. **P<0.01 (N=4–5). The data represent cumulative counts from anterior and posterior sections. Percent SOM or NPY with c-Fos neurons represent: (the total number of double-labeled neurons / total NPY or SOM-labeled neurons) * 100. Pictures are at 40× magnification. Scale bar represents approximately 50 microns. The solid arrow points to a single-labeled neuron (blue/black = c-Fos and brown = SOM or NPY), and the dotted arrow points to a double-labeled SOM/c-Fos neuron.
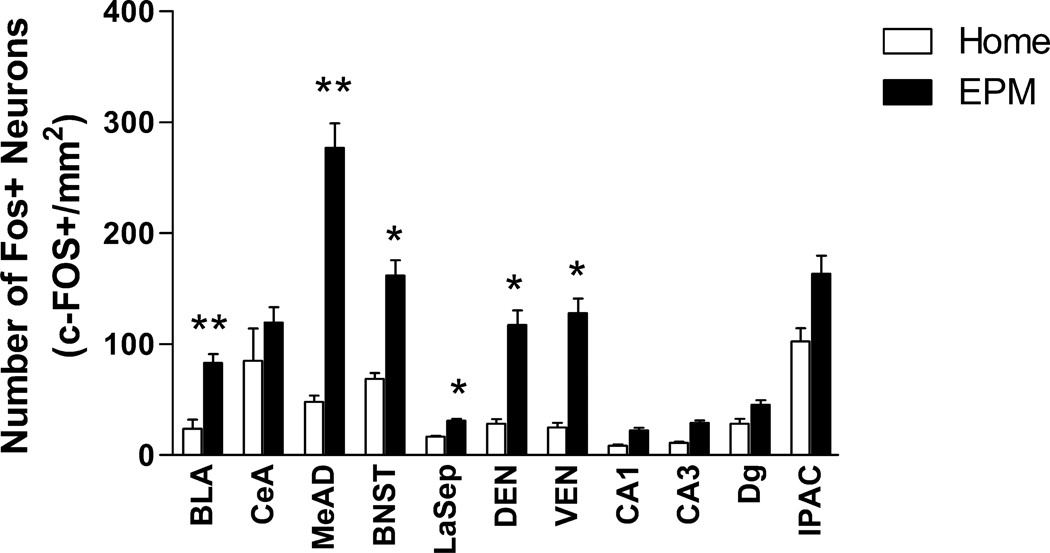
Figure 2.
Elevated plus maze exposure-induced changes in c-Fos expression in limbic brain regions. The data represent counts from single sections in the representative region. **P<0.01, *P<0.05 (N=7–8).
3. Results
3.1. Exposure to the elevated plus maze increased c-Fos expression in several limbic brain regions
Behavioral results from the animals with the EPM test show that rats averaged 1423 ± 94 cm (group 1) and 1535 ± 100 cm (group 2) of total distance traveled for groups 1 and 2, respectively. In addition, rats spent an average of 6.3 ± 2.5% (range of 0.4 to 31.9%; group 1) and 3.7 ± 1.2% (range of 3.1 to 27.1%; group 2) in the open arms of the EPM, averaged 4 ± 1 (group 1) and 7 ± 1 (group 2) entries with a frequency of 30.5 ± 5.1% (group 1) and 44.4 ± 4.5% (group 2).
To determine regions and markers for phenotypic analysis, single-labeled c-Fos was analyzed on the first group of animals exposed to the EPM test (N=8) and compared to home-cage controls (N=8). Cell counts from many regions of interest showed significantly greater c-Fos expression in EPM-exposed animals compared to control animals that remained in their home cages, including the BLA, MeAD, BNST, LaSep, DEN, and VEN (Figure 2). Similarly, exposure to the EPM resulted in a significant increase in the density of c-Fos in the amygdala areas from both SOM- and NPY-double-stained tissue (Table 2; SOM: P=0.0031, t=4.168 df=8; NPY: P=0.0057, t=3.256 df=14).
3.2. Ferret odor exposure and elevated plus maze exposure have opposite effects on the activation of somatostatin-positive neurons in the anterior basolateral amygdala
SOM-positive neurons in the anterior BLA are exclusively an interneuronal, GABAergic population (McDonald and Mascagni, 2002; McDonald and Pearson, 1989; Muller et al., 2007a). One-way ANOVA showed a significant effect of odor-exposure on the percentage of SOM-positive neurons with c-Fos in the anterior (F2,17=9.048; P=0.0026; Figure 3A), but not the posterior (F2,17=0.7548; P=0.4871; data not shown), portion of the BLA. Student-Newman-Keuls (SNK) post-hoc comparison revealed a significant reduction in SOM-positive neuronal activation following exposure to both butyric acid (P<0.01) and ferret odor (P<0.01) compared to control odor in the anterior BLA. Despite the fact that there is extensive co-localization of SOM with NPY in the BLA (McDonald, 1989), no effects of odor exposure on NPY-positive neuronal activation were observed in the BLA (F2,17=2.953; P=0.0829; Figure 3B).
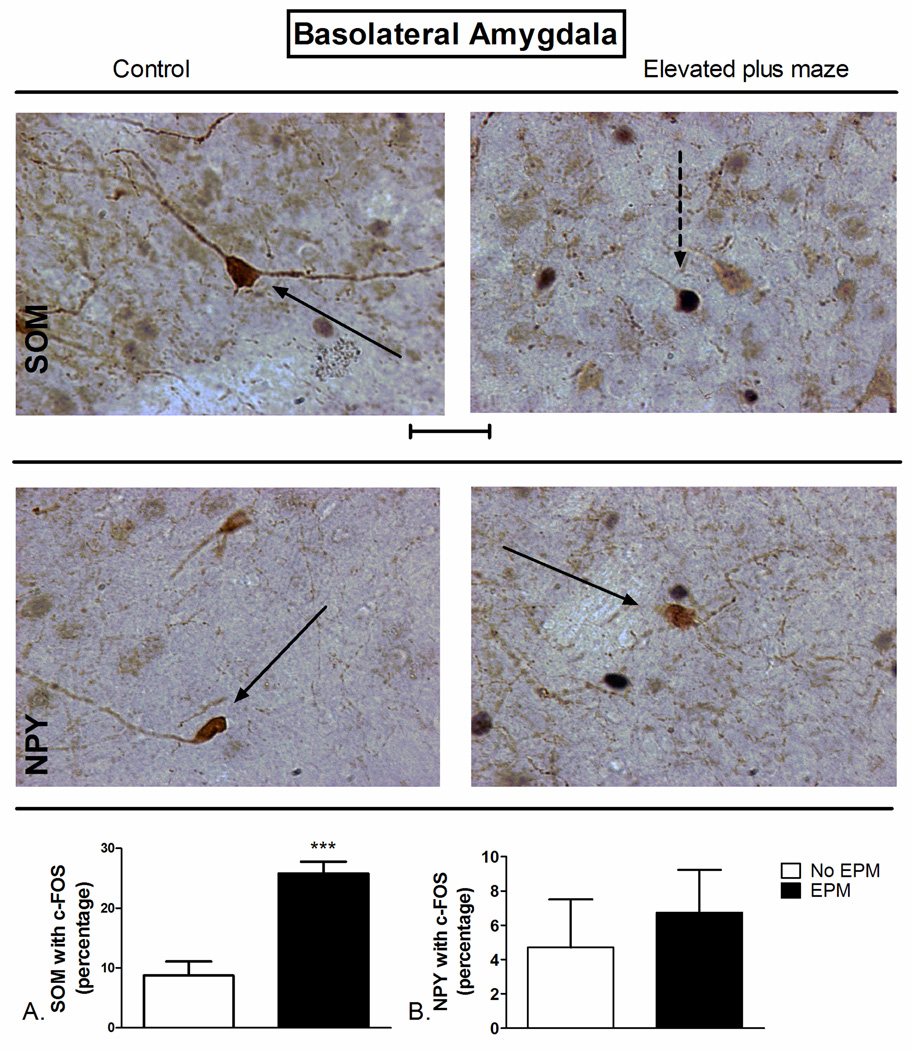
Exposure to the EPM significantly increased co-localization of somatostatin with c-Fos through the BLA (t=5.613 df=7, P=0.0008; Figure 4A). No significant effect of EPM exposure on NPY co-expression with c-Fos in the BLA was observed (t=0.545 df=17, P=0.272; Figure 4B).
Figure 4.
Elevated plus maze (EPM) exposure-induced changes in c-Fos expression of A) SOM+ and B) NPY+ neurons of the basolateral amygdala (BLA). (Top) Representative photomicrograph with SOM (brown) and c-Fos (blue/black) labeling in control (left) and EPM-exposed (right) rats. (Middle) Representative photomicrograph with NPY (brown) and c-Fos (blue/black) labeling in control (left) and EPM-exposed (right) rats. Exposure to the EPM increased the percentage of SOM-positive neurons with c-Fos in the BLA (Bottom, A) compared to controls. ***P<0.001 (N=4–5). The data represent cumulative counts from anterior and posterior sections. Percent SOM or NPY with c-Fos neurons represent: (the total number of double-labeled neurons / total NPY or SOM-labeled neurons) * 100. Pictures are at 40× magnification. Scale bar represents approximately 50 microns. The solid arrow points to a single-labeled neuron (blue/black = c-Fos and brown = SOM or NPY), and the dotted arrow points to a double-labeled SOM/c-Fos neuron.
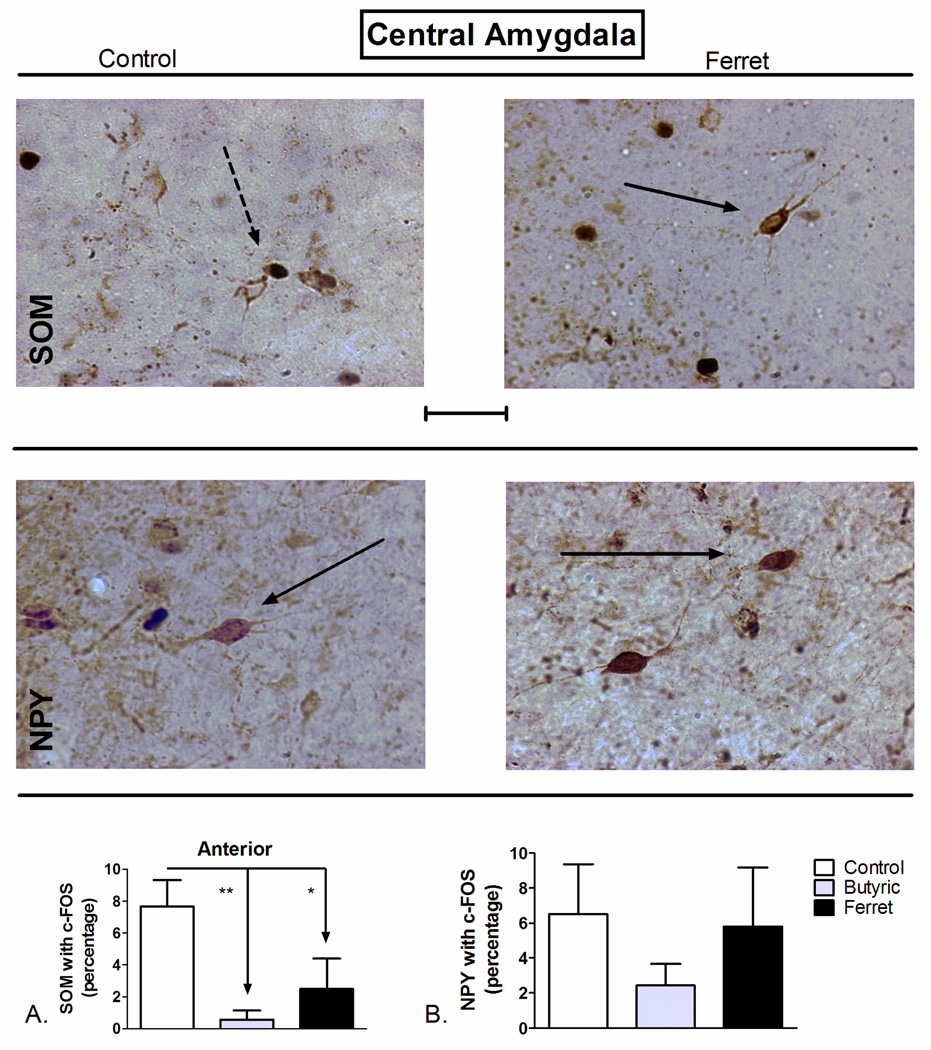
3.3. Exposure to ferret-odor reduced somatostatin-positive neuronal activation in the anterior central amygdala
SOM-positive neurons in the CEA project outside of the CEA to regions such as the parabrachial nucleus (Moga and Gray, 1985), BNST (McDonald, 1987), and the periaqueductal grey (Gray and Magnuson, 1992). One-way ANOVA of data from the anterior CEA (F2,16=8.407; P=0.004; Figure 5A), but not the posterior CEA (F2,17=0.4127; P=0.67; data not shown), revealed a significant effect of odor exposure on the percentage of SOM-positive neurons with c-Fos. SNK post-hoc comparison revealed a significant reduction in SOM-positive neuronal activation following exposure to butyric acid (P<0.01) and ferret odor (P<0.05) compared to control odor in the anterior CEA. Despite the fact that there is extensive co-localization of NPY and SOM in the CEA (McDonald, 1989), no effect of odor exposure on NPY-positive neuronal activation was observed in the CEA (F2,16=0.8555; P=0.44; Figure 5B).
Figure 5.
Odor exposure-induced changes in c-Fos expression of A) SOM+ and B) NPY+ neurons of the central amygdala (CEA). (Top) Representative photomicrograph with SOM (brown) and c-Fos (blue/black) labeling in control (left) and ferret odor-exposed (right) rats. (Middle) Representative photomicrograph with NPY (brown) and c-Fos (blue/black) labeling in control (left) and ferret odor-exposed (right) rats. Exposure to butyric acid or ferret odor decreased the percentage of SOM-positive neurons with c-Fos in the CEA (Bottom, A) compared to control odor. **P<0.01, *P<0.05 (N=5–7). The data represent counts from the anterior sections (A) and cumulative counts from anterior and posterior sections (B). Percent SOM or NPY with c-Fos neurons represent: (the total number of double-labeled neurons / total NPY or SOM-labeled neurons) * 100. Pictures are at 40× magnification. Scale bar represents approximately 50 microns. The solid arrow points to a single-labeled neuron (blue/black = c-Fos and brown = SOM or NPY), and the dotted arrow points to a double-labeled SOM/c-Fos neuron.
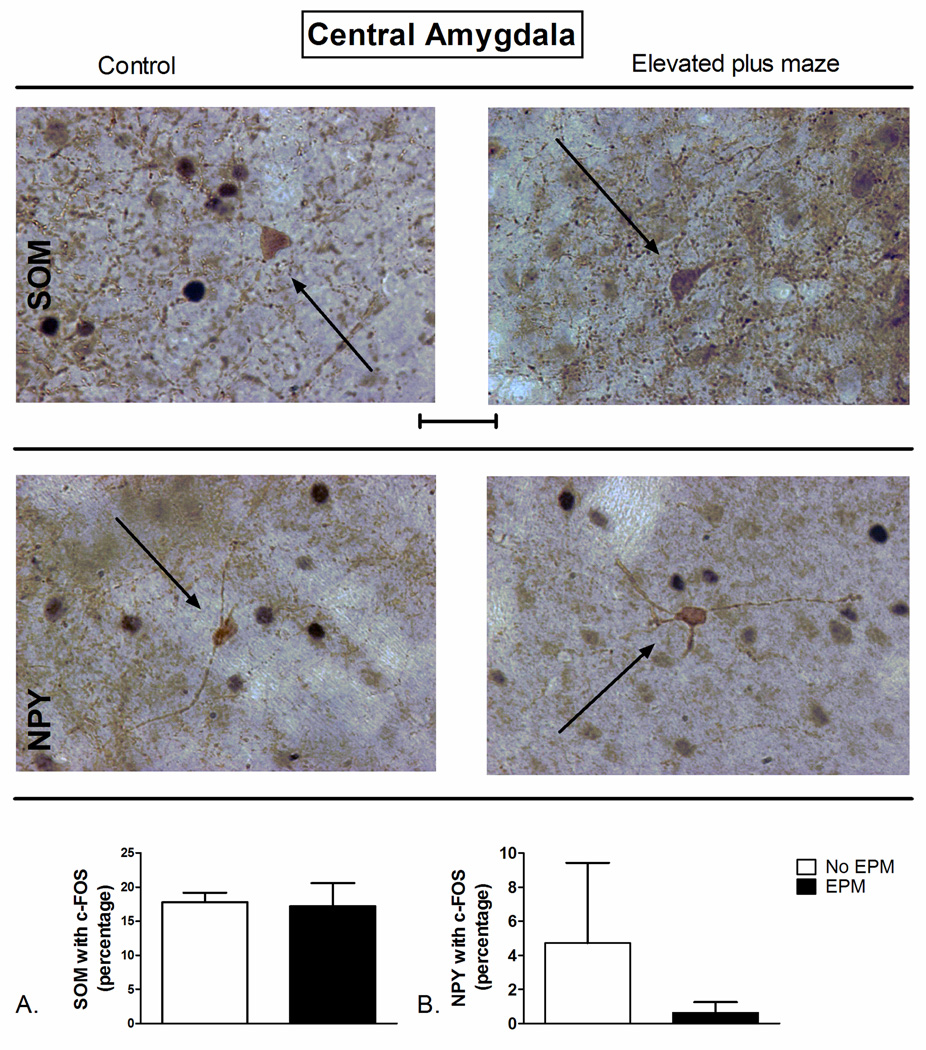
No significant difference in the co-localization of SOM (t=1.362 df=7, P=0.90; Figure 6A) or NPY (t=0.9217 df=13, P=0.37; Figure 6B) with c-Fos in the CEA between EPM exposed rats and controls was observed.
Figure 6.
Elevated plus maze (EPM) exposure-induced changes in c-Fos expression of A) SOM+ and B) NPY+ neurons of the basolateral amygdala (BLA). (Top) Representative photomicrograph with SOM (brown) and c-Fos (blue/black) labeling in control (left) and EPM-exposed (right) rats. (Middle) Representative photomicrograph with NPY (brown) and c-Fos (blue/black) labeling in control (left) and EPM-exposed (right) rats. Exposure to the EPM did not significantly alter percentage of SOM- or NPY-positive neurons with c-Fos in the CEA (Bottom, A and B) compared to controls. ***P<0.001 (N=4–5). The data represent cumulative counts from anterior and posterior sections (A and B). Percent SOM or NPY with c-Fos neurons represent: (the total number of double-labeled neurons / total NPY or SOM-labeled neurons) * 100. Pictures are at 40× magnification. Scale bar represents approximately 50 microns. The solid arrow points to a single-labeled neuron (blue/black = c-Fos and brown = SOM or NPY).
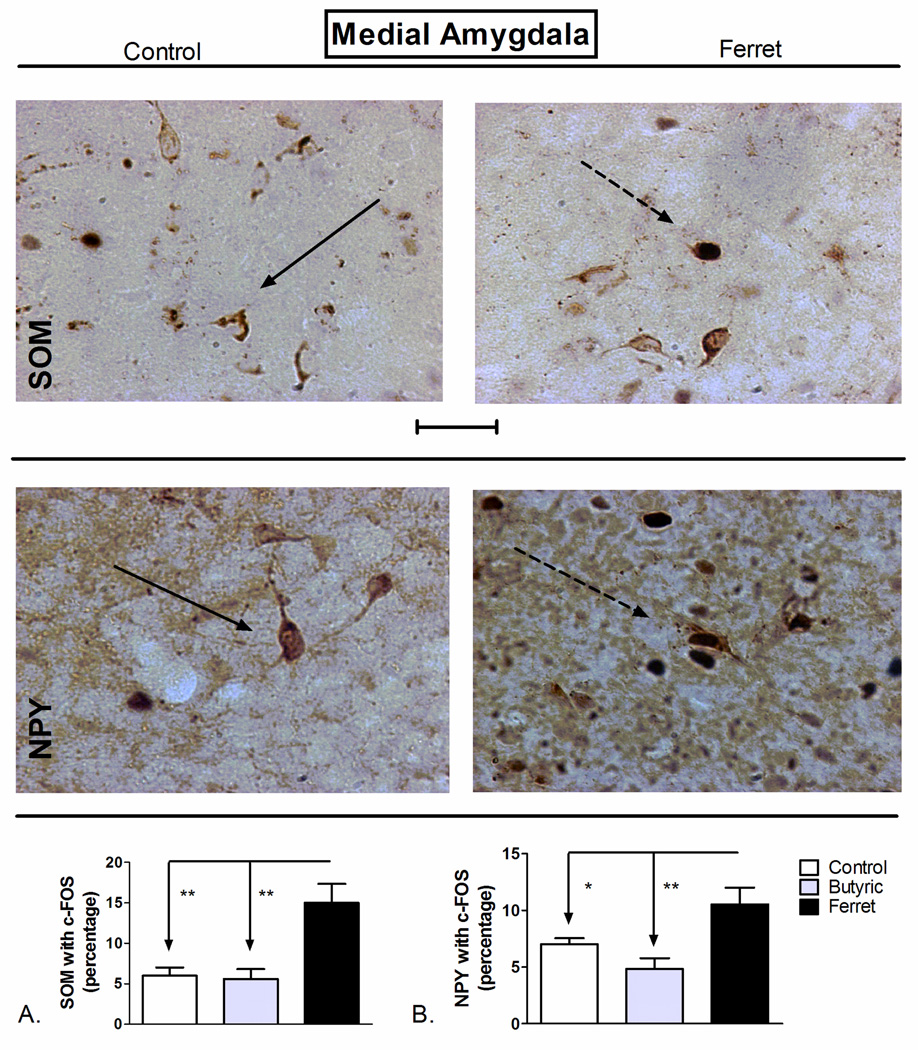
3.4. Exposure to ferret-odor increased SOM- and NPY-positive neuronal activation in the medial amygdala while elevated plus maze exposure increased SOM-positive neuronal activation
Although there is an increase in c-Fos expression in the MEA of rodents following exposure to predator odor (Butler et al., 2011; Dielenberg et al., 2001), the phenotypic characteristics of these activated neurons have not been adequately described. Here we report a significant effect of odor exposure in the MEA on SOM-positive neuronal co-localization with c-Fos (F2,17=10.55; P=0.0012; Figure 7A). SNK post-hoc analysis revealed a significant increase in the percentage of SOM-positive c-Fos expression following exposure to ferret odor (P<0.01) compared to butyric acid and to control odor. NPY-positive neurons in the MEA are mostly GABAergic (Oberto et al., 2001), and we found a significant effect of odor exposure on the activation of NPY positive neurons throughout the MEA (F2,14=7.731; P=0.007; Figure 7B). Exposure to ferret odor increased the co-expression of NPY with c-Fos compared to both butyric acid and control odor exposure throughout the MEA (P<0.001).
Figure 7.
Odor exposure-induced changes in c-Fos expression of A) SOM+ and B) NPY+ neurons of the medial amygdala (MEA). (Top) Representative photomicrograph with SOM (brown) and c-Fos (blue/black) labeling in control (left) and ferret odor-exposed (right) rats. (Middle) Representative photomicrograph with NPY (brown) and c-Fos (blue/black) labeling in control (left) and ferret odor-exposed (right) rats. Exposure to ferret odor increased the percentage of SOM- and NPY-positive neurons with c-Fos in the MEA (Bottom, A and B) compared to control odor and butyric acid. **P<0.01, *P<0.05 (N=5–7). The data represent cumulative counts from anterior and posterior sections (A and B). Percent SOM or NPY with c-Fos neurons represent: (the total number of double-labeled neurons / total NPY or SOM-labeled neurons) * 100. Pictures are at 40× magnification. Scale bar represents approximately 50 microns. The solid arrow points to a single-labeled neuron (blue/black = c-Fos and brown = SOM or NPY), and the dotted arrow points to a double-labeled SOM/c-Fos neuron.
Elevated plus maze exposure also resulted in a significant increase in the percentage of SOM-positive neurons co-localized with c-Fos compared to controls in the MEA (t=4.012 df=7, P=0.005; Figure 8A). Surprisingly, unlike ferret odor exposure, NPY-positive neurons in MEA did not exhibit any significant co-localization with c-Fos after EPM exposure (t=0.304 df=15, P=0.766; Figure 8B).
A significant increase in the density of double-labeled SOM/c-Fos neurons was observed in the MEA following either ferret odor-exposure compared to butyric acid and control odor (Table 1; F2,17=10.77; P=0.0011), or following EPM-exposure (Table 2; P=0.0052, t=3.806 df=8). A significant increase in the density of double-labeled NPY/c-Fos neurons was observed in the MEA following ferret odor-exposure (Table 1; F2,16=6.303; P=0.0112), but not EPM exposure.
Exposure to the different odors (butyric acid, ferret odor; Table 1) or the EPM (Table 2) did not significantly affect the densities of NPY- or SOM- containing neurons compared to controls in the BLA, CEA, or MEA (Tables 1 and 2).
4. Discussion
In the current study, we compared the pattern of activation of SOM- and NPY-positive neuronal populations in the amygdala following exposure to predator odor (ferret) and the elevated plus maze (EPM). Rats exposed to the EPM or predator odor displayed increased single-labeled c-Fos in limbic regions such as the BLA and MEA (and other non-amygdala limbic regions; see below; Butler et al. 2011). Dual-labeled immunohistochemistry showed that rat exposed to butyric acid or ferret odor displayed decreased co-localization of c-Fos with SOM in the BLA whereas rats exposed to the EPM displayed increased co-localization of c-Fos with SOM. In the CEA, ferret odor and butyric acid exposure, but not EPM exposure, decreased co-localization of c-Fos with SOM. In the MEA, ferret odor or EPM exposure, but not butyric acid exposure, resulted in increased co-localization of c-Fos with SOM. Also in the MEA, ferret odor exposure, but not butyric acid or EPM exposure, resulted in increased co-localization of NPY with c-Fos. Exposure of rats to a either a predator odor or a noxious control odor elicits aversive behaviors such as increased defensive burying and a decrease in the latency to bury, although the levels of burying are significantly greater in response to predator odor than butyric acid (Butler et al., 2011; Hebb et al., 2004). Similar to predator exposure, this study demonstrates that robust c-Fos expression occurs in amygdala subregions in response to EPM exposure. Specifically, EPM or ferret odor exposure both resulted in increased c-Fos expression in the MEA; however, in contrast, EPM exposure resulted in increased c-Fos expression in the BLA whereas predator odor exposure had previously been shown not to affect total c-Fos expression (Baisley et al., 2011; Butler et al., 2011). Neuron activation in response to EPM was seen in areas outside the amygdala, specifically the Cg, NAcC, and Cl, which expands upon and supports previous research in the area (Duncan et al., 1996). The exception to increased c-Fos expression in response to the EPM was the NAcS, for which the increase in c-Fos expression did not reach statistical significance. A prior study showed a very similar pattern of increased c-Fos expression in the BLA and MEA following EPM exposure (Salome et al., 2004) although others have shown no activation in these regions (Linden et al., 2004). However, the latter study differed from the present study in that animals received subcutaneous injection of saline prior to the test. The regions of interest in this study are also known to express c-Fos after other anxiogenic stimuli such as foot shock avoidance and air puff (Duncan et al., 1996), as well as predator odor (Baisley et al., 2011; Dielenberg et al., 2001; Janitzky et al., 2009). The reliable activation of these limbic areas by aversive stimuli supports their involvement in behavioral responses to emotional stimuli, although the activation of distinct neuronal populations is seen with different anxiogenic stressors.
This study begins the task of comparing which phenotypic populations of neurons in these regions are activated in response to different anxiogenic stimuli that have distinct behavioral responses, namely defensive burying responses to predator odor and novelty exposure on the EPM. Interestingly, despite the apparent relationship of NPY to anxiety and behavior (Kask et al., 2002; Primeaux et al., 2005), activation of neurons containing the neuropeptide was not altered in response to the EPM in the regions examined. Since NPY’s role in anxiety is thought to be regaining homeostasis (for review see Heilig, 2004), it is possible that the two-hour window allowed during the current study is not long enough to detect c-Fos in NPY neurons, since they become activated at a later time point after anxiogenic stimuli. Another possibility is that NPY neurons in these areas do not make c-Fos in response to a challenge and instead synthesize other immediate early genes such as zif-268 or arc. Regardless, given the large number of c-Fos-positive neurons in response to the EPM in some brain regions, the results of this study suggest that non-NPY containing neurons are activated in the limbic system as an immediate response to EPM stimuli.
Our data suggest that following EPM exposure, SOM-positive neurons in the BLA are activated. Interestingly, the opposite effect occurs following exposure to ferret odor in the BLA where a decrease in co-expression of c-Fos with SOM was observed. The opposing effects on SOM-positive neuronal activation of these two anxiogenic stimuli raise the possibility that BLA SOM-positive neurons play an important role in behavioral processing to predator and/or noxious odors versus environmental stress induced by a complex novel environment. The fact that both butyric acid and ferret odor exposure yielded similar behavior effects (defensive burying) and both resulted in a decrease in BLA SOM-positive neuronal activation further corroborates this hypothesis.
Additional populations of interest include enkephalin (ENK), and/or corticotrophin releasing factor (CRF) containing neurons, which are thought to comprise two distinct populations from NPY-containing neurons in the CEA (Cassell et al., 1986; Wray and Hoffman, 1983). Both opioid and CRF- amygdala systems are known to be involved with anxiety responses in general and behavior in the EPM in particular (Burghardt and Wilson, 2006; for reviews see Drolet et al., 2001; Koob and Heinrichs, 1999; Koob et al., 1993; Wilson and Junor, 2008). Future research will focus on characterizing these potential phenotypic neuron populations in an effort to determine which neurons are activated in response to anxiogenic stimuli.
Previous studies have shown that the MEA is an important relay region for the neuroendocrine response to ferret odor (Masini et al., 2009). As mentioned above, the data presented here shows that butyric acid and ferret odor exposure induce the same decrease in co-localization of somatostatin with c-Fos in the BLA and CEA. However, in the MEA, only ferret odor exposure induced activation of SOM and NPY containing neurons; this activation pattern was not observed following butyric acid exposure. Both ferret odor and butyric acid induce similar behavioral effects but they differ in magnitude (Butler et al., 2011). Because there is differential activation of neuronal populations to ferret odor and butyric acid in the MEA, the possibility exists that anxiogenic (predator) stimuli are processed by SOM- and NPY-positive neurons whereas noxious olfactory stimuli such as butyric acid are not processed by these subpopulations. This observation also suggests that c-Fos activation in MEA is not solely driven by the motor component of the response to an aversive odor.
The hypothesis that predator and noxious stimuli are processed by different amygdala neuronal populations adds to our prior study where we demonstrated that Ca2+/calmodulin-dependent protein kinase II (CAMKII)-positive projection neurons of the BLA are activated following predator odor stimuli whereas noxious stimuli such as butyric acid are processed by both projection neurons and CB-positive interneuronal populations of the BLA (Butler et al., 2011). In the prior study we showed that in the CEA, exposure to a ferret odor, but not high levels of butyric acid, resulted in an increase in CAMKII neuronal activation (Butler et al., 2011). In this study both butyric acid and ferret odor exposure have similar effects on the activation of SOM- and NPY-positive neurons in BLA and CEA. Elegant optogenetic studies have shown that the microcircuitry of the amygdala, specifically in the BLA and CEA, involve neurons which produce different behavioral outputs especially those related to conditioned fear and fear extinction (Haubensak et al., 2010; Herry et al., 2008). Taken together, the cumulative data shows that activation of certain neuronal populations are associated with the detection of anxiogenic stimuli, rather than the specific behavior output, while other neuronal populations in the amygdala help determine the behavioral output based on the environmental situation.
One of the major findings of this study is that exposure to butyric acid, ferret odor, or the EPM results in activation of different populations of neurons in the amygdala compared to control groups, although the control group for the predator odor and EPM tests differed considerably. In the predator odor experiment, control rats were exposed to the arena and an unscented cloth for 30 minutes whereas in the EPM test, control rats were not removed from their home cages. The rats in the predator odor experiment were, however, habituated to the novel arena for two days prior to testing. Although the baseline levels of activation of SOM- and NPY-positive neurons in the control groups between the two experiments were very similar, there is still the possibility that the difference in the control groups between the two experiments may influence the activation of certain regions of the amygdala and/or the level of SOM or NPY expression.
One of the rather surprising findings of this study is that the pattern of activation of SOM- and NPY-positive neurons in the same subregion differed for the same stressor. McDonald (1989) showed that SOM and NPY are extensively co-localized throughout the amygdala. Specifically, 90–100% of NPY neurons in the BLA, CEA, and MEA also express SOM. However, the McDonald study also showed that only 27–39% of BLA and 65–78% of MEA neurons expressing SOM also express NPY. In addition, this same study showed that co-localization does not extend to the lateral subdivision of the CEA. Thus, the small population of neurons in the BLA and MEA which contain either SOM or NPY, but not both, may play critical roles in the different behavior effects of these two tests. Another surprising result is the dramatic difference in density of SOM-positive neurons between the control groups of the two experiments in the BLA and CEA. This may be due to minor differences in staining between the two experiments or the fact that these analyses were done in different runs with slightly different staining times and different batches antiserum, plus sections differed in thickness. In addition, the control subjects between EPM and odor experiments are handled differently, and could have resulted in different levels of some neuronal phenotypes. However, the method for determining area was the same within each experiment and there were no treatment differences in SOM-positive neuronal density.
The study presented here enhances our knowledge of the amygdala circuitry involved in the expression of innate fear induced by the odor of a natural predator or to an environmental stressor. We show that distinct neuronal populations in the amygdala are activated by different stressors/anxiogenic stimuli, suggesting that these stimuli activated unique neuronal circuits through this brain area. Such knowledge is important for the fundamental understanding of fear and anxiety and could be used to determine viable targets for anxiety-related disorders.
Highlights.
Ferret odor decreased activation of rat basolateral amygdala somatostatin neurons
Elevated plus maze increased activity of basolateral amygdala somatostatin neurons
Ferret odor decreased activation of central amygdala somatostatin neurons
Elevated plus maze increased activation of medial amygdala somatostatin neurons
Ferret odor increased activation of medial amygdala somatostatin and NPY neurons
ACKNOWLEDGEMENTS
This work was funded by an NIMH RO1 MH063344 grant awarded to MAW and JRF. Additionally, we also acknowledge a NARSAD Young Investigator Award from the Brain & Behavior Research Foundation to RKB. A memorium to Pumpkin, Zooey, Valentine from Dr. John Hines (Yale University) for supplying ferret-scented towels.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Baisley SK, Cloninger CL, Bakshi VP. Fos expression following regimens of predator stress versus footshock that differentially affect prepulse inhibition in rats. Physiol Behav. 2011;104:796–803. doi: 10.1016/j.physbeh.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J.Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci.Biobehav.Rev. 2005;29:1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Buchan AM, Sikora LK, Levy JG, McIntosh CH, Dyck I, Brown JC. An immunocytochemical investigation with monoclonal antibodies to somatostatin. Histochemistry. 1985;83:175–180. doi: 10.1007/BF00495150. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Wilson MA. Microinjection of naltrexone into the central, but not the basolateral, amygdala blocks the anxiolytic effects of diazepam in the plus maze. Neuropsychopharmacology. 2006;31:1227–1240. doi: 10.1038/sj.npp.1300864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler RK, Sharko AC, Oliver EM, Brito-Vargas P, Kaigler KF, Fadel JR, Wilson MA. Activation of phenotypically-distinct neuronal subpopulations of the rat amygdala following exposure to predator odor. Neuroscience. 2011;175:133–144. doi: 10.1016/j.neuroscience.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci.Biobehav.Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann.N.Y.Acad.Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. J.Comp Neurol. 1986;246:478–499. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- Chieng B, Christie MJ. Somatostatin and nociceptin inhibit neurons in the central nucleus of amygdala that project to the periaqueductal grey. Neuropharmacology. 2010;59:425–430. doi: 10.1016/j.neuropharm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Chieng BC, Christie MJ, Osborne PB. Characterization of neurons in the rat central nucleus of the amygdala: cellular physiology, morphology, and opioid sensitivity. J.Comp Neurol. 2006;497:910–927. doi: 10.1002/cne.21025. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kim JJ. Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proc.Natl.Acad.Sci.U.S.A. 2010;107:21773–21777. doi: 10.1073/pnas.1010079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos.Trans.R.Soc.Lond B Biol.Sci. 1997a;352:1675–1687. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Ann.N.Y.Acad.Sci. 1997b;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. "When a rat smells a cat": the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Prog.Neuropsychopharmacol.Biol.Psychiatry. 2001;25:729–741. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Knapp DJ, Breese GR. Neuroanatomical characterization of Fos induction in rat behavioral models of anxiety. Brain Res. 1996;713:79–91. doi: 10.1016/0006-8993(95)01486-1. [DOI] [PubMed] [Google Scholar]
- File SE, Gonzalez LE, Gallant R. Role of the basolateral nucleus of the amygdala in the formation of a phobia. Neuropsychopharmacology. 1998;19:397–405. doi: 10.1016/S0893-133X(98)00035-9. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of 'fear'-motivated behaviour. Naunyn Schmiedebergs Arch.Pharmacol. 1984;327:1–5. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb AL, Zacharko RM, Gauthier M, Trudel F, Laforest S, Drolet G. Brief exposure to predator odor and resultant anxiety enhances mesocorticolimbic activity and enkephalin expression in CD-1 mice. Eur.J.Neurosci. 2004;20:2415–2429. doi: 10.1111/j.1460-9568.2004.03704.x. [DOI] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. Eur.J.Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Janitzky K, Stork O, Lux A, Yanagawa Y, Schwegler H, Linke R. Behavioral effects and pattern of brain c-fos mRNA induced by 2,5-dihydro-2,4,5-trimethylthiazoline, a component of fox feces odor in GAD67-GFP knock-in C57BL/6 mice. Behav.Brain Res. 2009;202:218–224. doi: 10.1016/j.bbr.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am.J.Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kask A, Harro J, von HS, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide. Y.Neurosci.Biobehav.Rev. 2002;26:259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, Britton KT. The role of corticotropin-releasing factor in behavioural responses to stress. Ciba Found.Symp. 1993;172:277–289. doi: 10.1002/9780470514368.ch14. [DOI] [PubMed] [Google Scholar]
- Linden AM, Greene SJ, Bergeron M, Schoepp DD. Anxiolytic activity of the MGLU2/3 receptor agonist LY354740 on the elevated plus maze is associated with the suppression of stress-induced c-Fos in the hippocampus and increases in c-Fos induction in several other stress-sensitive brain regions. Neuropsychopharmacology. 2004;29:502–513. doi: 10.1038/sj.npp.1300321. [DOI] [PubMed] [Google Scholar]
- Martinez RC, Carvalho-Netto EF, Ribeiro-Barbosa ER, Baldo MV, Canteras NS. Amygdalar roles during exposure to a live predator and to a predator-associated context. Neuroscience. 2011;172:314–328. doi: 10.1016/j.neuroscience.2010.10.033. [DOI] [PubMed] [Google Scholar]
- Masini CV, Sasse SK, Garcia RJ, Nyhuis TJ, Day HE, Campeau S. Disruption of neuroendocrine stress responses to acute ferret odor by medial, but not central amygdala lesions in rats. Brain Res. 2009;1288:79–87. doi: 10.1016/j.brainres.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Somatostatinergic projections from the amygdala to the bed nucleus of the stria terminalis and medial preoptic-hypothalamic region. Neurosci.Lett. 1987;75:271–277. doi: 10.1016/0304-3940(87)90533-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Coexistence of somatostatin with neuropeptide Y, but not with cholecystokinin or vasoactive intestinal peptide, in neurons of the rat amygdala. Brain Res. 1989;500:37–45. doi: 10.1016/0006-8993(89)90297-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Betette RL. Parvalbumin-containing neurons in the rat basolateral amygdala: morphology and co-localization of Calbindin-D(28k) Neuroscience. 2001;102:413–425. doi: 10.1016/s0306-4522(00)00481-4. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Res. 2002;943:237–244. doi: 10.1016/s0006-8993(02)02650-1. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Muller JF, Mascagni F. GABAergic innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. J.Comp Neurol. 2002;446:199–218. doi: 10.1002/cne.10204. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Pearson JC. Coexistence of GABA and peptide immunoreactivity in non-pyramidal neurons of the basolateral amygdala. Neurosci.Lett. 1989;100:53–58. doi: 10.1016/0304-3940(89)90659-9. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann.N.Y.Acad.Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- Moga MM, Gray TS. Evidence for corticotropin-releasing factor, neurotensin, and somatostatin in the neural pathway from the central nucleus of the amygdala to the parabrachial nucleus. J.Comp Neurol. 1985;241:275–284. doi: 10.1002/cne.902410304. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatincontaining interneurons in the rat basolateral amygdala. J.Comp Neurol. 2007a;500:513–529. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Serotonin-immunoreactive axon terminals innervate pyramidal cells and interneurons in the rat basolateral amygdala. J.Comp Neurol. 2007b;505:314–335. doi: 10.1002/cne.21486. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc.Natl.Acad.Sci.U.S.A. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberto A, Panzica GC, Altruda F, Eva C. GABAergic and NPY-Y(1) network in the medial amygdala: a neuroanatomical basis for their functional interaction. Neuropharmacology. 2001;41:639–642. doi: 10.1016/s0028-3908(01)00109-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. Ref Type: Generic. [Google Scholar]
- Primeaux SD, Wilson SP, Cusick MC, York DA, Wilson MA. Effects of altered amygdalar neuropeptide Y expression on anxiety-related behaviors. Neuropsychopharmacology. 2005;30:1589–1597. doi: 10.1038/sj.npp.1300705. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci.Biobehav.Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Donley MP. Animal studies of amygdala function in fear and uncertainty: relevance to human research. Biol.Psychol. 2006;73:49–60. doi: 10.1016/j.biopsycho.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez de AM, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Salome N, Salchner P, Viltart O, Sequeira H, Wigger A, Landgraf R, Singewald N. Neurobiological correlates of high (HAB) versus low anxiety-related behavior (LAB): differential Fos expression in HAB and LAB rats. Biol.Psychiatry. 2004;55:715–723. doi: 10.1016/j.biopsych.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Stock HS, Hand GA, Ford K, Wilson MA. Changes in defensive behaviors following olfactory bulbectomy in male and female rats. Brain Res. 2001;903:242–246. doi: 10.1016/s0006-8993(01)02421-0. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Junor L. The role of amygdalar mu-opioid receptors in anxiety-related responses in two rat models. Neuropsychopharmacology. 2008;33:2957–2968. doi: 10.1038/sj.npp.1301675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S, Hoffman GE. Organization and interrelationship of neuropeptides in the central amygdaloid nucleus of the rat. Peptides. 1983;4:525–541. doi: 10.1016/0196-9781(83)90059-1. [DOI] [PubMed] [Google Scholar]