Abstract
Chronic arsenic exposure through contaminated drinking water is a major environmental health issue. Chronic arsenic exposure is known to exert its toxic effects by a variety of mechanisms, of which generation of reactive oxygen species (ROS) is one of the most important. High level of ROS, in turn, leads to DNA damage that might ultimately culminate in cancer. In order to keep the level of ROS in balance, an array of enzymes is present, of which catalase (CAT) and myeloperoxidase (MPO) are important members. Hence, in this study, we determined the activites of these two enzymes in the sera and chromosomal aberrations (CA) in peripheral blood lymphocytes in individuals exposed and unexposed to arsenic in drinking water. Arsenic in drinking water and in urine was used as a measure of exposure. Our results show that individuals chronically exposed to arsenic have significantly higher CAT and MPO activity and higher incidence of CA. We found moderate positive correlations between CAT and MPO activities, induction of CA and arsenic in urine and water. These results indicate that chronic arsenic exposure causes higher CAT and MPO activity in serum that correlates with induction of genetic damage. We conclude that the serum levels of these enzymes might be used as biomarkers of early arsenic exposure induced disease much before the classical dermatological symptoms of arsenicosis begin to appear.
Keywords: Arsenic, Catalase, Myeloperoxidase, Reactive oxygen species, Chromosomal aberrations
Introduction
Chronic arsenic exposure primarily through contaminated drinking water is presently a global issue of colossal proportion. More than 35 countries of the world suffer from the ill effects of this environmental contaminant, especially the countries of Southeast Asia. However, the scenario is nowhere as bleak as it is in Bangladesh and West Bengal, India. In West Bengal alone, 9 out of the 19 districts have groundwater heavily laden with arsenic from geogenic sources, concentrations ranging far above the maximum permissible contamination level of 10 µg/L set both by WHO and USEPA (WHO Guidelines, 1996, US EPA, 2000). An imponderable 26 million individuals are exposed chronically to arsenic in this area (Chakraborti et al., 2009), rendering it as the largest mass poisoning in history. Long term exposure to low doses of arsenic might lead to the development of dermatological symptoms like raindrop pigmentation, hyperpigmentation, hyperkeratosis as well as skin cancers like Bowen’s Disease, squamous cell carcinoma and basal cell carcinoma, which are considered to be hallmarks of arsenic toxicity. However, mounting evidence suggests that exposed individuals who do not exhibit skin lesions are also susceptible to chronic arsenic toxicity, albeit to non-dermatological disorders like conjunctivitis, peripheral neuropathy and opportunistic infections (Ghosh et al, 2007; Baidya et al, 2006; Hossain et al., 2005; Soto-Pena et al., 2006). This implies that chronic arsenic exposure exerts its toxic effects much before the dermatological symptoms begin to appear, which typically appear after a latency period of about 10 years or more after first exposure (Haque et al. 2003).
Inorganic arsenic and its metabolites exert their toxic effects by a variety of mechanisms of which one of the most important is the generation of reactive oxygen species (ROS). Arsenic, as well as its mono- and dimethylated metabolic products generate ROS in biological systems, usually by inhibiting the enzymes which maintain ROS balance (Styblo et al., 1997; Lin et al., 1999). These ROS, if not neutralized, attack the genetic material, leading to the generation of DNA damage, chromosomal aberrations (CA), and DNA–protein cross links. This genetic damage can accumulate and lead to carcinogenesis.
In order to combat endogenous as well as exogenous oxidative stress, organisms have developed an intricate system of interacting enzymes. This array of enzymes works in a stepwise manner to maintain a low level of ROS in the body. Catalase and myeloperoxidase are two of the most important enzymes involved in this function. Catalase (EC 1.11.1.6), is a homotetrameric oxidoreductase, which facilitates the conversion of hydrogen peroxide into water and molecular oxygen. On the other hand, myeloperoxidase (EC 1.11.1.7) is another prominent member of the oxidoreductase family, which is composed of two identical dimers linked by a disulphide bridge (Nauseef et al., 1986), and is involved in the conversion of hydrogen peroxide into hypochlorous acid (HOCl) and chloride anion (Cl−), thereby adding to the ROS pool of the system. Thus, it is clear that the opposing activities of these two enzymes play a pivotal role in the maintainance of ROS balance in the physiological milieu. Since, it is well documented that chronic exposure to arsenic induced high levels of ROS (Banerjee N, et al., 2008), we wanted to look at the activity status of these enzymes in the serum of exposed individuals and to correlate them with chromosomal aberrations status, as ROS is known to induce genetic damage.
Materials and Methods
Study area and selection of subjects
The affected district of Murshidabad was selected as our study site and the details of the selection of study subjects were described previously (Basu et al, 2004; Ghosh et al., 2006). Physicians examined the study participants for detection of various types of arsenic related skin lesions and also other non-dermatological maladies. The criteria for identification of dermatological and non-dermatological diseases, as also the exclusion criteria were similar to that described in details in our earlier publication (Ghosh et al., 2006). As we were interested in determining the association between chronic arsenic exposure and serum oxidative stress enzymes activity, we selected arsenic exposed individuals irrespective of the presence or absence of arsenic-induced skin lesions as study participants. We recruited 50 individuals all of whom had been exposed to arsenic for more than 10 years through contaminated drinking water. Thirty four of these exposed individuals had developed arsenic-induced skin lesions, while 16 individuals did not have any arsenic-specific skin lesions even being exposed for 10 years or more. Forty one unexposed subjects with no history of arsenic contamination in their drinking water and age group between 15 and 70 years were selected from the East Midnapur district. Water and other biological samples such as blood, nail, hair and urine were collected from all the participants. The selected subjects provided informed consent to participate and fulfilled the inclusion criteria (Basu et al., 2004). This study was conducted in accord with the Helsinki II declaration and approved by our institutional ethics committee.
Arsenic exposure assessment
Study participants were provided with acid-washed [nitric acid-water (1:1)] plastic bottles for collection of drinking water (approx. 100 mL) samples into which nitric acid (1.0 mL/l) was added as preservative (Basu et al., 2004). First morning voids (approx. 100 mL) were collected in pre-coded polypropylene bottles for arsenic estimation. Water, urine, nail (~250–500 mg) and hair (~300–500 mg) samples were collected and arsenic was estimated as described earlier in details (Ghosh et al., 2006; Biswas et al., 2008). Flow injection-hydride generation-atomic absorption spectrometry (FI-HG-AAS) was used for estimation of arsenic content in different biological samples (urine, nail and hair) and drinking water, as described previously (Ghosh et al., 2006; Biswas et al., 2008). Quality control and quality assurance were ensured as elaborated elsewhere (Biswas et al., 2008).
Estimation of total protein
Total protein estimation in serum samples was carried out by Bradford’s Method (Bradford, 1976), using commercially available Bradford reagent (BioRad, Hercules, CA, USA) according to the manufacturer’s protocol. Briefly, 20 µl of the suitably diluted serum samples were mixed with 1 mL of Bradford Reagent and the mixture was incubated in the dark for 15 minutes at room temperature before absorbance of the colored product was measured at 595 nm using a UV-VIS spectrophotometer (Shimadzu Corporation, Kyoto, Japan).
Catalase assay
Catalase activity was measured following the method of Aebi (Aebi, 1984) as described elsewhere (Mittal and Flora, 2007) with minor modifications. Briefly, the activity was determined by measuring the decrease in absorbance at 240 nm of a reaction mixture consisting of H2O2, in phosphate buffer, pH 7.0, and requisite volume of serum sample. The molar extinction coefficient of 43.6 M cm−1 was used to determine catalase activity. The specific activity was calculated and was expressed as µmoles/min/mg of total protein.
Myeloperoxidase assay
Myeloperoxidase activity was measured following the method ofKlebanoff et al. (1984). The enzyme activity was assayed by determining the change in absorbance per minute of a guaiacol solution upon adding 10 µl of the diluted serum sample (5 times diluted) at 470 nm at 25°C using a UV-VIS spectrophotometer (Shimadzu Corporation, Kyoto, Japan). The molar extinction coefficient of guaiacol of 26.6 mM cm−1 was used to determine catalase activity. The specific activity was calculated and was expressed as nmoles/min/mg of total protein.
Chromosomal aberrations assay (CA)
From each subject 5 mL of venous blood was drawn and lymphocyte culture was carried out following standard protocol as described previously (Banerjee M et al., 2008). Whole blood (0.7 mL) was added to 7 mL of RPMI-1640 supplemented with L-glutamine, 15% FCS, penicillin (100 IU/ mL), streptomycin (100 µg/mL) and 2% PHA-M form. Duplicate cultures were maintained for each sample. The cultures were incubated at 37°C and harvested at 72 h (since arsenic is known to induce cell cycle delay, hence it is customary to carry out these cultures for 72 h instead of routine 48 h to get sufficient population of first and second division cells for scoring aberrations) (Banerjee M et al., 2008; Rasmussen and Menzel. 1997). All the slides of chromosomal aberration (CA) assay were coded and from each individual subject, depending on the availability of good scoring metaphases, 50–100 metaphases were randomly scored for CA (both chromatid type and chromosome type of aberrations) (Ghosh et al., 2006). Gaps were not included as aberrations. Results were expressed as CA per cell and also as percentage of aberrant cells.
Statistical analyses
Mean or median (as required) was used as measure of central tendency. Two tailed unpaired t test (Welch corrected) or Mann-Whitney Test was used to test if the differences in the central tendencies of different parameters between the two study groups were statistically significant or not. Two tailed Fishers exact test was used to analyze if the two study groups were different with respect to their gender distribution or smoking status. We also looked at the correlation between arsenic content in water and urine with specific activity of serum catalase and serum myeloperoxidase. Correlation between specific activity of serum catalase and serum myeloperoxidase with CA/Cell and % of aberrant cells were calculated, as also that between arsenic content in water and urine with CA/Cell and % of aberrant cells. Mean, median, standard deviation and 2-tailed p-values (for Welch corrected two tailed t test, Mann-Whitney Test and correlations) were calculated by using GraphPad InStat Software (Graphpad Software, San Diego). Scatter plots with trend lines were drawn and correlation coefficients (r) were determined using MS-Excel software. The risk of development of non-dermatological clinical outcomes were calculated and the results were expressed in terms of odds ratio (OR) and 95% confidence intervals (95% CI). OR and 95% CI were calculated taking unexposed group as the referent (risk of development of disease outcomes in each of the exposed subgroups compared to the unexposed individuals) as also, taking exposed individuals without arsenicinduced skin lesion as the referent group (risk of development of disease outcomes in exposed individuals with skin lesions compared to the exposed individuals without skin lesions). OR and 95% CI were calculated with the help of GraphPad InStat Software (Graphpad Software, San Diego). Further, the exposed group was stratified into 3 subgroups by exposure range with respect to water arsenic concentration (>10 – ≤100 µg/L, >100 – ≤200 µg/L and >200 µg/L) and urinary arsenic concentration (>50 – ≤200 µg/L, >200 – ≤500 µg/L and >500 µg/L). Activity levels of both serum catalase and serum myeloperoxidase of each of these subgroups were compared to the control as also among themselves by one sided Welch two sample t test to determine any dose effect of chronic arsenic exposure on the serum activity levels of the studied enzymes. The statistical software R was used for this purpose (R Development Core Team, 2006).
Results
Table I depicts the demographic characteristics of the study groups. Both the exposed and unexposed groups are well matched with respect to age, gender and smoking status. The arsenic-exposed group had significantly higher arsenic concentration in drinking water, and significantly higher urine arsenic concentration (p<0.001). Similarly, higher nail and hair arsenic content were observed in exposed individuals as in our previous study (Ghosh et al., 2006).
Table I.
Demographic characteristics of the study populations
| Parameters | Unexposed group | Exposed group |
|---|---|---|
| Total subjects | 41 | 50 |
| Age in years (mean ± SD) | 42.15 ± 13.27 | 43.12 ± 13.19 |
| Sex [N(%)] | ||
| Male | 27 (65.85) | 36 (72.00) |
| Female | 14 (34.15) | 14 (28.00) |
| Occupation [N (%)] | ||
| Males | 27 | 36 |
| Cultivation | 23 (85.18) | 21 (58.33) |
| Business | 0 (0) | 5 (13.89) |
| Daily wage earners | 2 (7.41) | 3 (8.33) |
| Service | 0 (0) | 2 (5.56) |
| Student | 2 (7.41) | 3 (8.33) |
| Unemployed | 0 (0) | 1 (2.78) |
| Retired | 0 (0) | 1 (2.78) |
| Female | 14 | 14 |
| Housewife | 14 (100.00) | 14 (100.00) |
| Smoking Status [N (%)] | ||
| Smoker | 18 (43.90) | 20 (40.00) |
| Non-smoker | 23(56.10) | 30 (60.00) |
|
Aresnic content (mean ± SD) |
||
| Drinking water (µg/l) | 6.92 ± 2.54 | 218.17 ± 195.23* |
| Urine (µg/l) | 12.52 ± 9.16 | 473.01 ± 430.91* |
| Nail (µg/g) | 0.49 ± 0.47 | 4.69 ± 2.98* |
| Hair | 0.31 ± 0.27 | 3.18 ± 2.39* |
p<0.0001 (Unpaired two-tailed Mann-Whitney Test)
The dermatological findings of the study populations are presented in Table II. Skin lesions were present only in arsenic exposed group but not all exposed individuals exhibited skin lesions. Raindrop pigmentation was the most prevalent skin lesion in arsenic-exposed group, followed by hyperkeratosis and hyperpigmentation. The non-dermatological outcomes of the study populations are presented in Table III. Similar to our previous report, the incidence of non-dermatological symptoms (conjunctivitis, respiratory illness and peripheral neuropathy) was much higher in the arsenic-exposed group both with and without arsenic-induced skin lesions (Table III). The exposed individuals with skin lesions have much higher incidence of eye diseases and peripheral neuropathy compared to those without arsenic-induced skin lesions (Table III), consistent with our previous results (Ghosh et al., 2006).
Table II.
Dermatological findings in the study populations
| Clinical Outcome | Unexposed Group (N = 41) |
Exposed Group (N = 50) | |
|---|---|---|---|
| Exposed with skin lesion (N = 34) |
Exposed without skin lesion (N = 16) |
||
| Raindrop Pigmentation N (%) | |||
| Present | 0 (0) | 33 (97.06) | 0 (0) |
| Absent | 41 (100) | 1 (2.94) | 16 (100) |
| Hyperkeratosis N (%) | |||
| Present | 0 (0) | 29 (85.29) | 0 (0) |
| Absent | 41 (100) | 5 (14.71) | 16 (100) |
| Hyperpigmentation N (%) | |||
| Present | 0 (0) | 8 (23.53) | 0 (0) |
| Absent | 41 (100) | 26 (76.47) | 16 (100) |
| Bowen’s Disease N (%) | |||
| Present | 0 (0) | 1 (2.94) | 0 (0) |
| Absent | 41 (100) | 33 (97.06) | 16 (100) |
Table III.
Non-dermatological clinical findings in the study populations
| Parameters | Unexposed Group (N=41) | Exposed without skin lesion group (N=16) |
Exposed with skin lesion group (N=34) | ||||
|---|---|---|---|---|---|---|---|
| N (%) | OR (Ref) |
N (%) | OR (95%CI)a | N (%) | OR (95%CI)a | OR (95%CI)b | |
| Eye Diseases | |||||||
| Present | 2 (4.88) | 1.0 | 5 (31.25) | 8.86 (1.51–52.12) |
22 (64.71) | 37.75 (7.32–174.60) |
4.03 (1.13–14.36) |
| Absent | 39 (95.12) |
11 (68.75) | 12 (35.29) | ||||
| Respiratory Illness | |||||||
| Present | 1 (2.44) | 1.0 | 4 (25.00) | 13.33 (1.36–130.99) |
8 (23.53) | 12.31 (1.45–104.32) |
0.92 (0.23–3.68) |
| Absent | 40 (97.56) |
12 (75.00) | 26 (76.47) | ||||
| Peripheral Neuropathy | |||||||
| Present | 1 (2.44) | 1.0 | 4 (25.00) | 13.33 (1.36–130.99) |
20 (58.82) | 57.14 (7.00–466.21) |
4.29 (1.14–16.08) |
| Absent | 40 (97.56) |
12 (75.00) | 14 (41.18) | ||||
OR and 95% CI calculated for with reference to Unexposed group;
OR and 95% CI calculated for with reference to exposed without skin lesion group.
Catalase (CAT) and myeloperoxidase (MPO) serum activities are presented in Table IV. The activities of both enzymes are significantly higher in the group chronically exposed to arsenic (p<0.0001).
Table IV.
Activity of serum catalase and myeloperoxidase in the study groups
| Enzyme | Unexposed | Exposed |
|---|---|---|
| (Mean ± SD) | (Mean ± SD) | |
| Catalase (µmoles/min/mg of total protein) |
0.631 ± 0.358 | 2.809 ± 2.926* |
| Myeloperoxidase (nmoles/min/mg of total protein) |
3.327 ± 2.318 | 10.970 ± 8.767* |
p <0.0001 (Unpaired two-tailed Mann-Whitney Test)
The CA status of the 2 study groups is presented in Table V. Here also, the exposed group has significantly higher incidence of CA (both CA/cell and percent aberrant cells) [p < 0.001] than the unexposed group.
Table V.
Frequency of chromosomal aberrations (CA/cell) and percentage of aberrant cells in two study groups
| Parameters | Unexposed group | Exposed group |
|---|---|---|
| CA/cell (Mean ± S.D.) |
0.021 ± 0.01 | 0.103 ± 0.03* |
| % of Aberrant cells (Mean ± S.D.) |
1.95 ± 0.85 | 10.25 ± 2.68* |
p<0.0001 (Unpaired two-tailed Mann-Whitney Test)
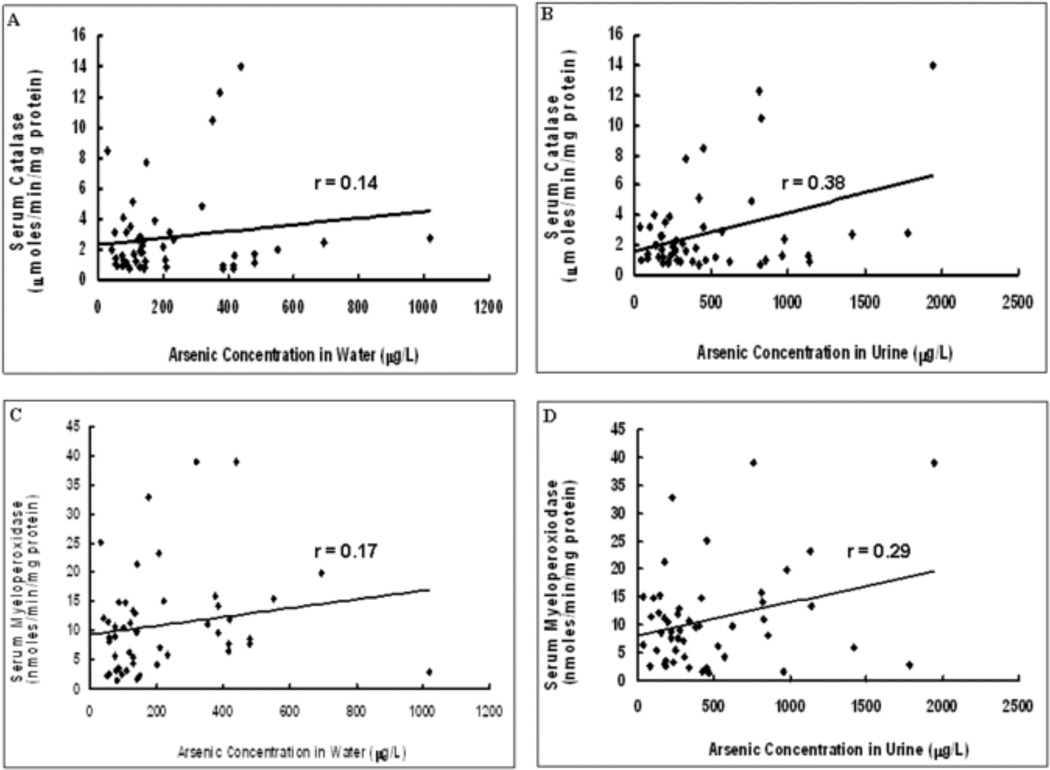
The scatter plots of the correlations between drinking water or urine arsenic concentration with specific activity of serum CAT or MPO in the arsenic-exposed group are shown in Figure 1. Panel A shows the correlation between drinking water arsenic concentration and CAT specific activity. The correlation coefficient is 0.14. Panel B shows the correlation between urine arsenic concentration and CAT specific activity. The correlation coefficient is 0.38. Panel C shows the correlation between drinking water arsenic concentration and MPO specific activity. The correlation coefficient is 0.17. Panel D shows the correlation between urine arsenic concentration and MPO specific activity. The correlation coefficient is 0.29. All of these correlations were significant (p<0.05).
Fig.1.
Correlation between arsenic concentration in water and urine and specific activities of the enzymes studied. 1A. Graph showing scatter plot of arsenic concentration in water vs. specific activity of serum catalase. 1B. Graph showing scatter plot of arsenic concentration in urine vs. specific activity of serum catalase. 1C. Graph showing scatter plot of arsenic concentration in water vs. specific activity of serum myeloperoxidase. 1D. Graph showing scatter plot of arsenic concentration in urine vs. specific activity of serum myeloperoxidase. Trendline has been provided in each graph as also the value of the correlation coefficient (r).
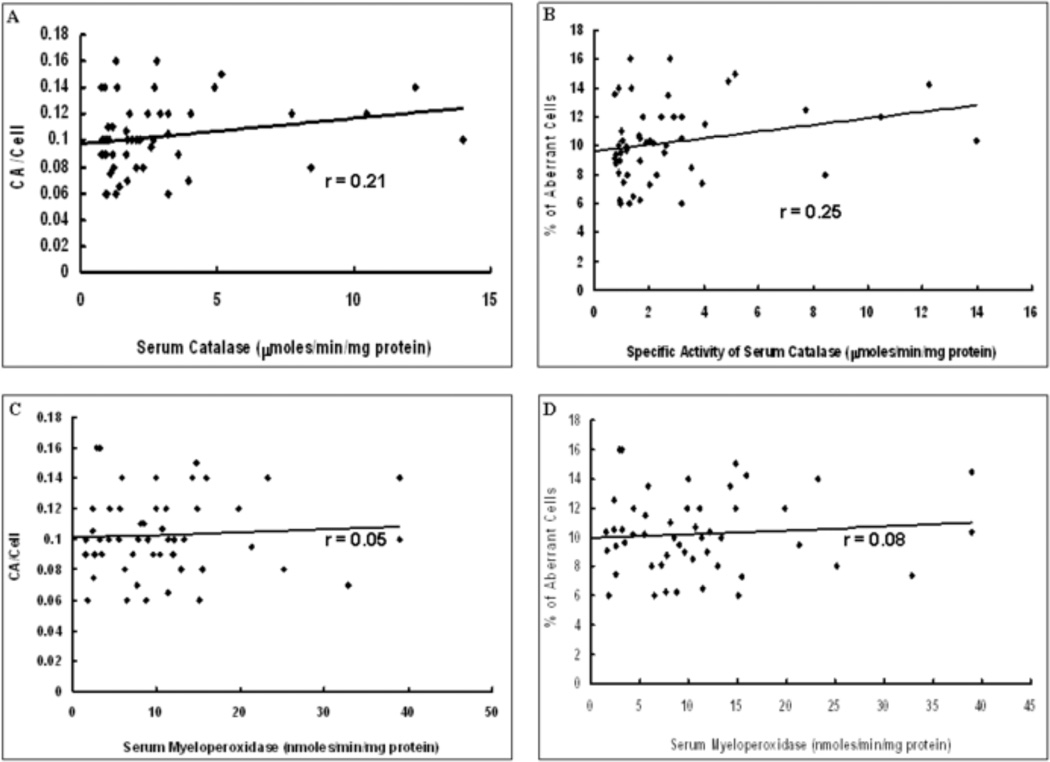
The scatter plots of the correlations between CAT or MPO specific activity and CA/Cell or % aberrant cells are shown in Figure 2. Panels A and B show the correlation between CA/Cell or % aberrant cells, respectively, and CAT specific activity; the correlation coefficients being 0.21 and 0.25 respectively. Both of these correlations were significant (p<0.05). Panels C and D show the correlations between CA/Cell or % aberrant cells, respectively and MPO specific activity; the correlation coefficients are 0.05 and 0.08 respectively and the p values (p>0.05) suggest that the correlations are not significant.
Fig.2.
Correlation between specific activities of the enzymes studied and induction of DNA damage. 2A. Graph showing scatter plot of specific activity of serum catalase vs. CA/cell. 2B. Graph showing scatter plot of specific activity of serum catalase vs. % of aberrant cells. 2C. Graph showing scatter plot of specific activity of serum myeloperoxidase vs. CA/cell. 2D. Graph showing scatter plot of specific activity of serum myeloperoxidase vs. 5 of aberrant cells. Trendline has been provided in each graph as also the value of the correlation coefficient (r).
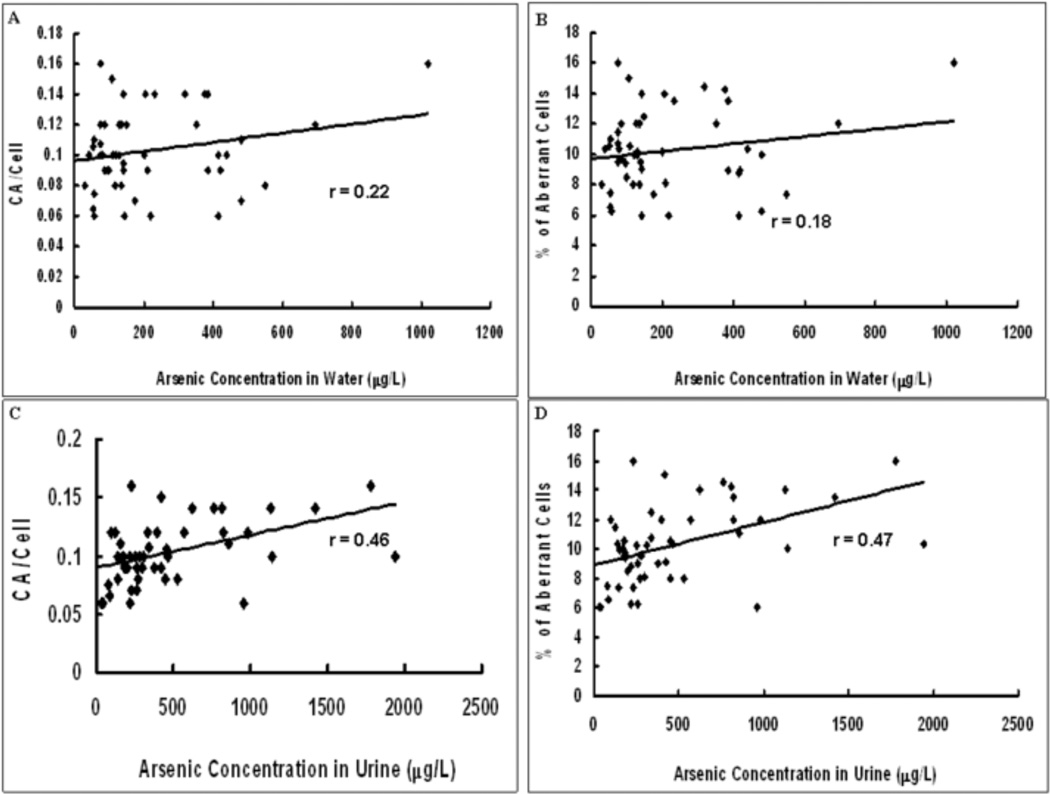
The scatter plots of the correlations between CA/cell or % aberrant cells and arsenic exposure parameters (water and urine arsenic concentration are shown in Figure 3. Panels A and B show the correlations between CA/Cell or % aberrant cells, respectively, and water arsenic concentration; the correlation coefficients are 0.22 and 0.18 respectively. Panels C and D show the correlation between CA/Cell or % of aberrant cells, respectively, and urine arsenic concentration; the correlation coefficients being 0.46 and 0.47 respectively. All of these correlations were significant (p<0.05).
Fig.3.
Correlation between arsenic concentration in water and urine and induction of DNA damage. 3A. Graph showing scatter plot of arsenic concentration in water vs. CA/cell. 3B. Graph showing scatter plot of arsenic concentration in water vs. % of aberrant cells. 3C. Graph showing scatter plot of arsenic concentration in urine vs. CA/cell. 3D. Graph showing scatter plot of arsenic concentration in urine vs. % of aberrant cells. Trendline has been provided in each graph as also the value of the correlation coefficient (r).
Table VI depicts the levels of enzyme activities (for both catalase and myeloperoxidase) when the study population was divided into different dosage regimes either according to water arsenic level (≤10 µg/L, >10 – ≤100 µg/L, >100 – ≤200 µg/L and >200 µg/L) or urinary arsenic level (≤50 µg/L, >50 – ≤200 µg/L, >200 – ≤500 µg/L and >500 µg/L). Results showed that the control group (≤10 µg/L in case of water arsenic content and ≤50 µg/L in case or urinary arsenic content) had significantly lower enzyme activity values for both catalase (p<0.01) and myeloperoxidase (p<0.01) compared to each of the exposed subgroups (both in case of groupings on the basis of water arsenic content and urinary arsenic content). The exposed subgroups, however, did not show any additional dosage effect within themselves (p>0.05).
Table VI.
Effect of different dose of chronic arsenic exposure on activity level of serum catalase and serum myeloperoxidase
| Unexposed | Exposed | |||
|---|---|---|---|---|
| Dose groups according to water arsenic content | ||||
| ≤10 µg/L (Mean ± SD) |
>10 – ≤100µg/L (Mean ± SD) |
>100 – ≤200µg/L (Mean ± SD) |
>200µg/L (Mean ± SD) |
|
| Catalase (µmoles/min/mg of total protein) |
0.631 ± 0.358 | 2.237 ± 1.97* | 2.483 ± 1.827* | 3.607 ± 4.151* |
| Myeloperoxidase (nmoles/min/mg of total protein) |
3.327 ± 2.318 | 8.244 ± 6.134* | 9.737 ± 8.299* | 14.488 ± 10.298* |
| Dose groups according to urinary arsenic content | ||||
| ≤50 µg/L (Mean ± SD) |
>50 – ≤200µg/L (Mean ± SD) |
>200 – ≤500µ g/L (Mean ± SD) |
>500µg/L (Mean ± SD) |
|
| Catalase (µmoles/min/mg of total protein) |
0.631 ± 0.358 | 2.102 ± 1.043* | 2.455 ± 2.236* | 3.987 ± 4.459* |
| Myeloperoxidase (nmoles/min/mg of total protein) |
3.327 ± 2.318 | 9.454 ± 5.821* | 9.455 ± 7.804* | 14.327 ± 11.712* |
p<0.01 compared to the unexposed group (One sided Welch two sample t test)
Discussions
In addition to its well known ability to induce a variety of cancers, arsenic exposure also gives rise to a plethora of precancerous and non-cancerous dermatological symptoms. However, these symptoms develop long after the initial exposure, sometimes requiring more than 10 years of chronic exposure (Haque et al., 2003). In addition, chronically exposed individuals develop a wide variety of nondermatological diseases, which might appear much earlier than the development of classical dermatological symptoms (Ghosh et al., 2006, Baidya et al., 2006). Thus, exposed individuals devoid of arsenic-induced skin lesions are at higher risk of developing non-dermatological symptoms (Ghosh et al., 2006). So, in addition to the classic dermatological biomarkers of chronic arsenic toxicity, novel biomarkers which will enable us to predict impending disease are needed.
Several mechanisms have been proposed to explain the mechanism of arsenic-induced toxicity and carcinogenicity. An ever accumulating body of evidence demonstrates that generation of ROS is one of the paramount mechanisms behind chronic arsenic toxicity and subsequent carcinogenicity. It has been shown that arsenic exposure leads to a rapid production of superoxide radicals in the system and also extracellular H2O2 (Barchowsky et al., 1999). It has been shown that during metabolism, arsenic can give rise to reactive intermediary products, such as (CH3)2AsOO. (Yamanaka et al., 1997) and reactive byproducts like superoxide radicals (Yamanaka and Okada, 1994). We have shown in an earlier publication that chronic exposure induces the formation of ROS in arsenic-exposed population of West Bengal (Banerjee N et al., 2008).
Depending on the level of the ROS generated, they can give rise to toxic and subsequently carcinogenic outcomes in a variety of ways. Usually, at low doses, ROS induce abnormal gene expression patterns by acting as second messengers, whereas at higher doses, ROS can directly cause cellular death through oxidative damage (Perkins et al., 2000). Another potent mechanism by which ROS induce their toxic effect is the generation of DNA damage. Arsenic induces DNA adducts through calcium-mediated production of peroxynitrite, hypochlorous acid and hydroxyl radicals (Wang et al., 2001). Also, it has been shown that chronic arsenic exposure gives rise to oxidative DNA adducts like 8-hydroxy-2-deoxyguanosine (8-OHdG) (Vizcaya-Ruiz et al., 2009). We have shown that chronic intake of arsenic leads to the generation of significantly higher levels of chromosomal aberrations in blood cells (including lymphocytes) compared to unexposed controls (Basu et al., 2005; Banerjee M et al., 2008). Pu et al (2006) has showed that arsenic induces the formation only of oxidative DNA adducts, employing a Fpg-mediated comet assay technique. Similar observations were found for trivalent and pentavalent arsenic as well as their methylated derivatives bySchwerdtle et al. (2003).Bau et al. (2002) showed that oxidative DNA adducts and DNA–Protein cross-links are the major DNA lesions induced by arsenite. The results have been confirmed in another study by Kligerman and Tennant. (2007). In addition, the excision of oxidative DNA adducts gives rise to DNA strand breaks (Tezuka et al., 1993). Such events, in the long run, are capable of giving rise to chromosomal aberrations (Vega et al., 1995). It has been speculated that such damage to DNA in the tumor suppressor genes or protooncogenes might lead to the initiation of cancer.
Cells have an arsenal of enzymes which keep ROS levels in balance. Catalase and myeloperoxidase are two such opposite acting enzymes, which help in keeping the level of ROS in check. Previous studies have used catalase to show that it can alleviate the toxic effects of chronic arsenic exposure on DNA damage (Wang et al., 1997). However, no other study, to our knowledge, has looked at the effect of chronic arsenic exposure on the activity of serum catalase and serum myeloperoxidase in human subjects. As the level of H2O2 increases in the system due to arsenic exposure, we expect that the activity levels of catalase will be induced by the increase in substrate concentration. Similarly, for myeloperoxidase as well, we expect a higher activity level with the increase in its substrate (H2O2) concentration. Our results are consistent with this hypothesis. We find that activities of both these enzymes in serum are significantly increased in subjects chronically exposed to arsenic. Also, it is interesting to note that there exists a moderate positive correlation between arsenic concentration in urine with specific activity levels of serum catalase and serum myeloperoxidase. The correlation, however, was not significant with arsenic content in drinking water. This is not an anomalous observation though, because urine arsenic represents body burden and drinking water arsenic merely the exposure. In order to strengthen our findings, we also studied induction of DNA damage in exposed and unexposed individuals inferred from CA analysis. Since individuals chronically exposed to arsenic have a higher amount of ROS in the system, we expect that they will have a greater amount of DNA damage as well. The results of our study demonstrate that indeed, the exposed individuals have a significantly higher CA/Cell as also % aberrant cells consistent with higher DNA damage. This observation is consistent with our previous findings (Mahata et al., 2003, Ghosh et al., 2006, Banerjee et al., 2007, Banerjee M et al., 2008). We find that activity of serum catalase (but not serum myeloperoxidase) is positively correlated with induction of CA (both CA/cell and % aberrant cells). Also, there is a positive correlation between arsenic concentration in urine and induction of CA (both CA/cell and % of aberrant cells) as expected. Analyses of demographic data show that the study populations are well matched in terms of age, sex and smoking distribution, thereby minimizing the possibility of generation of false positive results due to confounding by these factors. These data, taken together, suggest that chronic arsenic exposure induces an increased generation of ROS in the system (of which hydrogen peroxide is one of the most important species). This increased ROS, in turn, leads to increased activities of both serum catalase and myeloperoxidase and finally results in the generation of enhanced DNA damage which can be visualized and quantified as increased CA induction.
Thus, in conclusion, it can be said that chronic arsenic exposure increases the activity of both serum catalase and serum myeloperoxidase activity as a consequence of increased ROS. This elevated ROS, and also its active byproducts, subsequently cause elevated amounts of DNA damage reflected in high incidence of CA. It is also interesting to speculate that the serum activity levels of these enzymes might be used as putative biomarkers to detect the early effects of arsenic exposure in conjunction with other parameters like water and urinary arsenic content. The observation of a significant increase in activities even at low levels of chronic arsenic exposure (>10 – ≤100 µg/ water arsenic concentration; >50 – ≤200 µg/L urine arsenic concentration) indicates that these are early events in response to chronic arsenic exposure. Thus, activity levels of these enzymes in the serum could act as biomarkers of impending disease from chronic arsenic exposure, much before the appearance of typical dermatological symptoms, which are considered to be the hallmarks of arsenic toxicity.
Acknowledgements
This study is supported by grant (NWP – 0004) from Council of Scientific and Industrial Research (CSIR), Govt. of India. USPHS grants ES011314 and ES014443 provided support for J. Christopher States. Authors are grateful to Fogarty International Training Program, jointly with University of California, Berkeley, for providing training to MB, NB and PG for research on molecular epidemiology and environmental health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None
References
- Aebi H. In: Catalase in Vitro. Methods in Enzymology. Colowick SP, Kaplan NO, editors. Vol. 105. Florida: Acad. Press; 1984. pp. 114–121. [DOI] [PubMed] [Google Scholar]
- Baidya K, Raj A, Mondal L, Todani A. Persistent conjunctivitis associated with drinking arsenic contaminated water. J. Occul. Pharmacol. Ther. 2006;22:208–211. doi: 10.1089/jop.2006.22.208. [DOI] [PubMed] [Google Scholar]
- Banerjee M, Sarkar J, Das JK, Mukherjee A, Sarkar AK, Mondal L, Giri AK. Polymorphism in the ERCC2 codon 751 is associated with arsenicinduced premalignant hyperkeratosis and significant chromosome aberrations. Carcinogenesis. 2007;28:672–676. doi: 10.1093/carcin/bgl181. [DOI] [PubMed] [Google Scholar]
- Banerjee M, Sarma N, Biswas R, Roy J, Mukherjee A, Giri AK. DNA repair deficiency leads to susceptibility to develop arsenic-induced premalignant skin lesions. Int. J. Cancer. 2008;123:283–287. doi: 10.1002/ijc.23478. [DOI] [PubMed] [Google Scholar]
- Banerjee N, Banerjee M, Ganguly S, Bandyopadhyay S, Das JK, Bandyopadhay A, Chatterjee M, Giri AK. Arsenic-induced mitochondrial instabilityleading to programmed cell death in the exposed individuals. Toxicology. 2008;246:101–111. doi: 10.1016/j.tox.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Barchowsky A, Klei LR, Dudek EJ, Swartz HM, James PE. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Radic. Biol. Med. 1999;27:1405–1412. doi: 10.1016/s0891-5849(99)00186-0. [DOI] [PubMed] [Google Scholar]
- Basu A, Ghosh P, Das JK, Banerjee A, Ray K, Giri AK. Micronuclei as biomarkers of carcinogen exposure in populations exposed to arsenic through drinking water in West Bengal, India: a comparative study in three cell types. Cancer Epidemiol. Biomarkers Prev. 2004;13:820–827. [PubMed] [Google Scholar]
- Basu A, Som A, Ghoshal S, Mondal L, Chaubey RC, Bhilwade HN, Rahman MM, Giri AK. Assessment of DNA damage in peripheral blood lymphocytes of individuals susceptible to arsenic induced toxicity in West Bengal, India. Toxicol. Lett. 2005;159:100–112. doi: 10.1016/j.toxlet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Bau DT, Wang TS, Chung CH, Wang AS, Wang AS, Jan KY. Oxidative DNA adducts and DNA-protein cross-links are the major DNA lesions induced by arsenite. Environ. Health Perspect. 2002;110:753–756. doi: 10.1289/ehp.02110s5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas D, Banerjee M, Sen G, Das JK, Banerjee A, Sau TJ, Pandit S, Giri AK, Biswas T. Mechanism of erythrocyte death in human population exposed to arsenic through drinking water. Toxicol. Appl. Pharmacol. 2008;230:57–66. doi: 10.1016/j.taap.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chakraborti D, Das B, Rahman MM, Chowdhury UK, Biswas B, Goswami AB, Nayak B, Pal A, Sengupta MK, Ahamed S, Hossain A, Basu G, Roychowdhury T, Das D. Status of groundwater arsenic contamination in the state of West Bengal, India: A 20-year study report. Mol. Nutr. Food Res. 2009;53:542–551. doi: 10.1002/mnfr.200700517. [DOI] [PubMed] [Google Scholar]
- De Vizcaya-Ruiz A, Barbier O, Ruiz-Ramos R, Cebrian ME. Biomarkers of oxidative stress and damage in human populations exposed to arsenic. Mutat. Res. 2009;674:85–92. doi: 10.1016/j.mrgentox.2008.09.020. [DOI] [PubMed] [Google Scholar]
- EPA. Arsenic occurrence in public drinking water supplies (EPA – 815 – R – 00 – 023/December 2000) 2000. [Google Scholar]
- Ghosh P, Banerjee M, De Chaudhuri S, Chowdhury R, Das JK, Mukherjee A, Sarkar AK, Mondal L, Baidya K, Sau TJ, Banerjee A, Basu A, Chaudhuri K, Ray K, Giri AK. Comparison of health effects between individuals with and without skin lesions in the population exposed to arsenic through drinking water in West Bengal, India. J. Expo. Sci. Environ. Epidemiol. 2007;17:215–223. doi: 10.1038/sj.jes.7500510. [DOI] [PubMed] [Google Scholar]
- Haque R, Mazumder DN, Samanta S, Ghosh N, Kalman D, Smith MM, Mitra S, Santra A, Lahiri S, Das S, De BK, Smith AH. Arsenic in drinking water and skin lesions: dose-response data from West Bengal, India. Epidemiology. 2003;14:174–182. doi: 10.1097/01.EDE.0000040361.55051.54. [DOI] [PubMed] [Google Scholar]
- Hossain MK, Khan MM, Alam MA, Chowdhury AK, Delwar HM, Feroze AM, Kobayashi K, Sakauchi F, Mori M. Manifestation of arsenicosis patients and factors determining the duration of arsenic symptoms in Bangladesh. Toxicol. Appl. Pharmacol. 2005;208:78–86. doi: 10.1016/j.taap.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ, Waltersdorph AM, Rosen H. Antimicrobial activity of myeloperoxidase. Methods Enzymol. 1984;105:399–403. doi: 10.1016/s0076-6879(84)05055-2. [DOI] [PubMed] [Google Scholar]
- Kligerman AD, Tennant AH. Insights into the carcinogenic mode of action of arsenic. Toxicol. Appl. Pharmacol. 2007;222:281–288. doi: 10.1016/j.taap.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Lin S, Cullen WR, Thomas DJ. Methylarsenicals and arsinothiols are potent inhibitors of mouse liver thioredoxin reductase. Chem. Res. Toxicol. 1999;12:924–930. doi: 10.1021/tx9900775. [DOI] [PubMed] [Google Scholar]
- Mahata J, Basu A, Ghoshal S, Sarkar JN, Roy AK, Poddar G, Nandy AK, Banerjee A, Ray K, Natarajan AT, Nilsson R, Giri AK. Chromosomal aberrations and sister chromatid exchanges in individuals exposed to arsenic through drinking water in West Bengal, India. Mutat. Res. 2003;534:133–143. doi: 10.1016/s1383-5718(02)00255-3. [DOI] [PubMed] [Google Scholar]
- Mittal M, Flora SJ. Vitamin E supplementation protects oxidative stress during arsenic and fluoride antagonism in male mice. Drug Chem. Toxicol. 2007;30:263–281. doi: 10.1080/01480540701380075. [DOI] [PubMed] [Google Scholar]
- Nauseef WM, Malech HL. Analysis of the peptide subunits of human neutrophil myeloperoxidase. Blood. 1986;67:1504–1507. [PubMed] [Google Scholar]
- Perkins C, Kim CN, Fang G, Bhalla KN. Arsenic induces apoptosis of multidrug-resistant human myeloid leukemia cells that express Bcr-Abl or overexpress MDR, MRP, Bcl-2, or Bcl-x(L) Blood. 2000;95:1014–1022. [PubMed] [Google Scholar]
- Pu YS, Jan KY, Wang TC, Wang AS, Gurr JR. 8-Oxoguanine DNA glycosylase and MutY homolog are involved in the incision of arsenite-induced DNA adducts. Toxicol. Sci. 2007;95:376–382. doi: 10.1093/toxsci/kfl166. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2006. ISBN3-900051-07-0, http://www.rproject.org. [Google Scholar]
- Rasmussen RE, Menzel DB. Variation in arsenic-induced sister chromatid exchange in human lymphocytes and lymphoblastoid cell lines. Mutat. Res. 1997;386:299–306. doi: 10.1016/s1383-5742(97)00010-0. [DOI] [PubMed] [Google Scholar]
- Schwerdtle T, Walter I, Mackiw I, Hartwig A. Induction of oxidative DNA damage by arsenite and its trivalent and pentavalent methylated metabolites in cultured human cells and isolated DNA. Carcinogenesis. 2003;24:967–974. doi: 10.1093/carcin/bgg018. [DOI] [PubMed] [Google Scholar]
- Shi H, Hudson LG, Ding W, Wang SW, Copper KL, Liu SM, Shi XL, Liu KJ. Arsenite causes DNA damage in keratinocytes via generation of hydroxyl radicals. Chem. Res. Toxicol. 2004;17:871–878. doi: 10.1021/tx049939e. [DOI] [PubMed] [Google Scholar]
- Soto-Pena GA, Luna AL, Acosta-Saavedra L, Conde P, L´opez-Carrillo L, Cebri´an ME, Bastida M, Calder´on-Aranda ES, Vega L. Assessment of lymphocyte subpopulations and cytokine secretion in children exposed to arsenic. FASEB J. 2006;20:779–781. doi: 10.1096/fj.05-4860fje. [DOI] [PubMed] [Google Scholar]
- Styblo M, Serves SV, Cullen WR, Thomas DJ. Comparative inhibition of yeast glutathione reductase by arsenicals and arsenothiols. Chem. Res. Toxicol. 1997;10:27–33. doi: 10.1021/tx960139g. [DOI] [PubMed] [Google Scholar]
- Tezuka M, Hanioka K, Yamanaka K, Okada S. Gene damage induced in human alveolar type II (L-132) cells by exposure to dimethylarsinic acid. Biochem. Biophys. Res. Commun. 1993;191:1178–1183. doi: 10.1006/bbrc.1993.1341. [DOI] [PubMed] [Google Scholar]
- Vega L, Gonsebatt ME, Ostrosky-Wegman P. Aneugenic effect of sodium arsenite on human lymphocytes in vitro: an individual susceptibility effect detected. Mutat. Res. 1995;334:365–373. doi: 10.1016/0165-1161(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Wang TS, Hsu TY, Chung CH, Wang AS, Bau DT, Jan KY. Arsenite induces oxidative DNA adducts and DNA-protein cross-links in mammalian cells. Free Radic. Biol. Med. 2001;31:321–330. doi: 10.1016/s0891-5849(01)00581-0. [DOI] [PubMed] [Google Scholar]
- Wang TS, Shu YF, Liu YC, Jan KY, Huang H. Glutathione peroxidase and catalase modulate the genotoxicity of arsenite. Toxicology. 1997;121:229–237. doi: 10.1016/s0300-483x(97)00071-1. [DOI] [PubMed] [Google Scholar]
- WHO. WHO Guidelines for drinking water quality. 2nd Ed. vol. 2: Health criteria and other supporting information. Geneva: 1996. pp. 940–994. [Google Scholar]
- Yamanaka K, Hayashi H, Tachikawa M, Kato K, Hasegawa A, Oku N, Okada S. Metabolic methylation is a possible genotoxicity-enhancing process of inorganic arsenics. Mutat. Res. 1997;394:95–101. doi: 10.1016/s1383-5718(97)00130-7. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Okada S. Induction of lung-specific DNA damage by metabolically methylated arsenics via the production of free radicals. Envion. Health Perspect. 1994;102:37–40. doi: 10.1289/ehp.94102s337. [DOI] [PMC free article] [PubMed] [Google Scholar]