Abstract
In mammals, pigments are made by melanocytes within a specialized organelle, the melanosome. Mature, pigment-laden melanosomes are then transferred to keratinocytes to drive the visible pigmentation of the animal’s hair and skin. The dilute suppressor (dsu) locus encodes an extragenic suppressor of the pigmentation defect exhibited by mice lacking myosin Va (i.e. dilute mice). We recently showed that melanoregulin, the product of the dsu locus, functions as a negative regulator of a shedding mechanism that drives the intercellular transfer of melanosomes from the melanocyte to the keratinocyte. Here we address melanoregulin’s localization within the melanocyte, as well as the molecular basis for its localization. First, we confirm and extend recently published results using exogenous, GFP-tagged melanoregulin by showing that endogenous melanoregulin also targets extensively to melanosomes. Second, using site-directed mutagenesis, metabolic labeling with H3-palmitate, and an inhibitor of palmitoylation in vivo, we show that the targeting of melanoregulin to the limiting membranes of melanosomes in melanocytes and lysosomes in CV1 cells depends critically on the palmitoylation of one or more of six closely-spaced cysteine residues located near melanoregulin’s N-terminus. Finally, using Fluorescence Recovery after Photobleaching (FRAP), we show that melanoregulin-GFP exhibits little if any tendency to cycle in and out of the melanosome membrane. We conclude that multiple palmitoylation serves to stably anchor melanoregulin in the melanosome membrane.
Keywords: dilute suppressor, melanoregulin, palmitoylation, melanosome
INTRODUCTION
Genetically black mice that are homozygous for the myosin Va functional null allele dl20j appear grey [1]. When these mice are also made homozygous for a functional null allele at the dilute suppressor (dsu) locus, which encodes the protein melanoregulin, their coat color is restored almost completely (i.e. from grey back to near-black) [2]. We recently showed that melanoregulin negatively regulates a shedding mechanism that drives the transfer of melanosomes from the melanocyte to the keratinocyte [3]. To begin to define how, at the molecular level, melanoregulin regulates intercellular melanosome transfer, its localization within the melanocyte, as well as the molecular basis for this localization, must be determined. Recently, Ohbayashi et. al. [4] showed that GFP-tagged melanoregulin targets to melansomes in an immortal melanocyte cell line, and that purified melanosomes contain melanoregulin. Here we confirm and extend those observations in a number of ways. Most importantly, we show that melanoregulin, which is highly charged and lacks a transmembrane domain, binds to the limiting membrane of the melanosome because it is palmitoylated on one or more of six closely-spaced cysteine residues present near the protein’s N-terminus. We also show by FRAP that the protein is stably bound to the melanosome, most likely because it is multiply palmitoylated. These results represent an important first step in efforts to define at the molecular level how melanoregulin regulates intercellular melanosome transfer.
MATERIALS AND METHODS
Mouse breeding and genotyping, and the preparation and culture of primary mouse melanocytes, were performed as described previously [1]. Melan c melanocytes were a gift of Dorothy Bennet (St. Georges Hospital, London). GFP- and FLAG-tagged melanoregulin constructs were created in mEGFP-N1 (Clonetech) and pCMV-Tag4 (Stratagene) vectors using standard techniques. Site directed mutagenesis was performed using the Quick Change kit from Stratagene. All clones were confirmed by sequencing. Melanocytes were transfected using FuGene (Amersham). Cells were fixed and immunostained as described previously [1]. The anti- TRP-1 antibody MEL 5 was purchased from Signal Transduction Labs. The imaging of stained and live cells, as well as FRAP analyses, was performed on a Zeiss 510 LSM confocal microscope, as described previously [1]. For metabolic labeling, RPE and Cos7 cells in 60-mm dishes were labeled in 2 ml of DMEM containing 5% FBS and 600 μCi of H3-palmitate (60 Ci/mmol; Perkin Elmer) for 6 h at 37°C starting 24 h post-transfection. The GFP- and FLAG-tagged proteins were immunoprecipitated from Triton X-100-solubilized cells using anti-GFP antibody coated beads (Sigma) and anti-FLAG antibody coated beads (M2; Sigma), respectively, according to the manufactures protocols. Proteins were released from the beads by incubation at room temperature for 20 min in electrophoresis sample buffer containing 15 mM DTT and separated on a 4–20% SDS-PAGE minigel. The gel was then incubated with Amplify™ Fluorographic Reagent (GE Healthcare), dried, exposed to film (X-Omat AR, Eastman Kodak) for 15 days at −70°C, and the film developed. Bromo-palmitate and LysoTracker were purchased from Sigma and Molecular Probes, respectively.
RESULTS
Endogenous as well as exogenous melanoregulin localizes to end-stage melanosomes
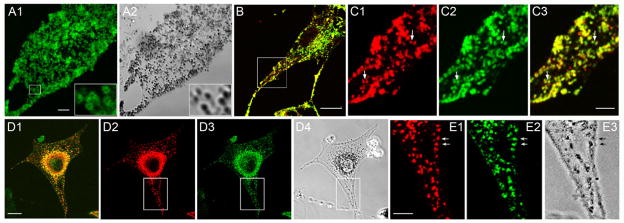
In general agreement with the recent results of Ohbayashi et. al. [4], Figure 1, Panels A1 and A2, show that exogenous, GFP-tagged melanoregulin (tagged here at the protein’s C-terminus, not its N-terminus as in Ohbayashi et. al. [4] for reasons discussed below) targets extensively to black, end-stage melanosomes present in primary wild type (WT) melanocytes. Time lapse imaging of such cells shows that the GFP signals move in precise register with the movement of the black pigment (Supplementary Movie 1), confirming that melanoregulin-GFP targets to end-stage melanosomes. Moreover, melanoregulin-GFP does not show obvious targeting to any other membrane structures, including the plasma membrane, Golgi, mitochondria or endoplasmic reticulum, indicating that it is targeted specifically to melanosomes in melanocytes. While we did not perform immuno electron microscopy to confirm that melanoregulin is on the surface of the melanosome (as opposed to being inside the organelle), images of melanoregulin-GFP-positive melanosomes often show a ring of fluorescence (see insets in Figure 1, Panels A1 and A2), consistent with melanoregulin accumulating on the perimeter of the organelle. This and other data below (e.g. palmitoylation) argue strongly that melanoregulin associates with the limiting membrane of the melanosome.
Figure 1. Both endogenous melanoregulin and melanoregulin tagged with GFP at its C-terminus exhibit striking co localization with melanosomes.
Panels A1 and A2 show a primary WT melanocyte that had been transfected with melanoregulin tagged with mGFP at its C-terminus. The insets show more clearly how melanoregulin-GFP forms a ring of florescence that surrounds the black pigment inside of end-stage melanosomes (see also Supplementary Movie 1). Panel B shows the overlaid image of a melan-C melanocyte (an immortal melanocyte cell line made from an albino mouse) that had been double-stained using a monoclonal antibody against the major melanosomal membrane protein TRP-1 (red) and a polyclonal antibody against melanoregulin (green). Panels C1–3 show enlargements of the boxed region in Panel B (C1, TRP-1; C2, melanoregulin; C3, overlay). Panels D1–4 show a dv/dv melanocyte that had been stained like the cell in Panel B (D1, TRP-1; D2, melanoregulin; D3, overlay: D4, transmitted image). Panels E1–3 show enlargements of the boxed region in Panels D1–4 (E1, TRP-1; E2, melanoregulin; E3, transmitted image; the arrows in these three panels point to black melanosomes that are positive for both TRP-1 and melanoregulin). The mag bars are 3.5 (A1), 8.5 (B), 2.5 (C3), 10 (D1) and 2.7 (E1) μm.
To determine if endogenous melanoregulin is also present on melanosomes, we double-stained melan C (i.e. albino) melanocytes with a previously-characterized polyclonal antibody to full length melanoregulin [2] and a monoclonal antibody to the glycoprotein TRP1 (Mel 5), which is present at high levels in the limiting membrane of melanosomes (including the unpigmented melanosomes present in melan C melanocytes; [1]). Figure 1, Panels B and C1–3, show a strong correspondence between the two signals, indicating that endogenous melanoregulin is present on melanosomes. Moreover, as with exogenous, GFP-tagged melanoregulin, endogenous melanoregulin appears to target specifically to melanosomes. To confirm these results, we double-stained melanocytes that contain black, end-stage melanosomes. In this case, we used dilute viral (dv) melanocytes, as these myosin Va-depleted melanocytes have a small number of well-separated melanosomes in their peripheral cytoplasm [1]. Figure 1, Panels D1–4 and E1–3, show that these well-separated, black melanosomes are uniformly positive for both TRP1 and melanoregulin. We conclude, therefore, that melanoregulin is a melanosome-associated protein. Parenthetically, we note that western blots performed on whole cell extracts prepared from pure cultures of primary, WT mouse keratinocytes (both undifferentiated and differentiated) show that they do not express melanoregulin (data not shown). This observation is in agreement with mouse chimera studies showing that melanoregulin functions cell autonomously within melanocytes to rescue the coat color defect of dilute mice [5].
Melanoregulin associates with melanosomes and lysosomes via palmitoylation
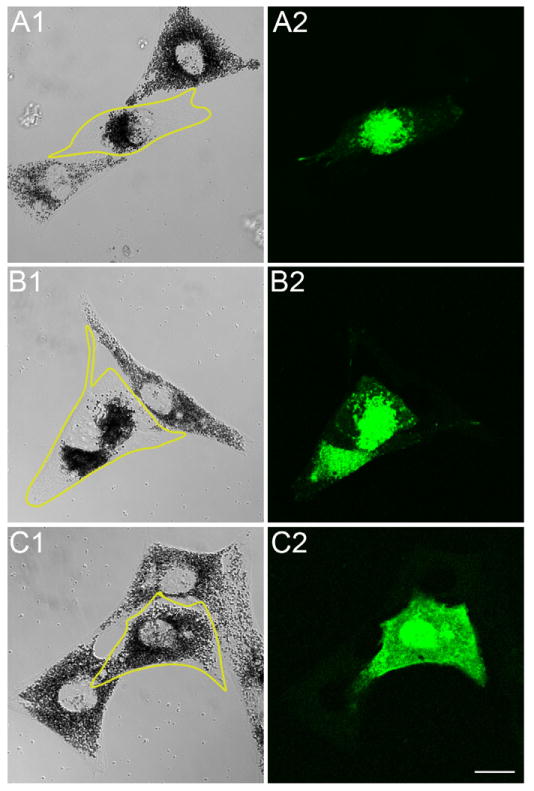
Consistent with the above localization data, the low speed pellet of osmotically-ruptured melanocytes, which contains essentially all of the cell’s melanosomes, also contains ~98% of cell’s total melanoregulin (data not shown). These results are somewhat surprising given that melanoregulin has no transmembrane domain and is highly charged (37 basic residues and 39 acidic residues out of a total of 220 residues) like typical soluble, cytosolic proteins. Melanoregulin does contain, however, a glycine residue immediately following its initiator methionine, as well as a stretch of eight residues that starts nine residues C-terminal to this glycine and that contains six cysteine residues (residues 11–14, 16 and 18). This type of sequence is typical of proteins that are strongly membrane associated as a consequence of being dually acylated, with the 14-carbon saturated fatty acid myristate being attached to the glycine residue and the 16-carbon saturated fatty acid palmitate being attached to one or more cysteine residues [6; 7]. To determine whether the glycine and/or the cysteines are necessary for the association of melanoregulin with the melanosome membrane, we created three melanoregulin-GFP mutants: (1) melanoregulin-GFP GΔA, in which the glycine was changed to an alanine, (2) melanoregulin-GFP CΔS, in which all six cysteines were changed to serine residues, and (3) melanoregulin-GFP GΔA+CΔS, which contains both of these changes. These three mutants were then compared to WT melanoregulin-GFP in terms of their ability to target to black melanosomes. For these experiments we used primary melanocytes from dv/dv, dsu/dsu mice for two reasons. First, by using melanocytes isolated from mice homozygous for the dsu allele, which lack melanoregulin [2], we eliminated the possibility that endogenous, WT melanoregulin might influence by possible self-association the targeting of the mutant versions of the protein. Second, by using melanocytes from mice homozygous for the dv allele, we were able to score one biological effect that melanoregulin has when it is over-expressed on this myosin Va mutant background. The intracellular distribution of melanosomes within these melanocytes, which are homozygous for the mutant myosin Va allele dilute viral (or dv) that allows very low level expression of myosin Va [8], lies somewhere between the distributions seen in WT melanocytes and in melanocytes from an animal homozygous for a true null allele for myosin Va like dilute lethal (dl20J) [1]. Specifically, the melanosomes are more spread intracellularly than in dl20J/dl20J melanocytes, but not as spread as in WT melanocytes. Importantly, while the degree of spreading is no different in dv/dv melanocytes whether or not melanoregulin is absent (i.e. on a dsu/dsu background) or present at normal levels (i.e. on a DSU/DSU background) [3], when melanoregulin is over expressed in dv/dv melanocytes it causes the melanosomes to become highly concentrated in the cell center. This biological effect is demonstrated for WT melanoregulin-GFP in Figure 2, Panels A1 and A2, where it can be seen to target to black melanosomes and to cause them to concentrate in the center of the cell (compare the transfected cell that is outlined in yellow to the adjacent, untransfected cells). Therefore, in addition to targeting to the melanosome, WT melanoregulin-GFP, when over expressed on a myosin Va mutant background, alters the distribution of melanosomes (see Discussion for the possible biological basis of this phenomena). Figure 2, Panels B1 and B2, show that putative myristoylation must not critical, as melanoregulin-GFP GΔA behaves just like WT melanoregulin-GFP, i.e. it targets to melanosomes and causes them to accumulate in the cell center. By contrast, Figure 2, Panels C1 and C2, show that melanoregulin-GFP CΔS neither targets to melanosomes nor alters their intracellular distribution, and is instead diffusely distributed throughout the cytoplasm. Similar results were obtained with melanoregulin-GFP GΔA+CΔS (data not shown). These results argue that palmitoylation is likely to play a key role in targeting melanoregulin to the melanosome membrane.
Figure 2. Clustered cysteine residues located near melanoregulin’s N-terminus are required for its targeting to melanosomes.
Panels A1 and A2 show phase and fluorescent images, respectively, of a dv/dv, dsu/dsu melanocyte that had been transfected with WT melanoregulin-GFP (the transfected cell is outlined in yellow in A2). Panels B1, B2, C1, and C2 show similar images for dv/dv, dsu/dsu melanocytes that had been transfected with melanoregulin-GFP containing either the GΔA (Panels B1 and B2) or CΔS (Panels C1 and C2) mutations (the transfected cells are outlined in yellow in B2 and C2). The mag bar is 10.2 μm.
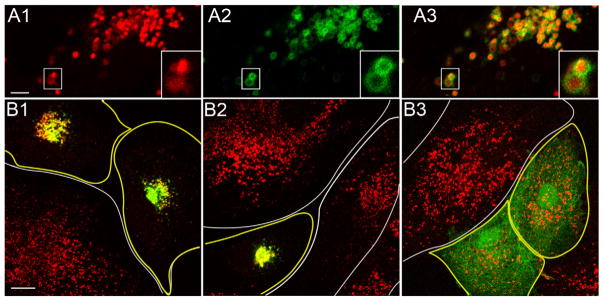
As a consequence of other efforts to define the function of melanoregulin, we found that melanoregulin-GFP targets dramatically and specifically in generic cell types like CV1 cells to late endosomes and/or lysosomes, as identified using LysoTracker (Figure 3, Panels A1–3; see also the insets in these panels and supplementary movie 2). Similar observations were made recently by Damek-Poprawa et.al. [9]. To extend the data in Figure 2, therefore, we examined the targeting of melanoregulin-GFP GΔA and melanoregulin-GFP CΔS to Lysostracker-positive structures in CV1 cells. Similar to the targeting of melanoregulin to melanosomes in melanocytes, WT melanoregulin-GFP (Figure 3, Panel B1) and melanoregulin-GFP GΔA (Figure 3, Panel B2) target to lysosomes (see the yellow signal), while melanoregulin-GFP CΔS (Figure 3, Panel B3) does not, being instead diffusely distributed in the cytoplasm. Therefore, the targeting of melanoregulin to lysosomes in CV1 cells, like its targeting to melanosomes in melanocytes, requires the presence of the putative palmitoylation sites (the cysteines) but not the putative myristoylation site (the glycine). Note also that, as with melanosomes, the over expression of melanoregulin results eventually in the central accumulation of lysosomes in CV1 cells when it is able to target to the organelle (i.e. WT melanoregulin-GFP (Panel B1) and melanoregulin-GFP GΔA (Panel B2)) (see Discussion).
Figure 3. Clustered cysteine residues located near melanoregulin’s N-terminus are required for its targeting to lysosomes.
Panels A1–3 show a still image of a portion of a CV1 cell that had been transfected with WT melanoregulin-GFP and then incubated in the presence of the live-cell lysosome marker LysoTracker-red for 30 minutes prior to imaging (A1, LysoTracker-red; A2, melanoregulin-GFP; A3, overlay) (see also the insets in these panels and Supplementary Movie 2). This cell was imaged 12 hours after transfection. Panels B1–3 show overlaid images of CV1 cells 30 hours after being transfected with WT melanoregulin-GFP (Panel B1), melanoregulin-GFP containing the GΔA mutation (Panel B2), or melanoregulin-GFP containing the CΔS mutations (Panel B3). The images were taken 30 minutes after addition of LysoTracker-red to the media. Transfected cells are outlined in yellow, while untransfected cells are outlined in white. The mag bars are 3.8 (A1) and 15.3 (B1) μm.
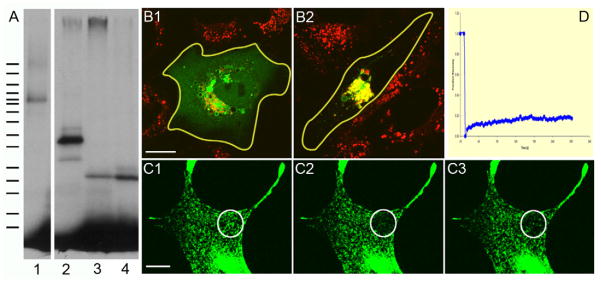
To demonstrate directly that melanoregulin is indeed palmitoylated, RPE and Cos7 cells were transfected with WT melanoregulin-GFP or WT melanoregulin-FLAG (melanoregulin with a FLAG tag at its C-terminus), the cells were grown for 6 hours in the presence of H3-palmitate (starting 24 hours post transfection), the melanoregulin proteins were pulled down using beads conjugated with the appropriate antibody (anti-GFP for WT melanoregulin-GFP and anti-FLAG for WT melanoregulin-FLAG), and the bead-bound material was subjected to SDS-PAGE followed by fluorography to detect the presence of H3-palmitate on melanoregulin. GFP-tagged mucolipin 3, which is known to be palmitoylated [10], was used as a positive control. Figure 4, Panel A, shows that, like GFP-mucolipin 3 (lane 1), both tagged versions of melanoregulin are positive for incorporated H3-palmitate (melanoregulin-GFP in lane 2, melanoregulin-FLAG in lanes 3 and 4). If melanoregulin is palmityolated in vivo, as indicated by this metabolic labeling experiment, then its targeting to lysosomes in CV1 cells should be abrogated or at least diminished significantly in cells incubated in the presence of bromo-palmitate, a cell-permeant, general inhibitor of palmitoyltransferases and, therefore, of palmitoylation in vivo [11]. Figure 4, Panels B1 and B2, show that the addition of bromo-palmitate to the media of CV1 cells transfected with WT melanoregulin-GFP does indeed reduce significantly the extent to which the protein targets to LysoTracker-positive organelles (compare Panel B1 (plus 100 μM bromo-palmitate), where the GFP signal is diffuse throughout the cell and not strongly co localized with the LysoTracker signal, to Panel B2 (no bromo-palmitate), where essentially all of the melanoregulin-GFP signal co-localizes with the centralized LysoTracker signal). We conclude, therefore, that melanoregulin is palmitoylated in vivo, and that this post translational modification plays a major role in it’s targeting to lysosomes in generic cell types and to melanosomes (which are secretory lysosomes) in melanocytes.
Figure 4. Palmitoylation of melanoregulin’s cysteines drives the protein’s stable association with the limiting membrane of lysosomes and melanosomes.
Panel A shows a fluorogram of a 4–20% SDS-PAGE gel of whole cell extracts from RPE or Cos7 cells that had been transfected with GFP-tagged mucolipin 3 (lane 1; estimated MR of 90 kDa), WT melanoregulin-GFP (lane 2; estimated MR of 50 kDa), or WT melanoregulin-FLAG (lanes 3 and 4; estimated MR of 26 kDa) and incubated for 6 hours in the presence of H3-palmitate starting 24 hours post-transfection (see Methods for details). The migration of the molecular weight markers is indicated by the hash marks to the left (from top to bottom: 220, 160, 120, 100, 90, 80, 70, 60, 50, 40, 30, 25, 20, 15, and 10 kDa). The extracts in lanes 1–3 were from RFE cells, while the extract in lane 4 was from a Cos7 cell. Panels B1 and B2 show fluorescent overlay images of CV1 cells that had been transfected with WT melanoregulin-GFP and incubated with (Panel B1) or without (Panel B2) 100 μM bromo-palmitate for 8 hours starting 16 hours post transfection. LysoTracker was added 30 minutes before image acquisition. Panels C1–3 show the fluorescence of melanosome-associated WT melanoregulin-GFP in melan-C (i.e. albino) melanocytes within the bleach target zone (white circle) immediately prior to bleaching (Panel C1), immediately after bleaching (Panel C2), and 5 minutes post-bleach (Panel C3). Panel D provides the quantitation of this FRAP experiment. The mag bars are 14 (B1) and 10 (C1) μm.
Palmitoylation is reversible [7], and for many proteins that are palmitoylated at a single cysteine residue, reversible palmitoylation and, as a consequence, reversible membrane association, plays a major role in their biological function (e.g. the alpha subunit of heterotrimeric G proteins). In contrast to such proteins are multiply palmitoylated proteins like cystiene string proteins, which seldom exit the membrane because at any moment in time they usually have at least one site palmitoylated [12]. Given that melanoregulin has multiple potential palmitoylation sites, and that very little of the protein is found in the cytoplasm at steady state, we hypothesized that the protein probably does not cycle in and out of the membrane to any significant extent. To test this, we transfected melan-C (albino) melanocytes with melanoregulin-GFP and subjected the melanosome-associated GFP signal to photo bleaching (note that these albino melanoccytes, which produce unpigmented melansomes, must be used for this experiment because pigmented melanocytes opitcute when subjected to the intense laser light required for photo bleaching). Consistent with our hypothesis, Figure 4, Panels C1–C3, show that melanoregulin-GFP exhibits very little recovery in the melanosome membrane in the five minutes following photo bleaching (Panel C1, pre-bleach; Panel C2, bleach; Panel C3, 5 minutes post-bleach). Figure 4, Panel D, which provides the quantitation of this FRAP experiment over a 15 minute time course, confirms that melanoregulin GFP does not cycle in and out of the melanosome membrane to an appreciable extent (the small degree of recovery by 15 minutes is probably due largely to the movement of unbleached melanosomes into the bleach zone). We conclude, therefore, that multiple palmitoylation probably serves to keep melanoregulin stably anchored in the limiting membrane of the melanosome.
DISCUSSION
Given that melanoregulin lacks a transmembrane domain and is highly charged, our main goal in this study was determine how the protein associates so dramatically with the limiting membrane of the melanosome. The answer is that the protein is palmitoylated, probably multiply, at a cluster of six cysteines located near the protein’s N-terminus. This conclusion is based primarily on the following results: (i) GFP-tagged melanoregulin in which these cysteines were changed to serines no longer targets to melanosomes in melanocytes and to lysosomes in CV1 cells, (ii) melanoregulin can be labeled metabolically with H3-palmitatic acid, and (iii) the addition of an inhibitor of palmitoylation to cultured CV1 cells disrupts the targeting of melanoregulin-GFP to lysosomes. Many palmitoylated proteins are also myristoylated at a glycine residue that follows the initiator methionine [6; 7]. Given that melanoregulin possesses a glycine at residue position 2, it is likely that it is also dually acylated. That said, melanoregulin in which this glycine was changed to an alanine still targets effectively to the melanosome. This is not surprising, however, as palmitoylation usually plays the dominant role in the membrane attachment of dually acylated proteins [6; 7]. Nevertheless, the addition of the myristate moiety often serves to facilitate subsequent palmitoyation [6; 7], so melanoregulin should be tagged at its C-terminus rather than at its N-terminus as in Ohbayashi et. al. [4] to avoid blocking myristate addition (the myristate is added only to the glycine when this glycine is at the very N-terminus following removal of the initiator methionine).
The fact that melanoregulin possesses many potential sites for palmitoylation suggests that it, like other multiply palmitoylated proteins (e.g. cysteine string proteins), may be stably bound to the melanosome membrane because at any one moment it would usually have at least one attached palmitate group. This would be in contrast to many dually acylated proteins that contain only one or two cysteines, which often cycle in and out of membranes as part of their life cycle [6; 7],. Consistent with these ideas, FRAP of melanoregulin-GFP present on melanosomes in melan C melanocytes showed essentially no recovery of fluorescence after five minutes. Overall, therefore, in terms of the site of action of melanoregulin in melanocytes, it appears to be largely if not entirely on the melanosome surface, where it is stably bound as a result of extensive palmitoylation.
We found that the over-expression of melanoregulin in dv/dv melanocytes causes melanosomes to concentrate more dramatically in the center of these cells. Similarly, the over-expression of melanoregulin in CV1 cells causes lysosomes to concentrate in the cell center. A similar melanoregulin over-expression phenotype in an immortal melanocyte cell line was recently reported by Ohbayashi et. al. [4]. They interpreted this over-expression phenotype in light of their data implicating melanoregulin, rather than Rab7 as previously reported [13; 14], as the anchor on the melanosome (and lysosome) surface for the complex of RILP, dynactin and dynein, which together drive the microtubule minus end-directed movements of these organelles. While the validity of their conclusion is not really relevant to our study here, our recent demonstration of the role played by melanoregulin in the intercellular transfer of melanosomes from the melanocyte to the keratinocyte [3] argues against melanoregulin serving as the melanosome receptor for dynein. Indeed, we think that the over expression of melanoregulin causes the central accumulation of melanosomes and lysosomes not by serving as the melanosome receptor for dynein, but rather by increasing the Rab7-dependent recruitment of dynein, and possibly the subsequent processivity of the motor. Moreover, we think the physical basis for this non-physiological, over expression effect is a significant shift from liquid-disordered to liquid-ordered lipid phases in the limiting membrane of the melanosome (and lysosome) that is caused by the presence of excess melanoregulin, and is a direct consequence of melanoregulin’s multiple attached palmitates, which partition into and promote liquid-ordered lipid microdomains [15]. Relevant to this hypothesis, other studies have linked increases in the content of cholesterol, another component of liquid-ordered lipid microdomains (i.e. rafts), in the limiting membrane of the lysosome to changes in the organelle’s dynein-dependent movement [16].
Supplementary Material
Bullet Points.
Melanoregulin-GFP targets extensively to the limiting membrane of the melanosome.
Endogenous melanoregulin also targets extensively to the melanosome.
Melanoregulin targeting to melanosomes and lysosomes requires N-terminal cysteines.
SDM, metabolic labeling and inhibitors show that melanoreguiln is palmitoylated.
FRAP shows that melanoregulin is stably targeted to the melanosome membrane.
Acknowledgments
We thank Juan Bonafacino for his suggestion that we explore the role of lipid acylation in the ecruitment of melanoregulin to the melanosome surface. We also thank Rosa Peurtollano for providing GFP-tagged mucolipin-3, and Maria Morasso for providing cultures of primary mouse keratinocytes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wu X, Bowers B, Rao K, Wei Q, Hammer JA., 3rd Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function In vivo. J Cell Biol. 1998;143:1899–918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Sullivan TN, Wu XS, Rachel RA, Huang JD, Swing DA, Matesic LE, Hammer JA, 3rd, Copeland NG, Jenkins NA. dsu functions in a MYO5A-independent pathway to suppress the coat color of dilute mice. Proc Natl Acad Sci U S A. 2004;101:16831–6. doi: 10.1073/pnas.0407339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu XS, Masedunskas A, Weigert R, Copeland NG, Jenkins NA, Hammer JA. Melanoregulin regulates a shedding mechanism that drives melanosome transfer from melanocytes to keratinocytes. Proc Natl Acad Sci U S A. 109:E2101–9. doi: 10.1073/pnas.1209397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohbayashi N, Maruta Y, Ishida M, Fukuda M. Melanoregulin regulates retrograde melanosome transport through interaction with the RILP-p150Glued complex in melanocytes. J Cell Sci. 125:1508–18. doi: 10.1242/jcs.094185. [DOI] [PubMed] [Google Scholar]
- 5.Moore KJ, Swing DA, Copeland NG, Jenkins NA. The murine dilute suppressor gene encodes a cell autonomous suppressor. Genetics. 1994;138:491–7. doi: 10.1093/genetics/138.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE. 2006;2006:re14. doi: 10.1126/stke.3592006re14. [DOI] [PubMed] [Google Scholar]
- 7.Resh MD. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 8.Seperack PK, Mercer JA, Strobel MC, Copeland NG, Jenkins NA. Retroviral sequences located within an intron of the dilute gene alter dilute expression in a tissue-specific manner. EMBO J. 1995;14:2326–32. doi: 10.1002/j.1460-2075.1995.tb07227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damek-Poprawa M, Diemer T, Lopes VS, Lillo C, Harper DC, Marks MS, Wu Y, Sparrow JR, Rachel RA, Williams DS, Boesze-Battaglia K. Melanoregulin (MREG) modulates lysosome function in pigment epithelial cells. J Biol Chem. 2009;284:10877–89. doi: 10.1074/jbc.M808857200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martina JA, Lelouvier B, Puertollano R. The calcium channel mucolipin-3 is a novel regulator of trafficking along the endosomal pathway. Traffic. 2009;10:1143–56. doi: 10.1111/j.1600-0854.2009.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Resh MD. Use of analogs and inhibitors to study the functional significance of protein palmitoylation. Methods. 2006;40:191–7. doi: 10.1016/j.ymeth.2006.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greaves J, Salaun C, Fukata Y, Fukata M, Chamberlain LH. Palmitoylation and membrane interactions of the neuroprotective chaperone cysteine-string protein. J Biol Chem. 2008;283:25014–26. doi: 10.1074/jbc.M802140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordens I, Westbroek W, Marsman M, Rocha N, Mommaas M, Huizing M, Lambert J, Naeyaert JM, Neefjes J. Rab7 and Rab27a control two motor protein activities involved in melanosomal transport. Pigment Cell Res. 2006;19:412–23. doi: 10.1111/j.1600-0749.2006.00329.x. [DOI] [PubMed] [Google Scholar]
- 14.Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, Olkkonen VM, Neefjes J. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol. 2007;176:459–71. doi: 10.1083/jcb.200606077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levental I, Grzybek M, Simons K. Greasing their way: lipid modifications determine protein association with membrane rafts. Biochemistry. 49:6305–16. doi: 10.1021/bi100882y. [DOI] [PubMed] [Google Scholar]
- 16.Lebrand C, Corti M, Goodson H, Cosson P, Cavalli V, Mayran N, Faure J, Gruenberg J. Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 2002;21:1289–300. doi: 10.1093/emboj/21.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.