Abstract
Background
Patient-initiated notification is a commonly used practice for notifying sex partners of possible exposure to a sexually transmitted infection (STI), yet 46% to 75% of partners are never treated. The Contraceptive CHOICE Project (CHOICE) is a longitudinal cohort study of women that provides no-cost contraception, STI testing, treatment to participants, and free partner treatment. Our objective was to evaluate characteristics of women who tested positive for chlamydia, gonorrhea, or trichomoniasis, and their association with successful partner treatment.
Methods
We analyzed baseline survey and STI testing, notification, and treatment data from the first 5,087 participants enrolled in CHOICE. We considered “treated partners” to be men who received antibiotic treatment at the study clinic or by a prescription through the study. Independent predictors of successful partner treatment were identified using univariate analysis and multivariable analysis using Poisson regression with robust error variance.
Results
Forty-four percent of male partners were successfully treated. Women whose partners were less likely to obtain treatment were black (RRadj=0.6; 95% CI: 0.5–0.8) or reported some concern about future STI with the partner (RRadj=0.6; 95% CI: 0.4, 0.8). Women whose partners were more likely to receive treatment were living with their partner (RRadj=1.4; 95% CI: 1.1–1.8) or reported recent inconsistent condom use (RRadj=1.5; 95% CI: 1.1–2.1).
Conclusions
The male partner treatment rate resulting from female patient-initiated partner notification in our study was low. Our findings highlight the need to develop novel notification interventions that yield higher partner treatment rates and consider patient-specific factors, such as race and relationship status.
Keywords: partner notification, sexually transmitted infections, treatment
INTRODUCTION
It is estimated that over 19 million cases of sexually transmitted infections (STIs) occur in the United States each year.1 According to the most recent report from the Centers for Disease Control and Prevention, the number of reported chlamydia cases increased nationally by 5.1% from 409.2 to 426.0 per 100,000 persons between 2009 and 2010, with continually high chlamydia rates in the Midwest.2 In St. Louis, Missouri, where this study takes place, rates of infections with Chlamydia trachomatis and Neisseria gonorrhoeae are consistently among the highest in the nation.2, 3 The short- and long-term health effects of bacterial STIs are substantial, including pelvic inflammatory disease, ectopic pregnancy, infertility, chronic pelvic pain, and adverse pregnancy outcomes such as preterm delivery and low birth weight infants.4 Consequently, STIs are associated with high direct and indirect healthcare costs, amounting to $17 billon annually.2
Partner notification is a public health intervention aimed at reducing the transmission of STIs and preventing re-infection. Partner notification consists of contacting the sex partners of infected individuals to notify them about their potential STI exposures, and offering healthcare services such as testing, treatment, and risk-reduction counseling.5, 6 Patient-initiated partner notification requires fewer resources than provider-initiated partner notification,7, 8 and has been central to most STI control efforts.6 Expedited partner therapy (EPT) provides patients with medication to give to their partners for STI treatment without an in-depth clinical assessment. Studies have demonstrated that EPT is feasible and cost-effectiveness,9 and is more effective than simple partner notification in reducing persistent or recurrent chlamydia or gonorrhea infection.10,11 Despite its potential benefits, EPT is used inconsistently used by a majority of physicians12,13 and it is fully permissible in only 30 states, some of which have specific restrictions.14 In the state of Missouri, EPT is authorized for chlamydia or gonorrhea treatment only, but not for trichomoniasis.14
Findings from observational and clinical trial studies examining female patient-initiated partner notification report that only 25% to 54% of male partners received treatment for N. gonorrhoeae, C. trachomatis, or T. vaginalis infection.8, 15, 16 Higher partner treatment rates of 53% to 79% are reported when based on the report of the female index patient.17–19 The large numbers of untreated partners with patient-initiated partner notification methods and the limited number of studies specifically examining female-initiated partner notification suggest that a more thorough understanding of the factors associated with successful partner STI treatment is necessary.20
The present study is a secondary data analysis that describes the demographic and behavioral characteristics associated with C. trachomatis (CT), N. gonorrhoeae (GC), and Trichomonas vaginalis (TV) infections among St. Louis women enrolled in a cohort study and examines the characteristics associated with successful male partner treatment following patient-initiated partner notification.
MATERIALS AND METHODS
The Contraceptive CHOICE Project (CHOICE) is an ongoing longitudinal study of 9,256 St. Louis women that seeks to promote the use of the most effective contraceptive methods to affect a population-level decrease in the number of unintended pregnancies.21 Women enrolled in CHOICE are followed for 2–3 years, during which time they are provided with contraception, STI screening, and STI treatment at no cost. Women are recruited to participate through provider referral, word-of-mouth, and while seeking care at specific recruitment locations including a university-based research clinic and community clinics that provide abortion care and general gynecologic and family planning services. Briefly, women are eligible to participate if they are 14–45 years of age, reside in St. Louis or seek clinical care in study-affiliated clinics, wish to avoid pregnancy for one year, have not had a hysterectomy or tubal ligation, have had sex with a male partner in the past 6 months or intend to have sex in the ensuing 6 months, and desire a new method of contraception. Eligible women undergo an enrollment session wherein they provide informed written consent and contact information, and respond to a staff-administered structured baseline questionnaire. The questionnaire collects information regarding the participant’s demographic characteristics and reproductive history, as well as the relationship characteristics and sexual behaviors with her main and other sexual partners. Following enrollment, women are contacted at 3- and 6-month post-enrollment and every 6 months thereafter with telephone surveys to measure contraceptive method continuation and satisfaction. For women under the age of 18, we obtained minor assent in addition to the informed consent of a parent or legal guardian. The CHOICE protocol was approved by the Washington University in St. Louis School of Medicine Human Research Protection Office prior to the initiation of recruitment.
At enrollment, participants submitted self-collected vaginal specimens for CT, GC, and TV screening. CT and GC infection were detected through DNA strand displacement amplification technology using BDProbeTec ET (Becton Dickson, Sparks, MD) instruments. The InPouch™ TV (BioMed Diagnostics, White City, Oregon) culture was used to detect TV infection. All participants with positive test results were notified by a staff clinician via telephone; participants with negative test results were notified by letter. Antibiotic treatment for STIs could be obtained from the study clinic or at a study-affiliated pharmacy at no cost to the participant.
During STI notification participants received standardized educational information regarding their infection and the need for treatment. The study clinician also encouraged participants to inform their partner(s) of their positive test result to prevent re-infection and to offer them free treatment through the study. If the participant arrived for treatment without her partner, the clinician again reminded her to notify her partner and recommend treatment through the study clinic. Partners were not screened for STIs, but were provided with treatment following clinician consultation with the male partner via a telephone prescription to a pharmacy or during a visit to the study clinic. The study clinic served only CHOICE participants and operated five days per week with evening hours and two Saturdays per month. STI treatment was considered a priority by the research team and every effort was made to accommodate appointments for patients and partners to receive treatment. The clinic was located on the perimeter of a medical school campus near public transportation lines with signage that stated it was a research building. Expedited partner therapy (EPT) was not offered as it was not permitted in Missouri at the time of initiation of this study; however, EPT was recently legalized in August 2010. STI treatment for both the patient and her partner(s) was documented on standardized forms.
We considered “treated partners” to be those who received antibiotic treatment either via a prescription through the study or at the study clinic. CHOICE participants who reported their partner(s) would or had sought STI treatment elsewhere were not considered “treated” for this analysis due to our inability to obtain objective treatment confirmation. However, we did review the 186 medical charts of participants whose partners did not receive treatment to determine if documentation regarding partner treatment elsewhere was noted by the study clinician.
Appropriate descriptive statistics were used to compare the demographic, behavioral, and relationship characteristics of women who (1) tested positive for any infection with women who did not test positive and (2) whose partner(s) obtained treatment with those of women whose partner(s) did not obtain treatment. We examined predictors of a woman testing positive using logistic regression and present relative risk estimates. Because the STI prevalence rate was low in our study, the odds ratio generated in logistic regression is considered a reliable estimate of the relative risk. To analyze the predictors of whether a participant’s partner obtained STI treatment, we used Poisson regression with robust error variance. This regression technique allows for an unbiased estimate of the relative risk when the outcome of interest occurs more than 10% of the time, as was the case for partner STI treatment in this analysis.22 Independent predictors of successful partner treatment were identified via univariate analyses and included in our multivariable analyses. Statistical analyses were conducted using SAS Software (v.9.2., SAS Institute, Cary, NC).
RESULTS
Of the first 5,087 women enrolled in CHOICE from August 2007 through December 2009, 70% enrolled at the university-based study clinic, 48% were black, 60% were 25 years or less, and over 50% reported difficulty paying for basic necessities or receiving public assistance. One in three women were living with a partner or spouse, more than half reported unprotected sex during the past month, and 40% reported a history of STI (Table 1).
TABLE 1.
Demographic and Behavioral Characteristics of Women Enrolled in the Contraceptive CHOICE Project by Baseline Sexually Transmitted Infection (STI) Status (n = 4691)
| Baseline STI Status | Univariate Regression | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Overall (n = 4691) | Negative (n = 4356) | Positive (n = 335) | Relative Risk (95% CI) | ||||
|
| |||||||
| n | % | n | % | n | % | ||
| Recruitment location | |||||||
| Abortion clinic | 697 | 14.9 | 621 | 14.3 | 76 | 22.7 | 1.8 (1.4, 2.4) |
| University research clinic | 3359 | 71.6 | 3149 | 72.3 | 210 | 62.7 | Referent |
| Community clinic | 635 | 13.5 | 586 | 13.4 | 49 | 14.6 | 1.3 (0.9, 1.7) |
| Demographic characteristics | |||||||
| Age (yr) | |||||||
| >18 | 189 | 4.0 | 177 | 4.1 | 12 | 3.6 | 1.1 (0.6, 2.0) |
| 18–20 | 765 | 16.3 | 679 | 15.6 | 86 | 25.7 | 2.0 (1.5, 2.7) |
| <21–25 | 1894 | 40.4 | 1767 | 40.5 | 127 | 37.9 | 1.1 (0.9, 1.5) |
| >25 | 1843 | 39.3 | 1733 | 39.8 | 110 | 32.8 | Referent |
| Race | |||||||
| Black | 2246 | 48.1 | 1973 | 45.5 | 273 | 81.5 | 5.7 (4.2, 7.8) |
| White | 2078 | 44.5 | 2029 | 46.8 | 49 | 14.6 | Referent |
| Other | 344 | 7.4 | 331 | 7.6 | 13 | 3.9 | 1.6 (0.9, 3.0) |
| Trouble paying for basic necessities in past 12 mo or currently receives public assistance* | |||||||
| Yes | 2660 | 56.7 | 2417 | 55.5 | 243 | 72.5 | 2.1 (1.7, 2.7) |
| No | 2031 | 43.3 | 1939 | 44.5 | 92 | 27.5 | Referent |
| Insurance | |||||||
| None | 2029 | 43.6 | 1850 | 42.8 | 179 | 53.9 | 2.2 (1.7, 2.8) |
| Private | 2058 | 44.2 | 1970 | 45.6 | 88 | 26.5 | Referent |
| Medicaid/Medicare | 567 | 12.2 | 502 | 11.6 | 65 | 19.6 | 2.9 (2.1, 4.1) |
| Social and Reproductive Characteristics | |||||||
| No. sexual partners in past 30 d | |||||||
| 0 | 931 | 19.9 | 857 | 19.7 | 74 | 22.1 | Referent |
| 1 | 3483 | 74.3 | 3266 | 75.1 | 217 | 64.8 | 0.8 (0.6, 1.0) |
| >1 | 270 | 5.8 | 226 | 5.2 | 44 | 13.1 | 2.3 (1.5, 3.4) |
| Self-reported history of STI† | |||||||
| Yes | 1889 | 40.3 | 1719 | 39.5 | 170 | 50.7 | 1.6 (1.3, 2.0) |
| No | 2802 | 59.7 | 2637 | 60.5 | 165 | 49.3 | Referent |
| Reported experiencing STI symptoms during past 7 d‡ | |||||||
| Yes | 708 | 15.1 | 629 | 14.4 | 79 | 23.6 | 1.8 (1.4, 2.4) |
| No | 3983 | 84.9 | 3727 | 85.6 | 256 | 76.4 | Referent |
| Relationship characteristics | |||||||
| Living with a partner or spouse | |||||||
| Yes | 1595 | 34.0 | 1536 | 35.3 | 59 | 17.6 | 0.4 (0.3, 0.5) |
| No | 3096 | 66.0 | 2820 | 64.7 | 276 | 82.4 | Referent |
| Length of time having sex with main partner | |||||||
| No sex or ≤3 mo | 701 | 17.2 | 645 | 17.0 | 56 | 19.4 | Referent |
| 4 mo to >1 yr | 794 | 19.4 | 723 | 19.0 | 71 | 24.7 | 1.1 (0.8, 1.6) |
| ≥1 yr | 2591 | 63.4 | 2430 | 64.0 | 161 | 55.9 | 0.8 (0.6, 1.0) |
| Any unprotected sex during past 30 d | |||||||
| Yes | 2410 | 54.2 | 2240 | 54.4 | 170 | 51.8 | 0.9 (0.7, 1.1) |
| No | 2037 | 45.8 | 1879 | 45.6 | 158 | 48.2 | Referent |
Note: Not all columns sum to 4691: 23 missing race, 37 missing insurance, 7 missing number sexual partners in past 30 d, 605 did not have a main partner, and 244 missing condom information during past 30 d.
Trouble paying for basic expenses: transportation, housing, health or medical care, or food; public assistance: current receipt of food stamps, WIC, welfare, or unemployment.
History of chlamydia, gonorrhea, syphilis, trichomoniasis, genital herpes, human papillomavirus, or HIV/AIDS.
Reported experiencing vaginal discharge, itching, or odor, pain with sexual intercourse, or pelvic pain during past 7 d at time of baseline interview and STI testing.
RR indicates relative risk; 95% CI, 95% confidence interval.
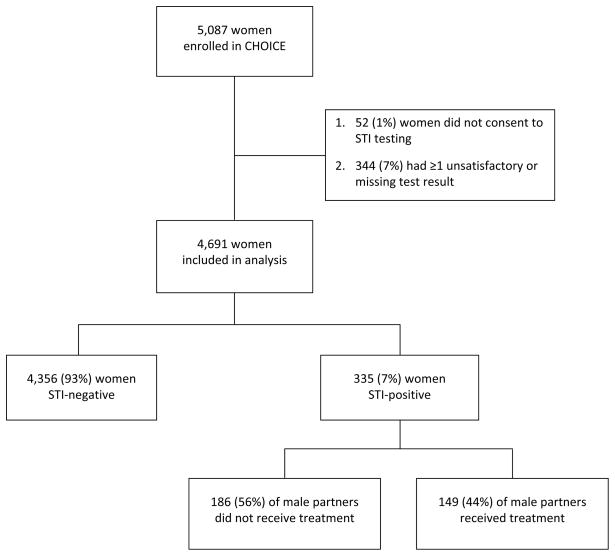
Fifty-two women (1%) did not consent to STI screening at enrollment and 344 women (7%) had one or more unsatisfactory or missing test results, therefore 396 were excluded from this analysis (Figure 1). Of the 4,691 women (92%) who consented to and successfully completed CT, GC, and TV screening, 335 women (7%) were STI-positive at enrollment. The baseline prevalence rate for CT, GC, and TV were 3% (n=134), 0.3% (n=12), and 4% (n=207), respectively. Of the participants testing positive, 18 (5%) were co-infected with more than one STI.
Figure 1.
Diagram of study participants and analysis outcomes
Participants who were black (OR=5.7, 95% CI 4.2, 7.8), aged 18–20 years (OR=2.0, 95% CI 1.5, 2.7), reported difficulty paying for basic necessities or currently received public assistance (OR=2.1, 95% CI 1.7, 2.7), had no insurance (OR=2.2, 95% CI 1.7, 2.8) or were publically insured (OR=2.9, 95% CI 2.1, 4.1) were more than twice as likely to test positive for an STI at baseline (Table 1). Compared to STI-negative women, women who were STI-positive at baseline were significantly more likely to report greater than one partner during the past 30 days (OR=2.3, 95% CI 1.5, 3.4) and a history of STIs (OR=1.6, 95% CI 1.3, 2.0). Women who lived with a partner or spouse were 60% less likely to be infected (OR=0.4, 95% CI 0.3, 0.5).
Of the 335 STI-positive participants, we successfully treated 100% of the women infected with CT and/or GC and 99% of women infected with TV. All but five women returned to the study clinic and received directly-observed therapy. For women who were unable to return to the clinic we called in a prescription to a pharmacy and subsequently called each woman to confirm she complied with treatment. However, only 44% of STI-positive participants had a partner successfully treated by CHOICE. Twenty-six women (8%) reported they were no longer with their partner or their partner was unavailable for treatment and an additional 8 women stated upon notification they would not notify their partner. Of the remaining 301 women who planned to tell their partner(s) about the STI infection and available treatment, 46% had all partners treated, 3% had some partners treated, and 51% had zero partners treated. Successful partner treatment did not vary significantly by type of infection (47%, 50%, 43% for CT, GC, and TV, respectively, p=0.69).
In Table 2, we compare the demographic, behavioral, and relationship characteristics of the 335 STI-positive participants whose partners did and did not obtain treatment. In univariate analyses, race, number of sexual partners in the past 30 days, living situation, length of sexual relationship, condom use, and likelihood of future STI with sexual partners were significantly associated with the likelihood of successful partner treatment. However, when considered simultaneously in the multivariable model, only four characteristics remained significantly associated. Compared to white women, black women were 40% less likely to have a partner treated through CHOICE (RRadj=0.6, 95% CI 0.5, 0.8). Women who reported some concern of becoming infected with an STI by a main and/or other partner in the next three months were also less likely to have a partner who obtained treatment (RRadj=0.6, 95% CI 0.4, 0.8). Conversely, women who lived with a partner or spouse (RRadj=1.4, 95% CI 1.1, 1.8) or who reported any unprotected sex during the past 30 days with any partner (RRadj=1.5, 95% CI 1.1, 2.1) were more likely to have a partner who obtained treatment. Recruitment location, a woman’s age, socioeconomic status, self-reported history of STI or report of STI symptoms at the time of STI screening, and lifetime history of abuse were not associated with whether or not a partner obtained treatment.
TABLE 2.
Characteristics of STI-Positive Female Study Participants Associated With Whether Male Partner Obtained Treatment From CHOICE Project (n = 335)
| Male Partner Treated | Regression Models | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| No (n = 186) | Yes (n = 149) | Univariate | Multivariable | |||
|
| ||||||
| n | % | n | % | RR (95% CI) | RR (95% CI) | |
| Recruitment location | ||||||
| Abortion clinic | 37 | 48.7 | 39 | 51.3 | 1.2 (0.9, 1.5) | |
| University research clinic | 118 | 56.2 | 92 | 43.8 | Referent | |
| Community clinic | 31 | 63.3 | 18 | 36.7 | 0.8 (0.6, 1.2) | |
| Demographic characteristics | ||||||
| Age (yr) | ||||||
| >18 | 9 | 75.0 | 3 | 25.0 | 0.6 (0.2, 1.6) | |
| 18–20 | 43 | 50.0 | 43 | 50.0 | 1.2 (0.9, 1.6) | |
| 21–25 | 70 | 55.1 | 57 | 44.9 | 1.1 (0.8, 1.4) | |
| >25 | 64 | 58.2 | 46 | 41.8 | Referent | |
| Race | ||||||
| Black | 163 | 60.0 | 110 | 40.0 | 0.6 (0.5, 0.8) | 0.6 (0.6, 0.8) |
| White | 16 | 32.7 | 33 | 67.4 | Referent | Referent |
| Other | 7 | 53.9 | 6 | 46.2 | 0.7 (0.4, 1.3) | 0.6 (0.3, 1.2) |
| Trouble paying for basic necessities in past 12 mo or currently receives public assistance* | ||||||
| Yes | 132 | 54.3 | 111 | 45.7 | 1.1 (0.8, 1.5) | |
| No | 54 | 58.7 | 38 | 41.3 | Referent | |
| Social and Reproductive Characteristics | ||||||
| No. sexual partners in past 30 d | ||||||
| 0 | 50 | 67.8 | 24 | 32.4 | Referent | Referent |
| 1 | 110 | 50.7 | 107 | 49.3 | 1.5 (1.1, 2.2) | 0.9 (0.5, 1.3) |
| >1 | 26 | 59.1 | 18 | 40.9 | 1.3 (0.8, 2.0) | 0.9 (0.4, 1.9) |
| Self-reported history of STI† | ||||||
| Yes | 93 | 56.4 | 77 | 43.6 | 1.0 (0.8, 1.3) | |
| No | 93 | 54.7 | 72 | 45.3 | Referent | |
| Reported experiencing STI symptoms during past 7 d‡ | ||||||
| Yes | 49 | 62.0 | 30 | 40.0 | 0.8 (0.6, 1.1) | |
| No | 137 | 53.5 | 119 | 46.5 | Referent | |
| History of abuse or violence in lifetime | ||||||
| Yes | 92 | 51.4 | 87 | 48.6 | 1.2 (0.9, 1.6) | |
| No | 94 | 60.3 | 62 | 39.7 | Referent | |
| Relationship characteristics | ||||||
| Living with partner or spouse | ||||||
| Yes | 17 | 28.8 | 42 | 71.2 | 1.8 (1.5, 2.3) | 1.4 (1.1, 1.8) |
| No | 169 | 61.2 | 107 | 38.8 | Referent | Referent |
| Length of time having sex with main partner | ||||||
| No sex or ≤3 mo | 36 | 64.3 | 20 | 35.7 | Referent | Referent |
| 4 mo to >1 yr | 39 | 54.9 | 32 | 45.1 | 1.3 (0.8, 2.0) | 1.1 (0.7, 1.7) |
| ≥1 yr | 76 | 47.2 | 85 | 52.8 | 1.5 (1.0, 2.2) | 1.2 (0.8, 1.9) |
| Any unprotected sex during past 30 d | ||||||
| Yes | 78 | 45.9 | 92 | 54.1 | 1.6 (1.2, 2.1) | 1.5 (1.1, 2.1) |
| No | 105 | 66.5 | 53 | 33.5 | Referent | Referent |
| Concerned about getting an STI from main and/or other partner in next 3 mo | ||||||
| Not at all concerned | 94 | 46.8 | 107 | 53.2 | Referent | Referent |
| Some concern | 60 | 65.2 | 32 | 34.8 | 0.7 (0.5, 0.9) | 0.6 (0.4, 0.8) |
Note: Not all columns sum to 186 or 149: 47 did not have a main partner and 7 missing condom information.
Trouble paying for basic expenses: transportation, housing, health or medical care, or food; Public assistance: current receipt of food stamps, WIC, welfare, or unemployment.
History of chlamydia, gonorrhea, syphilis, trichomoniasis, genital herpes, human papillomavirus, or HIV/AIDS.
Reported experiencing vaginal discharge, itching, or odor; pain with sexual intercourse; or pelvic pain during past 7 d at time of baseline interview and STI testing.
RR indicates relative risk; 95% CI, 95% confidence interval.
We reviewed 186 medical charts of participants whose partners were considered untreated. Thirty-three (18%) charts had written documentation stating the participant reported that her partner(s) had sought or would seek treatment elsewhere. When we repeated the analysis and considered the 33 instances as “treated” the findings did not appreciably change. The only significant difference was that unprotected sex in the past 30 days was no longer associated with successful partner treatment.
DISCUSSION
We found female patient-initiated partner notification resulted in the treatment of less than half of male partners, despite both exceptionally high treatment rates among female participants and removal of financial barriers. Our findings are comparable to male partner treatment rates of 25 to 54% resulting from patient-initiated notification reported in previous studies.8, 15, 16 Although previous research has shown that out-of-pocket expenses represent a significant barrier to obtaining STI treatment,23 perceived barriers other than cost may have contributed to the lower treatment outcomes found in this study.
We found that black female participants, those not living with a partner or spouse, and those reporting concern for contracting an STI from their main partner in the following three months were less likely to have their partner(s) treated. Women who do not live with their partner or have concerns about a future STI infection may be less willing to notify and recommend treatment to a partner who is perceived to be a casual partner.19 Interestingly, black participants and those not living with a partner or spouse were also more likely to be STI-positive. Gorbach et al. suggest that notification outcomes may be improved by tailoring the notification method to the type of patient, their partners, and their relationships.24 Our findings highlight the importance of developing notification interventions that take into consideration patient-specific factors, such as race and living or relationship status16, 18, 19, and that address the difficulties individuals face when informing their partners that they have an STI.25
Participants who reported any unprotected sex during the previous 30 days were more likely to have at least one partner treated. This finding supports that of an earlier study of female adolescents in which no condom use during the last sexual contact was associated with higher partner treatment rates.8 Women who do not use condoms may be more likely to believe they have an infection and therefore encourage treatment with their partner.
This study has several strengths. Male partners had the option of receiving treatment from a study-affiliated pharmacy or directly at the study clinic at no cost. These measures reduced the commonly-reported administrative, access, and financial barriers to treatment.23 Additional strengths include the study’s relatively large sample size and the racial and socioeconomic diversity of the female participants, all of whom were of reproductive-age, sexually active, and not recruited from an STI clinic.
However, a number of factors should be considered in the assessment of our findings. Although the cohort includes a large percent of women at risk of STI (e.g., young, black, low socioeconomic status), women in this study are participants of a contraception study which may decrease the generalizability of our findings to other clinic populations. We considered partners who received a prescription from CHOICE as treated, yet we do not know definitely whether they filled the prescription and took the medication. Furthermore, partners who intended to seek or received treatment at locations other than CHOICE study sites were not considered treated in this analysis due to our inability to obtain objective treatment confirmation. Consequently, treatment rates associated with partner notification may have been underestimated. We did not attempt to characterize how female participants notified their partners. Chacko et al. determined that young women use a number of communication methods and styles and present varying degrees of information when notifying their partners.17 Further studies are needed to assess whether different notification content and communication strategies predict successful male partner treatment. Finally, because female participant demographic and behavioral characteristics were the focus of this study, we did not collect and analyze male partner characteristics. An analysis of these characteristics may give greater insight into the factors associated with successful male partner referral and treatment.
Despite these limitations, low rates of verified partner treatment found in this and other studies substantiate the need for novel notification interventions yielding higher treatment rates. Increased permissibility and promotion of EPT may assist in improving partner notification and treatment rates but may not be an acceptable alternative to all infected patients and partners.18, 26 When we included self-reported treatment as successful partner treatment our study rate increased to 54% or approximately 0.5 partners treated per index case because the majority of study participants reported only one partner. This rate is similar to male partner treatment rates when women are randomized to public health disease intervention specialists (57%)15 or when adolescent women select provider notification (55%)8; both of which are staff-intensive notification methods. Therefore, the ability to identify and properly counsel woman less likely to have their partner treated may increase partner treatment rates resulting from a low-cost notification protocol.
In summary, our study is among the few to examine the demographic and behavioral characteristics of women that predict successful STI treatment in their male partners via patient-initiated partner notification. We found living with a partner and recent inconsistent condom use increased partner treatment whereas black race and concern for future STI were associated with decreased partner treatment. Our patient-initiated partner notification protocol resulted in less than half of male partners receiving directly observed treatment despite free and convenient treatment. Further studies are required to determine the impact of male partner demographic and behavioral characteristics, as well as female communication strategies, on STI treatment rates, especially through EPT. Based on the study results, other notification strategies are warranted in the at-risk population.
Acknowledgments
Financial support: Supported by an Anonymous Foundation and in part by award number K12HD001459 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), its contents are solely the responsibility of the authors and do not necessarily represent the official view NIH.
Shirley Shih, BA, for her careful editorial review of the manuscript.
Footnotes
Disclosures: None of the authors have any conflict of interest to disclose.
References
- 1.Weinstock H, Berman S, Cates W., Jr Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004 Jan-Feb;36(1):6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2010. Atlanta: U.S. Department of Health and Human Services; 2011. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2009. Atlanta: U.S. Department of Health and Human Services; 2010. [Google Scholar]
- 4.Aral SO. Sexually transmitted diseases: magnitude, determinants and consequences. Int J STD AIDS. 2001 Apr;12(4):211–215. doi: 10.1258/0956462011922814. [DOI] [PubMed] [Google Scholar]
- 5.Kissinger PJ, Niccolai LM, Magnus M, et al. Partner notification for HIV and syphilis: effects on sexual behaviors and relationship stability. Sex Transm Dis. 2003 Jan;30(1):75–82. doi: 10.1097/00007435-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Mathews C, Coetzee N, Zwarenstein M, et al. A systematic review of strategies for partner notification for sexually transmitted diseases, including HIV/AIDS. Int J STD AIDS. 2002 May;13(5):285–300. doi: 10.1258/0956462021925081. [DOI] [PubMed] [Google Scholar]
- 7.Fortenberry JD, Brizendine EJ, Katz BP, Orr DP. The role of self-efficacy and relationship quality in partner notification by adolescents with sexually transmitted infections. Arch Pediatr Adolesc Med. 2002 Nov;156(11):1133–1137. doi: 10.1001/archpedi.156.11.1133. [DOI] [PubMed] [Google Scholar]
- 8.Oh MK, Boker JR, Genuardi FJ, Cloud GA, Reynolds J, Hodgens JB. Sexual contact tracing outcome in adolescent chlamydial and gonococcal cervicitis cases. J Adolesc Health. 1996 Jan;18(1):4–9. doi: 10.1016/1054-139X(95)00109-6. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Expedited partner therapy in the management of sexually transmitted diseases. Atlanta, GA: US Department of Health and Human Services; 2006. [Google Scholar]
- 10.Shiely F, Hayes K, Thomas KK, et al. Expedited partner therapy: a robust intervention. Sex Transm Dis. 2010 Oct;37(10):602–607. doi: 10.1097/OLQ.0b013e3181e1a296. [DOI] [PubMed] [Google Scholar]
- 11.Trelle S, Shang A, Nartey L, Cassell JA, Low N. Improved effectiveness of partner notification for patients with sexually transmitted infections: systematic review. BMJ. 2007 Feb 17;334(7589):354. doi: 10.1136/bmj.39079.460741.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogben M, McCree DH, Golden MR. Patient-delivered partner therapy for sexually transmitted diseases as practiced by U.S. physicians. Sex Transm Dis. 2005 Feb;32(2):101–105. doi: 10.1097/01.olq.0000151417.43230.18. [DOI] [PubMed] [Google Scholar]
- 13.Golden MR, Hogben M, Handsfield HH, St Lawrence JS, Potterat JJ, Holmes KK. Partner notification for HIV and STD in the United States: low coverage for gonorrhea, chlamydial infection, and HIV. Sex Transm Dis. 2003 Jun;30(6):490–496. doi: 10.1097/00007435-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. [Accessed November 1, 2011];Legal Status of Expedited Partner Therapy (EPT) Available at: http://www.cdc.gov/std/ept/legal/default.htm.
- 15.Schwebke JR, Desmond RA. A randomized controlled trial of partner notification methods for prevention of trichomoniasis in women. Sex Transm Dis. 2010 Jun;37(6):392–396. doi: 10.1097/OLQ.0b013e3181dd1691. [DOI] [PubMed] [Google Scholar]
- 16.van de Laar MJ, Termorshuizen F, van den Hoek A. Partner referral by patients with gonorrhea and chlamydial infection. Case-finding observations. Sex Transm Dis. 1997 Jul;24(6):334–342. doi: 10.1097/00007435-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Chacko MR, Smith PB, Kozinetz CA. Understanding partner notification (Patient self-referral method) by young women. J Pediatr Adolesc Gynecol. 2000 Feb;13(1):27–32. doi: 10.1016/s1083-3188(00)00002-4. [DOI] [PubMed] [Google Scholar]
- 18.Gursahaney PR, Jeong K, Dixon BW, Wiesenfeld HC. Partner notification of sexually transmitted diseases: practices and preferences. Sex Transm Dis. 2011 Sep;38(9):821–827. doi: 10.1097/OLQ.0b013e31821c390b. [DOI] [PubMed] [Google Scholar]
- 19.Kissinger P, Schmidt N, Mohammed H, et al. Patient-delivered partner treatment for Trichomonas vaginalis infection: a randomized controlled trial. Sex Transm Dis. 2006 Jul;33(7):445–450. doi: 10.1097/01.olq.0000204511.84485.4c. [DOI] [PubMed] [Google Scholar]
- 20.St Lawrence JS, Montano DE, Kasprzyk D, Phillips WR, Armstrong K, Leichliter JS. STD screening, testing, case reporting, and clinical and partner notification practices: a national survey of US physicians. Am J Public Health. 2002 Nov;92(11):1784–1788. doi: 10.2105/ajph.92.11.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Secura GM, Allsworth JE, Madden T, Mullersman JL, Peipert JF. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010 Aug;203(2):115, e111–117. doi: 10.1016/j.ajog.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998 Nov 18;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 23.Tilson EC, Sanchez V, Ford CL, et al. Barriers to asymptomatic screening and other STD services for adolescents and young adults: focus group discussions. BMC Public Health. 2004 Jun 9;4:21. doi: 10.1186/1471-2458-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorbach PM, Aral SO, Celum C, et al. To notify or not to notify: STD patients’ perspectives of partner notification in Seattle. Sex Transm Dis. 2000 Apr;27(4):193–200. doi: 10.1097/00007435-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Mathews C, Coetzee D. Partner notification for the control of sexually transmitted infections. BMJ. 2007 Feb 17;334(7589):323. doi: 10.1136/bmj.39114.635405.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howard EJ, Xu F, Taylor SN, et al. Patient preference for patient-delivered partner therapy: exploratory findings from three sexually transmitted disease clinics. Sex Transm Dis. 2011 Feb;38(2):148–149. doi: 10.1097/OLQ.0b013e3182036d45. [DOI] [PubMed] [Google Scholar]