Abstract
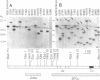
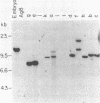
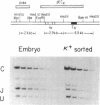
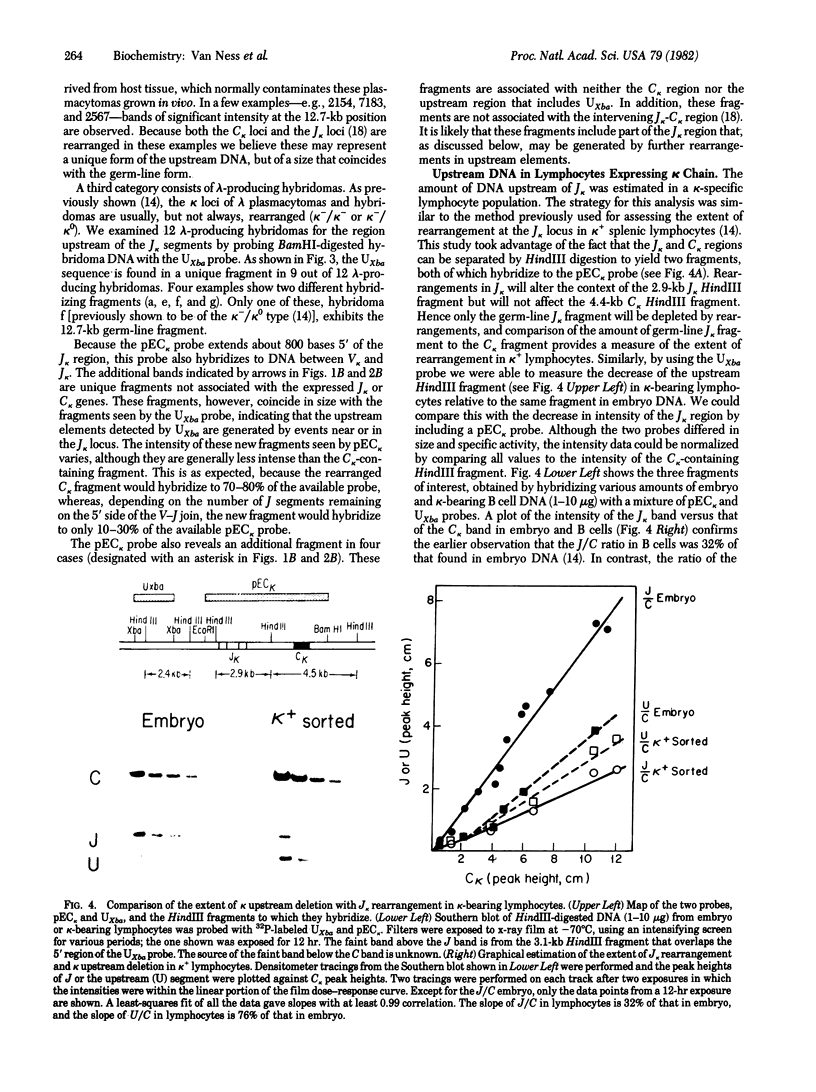
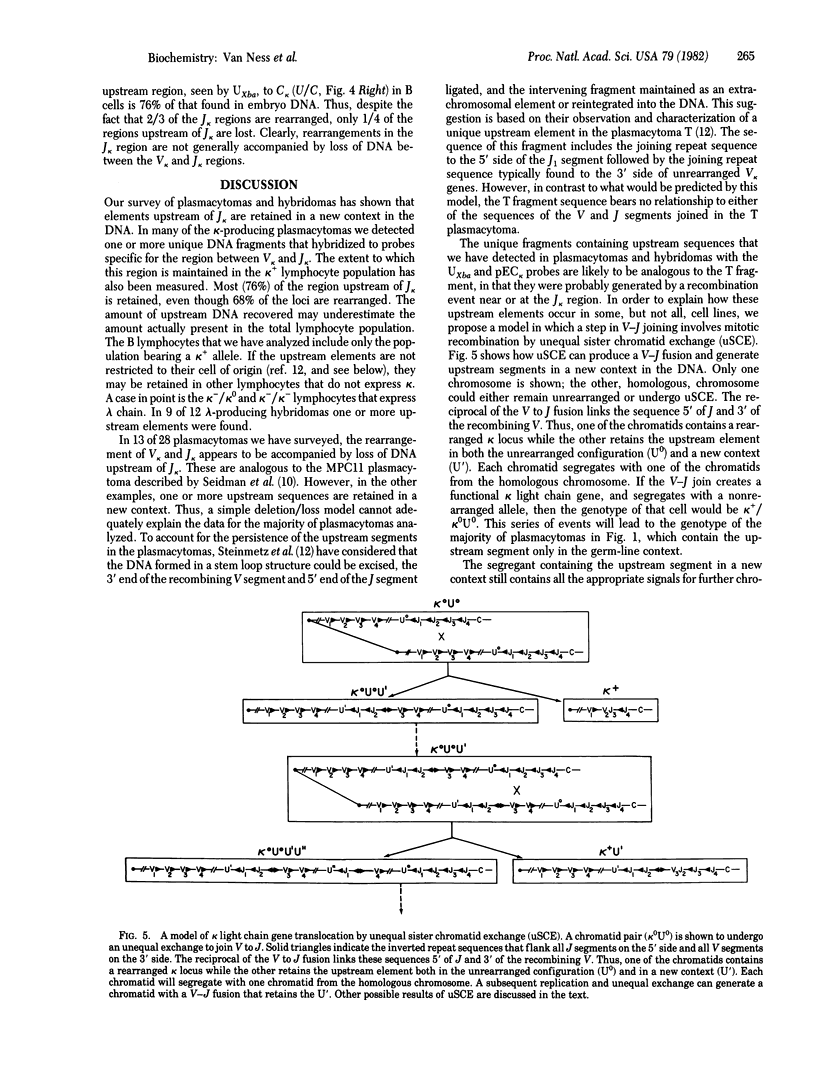
A systematic analysis of the fate of the DNA between kappa chain variable (V kappa) and joining (J kappa) genes in cells that have rearranged kappa loci was carried out. The DNA from a variety of kappa-producing plasmacytomas, lambda-producing hybridomas, and kappa-expressing lymphocytes was digested, fractionated by size, and analyzed with two probes containing sequences 5' of J kappa. In 13 of 28 plasmacytomas examined the rearrangement of V kappa and J kappa appears to be accompanied by loss of DNA upstream of J kappa. However, in the rest of the plasmacytomas one or more upstream sequences are retained in a new context. In 9 of 12 lambda-producing hybridomas (which frequently rearrange both kappa loci) one or more upstream segments were detected. These unique fragments were probably generated by a recombination event near or at the J kappa region. The extent to which the region between V and J is maintained in kappa-expression lymphocytes was also measured. Most (76%) of the region upstream of J kappa is retained in the population, even though 68% of the kappa loci are rearranged. In order to explain how these upstream elements occur in some, but not all, cell lines, and the significant occurrence in the lymphocyte population, we propose a model in which a step in V--J joining involves mitotic recombination by unequal sister chromatid exchange.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coleclough C., Perry R. P., Karjalainen K., Weigert M. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981 Apr 2;290(5805):372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Hozumi N., Tonegawa S. Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3628–3632. doi: 10.1073/pnas.73.10.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt T. J., Mandy W. J., Todd C. W. Association of allotypic specificities of group a with allotypic specificities A11 and A12 in rabbit immunoglobulin. Biochemistry. 1970 Apr 28;9(9):2028–2032. doi: 10.1021/bi00811a026. [DOI] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Miller H., Leder P. Variation in the crossover point of kappa immunoglobulin gene V-J recombination: evidence from a cryptic gene. Cell. 1980 Oct;21(3):793–799. doi: 10.1016/0092-8674(80)90442-0. [DOI] [PubMed] [Google Scholar]
- Obata M., Kataoka T., Nakai S., Yamagishi H., Takahashi N., Yamawaki-Kataoka Y., Nikaido T., Shimizu A., Honjo T. Structure of a rearranged gamma 1 chain gene and its implication to immunoglobulin class-switch mechanism. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2437–2441. doi: 10.1073/pnas.78.4.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Coleclough C., Weigert M. Reorganization and expression of immunoglobulin genes: status of allelic elements. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):925–933. doi: 10.1101/sqb.1981.045.01.109. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Coleclough C., Seidman J. G., Leder P., Tonegawa S., Matthyssens G., Weigert M. Transcription of mouse kappa chain genes: implications for allelic exclusion. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1937–1941. doi: 10.1073/pnas.77.4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Schibler U., Huebner K., Croce C. M. Selective suppression of the transcription of ribosomal genes in mouse-human hybrid cells. J Cell Physiol. 1979 Mar;98(3):553–559. doi: 10.1002/jcp.1040980313. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Kurosawa Y., Weigert M., Tonegawa S. Identification and nucleotide sequence of a diversity DNA segment (D) of immunoglobulin heavy-chain genes. Nature. 1981 Apr 16;290(5807):562–565. doi: 10.1038/290562a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Nau M. M., Norman B., Kwan S. P., Scharff M., Leder P. Immunoglobulin V/J recombination is accompanied by deletion of joining site and variable region segments. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6022–6026. doi: 10.1073/pnas.77.10.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Altenburger W., Zachau H. G. A rearranged DNA sequence possibly related to the translocation of immunoglobulin gene segments. Nucleic Acids Res. 1980 Apr 25;8(8):1709–1720. doi: 10.1093/nar/8.8.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S., Hozumi N., Matthyssens G., Schuller R. Somatic changes in the content and context of immunoglobulin genes. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):877–889. doi: 10.1101/sqb.1977.041.01.097. [DOI] [PubMed] [Google Scholar]
- Tosi S. L., Dubiski S., Mage R. G. Distribution of allotypic specificities A1, A2, A14, and A15 among immunoglobulin G molecules. J Immunol. 1970 Mar;104(3):641–647. [PubMed] [Google Scholar]
- Weigert M., Gatmaitan L., Loh E., Schilling J., Hood L. Rearrangement of genetic information may produce immunoglobulin diversity. Nature. 1978 Dec 21;276(5690):785–790. doi: 10.1038/276785a0. [DOI] [PubMed] [Google Scholar]