Summary
Autism has been described as a disorder of general neural processing, but the particular processing characteristics that might be abnormal in autism have mostly remained obscure. Here, we present evidence of one such characteristic: poor evoked response reliability. We compared cortical response amplitude and reliability (consistency across trials) in visual, auditory, and somatosensory cortices of high-functioning individuals with autism and controls. Mean response amplitudes were statistically indistinguishable across groups, yet trial-by-trial response reliability was significantly weaker in autism, yielding smaller signal-to-noise ratios in all sensory systems. Response reliability differences were evident only in evoked cortical responses and not in ongoing resting-state activity. These findings reveal that abnormally unreliable cortical responses, even to elementary non-social sensory stimuli, may represent a fundamental physiological alteration of neural processing in autism. The results motivate a critical expansion of autism research to determine whether (and how) basic neural processing properties such as reliability, plasticity, and adaptation/habituation are altered in autism.
Keywords: Autism, Autism spectrum disorders, fMRI, response variability, response reliability, sensory systems, visual, auditory, somatosensory, noise, neural reliability, neural noise
Introduction
Autism is a multi-faceted and heterogeneous developmental disorder, which is characterized by three “core” behavioral symptoms (social difficulties, communication problems, and repetitive behaviors) (DSM-IV-TR, 2000) and a long list of “secondary” symptoms (e.g., epilepsy, intellectual disability, motor clumsiness, and sensory sensitivities). Neurobiological studies of autism can be divided broadly into two general approaches. The first approach has focused on identifying brain areas that exhibit abnormal functional responses when individuals with autism perform particular social/cognitive tasks that are associated with the “core” symptoms (Chiu et al., 2008; Dapretto et al., 2006; Humphreys et al., 2008; Pelphrey et al., 2005; Redcay and Courchesne, 2008). The implicit assumption has been that specific behavioral impairments (e.g., difficulties imitating facial expressions) can be associated with dysfunctions in particular brain areas/modules (e.g., mirror system areas (Dapretto et al., 2006)) and that autism can be successfully described as a combination of perturbations in different social/cognitive brain systems. The second approach has focused on characterizing brain architecture in autism by assessing the integrity of anatomical connections and the strength of functional synchronization between neural populations located in different brain areas. Anatomical studies have reported widespread abnormalities in neural organization (Casanova et al., 2002), white matter integrity (Ben Bashat et al., 2007; Thomas et al., 2011), and cellular morphology (Bauman and Kemper, 2005), while functional studies have reported that the correlations in activity between functionally related brain areas is generally weaker in autism during the performance of tasks (Just et al., 2007) and during rest (Kennedy and Courchesne, 2008) or sleep (Dinstein et al., 2011). A clear conclusion from these investigations is that individuals with autism exhibit widespread functional and anatomical abnormalities in multiple brain systems.
This conclusion has led to proposals that autism might be better described as a general disorder of neural processing (Belmonte et al., 2004; Minshew et al., 1997), in which neural responses might be “noisier” or less reliable (Baron-Cohen and Belmonte, 2005; Dakin and Frith, 2005; Rubenstein and Merzenich, 2003; Simmons et al., 2009). An advantage of these theories is that they offer a more parsimonious explanation of autism: instead of considering multiple independent physiological abnormalities, each located in a distinct social/cognitive brain area, they explicitly state that all of the “core” and “secondary” behavioral symptoms of an individual emerge through development of a single pathological abnormality that has widespread developmental effects on multiple brain systems. These theories, however, have been rather vague and have largely based their arguments on behavioral observations or on speculations regarding the developmental effects of genetic abnormalities associated with autism. Only two previous studies have presented evidence of greater response variability in autism. The first reported that individuals with autism exhibited more variable fMRI responses in motor and visual brain areas during the execution and observation of hand movements (Dinstein et al., 2010) and the second documented more variable EEG responses in autism during the observation of Gabor patches (Milne, 2011). The purpose of the current study was to perform a systematic examination of response reliability in autism by testing multiple sensory systems in the same individuals and to better understand which components of brain activity contribute to the difference in response reliability across subject groups.
In the current study, we characterized cortical responses independently in visual, auditory, and somatosensory sensory systems of high-functioning adults with autism and matched controls using functional magnetic resonance imaging (fMRI). Evoked response amplitudes, on average, were statistically indistinguishable across groups, yet within-subject trial-by-trial response variability was significantly larger in individuals with autism, yielding significantly weaker signal-to-noise ratios in all three cortical sensory systems. Only the stimulus-evoked responses were unreliable in autism; variability of ongoing cortical activity in areas that did not respond to the sensory stimuli and variability of ongoing activity during a separate resting-state scan did not differ significantly across groups. We suggest that poor neural reliability is a widespread cortical characteristic of autism, evident in the evoked responses of multiple brain areas, and that this neural atypicality may be a consequence of altered synaptic development (Bourgeron, 2009; Gilman et al., 2011; Zoghbi, 2003) and/or imbalanced excitation/inhibition (Markram et al., 2007; Rubenstein and Merzenich, 2003). These findings support theories emphasizing the role of sensory abnormalities in autism development (Happe and Frith, 2006; Markram et al., 2007; Mottron et al., 2006) as well as theories that describe autism as a disorder characterized by greater neural “noise” (Baron-Cohen and Belmonte, 2005; Dakin and Frith, 2005; Rubenstein and Merzenich, 2003; Simmons et al., 2009).
Results
Each participant completed three event-related fMRI experiments, which enabled us to measure stimulus-evoked responses independently in the visual, auditory, and somatosensory systems. The visual stimulus consisted of moving white dots, presented in two circular apertures, one on either side of fixation, against a black background. The auditory stimulus consisted of pure tone beeps, which were presented to both ears. The somatosensory stimulus consisted of air puffs delivered through a hose to the back of the left hand. The experiments were designed to assess trial-by-trial response reliability as well as response adaptation/habituation (see Methods and Supplementary Figure 1). Here, we focused specifically on the reliability of responses across trials containing identical stimuli. In all experiments, subjects performed a letter repetition-detection task at fixation to divert attention from the sensory stimuli. The temporal structure of this task was unrelated to that of the sensory stimulus presentations, enabling us to measure the sensory-evoked activity and the task-related activity independently of one another. Thirteen out of the fourteen subjects in each group also completed a resting-state scan, which enabled us to compare variability of ongoing activity across groups.
Robust sensory responses in both groups
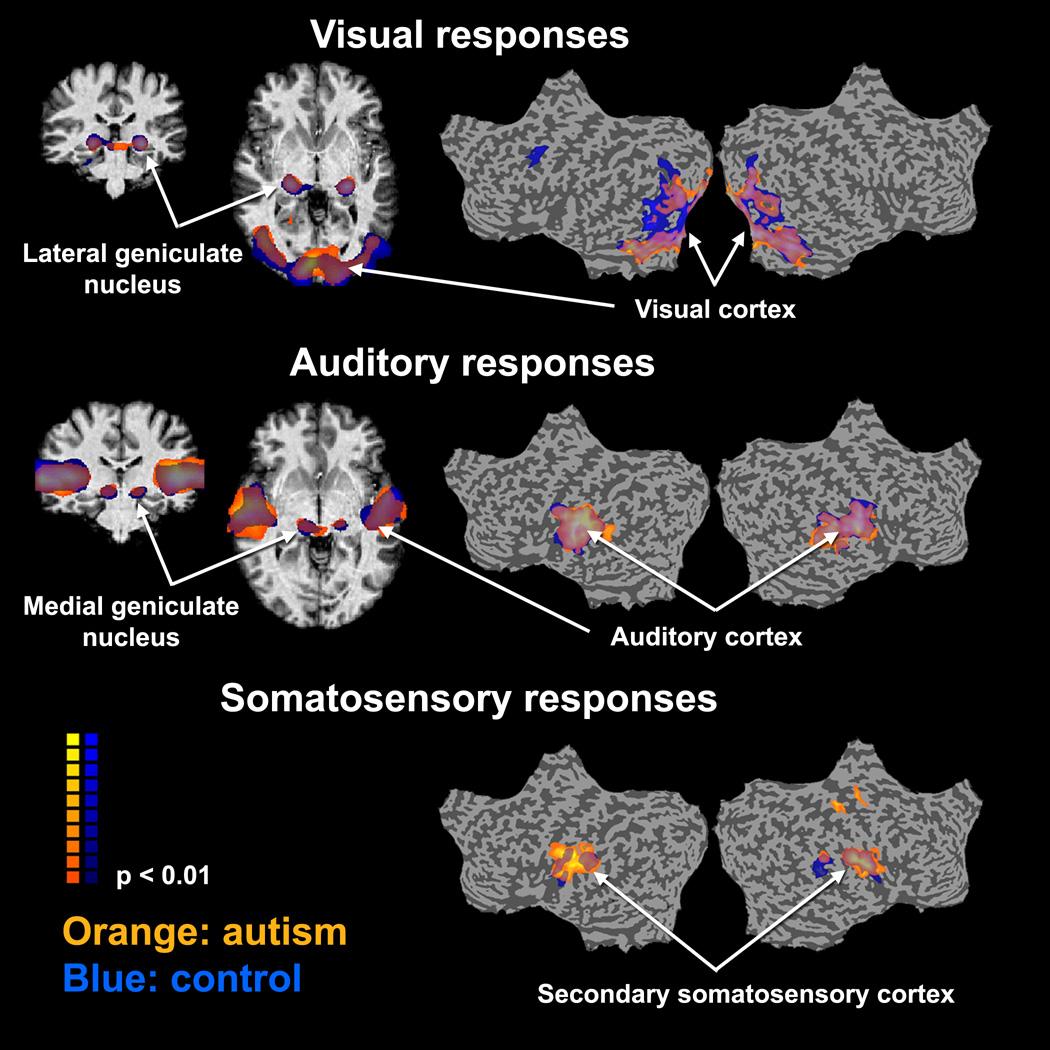
Both subject groups exhibited similar cortical and sub-cortical fMRI activations to the visual, somatosensory, and auditory stimuli (Figure 1). The visual stimulus elicited robust responses in lateral geniculate nucleus and in visual cortex. The auditory stimulus elicited robust responses in medial geniculate nucleus and auditory cortex. The somatosensory stimulus elicited strong bilateral responses in ventral post-central sulcus (secondary somatosensory cortex), which is dorsal to auditory cortex. We are confident that these were not auditory responses to the sound elicited by the air puffs, because we presented a masking white-noise auditory stimulus throughout the somatosensory experiment.
Figure 1.
Statistical parameter maps showing the significance of activation evoked by the unattended sensory stimuli. Orange: autism group. Blue: control group. The activation of both groups is presented on a flattened representation of a single subject’s cortical surface. Random effects analysis, p<0.01. Cluster size > 15 mm3 in diameter.
The strong sensory activations allowed us to define three bilateral cortical regions of interest (ROIs), individually for each subject: visual cortex, auditory cortex, and secondary somatosensory cortex. ROIs were identified using an automated procedure that selected 200 adjacent voxels in each hemisphere, which exhibited the most significant activation to the stimulus (see Supplementary Figure 2).
Cortical response amplitude and variability
Stimulus-evoked responses were less reliable in individuals with autism (Figure 2). To demonstrate this we show an example of response time-courses to the auditory stimuli, taken from one individual with autism and one control subject. While response amplitudes were equivalent across the two individuals, trial-by-trial response variability was larger in the individual with autism (Figure 2A, compare error bars between the two curves). An assessment across subjects revealed that although the mean response amplitudes in each of the three sensory systems were statistically indistinguishable across subject groups (Figure 2B, p>0.1, one tailed t-test), the trial-by-trial standard deviation was significantly larger in individuals with autism in all three sensory systems (Figure 2C, p<0.05, one tailed t-test). The resulting signal-to-noise ratios (response amplitude divided by response variability) were, consequently, significantly smaller in individuals with autism (Figure 2D, p<0.05, one tailed t-test) in all three independent experiments. To exclude gender affects, we also assessed these results in a subset of 10 subjects from each group, which contained only males. The results were equivalent to those presented for the entire group; significantly larger trial-by-trial standard deviation and significantly smaller signal-to-noise ratios across all three experiments (data not shown).
Figure 2.
Cortical response amplitudes and variability. A: Example of response time-courses from a single subject with autism and a single control subject in the auditory experiment. Error bars: standard error across trials. B: Mean response amplitudes, averaged across trials and across subjects in each group. C: Standard deviations of response amplitudes across trials. D: Signal-to-noise ratios. Each pair of bars presents responses in one sensory ROI during the relevant experiment (e.g., responses in visual cortex during the visual experiment). Responses are from an analysis of no-test trials, but similar results were found regardless of trial type examined (Supplementary Figure 4). Orange: autism group. Blue: control group. Red asterisks: significant difference between groups (p<0.05, one tailed t-test). Error bars: standard error across subjects.
We also performed a complementary linear regression analysis using a general linear model that contained a separate predictor for each trial (Supplementary Figure 5). We used fMRI data from one scan to identify the relevant ROIs in each subject, and performed the response amplitude and variability analyses on statistically-independent data from the second scan. Poor response reliability in autism was clearly evident in this analysis as well.
“Global” and “local” contributions to response variability
Larger response variability was evident in the autism group even when isolating the “local” activity that was unique to each sensory ROI (Figure 3). The trial-by-trial fMRI variability presented above (Figure 2) can be separated into two complementary components. The first is a “global” component, which corresponds to the variability of fMRI fluctuations that are common across the entire cortex. This component was estimated, separately in each experiment, by computing the average activity time-course of all cortical gray-matter voxels and determining its variance. The variance of the global time-course was larger in individuals with autism, as compared with controls, in all three independent experiments, although this difference was not statistically significant (0.05<p<0.13, one-tailed t-test, Figure 3A). The second component of variability is a “local” component, which corresponds to the trial-by-trial variability that remains after extracting the “global” time-courses from the data. The global time-course was removed from the time-course of each gray matter voxel, separately for each experiment, using orthogonal projection (Fox et al., 2006). This procedure ensures that there is no correlation between the global time-course and the time-course of each voxel, thereby extracting the fMRI fluctuations that are common across the entire cortex, while preserving the local fluctuations.
Figure 3.
Response characteristics after removing “global” average time-courses. A: Standard deviation of “global” time-course, averaged across subjects of each group. B-D: Same format as Figure 2, but after removing the “global” time-course. B: Mean response amplitudes. C: Standard deviations of response amplitude across trials. D: Signal-to-noise ratios. Orange: autism group. Blue: control group. Red asterisk: significant difference between groups after regressing out “global” average. Error bars: standard error across subjects. Light blue and dark orange lines show results from Figure 2 (before removing the “global” time-course) for comparison.
After removing the global time-course, auditory response amplitudes were significantly weaker in the autism group, trial-by-trial standard deviations were reduced by 20–35% in both subject groups, and signal-to-noise ratios increased by 50–80% in both subject groups (Figure 3). Most importantly, individuals with autism still exhibited significantly larger trial-by-trial variability, relative to controls, in the visual and somatosensory experiments (Figure 3C, p<0.05, one tailed t-test) and significantly smaller signal-to-noise ratios across all three experiments (Figure 3D, p<0.05, one tailed t-test).
Evoked responses and ongoing activity
Two complementary analyses revealed that larger variability in autism was evident only in cortical stimulus-evoked responses and not in ongoing activity fluctuations (Figure 4). In the first analysis, we selected forty non-responding cortical ROIs (e.g., anterior cingulate, superior frontal gyrus, and precuneus) separately in each subject, using an automated anatomical procedure (see Methods). For each of these ROIs, we performed an identical analysis to that presented above for the sensory ROIs; assessing their mean response amplitude, trial-by-trial response variability, and signal-to-noise ratios according to the stimulus presentations (Figure 4 A-C). Since none of these ROIs exhibited evoked responses to any of the stimuli, computing the trial-by-trial standard deviations offers a way of assessing the variability of background ongoing activity, which always fluctuates randomly. The standard deviation values from each ROI were averaged across the 40 ROIs and compared across groups, separately for each of the sensory experiments. All measures were statistically indistinguishable across groups.
Figure 4.
Variability of activity in the absence of stimulus-evoked responses (same format as Figure 2). A-C: Non-responding brain areas during the sensory experiments. Each pair of bars presents the mean across all 40 non-activated ROIs in each sensory experiment. D-F: Sensory brain areas during a resting-state experiment. Each pair of bars presents responses from a single sensory ROI during the resting-state experiment. A,C: Mean response amplitudes. B,E: Standard deviations across trials. C,F: Signal-to-noise ratios. Orange: autism group. Blue: control group. Error bars: standard error across subjects.
In a second analysis we assessed cortical activity in the three sensory ROIs during a resting-state experiment, which did not contain any stimulus or task (Figure 4 D-F). Applying the same logic, we computed mean response amplitudes, trial-by-trial standard deviation, and signal-to-noise ratios in each sensory ROI according to the trial sequences from the sensory experiments. Since no stimuli were presented in this resting-state experiment, there were no evoked responses in any of the sensory ROIs, and trial-by-trial standard deviations were used to assess the variability of the ongoing activity fluctuations. In agreement with the first analysis, all measures were statistically indistinguishable across groups. In both analyses we first removed the global mean time-course by orthogonal projection, so as to assess only local variance, but results were also statistically indistinguishable across groups when omitting this step.
Consistency across experiments and relationship with IQ and autism severity
Subjects who exhibited a low signal-to-noise ratio in one sensory modality tended to exhibit a low signal-to-noise ratio in the other two modalities as well (Figure 5 top). We computed the correlation between signal-to-noise ratios across pairs of modalities in each group separately as well as across all subjects from both groups. All correlations were positive and most were statistically significant as assessed by randomization tests (See Methods). Note that correlations across all subjects would be expected because of the signal-to-noise difference across groups, yet correlations within each group suggest a subject-by-subject correspondence of signal-to-noise ratios across sensory systems.
Figure 5.
Consistency of signal-to-noise ratios across experiments and relationship with IQ and autism severity. Top: Each panel depicts the association between signal-to-noise ratios for a pair of sensory experiments. Each point represents the signal-to-noise ratio of a single subject. Correlation r values are presented for each pair of experiments (blue: within control group; orange: within autism group; black: across both groups). Middle: Relationship between signal-to-noise ratios and IQ. Bottom: Relationship between signal-to-noise and ADOS. Each panel displays the relationship for a single sensory modality along with the relevant r value. Orange: autism group. Blue: control group. Red asterisks: significant correlation as assessed by a randomization test.
Signal-to-noise ratios in the autism group were positively correlated with IQ scores (Figure 5, middle) and negatively correlated with autism symptom severity as assessed by the ADOS test (Figure 5, bottom) in all three experiments. However, only the correlation between signal-to-noise and IQ in the visual experiment was statistically significant.
Sub-cortical responses
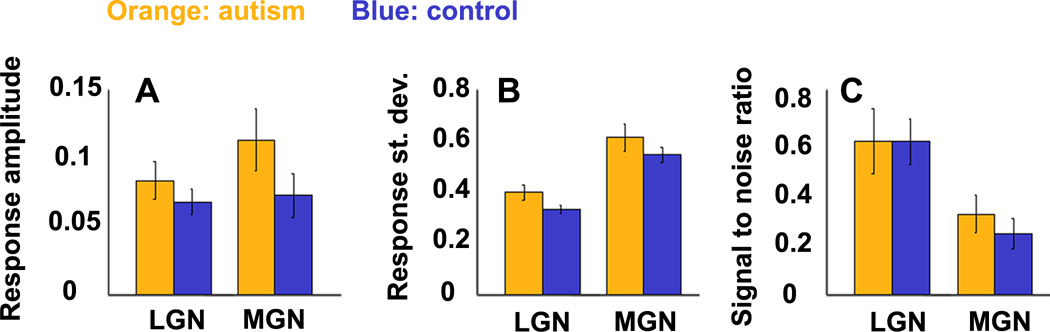
There was no evidence of signal-to-noise differences across subject groups in sub-cortical nuclei (Figure 6). We manually identified two sub-cortical ROIs – the lateral geniculate nucleus (LGN) and the medial geniculate nucleus (MGN) – using the average activation maps across all subjects in each group (Figure 1). Analyses of the responses in the two ROIs did not reveal any significant differences between groups in any of the measures (Figure 6 A-C).
Figure 6.
Sub-cortical responses (same format as Figure 2). A: Response amplitudes. B: Standard deviations across trials. C: Signal-to-noise ratios. Visual responses were assessed in the LGN (lateral geniculate nucleus) and auditory responses were assessed in the MGN (medial geniculate nucleus). Orange: autism group. Blue: control group. Error bars: standard error across subjects.
Cortical responses to letter repetition-detection task
Both subject groups exhibited robust motor responses when indicating letter repeats via a button press (Figure 7A). We used these responses to identify three motor ROIs (Supplementary Figure 2): left primary motor cortex (Mot), right and left anterior intraparietal sulcus (aIPS), and right and left ventral premotor cortex (vPM). Response amplitude, variability, and signal-to noise were statistically indistinguishable across the two groups across all three ROIs (Figure 7). In this analysis, we combined trials across all three experiments because the task at fixation was identical.
Figure 7.
Cortical responses to the letter repetition-detection task. A: SPM map showing activation during button presses in each group. White ellipses: approximate location of motor ROIs. B-D: ROI analysis B: Response amplitudes. C: Standard deviations across trials. D: Signal-to-noise ratios. Orange: autism group. Blue: control group. Error bars: standard error across subjects.
Task performance and response variability
Individuals with autism were significantly slower and less accurate in detecting letter repeats than controls. This raised a concern that the higher trial-by-trial sensory response variability reported in the autism group might be a consequence of the performance difference across groups. To address this issue, we excluded 8 experiments with the poorest performance in the autism group and 4 experiments with the best performance in the control group, so as to match mean accuracy and reaction times across groups (Figure 8 A,B). Cortical response signal-to-noise ratios remained significantly smaller in the autism group (Figure 8C) even when behavioral performance was statistically indistinguishable across groups.
Figure 8.
Task performance and cortical response reliability. A: Performance accuracy. B: Reaction times. C: Cortical signal-to-noise ratios after matching behavioral performance. Orange: autism group. Blue: control group. Darker orange lines show original performance in the autism group before equating it. Red asterisks: significant difference across groups after equating for performance. Error bars: standard error across subjects.
The behavioral analyses also revealed that trial-by-trial variability in reaction times was larger in individuals with autism when comparing across all experiments and when considering only the subset of experiments for which mean accuracy and reaction times were matched across groups (Supplementary Figure 6).
Control analyses
We performed several control analyses to ensure that larger trial-by-trial fMRI variability in the autism group was not caused by more variable head motion, heart rate, respiration, or eyefixation during the experiments. The variability of all six head motion estimates, derived during 3D motion correction, was statistically indistinguishable across groups as was the mean frameby- frame displacement (Supplementary Figure 7 A-B). Furthermore, we reanalyzed the fMRI responses after removing head motion parameters using orthogonal projection (Fox et al., 2006), and found that signal-to-noise ratios remained significantly smaller in autism (Supplementary Figure 7 C-E). A comparison of heart rate and respiration measurements collected during fMRI rest scans in a subgroup of participants (6 autism and 10 control subjects) revealed that the variability of both measures was not statistically different across the groups (Supplementary Figure 8). Finally, a comparison of eye tracking data collected from a subgroup of participants (6 autism and 3 control subjects) did not reveal any evidence for a difference in eye movement variability across groups (Supplementary Figure 8). These analyses re-assured us that the difference in trial-by-trial fMRI response reliability across groups was not due to alternative non-neural sources that may generate variability in fMRI measurements.
Discussion
Poor response reliability appears to be a fundamental neural characteristic of autism, which was evident in visual, auditory, and somatosensory responses. While mean response amplitudes were statistically indistinguishable across groups, within-subject trial-by-trial variability was significantly larger in individuals with autism, yielding significantly smaller signal-to-noise ratios in all three sensory systems (Figure 2). Subjects with autism exhibited larger response variability even though attention was diverted to an unrelated task, and even when we equated performance accuracy and reaction times across groups (Figure 6). Larger fMRI response variability in autism was evident only in sensory brain areas exhibiting evoked responses to the stimuli and there was no evidence of differences in the variability of ongoing fMRI activity across groups. This was true both for ongoing activity sampled from non-responding brain areas during the sensory experiments and for ongoing activity sampled from the sensory areas during a separate resting-state fMRI experiment (Figure 4).
It is notable that such a basic abnormality in brain activity is evident in early sensory responses to non-social stimuli even in high-functioning individuals with autism. These findings offer strong support for theories that describe autism as a disorder of general neural processing (Belmonte et al., 2004; Minshew et al., 1997) and more specifically as a disorder characterized by greater neural “noise” (Baron-Cohen and Belmonte, 2005; Dakin and Frith, 2005; Rubenstein and Merzenich, 2003; Simmons et al., 2009). The results may also support theories that suggest a role for sensory processing abnormalities in the development of autism (Happe and Frith, 2006; Markram et al., 2007; Mottron et al., 2006).
Larger cortical response variability in autism
Our results are compatible with two previous studies that have reported larger trial-by-trial response variability in autism. The first study reported that fMRI response variability was larger in visual and motor cortical areas of individuals with autism who were passively observing or actively executing hand movements (Dinstein et al., 2010) and the second study reported that EEG response variability was larger in individuals with autism who were observing Gabor patches (Milne, 2011). The current findings go beyond these initial results in several important ways. 1) We examined three sensory systems within the same subjects, thereby demonstrating the generality of findings across multiple sensory systems. 2) We dissociated variability evident in evoked responses from variability evident in ongoing activity. 3) We dissociated “local” variability that is specific to each sensory area from “global” variability that is shared across the entire cortex. 4) We dissociated trial-by-trial variability from task engagement and arousal by introducing a demanding letter repetition-detection task at fixation. Taken together, our results and the previous studies reveal that response variability is consistently larger in autism across multiple brain systems (sensory and motor), across multiple types of stimuli and tasks, across multiple experimental designs in which participants’ behavior is tightly controlled or not, and across experiments utilizing either EEG or fMRI measurements.
While poor signal-to-noise ratios in autism were evident in all cortical regions examined, signal-to-noise ratios in lateral and medial geniculate nuclei were statistically indistinguishable across subject groups (Figure 6). The distinction between the cortical and thalamic results is indicative of a possible dissociation whereby weak signal-to-noise may be a specific characteristic of cortical processing in autism. We do, however, suggest some caution in interpreting these results, because fMRI response amplitudes in thalamic nuclei were weaker than those in cortical areas, thereby limiting the statistical power for comparing cortical and sub-cortical responses.
“Global” and “Local” response variability
Larger response variability in autism was mostly due to larger “local variability”, which was unique to the responding sensory areas rather than common to the entire cortical gray matter. We separated the trial-by-trial variability, which was computed for each subject separately, into two components. One component, “global variability”, was defined as the variance of the average time-course across all gray matter voxels. This time-course contained the moment-by-moment fMRI fluctuations, which were common to the entire cortex. Such fluctuations may represent general changes in arousal, blood oxygenation levels, and other “global” contributors of variability (Birn & Bandettini 2006, Wise & Tracey 2004). The other component, “local variability”, was defined as the trial-by-trial variability that remained after the global time-course was removed (Figure 3). This component of variability represented the local trial-by-trial changes that were unique to each sensory area. Both components of variability were larger in the autism group, yet only “local variability” was significantly larger in autism. Determining how “local” these variability differences are – whether they are common to an entire sensory area or unique to the responding neurons – would be an interesting question that could be further addressed using electrophysiology techniques.
Removing the global time-course reduced trial-by-trial standard deviations by 20–30% and increased signal-to-noise ratios by 50–80%. This suggests that removing the global time-course may be a generally useful tool for reducing trial-by-trial variability in fMRI measurements (also see (Fox et al., 2006)).
Another notable characteristic of cortical response variability was its correlation across sensory systems in individuals of both groups (Figure 5, top). This finding suggests that the reliability of cortical activity may develop equivalently across all sensory systems of an individual rather than independently in each system.
Variability of evoked responses and ongoing activity
Larger variability in autism was evident only in evoked responses, not in ongoing cortical activity, which fluctuates continuously (Fox et al., 2006). We performed two complementary analyses to compare the variability of ongoing cortical activity across the two subject groups, while using the same trial-triggered average procedures that were used to assess the variability of evoked responses (Figure 4). In the first analysis we computed the mean response amplitudes and trial-by-trial standard deviations in 40 cortical ROIs that did not respond to the sensory stimuli (see Methods). Since none of these ROIs exhibited evoked responses, the standard deviations measured the variability of ongoing activity fluctuations. In the second analysis we computed the same measures in the three sensory ROIs during an independent resting-state experiment. Since this experiment did not contain any stimulus or task, there were no evoked responses in any of the sensory ROIs, and the trial-by-trial standard deviations were again used to measure of the variability of ongoing activity fluctuations. In neither of these analyses was there any evidence of a difference between the autism and control groups, suggesting that only the variability of evoked responses (Figure 2) was larger in autism.
Neural and non-neural sources of fMRI variability
It is unlikely that the results obtained here can be explained by trivial differences in non-neural sources of variability such as head motion or physiology. Since fMRI is a technique that measures changes in oxygenated blood rather than directly measuring neural activity, numerous non-neural sources may generate fMRI variability and need to be accounted for. The most important potential source is head motion, which can generate transient changes in fMRI image intensity that would cause an increase in fMRI variability (Van Dijk et al., 2012; Power et al., 2012). A possible alternative explanation of our results may, therefore, be that the larger trial-by-trial fMRI variability found in the autism group was a consequence of more frequent and/or larger head movements.
The most compelling evidence against this possibility is that the group variability differences were unique to sensory areas and were not evident in other brain areas. More frequent or larger head movements would not be able to generate such spatially specific effects, because head motion would increase fMRI variability similarly across the entire brain (fMRI image intensity changes transiently across the entire brain during a head movement). This same logic would apply to other possible sources of non-neural variability as well. For example, in theory, greater fMRI variability in autism could be a consequence of greater variability in neuro-vascular coupling rather than greater neural response variability. Such an alternative source of fMRI variability, however, would likely affect evoked responses and ongoing activity in a similar manner. The fact that larger fMRI variability in autism was evident only in evoked responses (Figure 4) and appeared mostly as “local variability” that remained after regressing out “global variability” (Figure 3), strongly suggests that it is a characteristic of the underlying stimulus-evoked neural activity.
To further address these issues, however, we performed several control analyses. First, we assessed the amount of head motion apparent in individuals of each group using two different analyses and found no significant differences across groups (Supplementary Figure 7, A & B).
Second, we regressed out the estimated head motion time-courses from the time-course of each voxel in the data of each subject, thereby eliminating the correlation between head motion fMRI time-courses. Performing the same analyses on this processed data revealed equivalent results – fMRI variability remained significantly larger in the autism than control group (Supplementary Figure 7, C). Note that regressing out the head motion time-course does not entirely eliminate the effects of small head movements (>1 mm) that also generate transient changes in fMRI image intensity (Van Dijk et al., 2012), but such head movements would not be able to generate spatially specific differences in response reliability (see above). Finally, we assessed variability of respiration and heart rate in each individual during the independent resting-state fMRI scan and found no evidence for differences across groups (Supplementary Figure 8, B & D).
Synaptic abnormalities and poor neural reliability
Our findings are compatible with genetic and animal model studies that describe autism as a disorder of synaptic development and function (Bourgeron, 2009; Gilman et al., 2011; Zoghbi, 2003) and/or an imbalance of excitation and inhibition (Markram et al., 2007; Rubenstein and Merzenich, 2003). Indeed, it has been reported that several animal models of autism exhibit abnormally high excitation-inhibition ratios (over-reactive responses) as well as noisy asynchronous neural firing patterns (Gibson et al., 2008; Penagarikano et al., 2011; Zhang et al., 2008). Our results argue against over-reactivity of neural responses, because mean response amplitudes were statistically indistinguishable across subject groups. We speculate, however, that neural circuits with abnormal excitation/inhibition balances may develop and adapt to maintain similar mean response amplitudes (since neural activity rates are strictly limited by energy availability (Lennie, 2003)), while sacrificing response reliability in the process, such that poor response reliability may represent a common developmental outcome of the different genetic and molecular abnormalities mentioned above.
Neural, perceptual, and behavioral variability in autism
Unreliable neural activity may be expected to degrade perception and generate variability in behavior. A common finding in autism is that individuals with autism exhibit enhanced perception of details and degraded perception of holistic/gestalt stimuli (Simmons et al., 2009). It may be difficult to understand how unreliable neural activity might improve perception of some stimuli and degrade perception of other stimuli. However, greater neural response variability in early visual cortex may enhance the perception of local details through stochastic resonance (McDonnell and Abbott, 2009) and at the same time degrade perception of gestalt stimuli (Simmons et al., 2009). Alternatively, greater response variability could alter neural plasticity and learning in a way that would favor over-classification of local details at the expense of gestalt perceptual organization (Cohen, 1994).
With regards to behavior, there is evidence that individuals with autism do exhibit greater trialby- trial motor variability, which is evident in the accuracy of both reaching movements (Glazebrook et al., 2006) and saccadic eye movements (Takarae et al., 2004). Greater trial-bytrial reaction time variability in autism is evident for a variety of tasks (Castellanos et al., 2005; Geurts et al., 2008) as was also the case in our letter repetition-detection task (Supplementary Figure 8).
Unreliable neural activity and the symptoms of autism
Determining the relationship between greater neural response variability and the behavioral symptoms of autism will clearly require additional research. It is notable that signal-to-noise ratios of individuals with autism exhibited a trend of positive correlations with IQ scores and negative correlations with autism severity scores (Figure 5), provocatively suggesting that cortical response reliability might be related to the level of behavioral abilities in autism. We speculate that poor response reliability may be directly related to the development of both secondary and core symptoms of autism. With respect to secondary symptoms, unreliable neural networks are susceptible to epileptic seizures (Rubenstein and Merzenich, 2003), which is one of the most prominent co-morbidities in autism (Tuchman and Rapin, 2002). Unreliable neural responses in sensory and motor cortices may also explain why the vast majority of individuals with autism exhibit debilitating sensory sensitivities (Marco et al., 2011), motor clumsiness, and balance problems (Whyatt and Craig, 2011). With respect to the core symptoms, unreliable neural activity early in development may create an unstable and unpredictable perception of the environment, which may be specifically accentuated in social situations that involve an added level of unpredictability (unlike objects, humans tend to exhibit variable behavior). Developing under such conditions might motivate an infant to retract from the environment, avoid social interaction, and focus instead on the performance of repetitive behaviors that generate more predictable neural responses. Even a small bias in this direction during early development may lead to dramatic and heterogeneous behavioral consequences later in life. While admittedly speculative, this hypothesis motivates further study of neural reliability in autism, particularly during early stages of development.
Specificity of poor response reliability to autism
Is poor response reliability unique to autism or might it also be apparent in other disorders such as epilepsy, developmental delay, and schizophrenia? At present, there is no evidence from any other disorder with which to compare the autism results. Poor neural reliability is a general physiological characteristic, which is likely to have profound developmental impact on the function and organization of many brain systems, potentially altering multiple components of typical neural processing including synaptic plasticity, neural connectivity, and neural selectivity. When considering such broad physiological changes, it seems possible that unreliable neural activity may underlie multiple cognitive and social abnormalities, which would not be limited to those found in autism. If poor response reliability were to be detected in other disorders, however, it would be critical to determine the developmental timing of its onset (which may differ across disorders). This highlights the need for comparative research to characterize the reliability of cortical activity in autism and other disorders across multiple developmental time-points. Such research may offer important insights not only into the neurobiology of autism, but also into the neurobiology of other disorders as well.
Conclusions
Accumulating evidence suggests that autism is a disorder of general neural processing (Belmonte et al., 2004; Minshew et al., 1997). Poor reliability of evoked responses may embody one specific neural processing abnormality, which is common in autism. We suggest that thorough characterization of other basic neural processing properties such as plasticity and selectivity are critical for understanding autism and for properly relating neurophysiological characteristics with possible underlying genetic and molecular mechanisms that likely involve widespread synaptic abnormalities (Bourgeron, 2009; Gilman et al., 2011; Zoghbi, 2003). Finally, determining the precise effects that poor neural reliability may have on the integrity of neural processing throughout development will offer important insights, which may be relevant not only for our understanding of autism, but also for our understanding of other psychiatric and neurological disorders, more generally.
Experimental procedures
Subjects
Twenty eight subjects (four female) participated in this study: fourteen with autism (mean age: 26.5, range: 19 to 39) and fourteen age, gender, and IQ matched controls (mean age: 26.4, range: 20 to 40). All subjects had normal or corrected-to-normal vision, provided written informed consent, and were paid for their participation in the study. The Institutional Review Board at Carnegie Mellon University and the University of Pittsburgh approved the experimental procedures, which were in compliance with the safety guidelines for MRI research. Autism diagnosis was established using the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000) and expert clinical evaluation. Full clinical details and inclusion/exclusion criteria are available in the supplementary materials (Supplementary Table 1).
MRI acquisition
Imaging was performed using a Siemens (Erlangen, Germany) 3T Verio MRI scanner located at the Carnegie Mellon Scientific Imaging & Brain Research Center in Pittsburgh. The scanner was equipped with a Siemens 12 channel birdcage head coil, which was used for RF transmit and receive. Blood oxygenation level-dependent (BOLD) contrast was obtained using a T2*-sensitive echo planar imaging pulse sequence (repetition time of 1500 ms, echo time = 30 ms, flip angle = 75°, 24 slices, 3×3×3 mm voxels, field of view = 192 mm). Anatomical volumes were acquired with a T1-weighted 3D-MPRAGE pulse sequence (1×1×1 mm). Each session included 1 or 2 runs of each sensory experiment, one resting-state experiment, and one anatomical scan. The entire scanning session lasted between 1 and 1.5 hours.
MRI preprocessing
fMRI data were processed with Brain Voyager (R. Goebel, Brain Innovation, Maastricht, The Netherlands) and with custom software written in Matlab (Mathworks, Natick, MA, USA). Preprocessing of fMRI data included 3D motion correction, temporal high-pass filtering with a cutoff frequency of 6 cycles per run, spatial smoothing using a Gaussian kernel with 8mm width at half height, alignment with the anatomical volume using trilinear interpolation, and transformation to the Talairach coordinate system (Talairach and Tournoux, 1988). The cortical surface was reconstructed from the anatomical scans, separately for each subject; the procedure included segmenting the gray and white matter and inflating/flattening the gray matter for visualization.
Experimental design
Subjects participated in three independent sensory experiments in the visual, auditory, and somatosensory domains as well as one resting-state experiment, which did not contain any stimulus or task. All three sensory experiments followed the same rapid event-related temporal structure (Supplementary Figure 1), which was designed to enable assessment of response amplitude, variability, and adaptation (although adaptation results are not reported in the current paper). Each trial contained an adapter followed by a test stimulus (Supplementary Figure 1). Each run contained 12 adapted trials, 12 un-adapted trials, and 12 trials of the adapter without a test condition. Most subjects participated in two runs of each experiment.
In the visual experiment, stimuli were presented in two circular apertures whose radius was 6 degrees and whose center was located approximately 8 degrees on either side of fixation. Each aperture contained 500 white dots that moved radially with 80% coherence either towards fixation or away from fixation. Dots moved continuously throughout the adapter, disappeared during the blank and reappeared during the test. Test stimuli moved either in the same (adapted trials) or opposite direction (un-adapted trials) to the adapter.
In the auditory experiment, identical stimuli were presented to both ears through the Siemens headphones. The adapter consisted of eleven 150 ms pure tone beeps (either 400 or 600 Hz) interleaved with 150 ms blanks, followed by 200 ms of blank and a test composed of 3 tones at either the same (adapted trials) or different pitch (un-adapted trials).
In the somatosensory experiment, air puffs were presented at two alternative spatial locations on the back of the left hand (about 5 cm apart). Air puffs were delivered through a manifold connected to a set of hoses (similar to (Huang and Sereno, 2007)). The manifold was controlled by a computer to achieve accurate stimulation timing. The adapter and test puffs followed the same timing as in the auditory experiment. Test puffs were presented either the same (adapted trials) or different location on the back of the left hand (un-adapted trials).
During all three experiments, subjects performed a demanding letter repetition-detection task at fixation. Capital letters presented within the fixation point changed every 500 ms, and subjects pressed a button with their right hand every time they detected a consecutive letter repeat (1-back). Subjects had 1 second to respond. Correct and incorrect responses were indicated by a change in the fixation spot background to green or red respectively.
In the resting-state experiment, subjects were instructed to lay still with their eyes closed, and the MRI room lights and projector were turned off for the duration of this scan (8 minutes).
fMRI data analysis
We performed a statistical parameter mapping (SPM) analysis (Friston et al., 1994) to assess brain activation associated with each experimental condition. Response amplitudes were computed separately for each voxel in each subject and then a “random-effects” analysis (Friston et al., 1999) was used (t-test across subjects) to test the significance of response across all subjects of each group.
ROI selection
We used a single functional run of each experiment to define bilateral regions of interest (ROIs) in visual, auditory, and secondary somatosensory cortices individually in each subject, based on the SPM analysis. The ROIs were defined using an automated procedure implemented in Matlab that selected 200 adjacent voxels in each hemisphere, which exhibited the most significant activation to the stimulus (Supplementary Figure 2). This method ensured that ROI size was identical across all subjects and that each ROI contained the voxels with the strongest activation in each subject (strongest activation = strongest responses and smallest variability across trials). Selecting ROIs that were smaller (150 voxels) or larger (300 voxels) yielded equivalent results to those presented in the manuscript, confirming that the results were not limited to a specific ROI size. The two sub-cortical ROIs (LGN and MGN) and the three motor ROIs were selected manually using the relevant SPM maps of each group (Figures 1&7). We used an identical statistical threshold across the two groups, which yielded similar ROI sizes and locations (Supplementary Table 2).
Assessment of evoked responses
We performed a trial-triggered average analysis across trials containing identical stimuli to determine mean response amplitude and standard deviation across trials for each sensory ROI in each sensory experiment (see Supplementary Figures 3). To demonstrate the robustness of this result we also calculated mean response amplitude and standard deviation across trials using a complementary GLM analysis where the GLM contained a separate predictor for each trial (see Supplementary Figure 5). In the GLM analysis, we estimated the responses only in the second run of each experiment, which was statistically independent of the first run used to define the ROIs.
Assessment of ongoing activity
We used the same trial-triggered average procedure described above (Supplementary Figure 3) to assess the variability of ongoing activity fluctuations in two different analyses. In the first analysis we sampled the average time-courses from each of the three sensory ROIs during a resting-state experiment, which did not contain any stimulus or task. We performed the trial-triggered average analysis according to the trial sequence in the sensory experiments (e.g. visual trial sequence for assessing the responses in the visual ROI). Since no stimuli were presented, the mean response amplitudes were indistinguishable from zero. The “trial-by-trial” standard deviations, however, were not zero and captured the variability of ongoing activity, which fluctuated continuously during rest.
In the second analysis we sampled the average time-courses from each of 40 ROIs that did not respond to any of the sensory stimuli. We used the sensory trial sequences (timing of stimulus onsets) to calculate the mean response amplitudes and standard deviations across trials in each ROI, separately for each experiment. We then averaged the results across ROIs to yield a single measure across all non-activated ROIs. The non-activated ROIs included the superior frontal cortex, medial frontal cortex, medial orbital frontal, anterior cingulate, precuneus, fusiform gyrus, parahipocampal gyrus, superior parietal cortex, pars opercularis, pars triangularis, pars orbitalis, inferior temporal gyrus, middle temporal gyrus, and insula, in each hemisphere (20 ROIs per hemisphere). ROIs were defined anatomically using the Freesurfer automated parcellation procedure and restricted to 200 adjacent functional voxels so as to match the size of the sensory ROIs.
Signal-to-noise ratios
Sensory and motor signal-to-noise ratios were computed separately for each subject in each experiment. The ratio was computed by dividing response amplitude by variance across trials as estimated by either a trial-triggered average (Figure 2, 6, &7, Supplementary Figures 4) or GLM (Supplementary Figure 5) analysis.
Subject-by-subject signal-to-noise values from each sensory experiment were correlated with signal-to-noise values from the other experiments (Figure 5) or with IQ/ADOS behavioral scores (Figure 5). A randomization test was used to assess the significance of each correlation value: a null distribution of 10,000 random correlation values was generated by randomly shuffling signal-to-noise values across individuals and statistical significance was defined as the 95th percentile of this distribution. Note that this is a more conservative statistical test than the Pearson’s correlation coefficient, which assumes a normal distribution.
Task performance
We computed accuracy on the letter repetition-detection task by determining the fraction of trials where letter repeats were accurately reported from all possible letter repeats. Reaction time was measured from the appearance of the repeating letter to the button press (Supplementary Figure 6).
Head motion analyses
Two complementary analyses were carried out on the six estimated head motion parameters (3 translations and 3 rotations) that were extracted from the Brainvoyager 3D motion correction analysis. The standard deviation of head motion parameters and the mean frame-by-frame head motion were statistically indistinguishable across groups. Furthermore, projecting out head motion estimates from the fMRI data did not alter the findings (see Supplementary Figure 7).
Physiological measurements
Heart rate and respiration were measured using Siemens hardware and software, which automatically identifies and marks time-points containing heart beats and peaks of respiration. Physiology was sampled simultaneously with fMRI during a separate rest experiment, which was performed within the same scanning session as the sensory experiments. We computed heart and respiration rates and compared their average and temporal variability across groups (Supplementary Figure 8).
Eye tracking
Eye position was acquired with an MRI compatible eye tracker (EyeTrac6, Applied Science Laboratories, Bedford, MA, USA). Successful eye tracking was performed in six subjects with autism and 3 controls. We compared the average variance of the x and y eye position traces both throughout the entire experiment and also specifically within windows starting at stimulus onset and ending 500 after stimulus offset (Supplementary Figure 8).
Supplementary Material
Acknowledgments
Supported by Simons Foundation SFARI grant 177638 (DJH, MB, and ID), ISF and Bikura grants (RM), Clore and Kahn postdoctoral fellowships (ID), Pennsylvania Department of Health SAP grant 4100047862 and NICHD/NIDCD PO1/U19 (MB). This research was also supported by the NIH/NICHD University of Pittsburgh Autism Center of Excellence HD055748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baron-Cohen S, Belmonte MK. Autism: a window onto the development of the social and the analytic brain. Annu Rev Neurosci. 2005;28:109–126. doi: 10.1146/annurev.neuro.27.070203.144137. [DOI] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005;23:183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Cook EH, Jr., Anderson GM, Rubenstein JL, Greenough WT, Beckel-Mitchener A, Courchesne E, Boulanger LM, Powell SB, Levitt PR, et al. Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol Psychiatry. 2004;9:646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, Even A, Levy Y, Ben Sira L. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Birn RM, Murphy K, Handwerker DA, Bandettini PA. fMRI in the presence of task-correlated breathing variations. Neuroimage. 2009;47:1092–1104. doi: 10.1016/j.neuroimage.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu PH, Kayali MA, Kishida KT, Tomlin D, Klinger LG, Klinger MR, Montague PR. Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron. 2008;57:463–473. doi: 10.1016/j.neuron.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IL. An artificial neural network analogue of learning in autism. Biol Psychiatry. 1994;36:5–20. doi: 10.1016/0006-3223(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Dakin S, Frith U. Vagaries of visual perception in autism. Neuron. 2005;48:497–507. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Pierce K, Eyler L, Solso S, Malach R, Behrmann M, Courchesne E. Disrupted neural synchronization in toddlers with autism. Neuron. 2011;70:1218–1225. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Thomas C, Humphreys K, Minshew N, Behrmann M, Heeger DJ. Normal movement selectivity in autism. Neuron. 2010;66:461–469. doi: 10.1016/j.neuron.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSM-IV-TR. Diagnostic and statistical manual of mental disorders. Washington DC: American Psychiatric Press Inc; 2000. [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Jezzard P, Turner R. Analysis of Functional MRI Time-Series. Hum Brain Mapp. 1994;1:153–171. [Google Scholar]
- Geurts HM, Grasman RP, Verte S, Oosterlaan J, Roeyers H, van Kammen SM, Sergeant JA. Intra-individual variability in ADHD, autism spectrum disorders and Tourette's syndrome. Neuropsychologia. 2008;46:3030–3041. doi: 10.1016/j.neuropsychologia.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol. 2008;100:2615–2626. doi: 10.1152/jn.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SR, Iossifov I, Levy D, Ronemus M, Wigler M, Vitkup D. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron. 2011;70:898–907. doi: 10.1016/j.neuron.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook CM, Elliott D, Lyons J. A kinematic analysis of how young adults with and without autism plan and control goal-directed movements. Motor Control. 2006;10:244–264. doi: 10.1123/mcj.10.3.244. [DOI] [PubMed] [Google Scholar]
- Happe F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 2006;36:5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Huang RS, Sereno MI. Dodecapus: An MR-compatible system for somatosensory stimulation. Neuroimage. 2007;34:1060–1073. doi: 10.1016/j.neuroimage.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Humphreys K, Hasson U, Avidan G, Minshew NJ, Behrmann M. Cortical patterns of category-selective activation for faces, places, and objects in adults with autism. Autism Research. 2008;1:52–63. doi: 10.1002/aur.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Lennie P. The cost of cortical computation. Curr Biol. 2003;13:493–497. doi: 10.1016/s0960-9822(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Marco EJ, Hinkley LB, Hill SS, Nagarajan SS. Sensory processing in autism: a review of neurophysiologic findings. Pediatr Res. 2011;69:48R–54R. doi: 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Rinaldi T, Markram K. The intense world syndrome - an alternative hypothesis for autism. Front Neurosci. 2007;1:77–96. doi: 10.3389/neuro.01.1.1.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MD, Abbott D. What is stochastic resonance? Definitions, misconceptions, debates, and its relevance to biology. PLoS Comput Biol. 2009;5:e1000348. doi: 10.1371/journal.pcbi.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne E. Increased intra-participant variability in children with autistic spectrum disorders: evidence from single-trial analysis of evoked EEG. Front Psychol. 2011;2:51. doi: 10.3389/fpsyg.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: profile of a complex information processing disorder. J Int Neuropsychol Soc. 1997;3:303–316. [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulieres I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2–3-year-old children with autism spectrum disorder. Biol Psychiatry. 2008;64:589–598. doi: 10.1016/j.biopsych.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vision Res. 2009;49:2705–2739. doi: 10.1016/j.visres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Takarae Y, Minshew NJ, Luna B, Sweeney JA. Oculomotor abnormalities parallel cerebellar histopathology in autism. J Neurol Neurosurg Psychiatry. 2004;75:1359–1361. doi: 10.1136/jnnp.2003.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Thomas C, Humphreys K, Jung KJ, Minshew N, Behrmann M. The anatomy of the callosal and visual-association pathways in high-functioning autism: a DTI tractography study. Cortex. 2011;47:863–873. doi: 10.1016/j.cortex.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchman R, Rapin I. Epilepsy in autism. Lancet Neurol. 2002;1:352–358. doi: 10.1016/s1474-4422(02)00160-6. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt CP, Craig CM. Motor Skills in Children Aged 7–10 Years, Diagnosed with Autism Spectrum Disorder. J Autism Dev Disord. 2011 doi: 10.1007/s10803-011-1421-8. [DOI] [PubMed] [Google Scholar]
- Zhang L, He J, Jugloff DG, Eubanks JH. The MeCP2-null mouse hippocampus displays altered basal inhibitory rhythms and is prone to hyperexcitability. Hippocampus. 2008;18:294–309. doi: 10.1002/hipo.20389. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY. Postnatal neurodevelopmental disorders: meeting at the synapse? Science. 2003;302:826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.