Abstract
Background
The Network for Pancreatic Organ donors with Diabetes was established to recover and characterize pancreata and related organs from cadaveric organ donors with various risk levels for type 1 diabetes (T1D). These biospecimens are available to investigators for collaborative studies aimed at addressing questions related to TID natural history and pathogenesis.
Research design and methods
Organ donors included T1D patients (new-onset to long-term), non-diabetic autoantibody positive subjects, non-diabetic controls, and individuals with disorders relevant to β-cell function. Pancreas recovery and transport met transplant-grade criteria. Additional samples recovered included serum, whole blood, spleen, and pancreatic and non-pancreatic lymph nodes. Biospecimens were processed for cryopreserved cells, fixed paraffin and fresh frozen blocks, and snap frozen samples. T1D autoantibodies, c-peptide levels, and high-resolution HLA genotyping for risk alleles were also determined.
Results
Over 160 donors have been enrolled (ages of 1 day to > 90 years). Standard operating procedures were established along with a quality management system. Donor demographics, laboratory assays, and histopathological characterizations were shared through an open online informatics system. Biospecimens were distributed to more than 60 investigators.
Conclusions
The nPOD program provides access to high quality biospecimens without cost to investigators. Collaborations and open data sharing are emphasized to maximize research potential of each donor. Based on initial successes, the nPOD program is expanding to recover additional organs relevant to T1D pathogenesis and complications from European countries (PanFin network).
Keywords: β-cell, autoantibodies, HLA, type 1 diabetes
Introduction
Classic T1D is considered a T-cell-mediated autoimmune disease resulting from a selective destruction of the pancreatic insulin-producing β-cells (reviewed in (1)). T1D is the most common cause of diabetes in children but the disorder can be diagnosed at essentially any age (2). The disease is also thought to result from a combination of genetic predispositions in association with unknown environmental factors including viruses, diet, and geography (3). Despite more than four decades of appreciating the autoimmune nature of T1D, multiple questions remain regarding the exact nature of β-cell destruction in T1D. Indeed, an improved understanding of the underlying mechanisms of insulitis development could have profound therapeutic and preventative implications. Beyond this, explanations for β-cell demise in T1D could represent infection due to a viral or microbial origin, alterations secondary to hyperglycemia, unknown toxin(s), and/or a primary immunological reaction. The possibility of an infectious cause has received considerable attention, particularly with the observation of increasing T1D incidences in several western countries (reviewed in (4)).
In 2007, the Juvenile Diabetes Research Foundation (JDRF) established the Network for Pancreatic Organ donors with Diabetes (nPOD) for the purpose of obtaining tissues from cadaveric organ donors to address important questions regarding the pathogenesis and natural history of T1D. To aid in understanding genetic and environmental factors, as well as identifying their role in influencing the immune response underlying β-cell destruction, nPOD recovers biospecimens from several donor groups including T1D patients (new-onset to >75 years of disease duration), those without diabetes (controls) but representing complementary ages, gender, and ethnicity groups, non-diabetic autoantibody-positive donors, and those with additional conditions that impact β-cell function (e.g., gestational diabetes, cystic fibrosis, type 2 diabetes). While each of these groups are vital for studies of T1D, the majority of nPOD efforts are directed at the recovery of tissues from donor groups that would be most difficult for individual investigators to obtain, namely those who are autoantibody positive without clinical signs of T1D, as well as those at the earliest stages of the disease.
It is generally accepted that the destructive processes underlying T1D begin months to years before the manifestation of clinical symptoms. Autoantibodies directed against different antigens of pancreatic β-cells, glutamic acid decarboxylase-65 antibodies (GADA), insulinoma-associated-2ic antibodies (IA2A), insulin autoantibodies (mIAA), and the recently identified zinc transporter 8 autoantibodies (ZnT8A), have been utilized to identify those in this prodromal period (5, 6). Epidemiological studies also suggest that persons having two or more islet-related autoantibodies are at highest risk of developing T1D (7). The availability of a reliable and rapid (i.e., 90 minute) ELISA-based assay for GADA and IA-2A allowed nPOD to screen organ donors with research consent for these markers during the same time frame that transplant suitability is being determined (8). Analysis of all four autoantibodies is of value, and as such, comprehensive screening is conducted by the nPOD Autoantibody Core. To be clear, much rationale in support of such screening was drawn from studies in Finland, Belgium, as well as in the U.S. (9–12). The major goal of the current report is to provide an over-view of nPOD protocols as well as preliminary clinical data for donor demographics and laboratory analyses.
Research design and methods
nPOD Organization
The nPOD program consists of administrative, organ processing and pathology core (OPPC), autoantibody, and data management cores. The OPPC was primarily responsible for donor sample processing, analysis, storage, and distribution. The Autoantibody Core performed autoantibody analyses of serum or plasma obtained from the donor screening processes and at the time of organ recovery. The Data Management Core acquired donor clinical data through the United Network for Organ Sharing (UNOS) and maintained an online database for autoantibody screening and autoantibody core results.
Organ donor recovery
The OPO coordinators evaluated potential donors for nPOD inclusion and exclusion criteria and autoantibody status for those participating in autoantibody screening. Donor recovery was performed either directly with the OPO or through subsequent referral to the National Disease Research Interchange or the International Institute for the Advancement of Medicine. In addition to T1D donors, donors with no history of diabetes (i.e., controls), autoantibody positive donors with no diabetes (screened for GADA and IA-2A), and those with other diseases (e.g., type two diabetes, gestational diabetes, cystic fibrosis) are recovered as deemed of research significance for T1D investigators. Control donors are accepted to match demographic characteristics (i.e., age, gender, ethnicity, BMI) of the T1D donors or for specific initiatives such as β-cell developmental studies.
Autoantibody Screening
nPOD screening labs utilized a modified, rapid ELISA kit (Kronus, Star, ID) with internal standard curve and recorded results as either positive or negative. The screening laboratory was responsible for contacting the OPO coordinators when positive. If the organ donor was autoantibody positive, nPOD was contacted by the OPO for research placement. As part of the quality assurance process, an aliquot of the positive screening serum sample was reanalyzed with a Kronus ELISA kit in Gainesville FL, and at the autoantibody core for GADA, IA-2A, mIAA, and ZnT8A by radioligand-binding assay (RIA) as previously described (7).
Donor sample processing
Pancreas recovery was performed as for a standard transplantation and included intra-operative flushing in the majority of cases. The pancreas with fat containing pancreatic lymph nodes and proximal duodenum (stapled closed) were placed in a sterile container with additional media, submerged in ice, and shipped by standard organ courier to the University of Florida. Spleen and non-pancreatic lymph nodes were also recovered as were serum and whole blood tubes and shipped with the pancreas. The donor processing team dissected tissues and prepared a variety of storage formats for each sample type. The pancreas was weighed then divided into regions (head, body, tail). Each region was transected into alternating sections for fixed (formalin) and fresh frozen blocks. Minced samples were snap frozen in liquid nitrogen with or without RNAse inhibitor (RNAlater, Ambion, CA). Spleen and lymph node samples were processed in two main formats, the first as for the pancreas and the second for cryopreserved cells. In addition, fresh spleen and pancreatic lymph nodes were also minced and placed into sterile media for immediate shipment to investigators. Whole blood was processed to peripheral blood mononuclear cells (PBMC) using Ficoll density gradient followed by cryopreservation (13). All samples and aliquots were inventoried in an online database with reservation of approximately 30% of each sample type to ensure availability for next generation analytics.
Histopathological analyses
Six pancreas (two from each region) and one spleen paraffin block from each donor were serially sectioned and stained by hematoxylin and eosin (H&E) and immunohistochemistry. Two double immunohistochemistry stains were used to identify cell replication (Ki67) and β-cells (insulin), or T cell infiltration (CD3) and alpha cells (glucagon) according to published methods (14). Histopathological review was conducted with an emphasis on islet morphology, size, and numbers, alpha- and β-cell composition, and presence of inflammation (i.e., insulitis, pancreatitis). Insulitis was distinguished by the presence of 6 or more CD3+ cells adjacent to or within an islet. Stained slides were digitized by whole slide scanning using an Aperio CS scanner and were organized by each donor using the Spectrum Plus information management system (version 11, Aperio, Vista, CA). All paraffin blocks from the autoantibody positive donors were reviewed for the presence of insulitis following H&E staining.
Laboratory analyses
Sera obtained from the screening process and/or at donor organ recovery were analyzed by the autoantibody core. Donor C-peptide levels were determined by radioimmunoassay at the Northwest Lipid Metabolism and Diabetes Research Laboratories (Dr. Santica Marcovina, University of Washington, Seattle, WA) with the lower limit of detection at <0.05 ng/ml. Genomic DNA was extracted from frozen spleen and quality and concentration assessed by spectrophometry and agarose gel electrophoresis. Genomic DNA was analyzed for T1D genetic susceptibility on human leukocyte antigen (HLA) genotypes at the Children’s Hospital Oakland Research Institute (Dr. Janelle Noble, Oakland, CA) using sequence-specific oligonucleotide hybridization, as previously described (15).
Disease Classification
Clinical history was obtained through current hospital admission records and the UNOS questionnaire for donation. Donors with T1D were classified based on the available clinical history (e.g., past medical history including diabetic ketoacidosis, pre-transplant HLA, medications, BMI, HbA1c levels). Autoantibody status, C-peptide levels, and the presence of high-risk HLA alleles were considered (reviewed in (16)).
Ethical aspects
All procedures were in accordance with federal guidelines for organ donation and the University of Florida Institutional Review Board.
Statistics
For descriptive statistics, number and % were reported for all categorical variables. The measure of central tendency was described using either mean (± 1 SD) or median (min, max) for all continuous variables, as noted. P-values were generated for continuous parametric variables using an analysis of variance (ANOVA). The Kruskall-Wallis test was used to determine the P-values for continuous non-parametric variables. P-values for nominal categorical variables were determined using Pearson’s chi square when values in all cells exceeded 5. Fisher’s exact test was used only when values from 1 or more cells in a contingency table was found to be less than 5. If values from 2 or more cells in any table were equal to 0, p-values were not reported. All statistical analysis was performed using SAS software version 9.2 (SAS Institute, Cary, NC).
Results
Organ donor autoantibody screening
The rapid screening assay was established at 5 laboratories during the initial phase of the nPOD program. A total of 1677 donor sera were screened of which 135 were autoantibody positive (average 8.1%, range 4%–10% between labs). Twenty-one donors were recovered for the nPOD program (15.6%, range 10%–45%). Analysis of donor sera obtained at organ recovery showed that 13 donors were positive for one or more autoantibodies by RIA (62%).
Organ donor characteristics and disease specific factors
Organ donors consisted of 161 patients and general characteristics are presented in Table 1 for the no diabetes, autoantibody positive, and T1D donor groups. Age, gender, ethnicity, weight, and body mass index showed no significant differences between groups. As well, donation after cardiac death, hypertension, heavy alcohol use, and cocaine or other drug use was not significant between groups. Cigarette use was significantly different between the groups with the highest proportion in autoantibody positive donors. Additional donors recovered included T1D Medalists (12), type 2 diabetes (15), gestational diabetes (1), cystic fibrosis-related diabetes (2) and others (6; Turner Syndrome, acute hyperglycemia (2), acute eclampsia, and unknown diabetes duration (2)).
Table 1.
General Organ Donor Characteristics of nPOD Cases.
| Factors | (%), Mean (± 1 SD), or Median (min, max)A
|
p-value | |||||
|---|---|---|---|---|---|---|---|
| n | No Diabetes | n | Auto AB+ Only | n | Type 1 Diabetes | ||
|
| |||||||
| Age (in yrs) | 59 | 26.6 (± 20.7) | 12 | 35.6 (± 23.6) | 46 | 28.0 (± 11.9) | 0.2950 |
|
| |||||||
| Gender | |||||||
| Female | 22 | 37% | 5 | 42% | 22 | 48% | 0.5545 |
| Male | 37 | 62% | 7 | 58% | 24 | 52% | |
|
| |||||||
| EthnicityB | |||||||
| Black/African Am. | 6 | 10% | 0 | 0% | 4 | 9% | 0.2473 |
| Hispanic/Latino | 6 | 10% | 3 | 25% | 2 | 4% | |
| White | 45 | 79% | 9 | 75% | 39 | 87% | |
|
| |||||||
| Weight (in kg) | 59 | 60.0 (± 32.3) | 12 | 66.3 (± 34.7) | 46 | 69.2 (± 16.8) | 0.2307 |
|
| |||||||
| Body Mass Index (kg/m2) | 59 | 23.5 (± 6.0) | 12 | 25.2 (± 5.8) | 46 | 25.2 (± 4.3) | 0.2540 |
|
| |||||||
| Donation After Cardiac Death | |||||||
| No | 47 | 80% | 9 | 75% | 42 | 91% | 0.1402 |
| Yes | 12 | 20% | 3 | 25% | 4 | 9% | |
|
| |||||||
| History of Hypertension | |||||||
| No | 47 | 80% | 9 | 75% | 28 | 62% | 0.1267 |
| Yes | 12 | 20% | 3 | 25% | 17 | 38% | |
|
| |||||||
| Heavy Alcohol Use | |||||||
| No | 53 | 90% | 11 | 92% | 42 | 91% | 1.0000 |
| Yes | 6 | 10% | 1 | 8% | 4 | 9% | |
|
| |||||||
| History of Cigarette Use | |||||||
| No | 51 | 88% | 8 | 67% | 44 | 96% | 0.0259 |
| Yes | 7 | 12% | 4 | 33% | 2 | 4% | |
|
| |||||||
| History of Cocaine Use | |||||||
| No | 50 | 88% | 11 | 92% | 36 | 82% | 0.6240 |
| Yes | 7 | 12% | 1 | 8% | 8 | 18% | |
|
| |||||||
| History of Other Drug Use | |||||||
| No | 40 | 70% | 8 | 67% | 33 | 75% | 0.7923 |
| Yes | 17 | 30% | 4 | 33% | 11 | 25% | |
Number and % are reported for all categorical variables. Use of mean or median for continuous variables based on evaluation of skewness, kurtosis, normal probability plots, and histogram visualization of the distribution of values.
Grouping based on UNOS coding
Disease specific factors in the three major donor groups are presented in Table 2. Duration of diabetes, age at onset, history of diabetes, and insulin dependence were only applicable to the T1D group resulting in an expected significant difference from the other two groups. Diabetes-related laboratory data were also significantly different between groups. C-peptide and hemoglobin A1c levels were similar between the no diabetes and autoantibody positive groups while the T1D group had significantly lower and higher levels, respectively. Autoantibodies were exclusive to the autoantibody positive (100%) and T1D groups (83%) (Tables 2–3). Within the autoantibody positive group, the majority were positive for a single autoantibody (GADA (8), mIAA (3), ZnT8A (1)) with only one double autoantibody donor (nPOD 6080; GADA, mIAA+). Autoantibody-positivity in T1D donors included seven with no autoantibodies and the remainder with 1–4 autoantibodies (Table 3). Diabetes risk HLA alleles were also significantly different between groups with the T1D group having the highest proportion of DR3 and/or DR4 alleles (Table 2). Of note, all T1D donors were lacking the HLA-DQB1*0602 protective allele while 75% of the autoantibody positive donors had the protective allele.
Table 2.
Disease Specific Factors of nPOD Cases.
| Factors | (%), Mean (± 1 SD), or Median (min, max)A
|
p-value | |||||
|---|---|---|---|---|---|---|---|
| n | No Diabetes | n | AutoAB + Only | n | Type 1 Diabetes | ||
|
| |||||||
| Duration of Diabetes (in yrs) | 64 | 0.0 (± 0.0) | 13 | 0.0 (± 0.0) | 48 | 15.9 (± 11.7) | <0.0001 |
|
| |||||||
| Age at Onset | 64 | N/A | 13 | N/A | 46 | 12.1 (± 7.3) | Not reported |
|
| |||||||
| C-peptide (ng/mL)B | 51 | 3.15 (0.05, 22.92) | 9 | 3.19 (0.05, 26.2) | 46 | 0.05 (0.05, 0.24) | <0.0001 |
|
| |||||||
| Hemoglobin A1c (%) | 14 | 5.7 (± 0.4) | 2 | 5.3 (± 0.1) | 9 | 9.8 (± 2.1) | <0.0001 |
|
| |||||||
| History of Diabetes | |||||||
| No | 57 | 98% | 12 | 100% | 1 | 2% | <0.0001 |
| Yes | 1 | 2% | 0 | 0% | 45 | 98% | |
|
| |||||||
| Insulin Dependent | |||||||
| No | 1 | 100% | 0 | 0% | 1 | 2% | Not reported |
| Yes | 0 | 0% | 0 | 0% | 41 | 98% | |
|
| |||||||
| Autoantibody Results | |||||||
| Negative | 49 | 100% | 0 | 0% | 7 | 16% | Not reported |
| Positive | 0 | 0% | 13 | 100% | 34 | 84% | |
|
| |||||||
| AutoantibodiesC | |||||||
| GADA | |||||||
| Negative | 49 | 100% | 4 | 31% | 33 | 77% | <0.0001 |
| Positive | 0 | 0% | 9 | 69% | 10 | 23% | |
| IA2A | |||||||
| Negative | 49 | 100% | 13 | 100% | 35 | 81% | Not reported |
| Positive | 0 | 0% | 0 | 0% | 8 | 19% | |
| ZnT8A | |||||||
| Negative | 49 | 100% | 12 | 92% | 34 | 79% | <0.0001 |
| Positive | 0 | 0% | 1 | 8% | 9 | 21% | |
| mIAA | |||||||
| Negative | 49 | 100% | 9 | 69% | 9 | 21% | <0.0001 |
| Positive | 0 | 0% | 4 | 31% | 34 | 79% | |
|
| |||||||
| No. of Positive Autoantibodies/Case | |||||||
| 0 | 49 | 100% | 0 | 0% | 7 | 16% | Not reported |
| 1 | 0 | 0% | 12 | 92% | 19 | 44% | |
| 2 | 0 | 0% | 1 | 8% | 12 | 28% | |
| 3 | 0 | 0% | 0 | 0% | 2 | 5% | |
| 4 | 0 | 0% | 0 | 0% | 3 | 7% | |
|
| |||||||
| HLA-DBR Genotype | |||||||
| DR3 alone | 4 | 9% | 1 | 17% | 8 | 24% | <0.0001 |
| DR4 alone | 12 | 26% | 2 | 33% | 6 | 18% | |
| Both Alleles | 1 | 2% | 0 | 0% | 12 | 35% | |
| Neither | 29 | 63% | 3 | 50% | 8 | 24% | |
|
| |||||||
| HLA-DQB1*0602 Allele | |||||||
| Absent | 33 | 72% | 2 | 33% | 34 | 100% | <0.0001 |
| Present | 13 | 18% | 4 | 67% | 0 | 0% | |
Number and % are reported for all categorical variables. Use of mean or median for continuous variables based on evaluation of skewness, kurtosis, normal probability plots, and histogram visualization of the distribution of values.
When C-peptide levels were reported below the lower limit of detection, i.e. <0.05, a fill value of 0.05 was used as a laboratory number.
All values converted to NIDDK units and defined as positive for GADA if ≥20, IA2A if ≥5, ZnT8A if ≥0.020, and mIAA if ≥0.010.
Table 3.
Autoantibody titers for organ donors. Autoantibody levels were determined by RIA as described in Methods.
| Donor Type | CaseID | Age (yr) | Gender | Race | AutoAb Summary (RIA) A | GADA | IA2A | ZnT8A | mIAA |
|---|---|---|---|---|---|---|---|---|---|
| No diabetes | 6117 | 0.33 | M | Caucasian | Negative | 1 | 0 | −0.012 | 0.000 |
| No diabetes | 6115 | 0.40 | M | Caucasian | Negative | 11 | 0 | −0.009 | 0.002 |
| No diabetes | 6125 | 0.42 | M | Caucasian | Negative | 0 | 1 | 0.001 | −0.001 |
| No diabetes | 6122 | 0.42 | F | Multiracial | Negative | 1 | 0 | −0.011 | 0.001 |
| No diabetes | 6092 | 0.50 | F | African Am | Negative | 0 | 0 | −0.001 | 0.001 |
| No diabetes | 6072 | 1.00 | F | Caucasian | No serum available | ||||
| No diabetes | 6107 | 2.00 | M | African Am | Negative | 0 | 0 | −0.003 | 0.004 |
| No diabetes | 6103 | 2.00 | M | Caucasian | Negative | 0 | 0 | −0.004 | 0.003 |
| No diabetes | 6014 | 2.00 | M | Caucasian | No serum available | ||||
| No diabetes | 6106 | 3.00 | M | Caucasian | Negative | 0 | 0 | −0.003 | 0.004 |
| No diabetes | 6094 | 3.00 | M | African Am | Negative | 0 | 0 | −0.005 | 0.002 |
| No diabetes | 6130 | 5.00 | M | Caucasian | Negative | 0 | 0 | −0.007 | 0.001 |
| No diabetes | 6005 | 5.00 | F | Caucasian | Negative | 11 | 0 | 0.017 | −0.003 |
| No diabetes | 6112 | 6.00 | F | Hispanic/Latino | Negative | 0 | 0 | −0.001 | 0.002 |
| No diabetes | 6047 | 8.00 | M | Caucasian | Negative | 0 | 0 | −0.002 | −0.001 |
| No diabetes | 6144 | 8.00 | F | Hispanic/Latino | Negative | 0 | 4 | 0.002 | 0.003 |
| No diabetes | 6137 | 9.00 | F | Hispanic/Latino | Negative | 1 | 0 | 0.001 | 0.000 |
| No diabetes | 6007 | 9.00 | M | No serum available | |||||
| No diabetes | 6099 | 14.00 | M | Caucasian | Negative | 0 | 0 | −0.004 | 0.002 |
| No diabetes | 6153 | 15.00 | M | Hispanic/Latino | Negative | 0 | 0 | −0.002 | 0.005 |
| No diabetes | 6096 | 16.00 | F | African Am | Negative | 0 | 0 | −0.003 | 0.003 |
| No diabetes | 6075 | 16.00 | M | African Am | Negative | 11 | 0 | 0.001 | 0.003 |
| No diabetes | 6098 | 18.00 | M | Caucasian | Negative | 0 | 0 | −0.004 | 0.000 |
| No diabetes | 6073 | 19.00 | M | Caucasian | Negative | 0 | 0 | −0.001 | 0.001 |
| No diabetes | 6024 | 21.00 | M | Caucasian | Negative | 0 | 0 | −0.017 | −0.001 |
| No diabetes | 6057 | 22.00 | M | Caucasian | Negative | 0 | 1 | −0.002 | 0.001 |
| No diabetes | 6001 | 22.00 | M | Caucasian | Negative | 9 | 0 | −0.006 | −0.001 |
| No diabetes | 6003 | 23.00 | F | Caucasian | No serum available | ||||
| No diabetes | 6029 | 24.00 | F | Hispanic/Latino | No serum available | ||||
| No diabetes | 6131 | 24.00 | M | Caucasian | Negative | 0 | 2 | 0.001 | −0.002 |
| No diabetes | 6060 | 24.00 | M | Caucasian | Negative | 0 | 0 | −0.005 | −0.001 |
| No diabetes | 6126 | 25.00 | M | Hispanic/Latino | Negative | 0 | 1 | −0.010 | 0.000 |
| No diabetes | 6053 | 25.00 | M | Caucasian | Negative | 0 | 0 | −0.002 | −0.002 |
| No diabetes | 6055 | 27.00 | M | Caucasian | Negative | 0 | 0 | −0.001 | 0.008 |
| No diabetes | 6058 | 27.00 | M | Hispanic/Latino | Negative | 0 | 0 | −0.004 | 0.000 |
| No diabetes | 6091 | 27.00 | M | Caucasian | Negative | 0 | 3 | 0.006 | 0.001 |
| No diabetes | 6134 | 27.00 | M | Caucasian | Negative | 0 | 0 | −0.005 | 0.001 |
| No diabetes | 6048 | 30.00 | M | Caucasian | Negative | 0 | 0 | −0.004 | 0.001 |
| No diabetes | 6030 | 30.00 | M | Caucasian | Negative | 0 | 0 | 0.001 | 0.001 |
| No diabetes | 6034 | 32.00 | F | Caucasian | Negative | 0 | 0 | −0.016 | −0.002 |
| No diabetes | 6004 | 33.00 | M | No serum available | |||||
| No diabetes | 6140 | 38.00 | M | Caucasian | Negative | 0 | 0 | 0.002 | 0.000 |
| No diabetes | 6015 | 39.00 | F | Caucasian | Negative | 0 | 0 | −0.016 | 0.000 |
| No diabetes | 6095 | 40.00 | M | Hispanic/Latino | No serum available | ||||
| No diabetes | 6104 | 41.00 | M | Caucasian | Negative | 0 | 0 | 0.001 | −0.001 |
| No diabetes | 6019 | 42.00 | M | Caucasian | Negative | 0 | 0 | −0.020 | 0.000 |
| No diabetes | 6129 | 43.00 | F | Caucasian | Negative | 2 | 1 | −0.004 | 0.001 |
| No diabetes | 6097 | 43.00 | F | Caucasian | Negative | 0 | 0 | 0.006 | 0.003 |
| No diabetes | 6009 | 45.00 | M | Caucasian | Negative | 0 | 0 | 0.012 | −0.003 |
| No diabetes | 6102 | 45.00 | F | Caucasian | Negative | 0 | 0 | −0.005 | 0.003 |
| No diabetes | 6011 | 46.00 | F | African Am | No serum available | ||||
| No diabetes | 6010 | 47.00 | F | Caucasian | No serum available | ||||
| No diabetes | 6008 | 50.00 | F | Caucasian | No serum available | ||||
| No diabetes | 6017 | 59.00 | F | Caucasian | Negative | 1 | 0 | −0.015 | 0.001 |
| No diabetes | 6020 | 60.00 | M | Caucasian | Negative | 0 | 0 | 0.014 | 0.001 |
| No diabetes | 6016 | 64.00 | F | Caucasian | No serum available | ||||
| No diabetes | 6013 | 65.00 | M | Caucasian | Negative | 0 | 0 | −0.013 | 0.000 |
| No diabetes | 6012 | 68.00 | F | Caucasian | Negative | 0 | 0 | −0.019 | 0.000 |
| No diabetes | 6021 | 72.00 | F | Hispanic/Latino | Negative | 0 | 0 | −0.026 | −0.001 |
| No diabetes | 6022 | 75.00 | M | Caucasian | Negative | 0 | 0 | −0.018 | 0.001 |
| Autoab Pos | 6116 | 0.17 | F | Hispanic/Latino | mIAA+ | 1 | 1 | 0.000 | 0.024 |
| Autoab Pos | 6090 | 2.00 | M | Hispanic/Latino | GADA+ | 490 | 0 | −0.001 | −0.001 |
| Autoab Pos | 6027 | 19.00 | M | Caucasian | ZnT8+ | 0 | 0 | 0.030 | 0.000 |
| Autoab Pos | 6123 | 23.00 | F | Caucasian | GADA+ | 32 | 0 | −0.003 | 0.000 |
| Autoab Pos | 6147 | 24.00 | F | Caucasian | GADA+ | 290 | 1 | 0.004 | 0.001 |
| Autoab Pos | 6151 | 30.00 | M | Caucasian | GADA+ | 53 | 0 | −0.009 | 0.004 |
| Autoab Pos | 6002 | 39.00 | M | Caucasian | mIAA+ | 4 | 0 | −0.002 | 0.051 |
| Autoab Pos | 6156 | 40.00 | M | Caucasian | GADA+ | 208 | 0 | −0.002 | 0.007 |
| Autoab Pos | 6044 | 41.00 | M | Hispanic/Latino | GADA+ | 748 | 0 | −0.003 | 0.001 |
| Autoab Pos | 6154 | 48.00 | F | Caucasian | GADA+ | 845 | 0 | −0.005 | 0.006 |
| Autoab Pos | 6101 | 65.00 | M | Caucasian | GADA+ | 161 | 0 | −0.005 | −0.001 |
| Autoab Pos | 6023 | 66.00 | M | Caucasian | mIAA+ | 2 | 4 | 0.001 | 0.362 |
| Autoab Pos | 6080 | 69.00 | F | Caucasian | GADA+ mIAA+ | 90 | 0 | 0.002 | 0.028 |
| T1D | 6063 | 4.00 | M | Caucasian | mIAA+ | 0 | 0 | 0.010 | 0.065 |
| T1D | 6062 | 11.00 | M | African Am | No serum available | ||||
| T1D | 6079 | 11.00 | F | Caucasian | Negative | 0 | 0 | 0.012 | 0.005 |
| T1D | 6052 | 12.00 | M | African Am | IA-2A+ mIAA+ | 0 | 140 | 0.010 | 0.063 |
| T1D | 6113 | 13.00 | F | Caucasian | mIAA+ | 0 | 0 | 0.001 | 0.873 |
| T1D | 6089 | 14.00 | M | Caucasian | mIAA+ | 0 | 0 | 0.002 | 0.416 |
| T1D | 6084 | 14.00 | M | Caucasian | mIAA+ | 1 | 0 | 0.000 | 2.155 |
| T1D | 6049 | 15.00 | F | African Am | GADA+ mIAA+ | 384 | 2 | 0.017 | 0.392 |
| T1D | 6083 | 15.00 | F | Caucasian | mIAA+ | 0 | 0 | −0.007 | 0.143 |
| T1D | 6148 | 17.00 | M | Caucasian | GADA+ mIAA+ | 568 | 1 | 0.013 | 0.043 |
| T1D | 6145 | 18.00 | M | Caucasian | GADA+ ZnT8(+) mIAA+ | 664 | 3 | 0.021 | 0.822 |
| T1D | 6087 | 18.00 | M | Caucasian | ZnT8+ mIAA+ | 0 | 0 | 0.071 | 0.016 |
| T1D | 6046 | 19.00 | F | Caucasian | IA-2A+ ZnT8+ | 0 | 69 | 0.045 | −0.003 |
| T1D | 6064 | 20.00 | F | Caucasian | GADA+ IA-2A+ ZnT8+ | 38 | 111 | 0.017 | 0.015 |
| T1D | 6051 | 20.00 | M | Caucasian | mIAA+ | 0 | 0 | −0.001 | 0.052 |
| T1D | 6026 | 22.00 | M | Caucasian | Negative | 0 | 0 | −0.009 | 0.031 |
| T1D | 6069 | 23.00 | M | African Am | No serum available | ||||
| T1D | 6070 | 23.00 | F | Caucasian | IA-2A+ mIAA+ | 11 | 245 | 0.002 | 0.097 |
| T1D | 6025 | 24.00 | M | Caucasian | mIAA+ | 0 | 0 | −0.012 | 0.019 |
| T1D | 6076 | 26.00 | M | Caucasian | GADA+ | 355 | 0 | 0.008 | 0.018 |
| T1D | 6045 | 26.00 | M | Caucasian | mIAA+ | 0 | 0 | 0.033 | 0.032 |
| T1D | 6041 | 26.00 | M | Caucasian | Negative | 0 | 0 | −0.001 | 0.001 |
| T1D | 6119 | 28.00 | M | Caucasian | GADA+ mIAA+ | 105 | 0 | 0.011 | 0.014 |
| T1D | 6071 | 28.00 | F | Caucasian | IA-2A+ mIAA+ | 0 | 12 | 0.004 | 4.190 |
| T1D | 6061 | 28.00 | M | Caucasian | mIAA+ | 0 | 0 | 0.029 | 0.022 |
| T1D | 6039 | 29.00 | F | Caucasian | GADA+ IA-2A+ ZnT8+ mIAA+ | 141 | 135 | 0.112 | 0.017 |
| T1D | 6152 | 30.00 | F | Caucasian | ZnT8(+) | 0 | 3 | 0.021 | 0.001 |
| T1D | 6088 | 31.00 | M | Caucasian | GADA+ IA-2A+ ZnT8+ mIAA+ | 28 | 37 | 0.070 | 0.364 |
| T1D | 6081 | 31.00 | M | Hispanic/Latino | Negative | 0 | 0 | −0.003 | −0.007 |
| T1D | 6035 | 32.00 | M | Caucasian | mIAA+ | 0 | 0 | −0.009 | 0.319 |
| T1D | 6077 | 33.00 | F | Caucasian | mIAA+ | 0 | 0 | −0.001 | 0.083 |
| T1D | 6067 | 33.00 | F | Hispanic/Latino | Negative | 0 | 0 | 0.002 | −0.003 |
| T1D | 6143 | 33.00 | F | Caucasian | IA-2A+ mIAA+ | 0 | 13 | 0.008 | 0.043 |
| T1D | 6121 | 34.00 | F | Caucasian | Negative | 9 | 0 | 0.017 | −0.001 |
| T1D | 6128 | 34.00 | F | Caucasian | mIAA+ | 1 | 0 | −0.016 | 1.358 |
| T1D | 6032 | 34.00 | M | Caucasian | mIAA+ | 0 | 0 | −0.012 | 0.287 |
| T1D | 6054 | 35.00 | F | Caucasian | mIAA+ | 0 | 0 | 0.002 | 0.404 |
| T1D | 6038 | 37.00 | F | Caucasian | Negative | 0 | 0 | −0.009 | −0.001 |
| T1D | 6141 | 37.00 | M | Caucasian | GADA+ IA-2A+ ZnT8+ mIAA+ | 22 | 15 | 0.041 | 0.360 |
| T1D | 6031 | 39.00 | M | Caucasian | mIAA+ | 0 | 0 | −0.012 | 0.143 |
| T1D | 6033 | 41.00 | M | Caucasian | Negative | 0 | 0 | −0.009 | 0.004 |
| T1D | 6150 | 41.00 | M | Caucasian | mIAA+ | 0 | 0 | −0.002 | 0.567 |
| T1D | 6135 | 44.00 | M | Caucasian | GADA+ mIAA+ | 149 | 2 | 0.004 | 0.023 |
| T1D | 6036 | 49.00 | F | African Am | mIAA+ | 0 | 0 | 0.008 | 0.055 |
| T1D | 6138 | 49.00 | F | Caucasian | mIAA+ | 0 | 2 | 0.000 | 0.168 |
| T1D | 6040 | 50.00 | F | Caucasian | mIAA+ | 0 | 0 | 0.013 | 0.200 |
| T1D | 6155 | 50.00 | F | Caucasian | mIAA+ | 3 | 1 | −0.003 | 0.061 |
All values converted to NIDDK units and defined as positive for GADA if ≥20, IA2A if ≥5, ZnT8A if ≥0.020, and mIAA if ≥0.010.
Morphological characterization
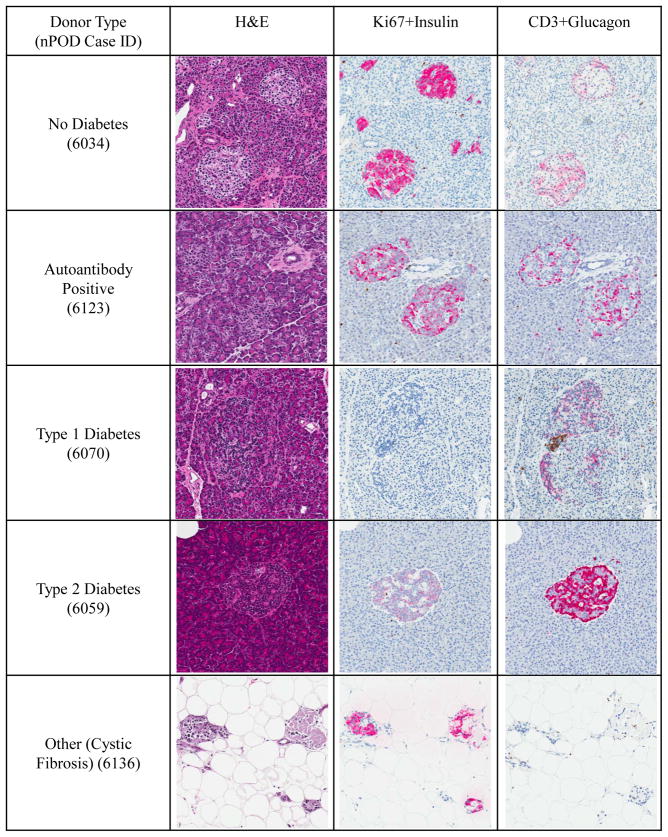
Pancreas cell morphology was excellent in organ donors with maximum preservation of cellular details while pancreata collected at autopsy (donors including T1D Medalists) showed autolysis in varying degrees. Histopathological characterization for pancreata provided a high degree of added value as investigators reviewed donor samples using the online pathology system and selected specific blocks for their studies. To show representative pancreas morphology, adult female Caucasian donors were randomly selected each donor group. Images were taken from the pancreas tail from H&E and immunohistochemistry stained slides (Figure 1). Donors without diabetes or autoantibody positive donors showed the expected predominance of β-cells in islets with fewer glucagon-positive α-cells and no CD3+ cells. Small islets and single β-cells were also present in these two donor groups. Donors with diabetes showed the absence (T1D) or decreased intensity (type 2 diabetes) of insulin immunopositivity in islets with a greater proportion of α-cells. A CD3+ infiltrate indicating insulitis is shown in the islet from the T1D donor. One of the two cystic-fibrosis related diabetes donors is shown demonstrating the profound absence of exocrine tissue and presence of infrequent, small islets. All donor digital images can be viewed through the online pathology system (http://www.jdrfnpod.org/online-pathology.php).
Figure 1.
Islets in nPOD donors. Donors were selected from adult female Caucasians from each of five donor groups (no diabetes, autoantibody positive, type 1 diabetes, type 2 diabetes, and cystic fibrosis). Islets were photographed from serially sections stained by H&E and two double immunohistochemistry assays (Ki67+Insulin, CD3+Glucagon, see Methods) using the online pathology system. Original magnifications: 10×, (1000 × 1000 pixel).
Discussion
The nPOD program demonstrated the feasibility of screening organ donors for diabetes-related autoantibodies and subsequent recovery of high quality pancreatic biospecimens suitable for a wide-range of investigations. This success was based on a highly interactive OPO and nPOD administrative and laboratory infrastructure and is exemplified in research projects by multiple investigators found worldwide (17–23).
As noted, a major effort for the nPOD program was involved in establishing a process for the recovery of organs from donors with preclinical (i.e., autoantibody positive only) T1D. Many OPOs use centralized laboratories to conduct transplant related serologies including HLA matching and infectious disease screening and several large laboratories were enrolled as screening sites. On-site training was initially provided and subsequent screening data monitored continuously for inter-lab variability. Autoantibody levels were further tested by the autoantibody core and those positive levels used to designate autoantibody positive status. The current nPOD screening data show the predominance of single GADA-positivity in organ donors, similar to current reports of the general US population (24). As well, different steps in the donor recovery process had issues that impacted overall recovery rates of the autoantibody positive donors. These issues included the screening laboratories (delayed contact of OPO), OPOs (inclusion/exclusion criteria adherence, recall by Medical Examiners), and operating room (pancreas used instead for transplantation or was damaged, staff not available).
An additional finding of note from the nPOD program has been that recoveries of donors with recent onset T1D were exceedingly rare, with only six T1D donors with disease duration ≤ 5 years. Difficulties in obtaining recent onset donors in the U.S. was multi-factorial and in part related to the majority of organ donors being middle aged adults compared to the younger average age of T1D onset (children and young adults). A second limitation was found in that patients with acute diabetic ketoacidosis (DKA) were only rarely considered for organ donation due to their inherent metabolic instability. Excellent medical emergency management for DKA in emergency departments has also made this devastating complication rare compared to past decades.
The nPOD program instituted several unique features uncommon to most biorepositories. Some of these features include the distribution of biospecimens at no cost and concerted efforts to distribute samples from the same donor to multiple investigators. A significant commitment is made to the end user to ensure that issues are addressed and recommendations for improvement are implemented in a timely manner. As for other large biobanking operations, the nPOD program required dedicated staffing (i.e., 24/7/365) for both donor acceptance and case processing on-call teams. Quality assurance and quality control were applied to all aspects of the project using best practices from national biorepository organizations (e.g., National Cancer Institute, International Society of Biological and Environmental Repositories) along with external audit and scientific advisory committee oversight. Support labs for autoantibody testing, C-peptide levels, and high resolution HLA genotyping were selected based on nationally established reputations and commitment to long-term participation to ensure assay consistency. nPOD established broad communication and outreach systems, including a highly interactive, continuously updated website, webinars that offer continuing education credit for OPO coordinators, continuously updated online pathology of each donor, and representation at multiple scientific meetings (e.g., American Diabetes Association, Association of Organ Procurement Organization, NATCO Organization for Transplant Processionals). Quarterly electronic newsletters were also provided to both OPO and investigator groups.
The growth of nPOD, in terms of the number of investigators it serves, has been remarkable. In 2007, nPOD provided biospecimens to seven investigators. nPOD currently provides biospecimens to over 60 investigators on four continents whose research has been supported by funding from the JDRF, National Institutes of Health, American Diabetes Association, Helmsley Foundation, the European Union, and from their host institution. nPOD’s online pathology system has over 200 registered users from around the world. Applications were reviewed for scientific merit and feasibility by the Tissue Prioritization Committee using current sample availability plus considerations for prospective donor recoveries. Here, a somewhat unique evaluation system was used wherein rather than a strictly critical evaluation, potential proposals were subjected to a process wherein improvements were suggested (i.e., try to make a project better). Indeed, potential use of data sharing through a “data cloud” network is underway to facilitate sharing of research data for each donor. Progress as deemed by numbers of publications is increasing and demonstrates new collaborations facilitated by the active role that nPOD has taking in promoting synergies.
nPOD’s efforts to screen referrals from OPO for T1D referrals have met with some challenges. These include, in some cases, lack of prior medical history, increasing rates of T2D in the adolescent population, and generalized confusion between T1D, T2D and “insulin dependent” diabetes. In addition, the donor’s representative may not have complete knowledge of the donor’s diabetes medical history yet completes the donor questionnaire. nPOD identified a need for greater training of OPO coordinating staff in distinguishing T1D from T2D as both were listed as “insulin-dependent” in the current UNOS organ donor questionnaire and addressed this need through online webinar training. As a result, OPO partners began obtaining additional clinical information, including insulin use at diagnosis, history of anti-diabetic oral medication, and type and dose of current insulin regimen. The nPOD program endeavored to provide strict characterization of each donor’s clinical phenotype; however, it was well appreciated that without a complete medical history, correct donor classification would be inexact in certain donors just as it may be for certain patients in the clinical setting.
In sum, this review details the current organization and donors of the nPOD biorepository. The ultimate mission of this network is to accelerate the discovery process for many key issues for T1D. Additional programmatic expansions are underway, including the implementation of mechanisms to promote the collaborative, multidisciplinary and comprehensive review of the nPOD cases, new efforts for autoantibody positive and recent onset T1D donor recovery in Europe (nPOD-E), studies of native and transplanted pancreas from transplanted patients (nPOD-T), and of diabetic complications from donors enrolled in clinical trials (nPOD-C). Amongst the many significant observations emanating from nPOD, our initial analyses highlighted the diversity of histopathology of T1D and the paucity of insulitis in humans contrasts with its remarkable severity in mouse models of T1D (1, 19). Researchers can be encouraged, and aided by access to rare biospecimens, to add studies of human tissues alongside of those of animal models for the disease. The entire community of T1D researchers is encouraged to utilize this resource and additional information can be found at the nPOD website (www.jdrfnpod.org).
Acknowledgments
The nPOD program thanks the donors and their families and the OPOs and other nPOD staff members for excellent administrative and technical support. Organ donor information was supplied by UNOS as the contractor for the Organ Procurements and Transplantation Network. The interpretation and reporting of such data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government. This work was supported, in part, by funding obtained from the National Institutes of Health (P01 AI42288), Juvenile Diabetes Research Foundation, Brehm Coalition for Type 1 Diabetes Research, and Jeffrey Keene Family Professorship.
Grant support: This work was supported by the Juvenile Diabetes Research Foundation (M.C-T., M.A.A.).
Abbreviations
- DKA
Acute diabetic ketoacidosis
- GADA
GAD autoantibodies
- HLA
Human leukocyte antigen
- IA2A
IA-2ic autoantibodies
- JDRF
Juvenile Diabetes Research Foundation
- mIAA
Insulin autoantibodies
- nPOD
Network for Pancreatic Organ donors with Diabetes
- OPO
Organ Procurement Organization
- T1D
Type 1 diabetes
- UNOS
United Network for Organ Sharing
- ZnT8A
ZnT8A autoantibodies
Footnotes
Conflict of interest
None declared.
References
- 1.Atkinson MA, Gianani R. The pancreas in human type 1 diabetes: providing new answers to age-old questions. Curr Opin Endocrinol Diabetes Obes. 2009;16(4):279–85. doi: 10.1097/MED.0b013e32832e06ba. Epub 2009/06/09. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Bell RA, D’Agostino RB, Imperatore G, Johansen JM, Linder B, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297(24):2716–24. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 3.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91(1):79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 4.von Herrath M. Diabetes: A virus-gene collaboration. Nature. 2009;459(7246):518–9. doi: 10.1038/459518a. [DOI] [PubMed] [Google Scholar]
- 5.Orban T, Sosenko JM, Cuthbertson D, Krischer JP, Skyler JS, Jackson R, et al. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2009;32(12):2269–74. doi: 10.2337/dc09-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winter WE, Schatz DA. Autoimmune Markers in Diabetes. Clin Chem. 2010 doi: 10.1373/clinchem.2010.148205. [DOI] [PubMed] [Google Scholar]
- 7.Bonifacio E, Yu L, Williams A, Eisenbarth G, Bingley P, Marcovina S, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for National Institute of Diabetes and Digestive and Kidney Diseases consortia. J Clin Endocrinol Metab. 2010;95(7):3360–7. doi: 10.1210/jc.2010-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maniatis AK, Yu L, Miao D, Nelson K, Eisenbarth GS. Rapid assays for detection of anti-islet autoantibodies: implications for organ donor screening. J Autoimmun. 2001;16(1):71–6. doi: 10.1006/jaut.2000.0457. [DOI] [PubMed] [Google Scholar]
- 9.Tauriainen S, Salmela K, Rantala I, Knip M, Hyöty H. Collecting high-quality pancreatic tissue for experimental study from organ donors with signs of β-cell autoimmunity. Diabetes Metab Res Rev. 2010;26(7):585–92. doi: 10.1002/dmrr.1129. [DOI] [PubMed] [Google Scholar]
- 10.In’t Veld P, Lievens D, De Grijse J, Ling Z, Van der Auwera B, Pipeleers-Marichal M, et al. Screening for insulitis in adult autoantibody-positive organ donors. Diabetes. 2007;56(9):2400–4. doi: 10.2337/db07-0416. [DOI] [PubMed] [Google Scholar]
- 11.Diamantopoulos S, Allende G, Ferreira J, Ciancio G, Burke G, Pugliese A. Retrospective assessment of islet cell autoantibodies in pancreas organ donors. Diabetes Care. 2008;31(9):1741–2. doi: 10.2337/dc08-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gianani R, Putnam A, Still T, Yu L, Miao D, Gill RG, et al. Initial results of screening of nondiabetic organ donors for expression of islet autoantibodies. J Clin Endocrinol Metab. 2006;91(5):1855–61. doi: 10.1210/jc.2005-1171. Epub 2006/02/16. [DOI] [PubMed] [Google Scholar]
- 13.Mallone R, Mannering SI, Brooks-Worrell BM, Durinovic-Belló I, Cilio CM, Wong FS, et al. Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: position statement of the T-Cell Workshop Committee of the Immunology of Diabetes Society. Clin Exp Immunol. 2011;163(1):33–49. doi: 10.1111/j.1365-2249.2010.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell-Thompson M, Dixon LR, Wasserfall C, Monroe M, McGuigan JM, Schatz D, et al. Pancreatic adenocarcinoma patients with localised chronic severe pancreatitis show an increased number of single beta cells, without alterations in fractional insulin area. Diabetologia. 2009;52(2):262–70. doi: 10.1007/s00125-008-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noble J, Valdes A, Cook M, Klitz W, Thomson G, Erlich H. The role of HLA class II genes in insulin-dependent diabetes mellitus: molecular analysis of 180 Caucasian, multiplex families. Am J Hum Genet. 1996;59(5):1134–48. [PMC free article] [PubMed] [Google Scholar]
- 16.Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, et al. Care of children and adolescents with type 1 diabetes - A statement of the American Diabetes Association. Diabetes Care. 2005;28(1):186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 17.Keenan H, Sun J, Levine J, Doria A, Aiello L, Eisenbarth G, et al. Residual Insulin Production and Pancreatic {beta} Cell Turnover after 50 Years of Diabetes: Joslin Medalist Study. Diabetes. 2010 doi: 10.2337/db10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yip L, Su L, Sheng D, Chang P, Atkinson M, Czesak M, et al. Deaf1 isoforms control the expression of genes encoding peripheral tissue antigens in the pancreatic lymph nodes during type 1 diabetes. Nat Immunol. 2009;10(9):1026–33. doi: 10.1038/ni.1773. Epub 2009/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowe PA, Campbell-Thompson ML, Schatz DA, Atkinson MA. The pancreas in human type 1 diabetes. Semin Immunopathol. 2011;33(1):29–43. doi: 10.1007/s00281-010-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green-Mitchell SM, Cazares LH, Semmes OJ, Nadler JL, Nyalwidhe JO. On-tissue identification of insulin: In situ reduction coupled with mass spectrometry imaging. Proteomics Clin Appl. 2011 doi: 10.1002/prca.201000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gianani R, Campbell-Thompson M, Sarkar SA, Wasserfall C, Pugliese A, Solis JM, et al. Dimorphic histopathology of long-standing childhood-onset diabetes. Diabetologia. 2010;53(4):690–8. doi: 10.1007/s00125-009-1642-y. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez NA, Liang T, Gaisano HY. Live Pancreatic Acinar Imaging of Exocytosis Using Syncollin-pHluorin. Am J Physiol Cell Physiol. 2011 doi: 10.1152/ajpcell.00433.2010. [DOI] [PubMed] [Google Scholar]
- 23.Penaranda C, Tang Q, Ruddle NH, Bluestone JA. Prevention of diabetes by FTY720-mediated stabilization of peri-islet tertiary lymphoid organs. Diabetes. 2010;59(6):1461–8. doi: 10.2337/db09-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vehik K, Beam CA, Mahon JL, Schatz DA, Haller MJ, Sosenko JM, et al. Development of Autoantibodies in the TrialNet Natural History Study. Diabetes Care. 2011 doi: 10.2337/dc11-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]