Abstract
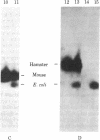
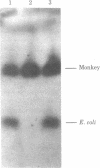
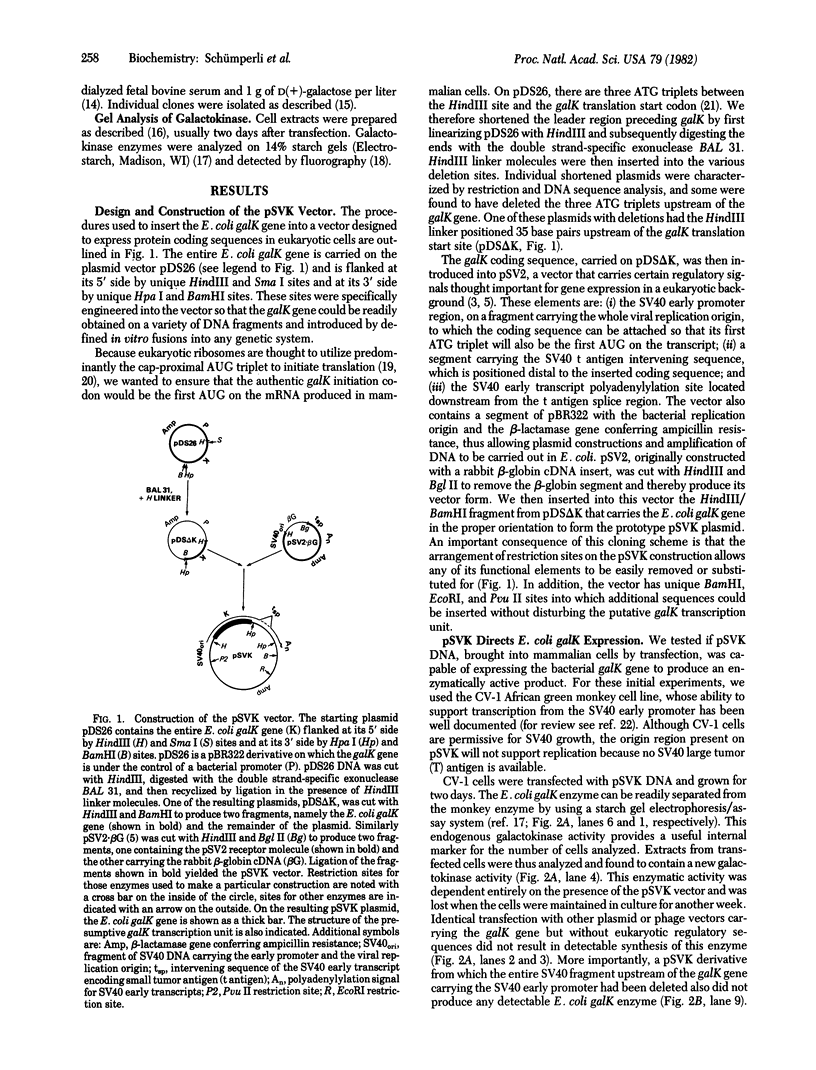
The Escherichia coli galactokinase gene (galK) was inserted into a modified early region transcription unit of simian virus 40 (SV40) contained on a bacterial plasmid. Introduction of this pSVK vector into monkey, mouse, and hamster cell lines by transfection resulted in efficient expression of the bacterial galK gene. This expression was shown to be dependent upon fusion of the galK gene to the early promoter of SV40 and did not appear to require SV40 splice signals. Moreover, expression in these cells could be obtained either transiently, 24--72 hr after transfection, or continuously, after stable transformation. In particular, pSVK-dependent galK expression was obtained in a hamster cell line genetically deficient in galactokinase activity. Expression of the bacterial enzyme was shown to complement the galactosemic defect of these cells, thereby allowing their selective survival and growth on galactose as the only carbon source. The ability to readily assay, select for, and potentially select against galK expression from pSVK and its derivatives should prove extremely useful in studying eukaryotic gene regulatory signals.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Büchsel R., Hassels-Vischer B., Tauber R., Reutter W. 2-Deoxy-D-galactose impairs the fucosylation of glycoproteins of rat liver and Morris hepatoma. Eur J Biochem. 1980 Oct;111(2):445–453. doi: 10.1111/j.1432-1033.1980.tb04959.x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitzelmann R. Deficiency of erythrocyte galactokinase in a patient with galactose diabetes. Lancet. 1965 Oct 2;2(7414):670–671. doi: 10.1016/s0140-6736(65)90400-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Legerski R. J., Hodnett J. L., Gray H. B., Jr Extracellular nucleases of pseudomonas BAL 31. III. Use of the double-strand deoxyriboexonuclease activity as the basis of a convenient method for the mapping of fragments of DNA produced by cleavage with restriction enzymes. Nucleic Acids Res. 1978 May;5(5):1445–1464. doi: 10.1093/nar/5.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehaug J. R., Kleppe K. Effect of salts and polyamines on T4 polynucleotide kinase. Biochemistry. 1975 Mar 25;14(6):1225–1229. doi: 10.1021/bi00677a021. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols E. A., Elsevier S. M., Ruddle F. H. A new electrophoretic technique for mouse, human, and Chinese hamster galactokinase. Cytogenet Cell Genet. 1974;13(3):275–278. doi: 10.1159/000130279. [DOI] [PubMed] [Google Scholar]
- O'Hare K., Benoist C., Breathnach R. Transformation of mouse fibroblasts to methotrexate resistance by a recombinant plasmid expressing a prokaryotic dihydrofolate reductase. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1527–1531. doi: 10.1073/pnas.78.3.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Rosenberg M. Efficient translation of prokaryotic mRNAs in a eukaryotic cell-free system requires addition of a cap structure. Nature. 1979 Jun 21;279(5715):692–696. doi: 10.1038/279692a0. [DOI] [PubMed] [Google Scholar]
- Peterson J. L., McBride O. W. Cotransfer of linked eukaryotic genes and efficient transfer of hypoxanthine phosphoribosyltransferase by DNA-mediated gene transfer. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1583–1587. doi: 10.1073/pnas.77.3.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Paterson B. M. Efficient cap-dependent translation of polycistronic prokaryotic mRNAs is restricted to the first gene in the operon. Nature. 1979 Jun 21;279(5715):696–701. doi: 10.1038/279696a0. [DOI] [PubMed] [Google Scholar]
- Thirion J. P., Banville D., Noel H. Galactokinase mutants of Chinese hamster somatic cells resistant to 2-deoxygalactose. Genetics. 1976 May;83(1):137–147. doi: 10.1093/genetics/83.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]