Abstract
Activation of either the A1 or the A3 adenosine receptor (A1R or A3R, respectively) elicits delayed cardioprotection against infarction, ischemia, and hypoxia. Mitochondrial contribution to the progression of cardiomyocyte injury is well known; however, the protective effects of adenosine receptor activation in cardiac cells with a respiratory chain deficiency are poorly elucidated. The aim of our study was to further define the role of A1R and A3R activation on functional tolerance after inhibition of the terminal link of the mitochondrial respiratory chain with sodium azide, in a state of normoxia or hypoxia, compared with the effects of the mitochondrial ATP-sensitive K+ channel opener diazoxide. Treatment with 10 mM sodium azide for 2 h in normoxia caused a considerable decrease in the total ATP level; however, activation of adenosine receptors significantly attenuated this decrease. Diazoxide (100 µM) was less effective in protection. During treatment of cultured cardiomyocytes with hypoxia in the presence of 1 mM sodium azide, the A1R agonist 2-chloro-N6-cyclopentyladenosine was ineffective, whereas the A3R agonist 2-chloro-N6-iodobenzyl-5′-N-methylcarboxamidoadenosine (Cl-IB-MECA) attenuated the decrease in ATP level and prevented cell injury. Cl-IB-MECA delayed the dissipation in the mitochondrial membrane potential during hypoxia in cells impaired in the mitochondrial respiratory chain. In cells with elevated intracellular Ca2+ concentration after hypoxia and treatment with NaN3 or after application of high doses of NaN3, Cl-IB-MECA immediately decreased the elevated intracellular Ca2+ concentration toward the diastolic control level. The A1R agonist was ineffective. This may be especially important for the development of effective pharmacological agents, because mitochondrial dysfunction is a leading factor in the pathophysiological cascade of heart disease.
Keywords: Ca2+ transience, hypoxia, ATP-sensitive K+ channel, sodium azide, heart disease, ischemia
The purine nucleoside adenosine is recognized as a major local (autocrine and paracrine) regulator of tissue function, especially when the energy supply acutely fails to meet the cellular energy demand (27). A brief ischemic episode is able to protect the heart against injury during a subsequent period of prolonged ischemia, which results in a reduction in infarct size (ischemic preconditioning). Exposure of the heart to adenosine instead of ischemia can also induce a preventive effect against subsequent ischemia-induced damage. Known as pharmacological preconditioning, this effect of adenosine has been the subject of much investigative interest. The released adenosine interacts with sarcolemmal membrane receptors. Adenosine receptors (ARs) exist in at least four different subtypes including A1, A2A, A2B, and A3 (28, 34). The recently identified A3 AR (A3R), like the A1R, negatively couples to adenylyl cyclase and displays significant cardioprotective activity (2, 36, 38, 45–47, 49).
Results of many investigations indicate that activation of either A1Rs or A3Rs (but not A2ARs) elicits a delayed defense against ischemia, hypoxia, or infarction and that both A1Rs and A3Rs induce cardioprotection through the opening of ATP-sensitive K+ (KATP) channels (38, 43, 46). However, recently it was shown that protection by exogenous adenosine in the ischemic reperfused mouse heart involves purine salvage and activation of A3Rs but not A1Rs or A2ARs (33). Similarly, cardioprotection against doxorubicin toxicity was achieved through activation of A3Rs, but A1R activation was ineffective (40). Comparison of findings from A3R-overexpressing mice (7, 41) with results from A1R-overexpressing mice (18) indicates that A1Rs and A3Rs differently control the heart rate (25). Thus despite the fact that activation of both the A1 and the A3 subtypes of the ARs can mimic the preventive effects of ischemic preconditioning, the specific protective functions mediated by each receptor remain to be delineated. Now it is generally accepted that cardiomyocyte protection is mediated via mitochondrial rather than sarcolemmal KATP channels (29), and the protective action of AR activation is abolished by KATP channel blockade (38). However, a cardioprotective role of KATP channels and ARs remains controversial and is not fully clarified. Moreover, the function of opening of mitochondrial KATP (mitoKATP) channels is also unclear (8).
A possible explanation for how opening of mitoKATP channels might protect myocytes against ischemic damage is that in decreasing the mitochondrial membrane potential (Δψ), the binding of the endogenous ATPase inhibitor IF1 is promoted, and thus ATP is conserved during ischemia (35). Another possibility is that dissipation of Δψ decreases the driving force for Ca2+ influx through the Ca2+ uniporter (24). It has been reported (14, 15) that the mitoKATP channel opener diazoxide can prevent mitochondrial Ca2+ accumulation.
Recently, we (42) have shown that regulation of Ca2+ levels in cultured cardiomyocytes may be a function of one AR subtype, A3R, having a signal transduction pathway distinct from the closely related A1R subtype. Activation of A3Rs decreased intracellular Ca2+ concentration ([Ca2+]i) and may attenuate mitochondrial Ca2+ accumulation by a mechanism independent from the mitoKATP channel. Ischemic and pharmacological preconditioning exerts cardioprotection by up-regulating endogenous protective mechanisms and may be fully achieved in undamaged, intact cells. The aim of this study was to elucidate the protective effects of AR activation and mitoKATP channel opening in cardiac cells with respiratory chain deficiency. We investigated the roles of A1R and A3R activation on functional tolerance after inhibiting the terminal link of the mitochondrial respiratory chain with sodium azide, which is an inhibitor of cytochrome c oxidase, during a state of normoxia or hypoxia. A comparison of the effects of the mitoKATP channel opener diazoxide and the Ca2+ response after activation of the A1R and A3R might shed light on the pathways of AR signaling in protecting the cardiac cells from conditions of stress.
MATERIALS AND METHODS
The experimental protocol was approved by the Animal Care and Use Committee of Bar-Ilan University. This investigation also conforms with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Pub. No. 85-23, Revised 1996).
Cell culture
Rat hearts (1–2 days old) were removed under sterile conditions and washed three times in phosphate-buffered saline (PBS) to remove excess blood cells. The hearts were minced and then gently agitated in a solution of proteolytic enzymes (RDB; Biological Institute; Ness-Ziona, Israel), which was prepared from a fig tree extract. The RDB was diluted 1:100 in Ca2+- and Mg2+-free PBS at 25°C for a few cycles of 10 min each as described previously (36, 40). Dulbecco’s modified Eagle’s medium that contained 10% horse serum (Biological Industries; Kibbutz Beit Haemek, Israel) was added to supernatant suspensions that contained dissociated cells. The mixture was centrifuged at 300 g for 5 min. The supernatant phase was discarded, and the cells were resuspended. The suspension of the cells was diluted to 1.0 × 106 cells/ml, and 1.5 ml of the suspension was placed in 35-mm plastic culture dishes on collagen-gelatin-coated coverslips. The cultures were incubated in a humidified 5% CO2-95% air atmosphere at 37°C. Confluent monolayers exhibiting spontaneous contractions were developed in culture within 2 days. Myocyte cultures were washed in serum-free BIO-MPM-1 medium (Biological Industries) that contained 5 mg/ml glucose and were incubated in this medium for an additional 48 h before the experiments were performed.
Hypoxic conditions
Myocyte cultures were washed in serum- and glucose-free medium before incubation in the presence of AR ligands under hypoxic conditions. A 60- or 90-min exposure to N2 (100%) in glucose-free media within a hypoxic chamber was used to simulate ischemic conditions in primary cardiac myocyte cultures. The hypoxic damage was characterized at the end of the hypoxic period by morphological and biochemical evaluations. Sodium azide (Sigma; St. Louis, MO) was freshly prepared in culture medium for each experiment. Continuous monitoring of [Ca2+]i or mitochondrial membrane potential during hypoxia was realized in a special barrier well, where cells were protected from oxygen by a laminar counterflowing layer of the inert gas argon (100%; Ref. 44). Coverslips with cultured cells were placed at the bottom of the well. This chamber was mounted on a specially modified Zeiss inverted epifluorescence microscope (Carl Zeiss; Oberkochen, Germany).
Experiments with A1R and A3R ligands
The A1R agonist 2-chloro-N6-cyclopentyladenosine (CCPA), the A3R agonist 2-chloro-N6-iodobenzyl-5′-N-methylcarboxamidoadenosine (Cl-IB-MECA), the A1R antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), and the A3R antagonist 5-propyl-2-ethyl-4-propyl-3-(ethylsulfanylcarbonyl)-6-phenylpyridine-5-carboxylate (MRS-1523) were added to cell cultures 10 min before the experimental treatment.
Lactate dehydrogenase assay
Cytotoxicity was assessed by spectrophotometric measurement of lactate dehydrogenase (LDH) released into the culture medium. Protein content and LDH activity were determined according to the methods of El-Ani et al. (6). Briefly, 25 µl of the supernatant were transferred to a 96-well dish, and the LDH activities were determined by using LDH-L kits (Sigma) as described by the manufacturer. The results are expressed as a fold of the control in the same experiment. Experiments were done in four to eight replicates each and were repeated at least six times.
Cell death assay
This assay was performed using a modification of the procedure used in our previous work (36). Cells were loaded with propidium iodide, which only stains the nuclei of membrane-compromised cells. To facilitate cell counting, Hoechst-33342 (10 µM) was included to stain the nuclei of all cells. Cell loss (percentage of cell death) was presented as the number of dead (propidium iodide stained) cells/total number of cells (Hoechst-33342 stained).
Measurement of ATP concentration
Cells were washed with ice-cold PBS, frozen in liquid N2, and stored at −80°C until analysis. Cells were resuspended in ice-cold homogenization buffer that consisted of 50 mM potassium fluoride, 10 mM EDTA, and 30% glycerol, pH 7.0. The cell extract was used to measure ATP content with the luciferin-luciferase bioluminescence kit (ATP Bioluminescence Assay Kit CLSII; Boehringer Mannheim) following the manufacturer’s protocol. Values are expressed as nanomoles per milligram of protein (41).
Monitoring mitochondrial retention of DASPMI
Living cells grown on coverslips were exposed to 4-[4-(dimethylamino)styryl]-N-methylpyridinium iodide (DASPMI) dissolved in PBS at a final concentration of 10 µg/ml for 15 min. The coverslips were then washed and mounted on chambers that contained dye-free medium. DASPMI fluorescence was elicited by excitation at 460 nm, and emission was measured using a long-pass filter at 540 nm. For registration of kinetic curves of DASPMI fluorescence, the emitted light was split on the path to the photomultiplier by a dichroic mirror with an input filter at 590 nm. The fluorescence intensity was fed to a SAMPLE program written by Dr. Doron Kaplan (Israel Institute for Biological Research; Ness-Ziona, Israel). DASPMI fluorescence intensity corresponds to a relative polarization of Δψ. It was shown that distribution of DASPMI ions on the inner mitochondrial membrane occurs in accordance with the Nernst equation. Mitochondrial membrane hyperpolarization and depolarization were induced by sodium succinate (20) and the mitochondrial uncoupler carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP; Ref. 39), respectively.
[Ca2+]i measurements
We estimated [Ca2+]i from indo-1 fluorescence using a ratio method described elsewhere (41). Continuous monitoring of [Ca2+]i during hypoxia was performed in a special barrier well where cells were protected from oxygen by a laminar counterflow layer of inert argon (100%) gas.
Chemicals
DASPMI and indo-1 were acquired from Molecular Probes (Eugene, OR). The highly selective A3R agonist Cl-IB-MECA was a gift from the National Institute of Mental Health Chemical Synthesis and Drug Supply Program. The highly selective A1R agonist CCPA and the selective A3R antagonist MRS-1523 were purchased from Sigma. Other reagents were purchased from Sigma.
Statistics
Results are expressed as means ± SE. Data were analyzed by ANOVA with application of a post hoc Tukey-Kramer test. P < 0.05 was accepted as indicating statistical significance.
RESULTS
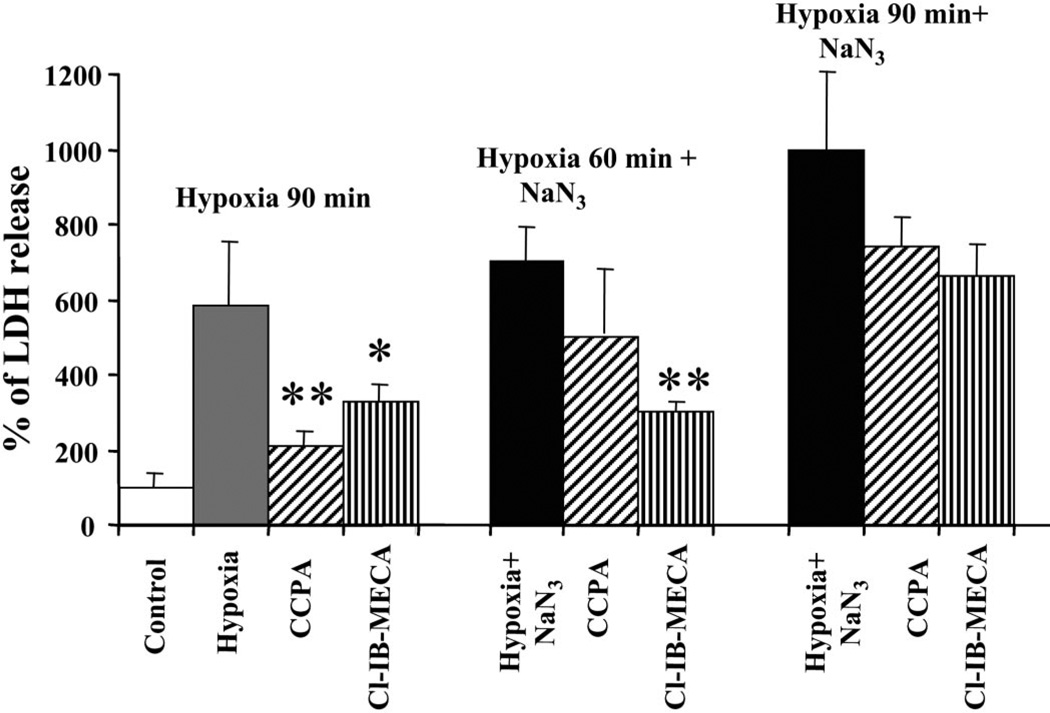
To investigate the role of AR agonists in attenuating myocyte injury during prolonged (90 min) hypoxia, cultured cardiomyocytes were incubated with A1R- and A3R-specific agonists (CCPA and Cl-IB-MECA, respectively) for 15 min before and during hypoxia. These agonists prevented injury produced by hypoxia according to the level of LDH released from the cells (Fig. 1). Decrease in LDH release suggested that both A1R and A3R agonists reduced hypoxia-induced injury in intact cardiomyocytes. Thus, when intact cardiomyocytes were exposed to hypoxia, the protective effects of A1R and A3R agonists against damage were evident, and protection by A1R activation was more effective. These data agreed well with our earlier findings that CCPA and Cl-IB-MECA attenuated cultured rat cardiomyocyte injury and prevented cell death during hypoxia through activation of ARs (36).
Fig. 1.
Effects of adenosine receptor (AR) activation on lactate dehydrogenase (LDH) release from cardiomyocytes treated with 1 mM sodium azide and exposed to hypoxia for 90 or 60 min. The A1 AR subtype (A1R) agonist 2-chloro-N6-cyclopentyladenosine (CCPA, 100 nM) or the A3 AR subtype (A3R) agonist 2-chloro-N6-iodobenzyl-5′-N-methylcarboxamidoadenosine (Cl-IB-MECA, 100 nM) were given 15 min before the insults. LDH release was determined immediately after hypoxia. Release in the control cultures was considered to be 100%. *P < 0.05; **P < 0.01 compared with hypoxia, according to ANOVA and a post hoc Tukey-Kramer test.
The efficacy of A1R and A3R agonists in protecting damaged cardiomyocytes was studied in the presence of sodium azide, which inhibits the mitochondrial respiratory chain through action at cytochrome c oxidase. Results of LDH release from cardiac myocytes after treatment with 1 mM sodium azide and hypoxia for 60 and 90 min are shown in Fig. 1. Neither the A1R agonist nor the A3R agonist was able to prevent the detrimental effects of sodium azide during 90 min of hypoxia. However, when the cultures were exposed to hypoxia for 60 min in the presence of 1 mM sodium azide, activation of A3Rs abolished the effects of azide, whereas activation of A1Rs was significantly less effective.
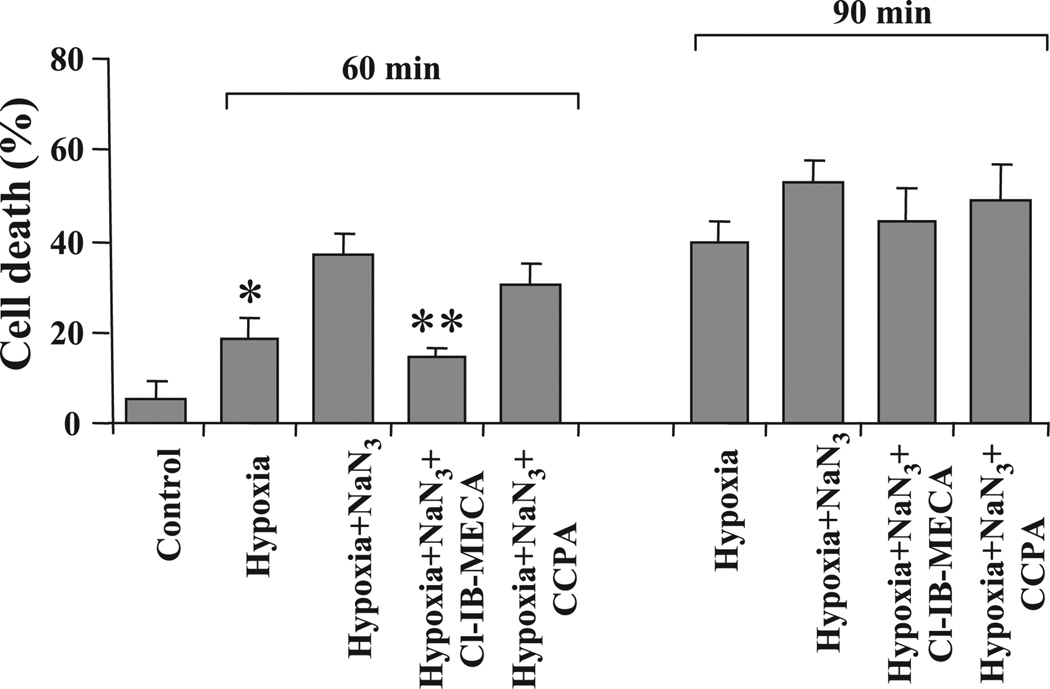
Cell death after 60 min of hypoxia was 18 ± 2%. In cardiocytes treated with sodium azide and hypoxia for 60 min, cell death increased to 37.2 ± 3.1%. Activation of A3Rs with Cl-IB-MECA attenuated cell loss to 14.3 ± 2.4%, and activation of A1Rs with CCPA attenuated cell loss only to 30.1 ± 6.1% (Fig. 2). Activation of ARs was not effective in preventing cell death after 90 min of hypoxia with sodium azide (Fig. 2).
Fig. 2.
Effects of activation of ARs on cardiomyocyte death. Effects of the A1R agonist CCPA (100 nM) and A3R agonist Cl-IB-MECA (100 nM) were studied after treatment of the cells with sodium azide (1 mM) and exposure to hypoxia for 60 or 90 min. *P < 0.01 compared with cells treated with sodium azide and hypoxia.
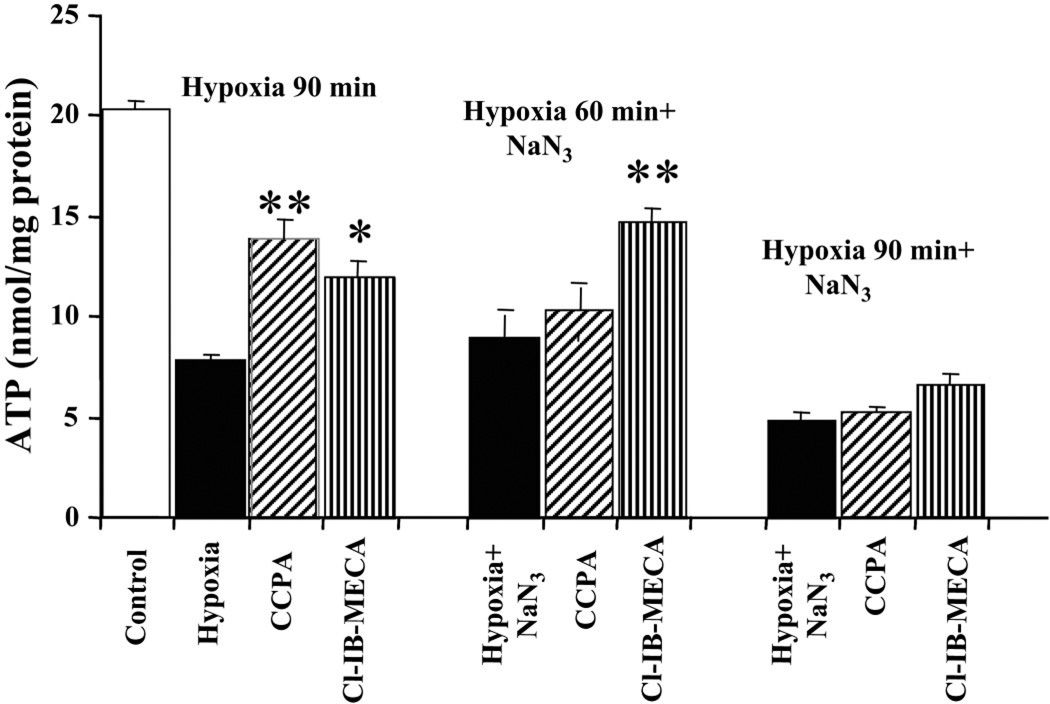
The total level of ATP decreased considerably after hypoxia (Fig. 3). However, treatment with 100 nM of the A1R agonist CCPA or 100 nM of the A3R agonist Cl-IB-MECA restricted the decrease in ATP level. Average values for total ATP content after 90 min of hypoxia were 13.54 ± 0.89 nmol/mg protein in the presence of CCPA and 11.18 ± 0.74 nmol/mg protein in the presence of Cl-IB-MECA compared with 7.86 ± 0.36 nmol/mg protein in the hypoxic group. The ATP level of control (untreated) cells was 21.36 ± 2.89 nmol/mg protein.
Fig. 3.
Effects of AR activation on ATP levels in cardiomyocytes treated with 1 mM sodium azide and exposed to hypoxia for 90 or 60 min. Effects of the A1R agonist CCPA (100 nM) or the A3R agonist Cl-IB-MECA (100 nM) were studied on ATP levels in cell homogenates. *P < 0.05; **P < 0.01 compared with hypoxia.
In cultures treated with sodium azide (1 mM) and hypoxia for 60 min, the average values for total ATP content were 10.01 ± 1.06 nmol/mg protein in the presence of CCPA (100 nM) and 14.06 ± 0.80 nmol/mg protein in the presence of Cl-IB-MECA (100 nM) compared with 8.57 ± 1.23 nmol/mg protein in cells treated with hypoxia and sodium azide together (Fig. 3). Again, activation of A3Rs in the cardiocytes exposed to hypoxia for 60 min in the presence of sodium azide was significantly more effective in protection of the cells. No effects were found when these insults were for 90 min (Fig. 3).
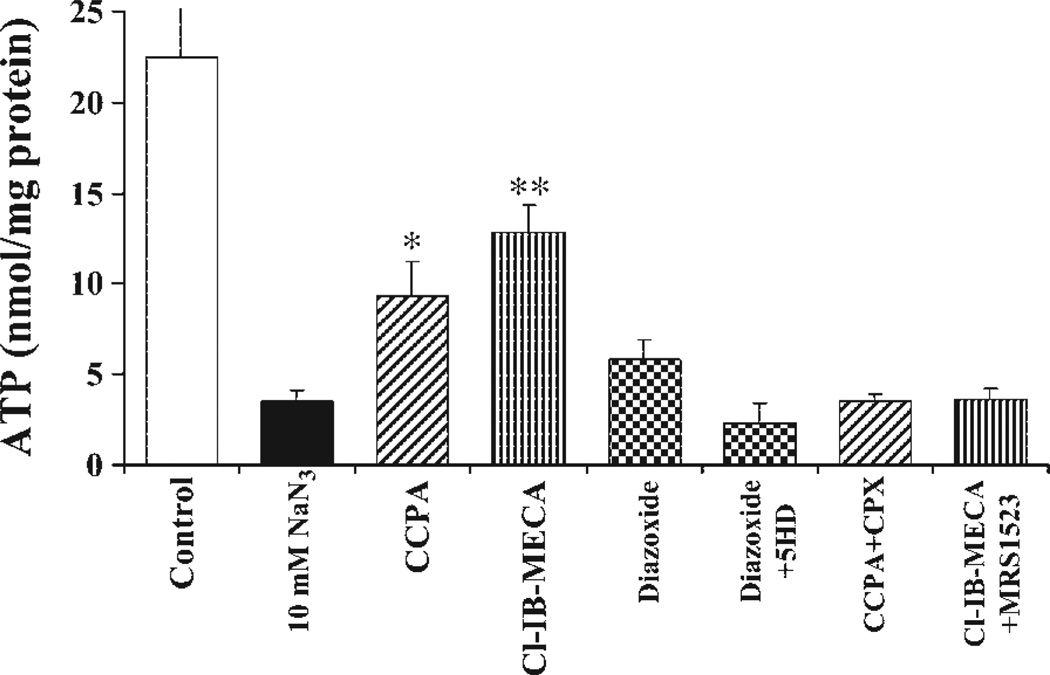
In experiments with cultured cardiomyocytes, sodium azide concentrations of 1–100 mM are usually used (3), which effectively inhibit respiratory activity in a concentration-dependent manner. Treatment with 10 mM sodium azide for 2 h under normoxic conditions induced only moderate LDH release (not shown). However, this treatment caused a considerable decrease in the total ATP level (Fig. 4). Blockade of cytochrome c oxidase with 10 mM sodium azide excessively decreased ATP content in cultured cardiomyocytes (3.56 ± 0.52 compared with 21.26 ± 0.69 nmol/mg protein in control cells after a 2-h incubation). Activation of ARs showed a protective action (13.23 ± 1.44 nmol/mg protein after A3R activation and 9.38 ± 1.90 nmol/mg protein after A1R activation). The A1R antagonist DPCPX (1 µM) abolished the protection by CCPA, and the A3R antagonist MRS-1523 (1 µM) abolished the protection by Cl-IB-MECA (Fig. 4). The contribution of mitoKATP channels in injured cells to the protective effects of ARs was examined by assessment of ATP levels in the presence of the mitoKATP channel opener diazoxide. Pretreatment with diazoxide (100 µM) attenuated the decrease in ATP level in cardiomyocytes after 120 min of incubation with sodium azide; however, this protection was less effective than activation of either A1Rs or A3Rs (Fig. 4).
Fig. 4.
Effects of AR activation and ATP-sensitive K+ (KATP) channels on ATP levels in cardiomyocytes treated for 2 h with 10 mM sodium azide. Effects of the A1R agonist CCPA (100 nM), the A3R agonist Cl-IB-MECA (100 nM), the A1R antagonist 8-cyclopentyl-1,3-dipropylxanthine (CPX, 1 µM), the A3R antagonist 5-propyl-2-ethyl-4-propyl-3-(ethylsulfanylcarbonyl)-6-phenylpyridine-5-carboxylate (MRS-1523, 1 µM), and the mitochondrial KATP channel blocker 5-hyrdoxydecanoate (5-HD, 0.3 mM) were studied on ATP levels in cultured cardiomyocytes. *P < 0.05; **P < 0.01 compared with cells treated with sodium azide.
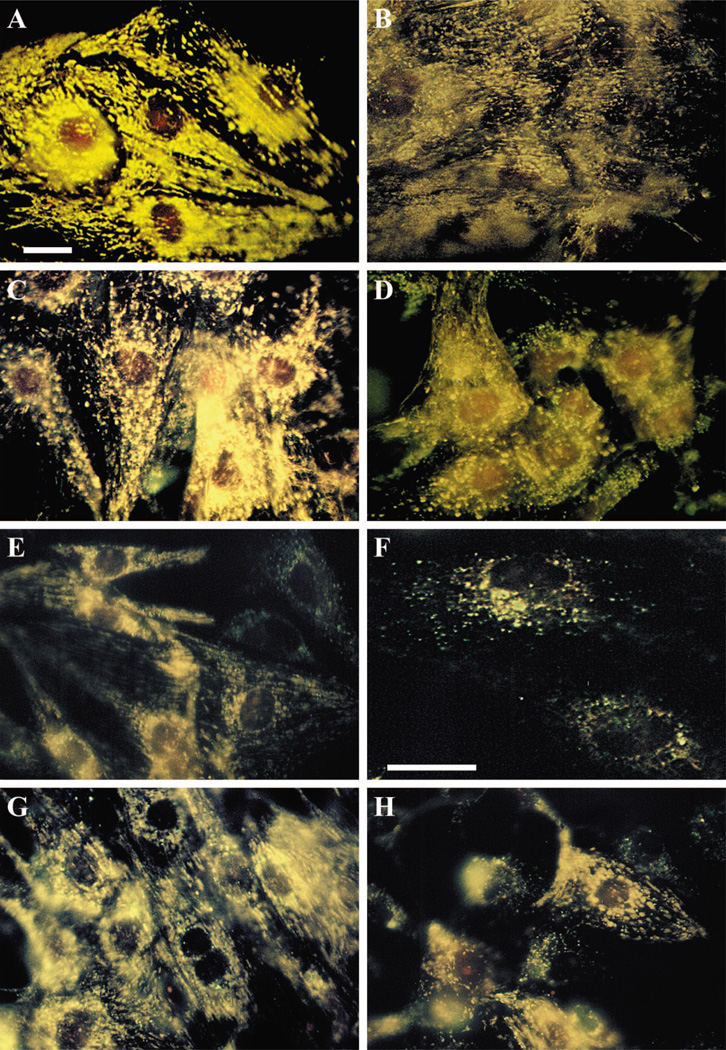
Microscopic observation of mitochondria in cultured cardiomyocytes with the use of the membrane potential indicator DASPMI showed two types of mitochondrial patterns in normoxic conditions. The first displayed longitudinally oriented and stretched mitochondria in subsarcolemmal areas in the cytoplasm, and the second featured oval-shaped mitochondria in the perinuclear and intramyofibrillar regions. Individual mitochondria were clearly identifiable in very thinly spread cells (Fig. 5A). High fluorescence associated with both mitochondrial patterns indicated a Δψ of high magnitude, which is characteristic of resting mitochondria. The mitochondrial damage arising after incubation with 10 mM sodium azide for 2 h was characterized by the loss of the intensive fluorescence staining exhibited by DASPMI dye, which represents the dissipation of Δψ. The longitudinal striated patterns observed in untreated cells disappeared completely, and prominent conglomerates of rounded mitochondrial patterns appeared in the perinuclear space. In many cells, a diffuse distribution of weakly fluorescent matter was evident (Fig. 5B). Pretreatment of the cells with the A3R agonist Cl-IB-MECA for 15 min before and during a 2-h incubation with sodium azide protected mitochondria from the loss of Δψ (Fig. 5C). Pretreatment of the cells with the A1R agonist CCPA for 15 min before and during a 2-h incubation with sodium azide did not prevent the decrease of Δψ (Fig. 5D).
Fig. 5.
Effects of A1R and A3R activation on 4-[4-(dimethylamino)styryl]-N-methylpyridinium iodide (DASPMI) fluorescence in mitochondria of cardiomyocytes treated with sodium azide. Four-day-old cardiomyocytes were exposed to 10 mM sodium azide for 120 min. A: control cells. B: treatment with 10 mM NaN3 in the absence of Cl-IB-MECA. C: treatment with 10 mM NaN3 in the presence of 100 nM Cl-IB-MECA. D: treatment with 10 mM NaN3 and 100 nM CCPA. E and F: DASPMI fluorescence in mitochondria of cardiomyocytes treated with sodium azide (1 mM) and exposed to hypoxia for 60 min (E) or 90 min (F). G and H: DASPMI fluorescence during 60 min of hypoxia and sodium azide (1 mM) shows the effects of A3R activation with 100 nM Cl-IB-MECA (G) and the effects of A1R activation with 100 nM CCPA (H). Bar = 10 µm. Each image is representative of six experiments.
In cardiac myocytes treated with 1 mM sodium azide and 60 or 90 min of hypoxia, the DASPMI fluorescence was decreased, and many myocytes exhibited signs of destructive oncotic alterations with a collapse of Δψ (Fig. 5, E and F). Neither the A1R agonist CCPA nor the A3R agonist Cl-IB-MECA was able to prevent mitochondrial damage after 90 min of hypoxia and blockade of the terminal link of the respiratory chain (not shown). When hypoxia with 1 mM sodium azide was applied for 60 min, activation of A3Rs prevented dissipation of Δψ (Fig. 5G); however, activation of A1Rs only partly protected mitochondrial bioenergetics (Fig. 5H).
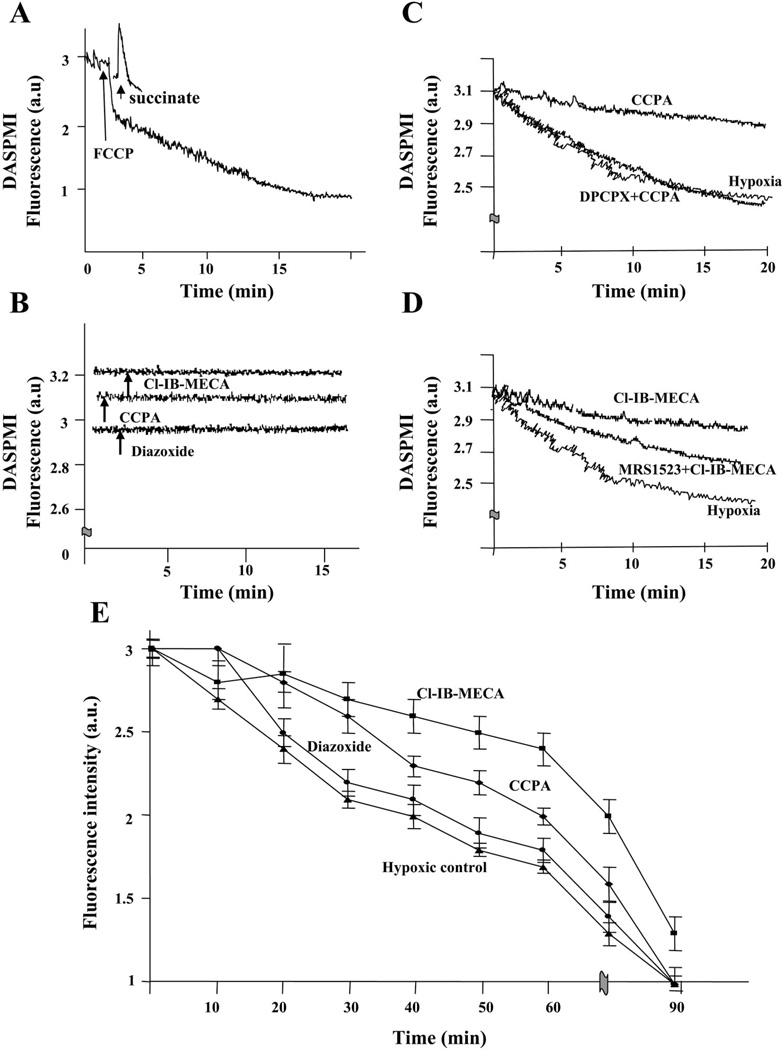
For elucidation of the kinetics of DASPMI fluorescence intensity, a microspectrofluorimetric method was used. Succinate and FCCP were applied as standards for mitochondrial energy generation and dissipation. Maximal increase of Δψ was evoked after the addition of 10 mM sodium succinate (Fig. 6A). The protonophore FCCP is able to efficiently collapse the Δψ in intact cells. Upon addition of 5 µM FCCP, the mitochondrial fluorescence decreased monotonically within 20 min (Fig. 6A). Addition of the K+ channel opener diazoxide (100 µM), the A1R agonist CCPA (100 nM), or the A3R agonist Cl-IB-MECA (100 nM) did not change DASPMI fluorescence and, hence, Δψ during 20 min of observations under normoxic conditions (Fig. 6B). When cultures were placed in hypoxic chambers under a stream of argon (see MATERIALS AND METHODS), the A1R agonist CCPA and the A3R agonist Cl-IB-MECA were effective in retarding a decrease in DASPMI fluorescence and, hence, dissipation in Δψ. Pretreatment of the cells with DPCPX before the addition of CCPA or with MRS-1523 before the addition of Cl-IB-MECA abolished the protective effects of these agonists (Fig. 6, C and D).
Fig. 6.
Changes in mitochondrial membrane potential (Δψ) after AR activation. A: sodium succinate (10 mM) and carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP, 5 µM) were applied as standards for mitochondrial energy generation and dissipation. B: effects of A1R agonist CCPA (100 nM), A3R agonist Cl-IB-MECA (100 nM), and mitochondrial KATP channel opener diazoxide (100 µM) on DASPMI fluorescence during normoxia. C and D: A1R agonist CCPA (100 nM) and A3R agonist Cl-IB-MECA (100 nM), respectively, were effective in retarding a decrease in DASPMI fluorescence and, hence, dissipation of Δψ during hypoxia. Pretreatment of the cells with DPCPX (1 µM) before addition of CCPA or with MRS-1523 (1 µM) before addition of Cl-IB-MECA abolished the protective effects of these agonists. E: effects of AR activation and diazoxide on kinetics of Δψ. Effects of the A1R agonist CCPA (100 nM), A3R agonist Cl-IB-MECA (100 nM), and diazoxide (100 µM) on DASPMI fluorescence in cardiomyocytes treated with sodium azide (1 mM) and exposed to hypoxia. Readings were obtained every 10 min. Each graph is representative of six experiments.
In single living cardiomyocytes exposed to hypoxia in the presence of sodium azide, Δψ was monitored for 20 s every 10 min during 90 min of the insults (Fig. 6E). The complete depression of Δψ by treatment with 1 mM sodium azide during hypoxia took ~90 min. Diazoxide was not effective in protecting the bioenergetics of cardiomyocytes with damaged mitochondria (Fig. 6E). The A1R agonist CCPA retarded the decrease in DASPMI fluorescence during the first 20–25 min of hypoxia. The A3R agonist Cl-IB-MECA was more effective in protection of Δψ. In the presence of 100 nM Cl-IB-MECA, Δψ was maintained during 60 min of hypoxia in cells treated with sodium azide (Fig. 6E).
The effects of A1R and A3R activation on Ca2+ transients were estimated from indo-1 fluorescence using the ratio method. Control myocytes demonstrated spontaneous, regular beating activity and [Ca2+]i transients in indo-1-loaded cells.
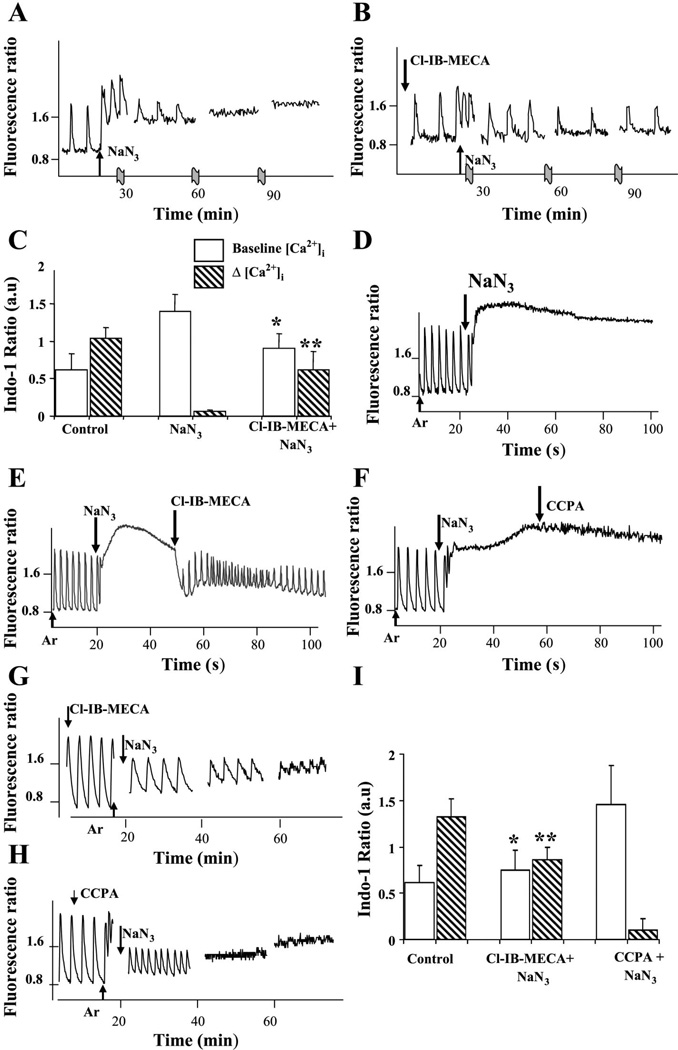
Treatment with sodium azide induced a transient rise in [Ca2+]i in a dose-dependent manner. Sodium azide at a concentration of 1 mM during normoxia induced only transient acceleration of the beating rate and [Ca2+]i elevation that lasted 10–20 s. Treatment with 10 mM sodium azide induced transient accelerations of the beating rate, elevation of baseline (diastolic) [Ca2+]i and termination of beating activity after 1–2 h of treatment (Fig. 7A). Pretreatment of cultures with 100 nM Cl-IB-MECA prevented the basal Ca2+ elevation caused by sodium azide and maintained myocyte contractility (Fig. 7B). The same experiment with the A1R agonist CCPA did not reveal any protective efficacy of A1R activation (not shown). To confirm the representative data shown in Fig. 7, A and B, we averaged results obtained in six experiments (Fig. 7C). Treatment of cultured cardiac muscle cells with 10 mM NaN3 led to elevation of baseline [Ca2+]i (to 1.40 ± 0.22 vs. 0.62 ± 0.21 in control cells) and disappearance of the [Ca2+]i transient amplitude (0.06 ± 0.02 vs. 1.04 ± 0.14 in control cells). Pretreatment with the A3R agonist restricted elevation of baseline [Ca2+]i and maintained muscle cell contractility (0.90 ± 0.20 and 0.62 ± 0.24, accordingly).
Fig. 7.
Effects of AR activation on intracellular Ca2+ concentration ([Ca2+]i) in cultured cardiomyocytes. A: sodium azide (10 mM) induced transient accelerations of the beating rate, elevation of diastolic [Ca2+]i, and termination of beating activity after 1.5–2 h of treatment. B: pretreatment of cultures with 100 nM Cl-IB-MECA abolished [Ca2+]i elevation after treatment with azide and maintained myocyte contractility. C: averaged data obtained from six experiments. Treatment of cultured cardiac muscle cells with 10 mM NaN3 led to elevation of baseline [Ca2+]i and disappearance of [Ca2+]i transient amplitude. Cl-IB-MECA (100 nM) restricted elevation of baseline [Ca2+]i (*P < 0.05 vs. NaN3 group; n = 18 cells) and maintained muscle cell contractility (**P < 0.01 vs. NaN3 group; n = 18 cells). D: continuous monitoring of [Ca2+]i during hypoxia (Ar) in cultures pretreated with 1 mM NaN3. E: application of 100 nM Cl-IB-MECA after increase of the basal level [Ca2+]i returned it to normal diastolic level, and beating activity was restored. F: A1R agonist CCPA (100 nM) in the same experiment was ineffective. G: application of Cl-IB-MECA (100 nM) for 15 min before sodium azide application (NaN3) maintained contractile activity and Ca2+ oscillations during 40–60 min. H: protective effect of A1R agonist CCPA (100 nM) was observed during 15–20 min of hypoxia after application of 1 mM NaN3. I: averaged data obtained from six experiments. Exposure of cultured cardiomyocytes to hypoxia with 1 mM NaN3 led at 40 min to elevation of baseline [Ca2+]i and disappearance of [Ca2+]i transient amplitude. Pretreatment with Cl-IB-MECA restricted elevation of baseline [Ca2+]i (*P < 0.05 vs. NaN3 group; n = 18 cells) and maintained muscle cell contractility (**P < 0.01 vs. NaN3 group; n = 18 cells).
Continuous monitoring of [Ca2+]i during hypoxia in cultures pretreated with 1 mM NaN3 revealed very fast (3–5 min) elevation of [Ca2+]i, decrease of amplitude in [Ca2+]i transients, and cessation of [Ca2+]i oscillations (Fig. 7D). We studied effects of adenosine agonists in cultures pretreated with 1 mM NaN3 during hypoxia when the basal level of [Ca2+]i increased considerably. If at that stage 100 nM Cl-IB-MECA was applied to the cells, [Ca2+]i returned to its normal basal level and beating activity was restored (Fig. 7E). The A1R agonist CCPA in this case was ineffective (Fig. 7F).
In cells pretreated with Cl-IB-MECA 15 min before the addition of sodium azide together with application of hypoxia, contractile activity and [Ca2+]i oscillations, with gradual decrease in oscillation amplitude, were observed during 40–60 min (Fig. 7G). A protective effect in this experiment was also achieved when A1Rs were activated with 100 nM CCPA but only during 15–20 min of hypoxia (Fig. 7H). In a total of six experiments, exposure to hypoxia with 1 mM NaN3 led at 40 min to elevation of baseline [Ca2+]i (to 1.40 ± 0.22 vs. 0.62 ± 0.21 in control cells) and disappearance of [Ca2+]i transient amplitude (0.06 ± 0.02 vs. 1.04 ± 0.14 in control cells). Pretreatment with the A3R agonist restricted elevation of baseline [Ca2+]i (0.90 ± 0.20 vs. 0.62 ± 0.24) and maintained muscle cell contractility (Fig. 7I).
DISCUSSION
A crucial mechanism for living cells is mitochondrial oxidative phosphorylation coupled to an electrochemical gradient of H+ (or OH−) across the inner membrane. Mitochondria support the energy-dependent regulation of several cell functions, e.g., intermediary metabolism and cardiomyocyte contraction. Animal cells derive >90% of their energy from oxidative phosphorylation associated with the inner mitochondrial membrane (26). Thus hypoxia, leading to deprivation of the main electron acceptor, causes perturbation of mitochondrial membrane potentials and decreases the coupling efficiency between oxidation and phosphorylation. This promotes large bioenergetic deficits that lead to the loss of several functions that are vital to the survival of the cell and the organism. The role of adenosine in mediating preconditioning is well recognized (27, 28). In rat and rabbit hearts, protection induced with both A1R and A3R agonists is similar to that obtained with adenosine pretreatment (17). Protection of the mitochondrial respiratory chain and its impact on mitochondrial bioenergetics after AR activation may be an important factor associated with increased resistance to hypoxia. As shown in this study, activation of both subtypes of ARs promotes preservation of adequate amounts of ATP and maintenance of mitochondrial metabolism on a level sufficient for cell survival (see Fig. 3).
A possible explanation for the ischemic protection associated with the opening of myocyte mitoKATP channels is that decreasing Δψ promotes the binding of the endogenous ATPase inhibitor IF1 and hence, the conservation of ATP during ischemia (35). In intact cells, administration of mitoKATP openers or endogenous signaling may lead to moderate K+ influx into the mitochondrial matrix. In low-work state cardiomyocytes (high Δψ), influx of K+ would cause matrix swelling, matrix alkalization, and increased production of reactive oxygen species (ROS; Ref. 9) during the transition to active mitochondria. In the high-work state, or during ischemia or hypoxia, K+ influx through mitoKATP channels will compensate for the decrease in K+ diffusion at the lower Δψ, so that matrix and intermembrane space volumes in mitochondria are maintained (9). The proton pump establishes Δψ, and the terminal sequence of respiratory pump enzymes is critical for ATP production in a state of shortage of the final electron acceptors. However, as shown in this study, activation of the A1Rs or A3Rs or application of diazoxide does not cause essential dissipation of transmembrane Δψ. Most of the evidence for the involvement of mitoKATP channels is pharmacological, based on the selectivity of the openers or blockers for mitoKATP channels and their similarities to effects of AR activation. Our finding with diazoxide agrees with other observations (21, 22) showing that diazoxide protects cardiac myocytes during metabolic inhibition without causing mitochondrial depolarization. Moreover, Hanley et al. (11) have shown that diazoxide (100 µM) or the nonselective KATP channel opener pinacidil (100 µM) did not change Δψ in isolated ventricular myocytes (11). They found that diazoxide dose dependently decreased succinate oxidation without affecting NADH oxidation, whereas pinacidil did not inhibit succinate oxidation but selectively inhibited NADH oxidation. Some authors (5, 11, 23, 32) have suggested that partial inhibition of electron transport may explain pharmacological preconditioning and thereby provide an alternative explanation for the preconditioning process (metabolic concept) without assuming the existence of mitoKATP channels. Downey et al. (4, 31) have found that most Gi-coupled receptors trigger protection through the mitoKATP-ROS pathway except for the ARs, which use some other as-yet-unidentified pathway and bypass the mitoKATP-ROS path.
The activity of KATP channels is tightly regulated by the metabolic state of the cell. The agents that interfere with ATP production via inhibition of energy metabolism are commonly used to activate KATP channels (12). Indeed, metabolic inhibition by sodium azide or cyanide has been reported to activate KATP channels in many cells including cardiac myocytes (16) and skeletal muscle (1). It is well known that cyanide or sodium azide inhibits oxidative phosphorylation via inhibition of cytochrome c oxidase, which is the final enzyme in the mitochondrial electron transport chain, and thereby results in a rapid depletion of ATP and leads to activation of KATP channels (12); however, sodium azide lacks the unfavorable characteristics associated with cyanide (3). It was shown (3) that sodium azide-treated myocytes (1 mM for 12–18 h) remain fully viable after removal of sodium azide from culture medium. Thus, under our conditions, the KATP channels were already opened. Therefore, diazoxide or AR activation could not act through modulating this channel activity. The efficiency of A3Rs while in a state of respiratory chain damage points to different or additional pathways of this receptor signaling. Recently, a similar effect was achieved in cardiomyocyte cultures treated with doxorubicin. Activation of the A3 subtype but not the A1 subtype of ARs attenuated doxorubicin-induced cardiotoxicity (40, 41). It is plausible that the cardioprotective effects of A3R activation may also be mediated via activation of KATP channels if the A3Rs are similar to the A1Rs in signal transduction downstream of protein kinase C (27, 28). However, the affinity of A3Rs for adenosine is roughly two orders of magnitude lower than for A1Rs (48).
Another possibility is that dissipation of Δψ decreases the driving force for Ca2+ influx through the Ca2+ uniporter (24). Prevention of Ca2+ accumulation in mitochondria may be a very important mechanism in the protection of mitochondrial structure and function and may be achieved not only through a decrease in mitochondrial energetics. It was shown in several publications that inhibition of Ca2+ influx into the cells by Ca2+ antagonists is beneficial for protecting the heart against mitochondrial disorders. Chen et al. (3) reported that in cultured neonatal rat cardiac myocytes, the Ca2+ antagonist nifedipine inhibited NaN3-induced cardiac cell death. Protective effects against cellular and tissue damages induced by this drug were obtained with diltiazem and verapamil (30, 37). Recently, Inomata and Tanaka (13) have shown that Ca2+ antagonists of all groups may protect against NaN3-induced cardiac cell death. We (42) have shown that A3R activation (and not A1R or A2AR activation) leads to an increase in cytosolic Ca2+ and its further extrusion. It was shown that extrusion of the elevated cytosolic Ca2+ was achieved via activation of sarcoplasmic reticulum (SR) Ca2+ reuptake and the sarcolemmal Na+/Ca2+ exchanger. The increase in SR Ca2+ uptake and Na+/Ca2+ exchanger Ca2+ efflux were sufficient not only for compensation of Ca2+ release from SR after A3R activation but also for effective prevention of extensive increase in intracellular Ca2+ and may provide a mechanism against cellular Ca2+ overload. It was shown that Ca2+ unloading of cultured cardiomyocytes after A3R activation is mainly achieved by Ca2+ uptake into the SR Ca2+ pool. We have shown that Ca2+/calmodulin-dependent protein kinase II-dependent phosphorylation was the only mechanism for sarco-(endo)plasmic reticulum Ca2+-ATPase 2a reactivation induced by A3R signaling (42). In this study, we added Cl-IB-MECA or CCPA to cells with elevated [Ca2+]i after hypoxia and treatment with NaN3. Cl-IB-MECA immediately decreased the [Ca2+]i toward diastolic control levels, whereas the A1R agonist was ineffective. This selective activation of A3Rs may be very important for prevention of irreversible damage in cardiomyocytes.
In cardiac myocytes, intracellular Ca2+ overload leads to activation of the proteolytic cleavage of some key cytoskeletal proteins and cell death (10). We showed that A3R signals to increase Ca2+ extrusion mechanisms, and this property allows prevention of the disorders in desmin cytoskeleton and maintenance of the contractile functions of cardiomyocytes after prolonged incubation in high extracellular Ca2+ concentration (42). Activation of both the A1 and A3 subtypes of the ARs can mimic the preventive effects of ischemic preconditioning, whereas the specific protective functions mediated by each receptor remain to be ascertained. It seems to be very important that protective effects of ARs may be achieved not only in intact cells but in cells where the terminal link of the mitochondrial respiratory chain is injured. For a long time, this state of cellular pathophysiology was considered to be “irreversible damage;” therefore, our findings concerning A3R signaling provide new insights into cellular adaptive properties. These properties of A3R signaling may be favorable in protecting heart muscle cells in many diseases accompanied by endotoxemia (shock, hemorrhage, ischemia, coronary artery bypass surgery, and others), which promotes mitochondrial disorders. These results support our earlier observations that A3R activation protects cardiomyocytes treated with doxorubicin via inhibition of Ca2+ overload (40), and prevents cardiomyocyte death during incubation in high extracellular Ca2+ concentrations (42).
In a recent review, Kloner and Rezkalla (19) ask, “Cardiac protection during acute myocardial infarction: Where do we stand in 2004?” The authors point out that adenosine and AR agonists belong to those classes of pharmacological agents that show promise as adjunctive therapies. Our data establish that adenosine can mediate myocardial protection by acting on A1Rs and A3Rs. Activation of both receptors leads to beneficial effects on high-energy phosphate production and on preservation of mitochondrial integrity. However, the cascade of events involved in cardioprotection may also be distinct for A1R and A3R signaling, and this seems especially important for the development of effective pharmacological agents against ischemia.
ACKNOWLEDGMENTS
The authors are indebted to Sharon Victor for helping to prepare this manuscript and to Ahuva Isaac for valuable technical assistance.
GRANTS
This research was partially supported by the Horowitz Foundation of Bar-Ilan University and the Israel Ministry of Health.
REFERENCES
- 1.Allard B, Lazdunski M, Rougier O. Activation of ATP-dependent K+ channels by metabolic poisoning in adult mouse skeletal muscle: role of intracellular Mg2+ and pH. J Physiol. 1995;485:283–296. doi: 10.1113/jphysiol.1995.sp020730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auchampach JA, Bolli R. Adenosine receptor subtypes in the heart: therapeutic opportunities and challenges. Am J Physiol Heart Circ Physiol. 1999;276:H1113–H1116. doi: 10.1152/ajpheart.1999.276.3.H1113. [DOI] [PubMed] [Google Scholar]
- 3.Chen SJ, Bradley ME, Lee TC. Chemical hypoxia triggers apoptosis of cultured neonatal rat cardiac myocytes: modulation by calcium-regulated proteases and protein kinases. Mol Cell Biochem. 1998;178:141–149. doi: 10.1023/a:1006893528428. [DOI] [PubMed] [Google Scholar]
- 4.Downey JM. ISHR Satellite: Cellular Injury in Ischaemia. Kruger National Park, South Africa: Berg en Dal; 2004. Aug 13–16, The cellular mechanisms of ischemic and pharmacological preconditioning (Abstract 13) [Google Scholar]
- 5.Dzeja PP, Bast P, Ozcan C, Valverde A, Holmuhamedov EL, Van Wylen DG, Terzic A. Targeting nucleotide-requiring enzymes: implications for diazoxide-induced cardioprotection. Am J Physiol Heart Circ Physiol. 2003;284:H1048–H1056. doi: 10.1152/ajpheart.00847.2002. [DOI] [PubMed] [Google Scholar]
- 6.El-Ani D, Jacobson KA, Shainberg A. Characterization of adenosine receptors in intact cultured heart cells. Biochem Pharmacol. 1994;48:727–735. doi: 10.1016/0006-2952(94)90050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabritz L, Kirchhof P, Fortmuller L, Auchampach JA, Baba HA, Breithardt G, Neumann J, Boknik P, Schmitz W. Gene dose-dependent atrial arrhythmias, heart block, and brady-cardiomyopathy in mice overexpressing A3 adenosine receptors. Cardiovasc Res. 2004;62:500–508. doi: 10.1016/j.cardiores.2004.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garlid KD. Opening mitochondrial KATP in the heart–what happens, and what does not happen. Basic Res Cardiol. 2000;95:275–279. doi: 10.1007/s003950070046. [DOI] [PubMed] [Google Scholar]
- 9.Garlid KD, Paucek P. The mitochondrial potassium cycle. IUBMB Life. 2001;52:153–158. doi: 10.1080/15216540152845948. [DOI] [PubMed] [Google Scholar]
- 10.Gorza L, Menabo R, Vitadello M, Bergamini CM, Di Lisa F. Cardiomyocyte troponin T immunoreactivity is modified by cross-linking resulting from intracellular calcium overload. Circulation. 1996;93:1896–1904. doi: 10.1161/01.cir.93.10.1896. [DOI] [PubMed] [Google Scholar]
- 11.Hanley PJ, Mickel M, Loffler M, Brandt U, Daut J. KATP channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol. 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvey J, Hardy SC, Ashford ML. Dual actions of the metabolic inhibitor, sodium azide on KATP channel currents in the rat CRI-G1 insulinoma cell line. Br J Pharmacol. 1999;126:51–60. doi: 10.1038/sj.bjp.0702267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inomata K, Tanaka H. Protective effect of benidipine against sodium azide-induced cell death in cultured neonatal rat cardiac myocytes. J Pharmacol Sci. 2003;93:163–170. doi: 10.1254/jphs.93.163. [DOI] [PubMed] [Google Scholar]
- 14.Ishida H, Higashijima N, Hirota Y, Genka C, Nakazawa H, Nakaya H, Sato T. Nicorandil attenuates the mitochondrial Ca2+ overload with accompanying depolarization of the mitochondrial membrane in the heart. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:192–197. doi: 10.1007/s00210-003-0851-z. [DOI] [PubMed] [Google Scholar]
- 15.Ishida H, Hirota Y, Genka C, Nakazawa H, Nakaya H, Sato T. Opening of mitochondrial KATP channels attenuates the ouabain-induced calcium overload in mitochondria. Circ Res. 2001;89:856–858. doi: 10.1161/hh2201.100341. [DOI] [PubMed] [Google Scholar]
- 16.Kakei M, Noma A. Adenosine-5′-triphosphate-sensitive single potassium channel in the atrioventricular node cell of the rabbit heart. J Physiol. 1984;352:265–284. doi: 10.1113/jphysiol.1984.sp015290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilpatrick EL, Narayan P, Mentzer RM, Jr, Lasley RD. Adenosine A3 agonist cardioprotection in isolated rat and rabbit hearts is blocked by the A1 antagonist DPCPX. Am J Physiol Heart Circ Physiol. 2001;281:H847–H853. doi: 10.1152/ajpheart.2001.281.2.H847. [DOI] [PubMed] [Google Scholar]
- 18.Kirchhof P, Fabritz L, Fortmuller L, Matherne GP, Lankford A, Baba HA, Schmitz W, Breithardt G, Neumann J, Boknik P. Altered sinus nodal and atrioventricular nodal function in freely moving mice overexpressing the A1 adenosine receptor. Am J Physiol Heart Circ Physiol. 2003;285:H143–H153. doi: 10.1152/ajpheart.01036.2002. [DOI] [PubMed] [Google Scholar]
- 19.Kloner RA, Rezkalla SH. Cardiac protection during acute myocardial infarction: where do we stand in 2004? J Am Coll Cardiol. 2004;44:276–286. doi: 10.1016/j.jacc.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 20.Kolosova NG, Kolpakov AR. Measurement of mitochondrial trans-membrane electric potential using the fluorescent probe DSM. Biofizika. 1991;36:802–804. [PubMed] [Google Scholar]
- 21.Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD. Bioenergetic consequences of opening the ATP-sensitive K+ channel of heart mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H649–H657. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence CL, Billups B, Rodrigo GC, Standen NB. The KATP channel opener diazoxide protects cardiac myocytes during metabolic inhibition without causing mitochondrial depolarization or flavoprotein oxidation. Br J Pharmacol. 2001;134:535–542. doi: 10.1038/sj.bjp.0704289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim KH, Javadov SA, Das M, Clarke SJ, Suleiman MS, Halestrap AP. The effects of ischaemic preconditioning, diazoxide and 5-hydroxydecanoate on rat heart mitochondrial volume and respiration. J Physiol. 2002;545:961–974. doi: 10.1113/jphysiol.2002.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Sato T, O’Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- 25.Mangoni ME, Barrere-Lemaire S. Adenosine receptors, heart rate, and cardioprotection. Cardiovasc Res. 2004;62:447–449. doi: 10.1016/j.cardiores.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell P. Coupling of phosphorylation to electron and hydrogen transport by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 27.Mubagwa K, Flameng W. Adenosine, adenosine receptors and myocardial protection: an updated overview. Cardiovasc Res. 2001;52:25–39. doi: 10.1016/s0008-6363(01)00358-3. [DOI] [PubMed] [Google Scholar]
- 28.Mubagwa K, Mullane K, Flameng W. Role of adenosine in the heart circulation. Cardiovasc Res. 1996;32:797–813. [PubMed] [Google Scholar]
- 29.Nakai Y, Horimoto H, Mieno S, Sasaki S. Mitochondrial ATP-sensitive potassium channel plays a dominant role in ischemic preconditioning of rabbit heart. Eur Surg Res. 2001;33:57–63. doi: 10.1159/000049695. [DOI] [PubMed] [Google Scholar]
- 30.Nishida M, Urushidani T, Sakamoto K, Nagao T. L-cis diltiazem attenuates intracellular Ca2+ overload by metabolic inhibition in guinea pig myocytes. Eur J Pharmacol. 1999;385:225–230. doi: 10.1016/s0014-2999(99)00709-8. [DOI] [PubMed] [Google Scholar]
- 31.Oldenburg O, Cohen MV, Yellon DM, Downey JM. Mitochondrial KATP channels: role in cardioprotection. Cardiovasc Res. 2002;55:429–437. doi: 10.1016/s0008-6363(02)00439-x. [DOI] [PubMed] [Google Scholar]
- 32.Ovide-Bordeaux S, Ventura-Clapier R, Veksler V. Do modulators of the mitochondrial KATP channel change the function of mitochondria in situ? J Biol Chem. 2000;275:37291–37295. doi: 10.1074/jbc.M005772200. [DOI] [PubMed] [Google Scholar]
- 33.Peart J, Flood A, Linden J, Matherne GP, Headrick JP. Adenosine-mediated cardioprotection in ischemic-reperfused mouse heart. J Cardiovasc Pharmacol. 2002;39:117–129. doi: 10.1097/00005344-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 35.Rouslin W. Regulation of the mitochondrial ATPase in situ in cardiac muscle: role of the inhibitor subunit. J Bioenerg Biomembr. 1991;23:873–888. doi: 10.1007/BF00786006. [DOI] [PubMed] [Google Scholar]
- 36.Safran N, Shneyvays V, Balas N, Jacobson KA, Shainberg A. Cardioprotective effects of adenosine A1 and A3 receptor activation during hypoxia in isolated rat cardiac myocytes. Mol Cell Biochem. 2001;217:143–152. doi: 10.1023/a:1007209321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santostasi G, Kutty RK, Bartorelli AL, Yasumoto T, Krishna G. Maitotoxin-induced myocardial cell injury: calcium accumulation followed by ATP depletion precedes cell death. Toxicol Appl Pharmacol. 1990;102:164–173. doi: 10.1016/0041-008x(90)90093-a. [DOI] [PubMed] [Google Scholar]
- 38.Sato T, Sasaki N, O’Rourke B, Marban E. Adenosine primes the opening of mitochondrial ATP-sensitive potassium channels: a key step in ischemic preconditioning? Circulation. 2000;102:800–805. doi: 10.1161/01.cir.102.7.800. [DOI] [PubMed] [Google Scholar]
- 39.Scaduto RC, Jr, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999;76:469–477. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shneyvays V, Mamedova L, Zinman T, Jacobson K, Shainberg A. Activation of A3 adenosine receptor protects against doxorubicin-induced cardiotoxicity. J Mol Cell Cardiol. 2001;33:1249–1261. doi: 10.1006/jmcc.2001.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shneyvays V, Mamedova LK, Korkus A, Shainberg A. Cardiomyocyte resistance to doxorubicin mediated by A3 adenosine receptor. J Mol Cell Cardiol. 2002;34:493–507. doi: 10.1006/jmcc.2002.1532. [DOI] [PubMed] [Google Scholar]
- 42.Shneyvays V, Zinman T, Shainberg A. Analysis of calcium responses mediated by the A3 adenosine receptor in cultured newborn rat cardiac myocytes. Cell Calcium. 2004;36:387–396. doi: 10.1016/j.ceca.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Stambaugh K, Elliott GT, Jacobson KA, Liang BT. Additive effects of late preconditioning produced by monophosphoryl lipid A and the early preconditioning mediated by adenosine receptors and KATP channel. Circulation. 1999;99:3300–3307. doi: 10.1161/01.cir.99.25.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stern MD, Silverman HS, Houser SR, Josephson RA, Capogrossi MC, Nichols CG, Lederer WJ, Lakatta EG. Anoxic contractile failure in rat heart myocytes is caused by failure of intracellular calcium release due to alteration of the action potential. Proc Natl Acad Sci USA. 1988;85:6954–6958. doi: 10.1073/pnas.85.18.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strickler J, Jacobson KA, Liang BT. Direct preconditioning of cultured chick ventricular myocytes. Novel functions of cardiac adenosine A2a and A3 receptors. J Clin Invest. 1996;98:1773–1779. doi: 10.1172/JCI118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thourani VH, Nakamura M, Ronson RS, Jordan JE, Zhao ZQ, Levy JH, Szlam F, Guyton RA, Vinten-Johansen J. Adenosine A3-receptor stimulation attenuates postischemic dysfunction through KATP channels. Am J Physiol Heart Circ Physiol. 1999;277:H228–H235. doi: 10.1152/ajpheart.1999.277.1.H228. [DOI] [PubMed] [Google Scholar]
- 47.Tracey WR, Magee W, Masamune H, Kennedy SP, Knight DR, Buchholz RA, Hill RJ. Selective adenosine A3 receptor stimulation reduces ischemic myocardial injury in the rabbit heart. Cardiovasc Res. 1997;33:410–415. doi: 10.1016/s0008-6363(96)00240-4. [DOI] [PubMed] [Google Scholar]
- 48.von Lubitz DK, Ye W, McClellan J, Lin RC. Stimulation of adenosine A3 receptors in cerebral ischemia. Neuronal death, recovery, or both? Ann NY Acad Sci. 1999;890:93–106. doi: 10.1111/j.1749-6632.1999.tb07984.x. [DOI] [PubMed] [Google Scholar]
- 49.Zucchi R, Yu G, Ghelardoni S, Ronca F, Ronca-Testoni S. A3 adenosine receptor stimulation modulates sarcoplasmic reticulum Ca2+ release in rat heart. Cardiovasc Res. 2001;50:56–64. doi: 10.1016/s0008-6363(00)00318-7. [DOI] [PubMed] [Google Scholar]