Abstract
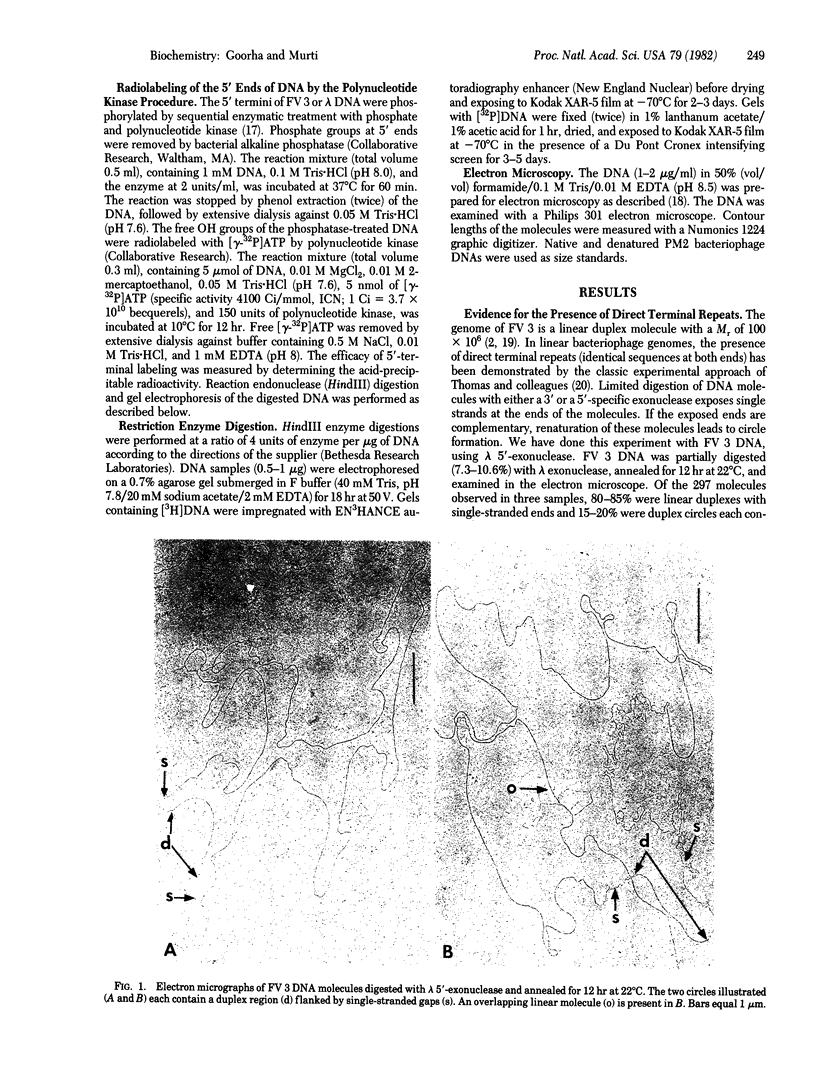
We examined the structure of the frog virus 3 (FV 3) genome by using electron microscopic and biochemical techniques. The linear FV 3 DNA molecules (Mr approximately 100 x 10(6) formed circles when partially degraded with bacteriophage lambda 5'-exonuclease and annealed, but not when the annealing was done without prior exonuclease digestion. The results suggest that the DNA molecules contain direct terminal repeats. The repeated region composed about 4% of the genome. Complete denaturation of native FV 3 DNA molecules followed by renaturation produced duplex circles each bearing two single-stranded tails at different points along the circumference. The tails presumably represent the terminal repeats. The formation of duplex circles suggests that the FV 3 genome is circularly permuted. This is further borne out by (i) failure to identify a specific restriction endonuclease fragment containing the label when the molecular ends were radiolabeled by using the polynucleotide kinase procedure, and (ii) similarity in the restriction patterns of virion DNA and large concatemeric replicating viral DNA as revealed by endonucleolytic cleavage of both DNAs with HindIII. From the above data, we conclude that the FV3 genome is both circularly permuted and terminally redundant--unique features for an animal virus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berns K. I., Silverman C. Natural occurrence of cross-linked vaccinia virus deoxyribonucleic acid. J Virol. 1970 Mar;5(3):299–304. doi: 10.1128/jvi.5.3.299-304.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carusi E. A. Evidence for blocked 5'-termini in human adenovirus DNA. Virology. 1977 Jan;76(1):380–394. doi: 10.1016/0042-6822(77)90310-5. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Haseltine W. A. Terminal redundancy and the origin of replication of Rous sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 May;74(5):1908–1912. doi: 10.1073/pnas.74.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorha R., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. I. Virus-specific protein synthesis and its regulation. Virology. 1974 Jul;60(1):237–250. doi: 10.1016/0042-6822(74)90381-x. [DOI] [PubMed] [Google Scholar]
- Goorha R., Murti G., Granoff A., Tirey R. Macromolecular synthesis in cells infected by frog virus 3. VIII. The nucleus is a site of frog virus 3 DNA and RNA synthesis. Virology. 1978 Jan;84(1):32–50. doi: 10.1016/0042-6822(78)90216-7. [DOI] [PubMed] [Google Scholar]
- Goorha R., Willis D. B., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. VI. Frog virus 3 replication is dependent on the cell nucleus. J Virol. 1977 Feb;21(2):802–805. doi: 10.1128/jvi.21.2.802-805.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorha R., Willis D. B., Granoff A., Naegele R. F. Characterization of a temperature-sensitive mutant of frog virus 3 defective in DNA replication. Virology. 1981 Jul 15;112(1):40–48. doi: 10.1016/0042-6822(81)90610-3. [DOI] [PubMed] [Google Scholar]
- Grafstrom R. H., Alwine J. C., Steinhart W. L., Hill C. W., Hyman R. W. The terminal repetition of herpes simplex virus DNA. Virology. 1975 Sep;67(1):144–157. doi: 10.1016/0042-6822(75)90412-2. [DOI] [PubMed] [Google Scholar]
- Houts G. E., Gravell M., Darlington R. W. Base composition and molecular weight of DNA from a frog polyhedral cytoplasmic deoxyribovirus. Proc Soc Exp Biol Med. 1970 Nov;135(2):232–236. doi: 10.3181/00379727-135-35026. [DOI] [PubMed] [Google Scholar]
- Jacob R. J., Morse L. S., Roizman B. Anatomy of herpes simplex virus DNA. XII. Accumulation of head-to-tail concatemers in nuclei of infected cells and their role in the generation of the four isomeric arrangements of viral DNA. J Virol. 1979 Feb;29(2):448–457. doi: 10.1128/jvi.29.2.448-457.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. C., Avery R. J. Frog virus 3 deoxyribonucleic acid. J Gen Virol. 1974 Aug;24(2):339–348. doi: 10.1099/0022-1317-24-2-339. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Davis R. W., Davidson N. A physical study by electron microscopy of the terminally reptitious, circularly permuted DNA from the coliphage particles of Escherichia coli 15. J Mol Biol. 1970 Feb 28;48(1):1–22. doi: 10.1016/0022-2836(70)90215-9. [DOI] [PubMed] [Google Scholar]
- MACHATTIE L. A., THOMAS C. A., Jr DNA FROM BACTERIOPHAGE LAMBDA: MOLECULAR LENGTH AND CONFORMATION. Science. 1964 May 29;144(3622):1142–1144. doi: 10.1126/science.144.3622.1142. [DOI] [PubMed] [Google Scholar]
- MacHattie L. A., Ritchie D. A., Thomas C. A., Jr, Richardson C. C. Terminal repetition in permuted T2 bacteriophage DNA molecules. J Mol Biol. 1967 Feb 14;23(3):355–363. doi: 10.1016/s0022-2836(67)80110-4. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Jr Replication and molecular recombination of T-phage. Annu Rev Microbiol. 1975;29:355–376. doi: 10.1146/annurev.mi.29.100175.002035. [DOI] [PubMed] [Google Scholar]
- Ortín J., Enjuanes L., Viñuela E. Cross-links in African swine fever virus DNA. J Virol. 1979 Sep;31(3):579–583. doi: 10.1128/jvi.31.3.579-583.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghow R., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. XIV. Characterization of the methylated nucleotide sequences in viral messenger RNAs. Virology. 1980 Nov;107(1):283–294. doi: 10.1016/0042-6822(80)90293-7. [DOI] [PubMed] [Google Scholar]
- STRACK H. B., KAISER A. D. ON THE STRUCTURE OF THE ENDS OF LAMBADA DNA. J Mol Biol. 1965 May;12:36–49. doi: 10.1016/s0022-2836(65)80280-7. [DOI] [PubMed] [Google Scholar]
- STREISINGER G., EDGAR R. S., DENHARDT G. H. CHROMOSOME STRUCTURE IN PHAGE T4. I. CIRCULARITY OF THE LINKAGE MAP. Proc Natl Acad Sci U S A. 1964 May;51:775–779. doi: 10.1073/pnas.51.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov I., Friedman A. Electron microscopy of human cytomegalovirus DNA. Arch Virol. 1976;50(4):343–347. doi: 10.1007/BF01317960. [DOI] [PubMed] [Google Scholar]
- Skalka A. M. DNA replication--bacteriophage lambda. Curr Top Microbiol Immunol. 1977;78:201–237. [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G., Emrich J., Stahl M. M. Chromosome structure in phage t4, iii. Terminal redundancy and length determination. Proc Natl Acad Sci U S A. 1967 Feb;57(2):292–295. doi: 10.1073/pnas.57.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS C. A., Jr, MACHATTIE L. A. CIRCULAR T2 DNA MOLECULES. Proc Natl Acad Sci U S A. 1964 Nov;52:1297–1301. doi: 10.1073/pnas.52.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. A., Jr, Kelly T. J., Jr, Rhoades M. The intracellular forms of T7 and P22 DNA molecules. Cold Spring Harb Symp Quant Biol. 1968;33:417–424. doi: 10.1101/sqb.1968.033.01.048. [DOI] [PubMed] [Google Scholar]
- Thomas C. A., Jr The rule of the ring. J Cell Physiol. 1967 Oct;70(2 Suppl):13–33. doi: 10.1002/jcp.1040700404. [DOI] [PubMed] [Google Scholar]
- Tye B. K., Chan R. K., Botstein D. Packaging of an oversize transducing genome by Salmonella phage P22. J Mol Biol. 1974 Jan 5;85(4):485–500. doi: 10.1016/0022-2836(74)90311-8. [DOI] [PubMed] [Google Scholar]
- Tye B. K., Huberman J. A., Botstein D. Non-random circular permutation of phage P22 DNA. J Mol Biol. 1974 Jan 5;85(4):501–528. doi: 10.1016/0022-2836(74)90312-x. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Goorha R., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. XI. A ts mutant of frog virus 3 that is defective in late transcription. Virology. 1979 Oct 30;98(2):328–335. doi: 10.1016/0042-6822(79)90556-7. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Goorha R., Miles M., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. VII. Transcriptional and post-transcriptional regulation of virus gene expression. J Virol. 1977 Oct;24(1):326–342. doi: 10.1128/jvi.24.1.326-342.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. B., Granoff A. Frog virus 3 DNA is heavily methylated at CpG sequences. Virology. 1980 Nov;107(1):250–257. doi: 10.1016/0042-6822(80)90290-1. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Granoff A. Macromolecular synthesis in cells infected with frog virus 3. V. The absence of polyadenylic acid in the majority of frog virus 3-specific mRNA species. Virology. 1976 Sep;73(2):543–547. doi: 10.1016/0042-6822(76)90417-7. [DOI] [PubMed] [Google Scholar]






