Abstract
Nineteen medium-chain-length (mcl) poly(3-hydroxyalkanoate) (PHA)-degrading microorganisms were isolated from natural sources. From them, seven Gram-positive and three Gram-negative bacteria were identified. The ability of these microorganisms to hydrolyze other biodegradable plastics, such as short-chain-length (scl) PHA, poly(ε-caprolactone) (PCL), poly(ethylene succinate) (PES), and poly(l-lactide) (PLA), has been studied. On the basis of the great ability to degrade different polyesters, Streptomyces roseolus SL3 was selected, and its extracellular depolymerase was biochemically characterized. The enzyme consisted of one polypeptide chain of 28 kDa with a pI value of 5.2. Its maximum activity was observed at pH 9.5 with chromogenic substrates. The purified enzyme hydrolyzed mcl PHA and PCL but not scl PHA, PES, and PLA. Moreover, the mcl PHA depolymerase can hydrolyze various substrates for esterases, such as tributyrin and p-nitrophenyl (pNP)-alkanoates, with its maximum activity being measured with pNP-octanoate. Interestingly, when poly(3-hydroxyoctanoate-co-3-hydroxyhexanoate [11%]) was used as the substrate, the main hydrolysis product was the monomer (R)-3-hydroxyoctanoate. In addition, the genes of several Actinobacteria strains, including S. roseolus SL3, were identified on the basis of the peptide de novo sequencing of the Streptomyces venezuelae SO1 mcl PHA depolymerase by tandem mass spectrometry. These enzymes did not show significant similarity to mcl PHA depolymerases characterized previously. Our results suggest that these distinct enzymes might represent a new subgroup of mcl PHA depolymerases.
INTRODUCTION
Biodegradability of polymers has drawn much attention as a solution to problems concerning the global environment and biomedical technologies. Several aliphatic polyesters showing properties comparable to those of conventional plastics have been developed and used as biodegradable plastics, such as poly(3-hydroxyalkanoate) (PHA), poly(ε-caprolactone) (PCL), poly(l-lactide) (PLA), and poly(ethylene succinate) (PES). They can be synthesized from petrochemicals (PES and PCL) or from renewable resources (PLA and PHA) (58). Among these biodegradable plastics, PHA is the only one that is completely synthesized by microorganisms and accumulates intracellularly during unbalanced growth conditions (30). Additionally, PHA is suitable for a broad range of applications in medicine, the pharmaceutical industry, and industry due to its biocompatibility and biodegradability (2). Moreover, all of the PHA monomers are enantiomerically pure and in the R configuration (3, 40, 44). More than 150 hydroxyalkanoic acids (HAs) have been identified as constituents of these microbial polyesters (6, 57). Interestingly, these monomers are valuable intermediates that can be used as starting materials for the synthesis of antibiotics, vitamins, flavors, and pheromones (1). Since chiral (R)-HAs are normally difficult to synthesize by chemical means (2), the study of enzymatic PHA hydrolysis has attracted much attention.
The ability to degrade extracellular PHA in the environment depends on the release of extracellular PHA depolymerases (17) that could be specific for either short-chain-length (scl) PHA (3 to 5 carbon atoms) (EC 3.1.1.75) or medium-chain-length (mcl) PHA (6 to 14 carbon atoms) (EC 3.1.1.76) (17). Depending on the depolymerase activity, the end products are only monomers, both monomers and dimers, or a mixture of oligomers as a result of the enzymatic PHA degradation (17).
Extracellular PHA depolymerase-producing microorganisms are widely distributed and have been isolated from various environments (32, 51). Currently, very few mcl PHA depolymerases have been characterized in comparison to the number of scl PHA depolymerases studied (26). To date, most of the mcl PHA depolymerases reported belong to Gram-negative bacteria, predominantly Pseudomonas species (22, 31). The poly(3-hydroxyoctanoate) depolymerase from Pseudomonas fluorescens GK13 (PhaZPflGK13) was the first mcl PHA depolymerase studied in detail at the molecular level (49–51). Additionally, several biotechnological applications of this enzyme have been reported, including the construction of fusion proteins with affinity to mcl PHAs (13), the production of (R)-3HAs (8), and the synthesis of polyesters (48). Thus, this enzyme is considered the prototype enzyme of extracellular mcl PHA depolymerases. In general, these enzymes consist of a signal peptide, an N-terminal substrate binding domain, and a C-terminal catalytic domain (15, 22). In a recent study, the identification of a significantly different mcl PHA depolymerase gene from the thermophilic bacterium Thermus thermophilus HB8 has been reported (36). Recently, the isolation and identification of Streptomyces venezuelae SO1 as a novel mcl PHA depolymerase (PhaZSveSO1) producer have been reported by our group (47). However, the molecular characteristics of the genes encoding mcl PHA depolymerases from Streptomyces origins have not yet been cleared. In this paper, we report the isolation of several novel extracellular mcl PHA-degrading microorganisms, predominantly Streptomyces species. Two of the isolates, SL3 and SO2, have been identified to be Streptomyces roseolus and Streptomyces omiyaensis, respectively. Furthermore, the mcl PHA depolymerase from S. roseolus SL3 (PhaZSroSL3) has been biochemically characterized. In addition, we provide for the first time information about the primary structure of the mcl PHA depolymerases from Streptomyces bacteria.
MATERIALS AND METHODS
Chemicals.
Poly(3-hydroxyoctanoate-co-3-hydroxyhexanoate [11%]) [P(3HO) or mcl PHA] was supplied by Biopolis, S.A. (Valencia, Spain), and CPI (Newcastle, United Kingdom). Accurel MP-1000 was purchased from Membrana GmbH (Obenburg, Germany). Poly(3-hydroxypropionic acid) [P(3HP)] was donated by CIBA (Manchester, United Kingdom). Chromatography media were obtained from GE Healthcare (Uppsala, Sweden). Molecular weight standards, p-nitrophenyl (pNP)-alkanoates, poly(3-hydroxybutyric acid) [P(3HB)], poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) {P(3HB-HV[12%])}, PCL, PES, and PLA were obtained from Sigma-Aldrich (St. Louis, MO). All other chemicals were supplied by Merck (Darmstadt, Germany).
Preparation of biopolymer suspensions.
Latex suspensions of PCL and P(3HO) were prepared as described by Schirmer and Jendrossek (50). In the case of PES and PLA, 4 volumes of water were poured into 1 volume of polymer suspension in methylene chloride with stirring. The suspensions were emulsified by an ultrasonic homogenizer, and the solvent was then evaporated. P(3HB), P(3HP), and P(3HB-HV[12%]) suspensions of similar concentration (10 mg/ml) were prepared by dispersing each polymer powder in water by ultrasonic treatment.
Isolation and identification of mcl PHA-degrading microorganisms.
Several mcl PHA-degrading bacterial strains were isolated in our laboratory from natural environmental samples (soil, sludge, and water) taken at different places on the Leioa campus of the University of the Basque Country, Vizcaya, Spain. Serial dilutions of the homogenized samples were spread on P(3HO)-mineral agar plates consisting of P(3HO) latex-covered petri plates with mineral medium such as M9 medium (45) and E medium (24). The plates were incubated for 2 to 3 days at 30°C. Those strains which showed clearing of the P(3HO) latex were selected and isolated.
The bacteria were identified by sequence analysis of the 16S rRNA gene.
For further identification, the cultural, morphological, and physiological characteristics of the SL3 and SO2 strains were obtained by following the methods given in the International Streptomyces Project (ISP) (54). Aerial spore mass color and substrate mycelium color were recorded using Inter-Society Color Council, National Bureau of Standards (NBS), color name charts (18), after incubation for 20 days at 30°C in oatmeal agar medium (ISP medium 3). Morphological observations of spores and mycelia were made by light microscopy (Nikon Eclipse 50i A light microscope) and scanning electron microscopy (model JEOL 6100 scanning electron microscope). The carbon utilization test was performed in ISP9 medium with the addition of d-glucose (positive control), l-arabinose, sucrose, d-xylose, myo-inositol, d-mannitol, d-fructose, rhamnose, or raffinose and in the absence of a carbon source (negative control), as described by Shirling and Gottlieb (54).
Strains SL3 and SO2 were identified from ISP (55, 56). The identified mcl PHA-degrading strains have been deposited in the Spanish Type Culture Collection (CECT, Valencia, Spain; www.cect.org) as Streptomyces roseolus SL3 CECT 7919 and Streptomyces omiyaensis SO2 CECT 7923.
Microorganisms and growth conditions.
The following microorganisms were used in this study: S. venezuelae SO1 CECT 7920, S. omiyaensis SO2 CECT 7923, and S. roseolus SL3 CECT 7919. All other strains are listed in Table 1. Polymer-degrading bacteria were routinely grown in solid M9 mineral medium (45) containing 1.5% (wt/vol) agar with the carbon sources indicated in the text. For enzyme production, S. roseolus SL3 and S. venezuelae SO1 cells were grown at 30°C in 250-ml Erlenmeyer flasks containing 100 ml of mineral medium supplemented with a film (0.15 g) of P(3HO) as the sole carbon and energy source, as described by Santos et al. (47). The strains were maintained as frozen spore suspensions in 15% (vol/vol) glycerol at −20°C, as described by Kieser et al. (19).
Table 1.
Microbial strains isolated, their closest relative bacteria based on 16S rRNA analysis, and their polyester-degrading abilities
| Strain | Closest relative (GenBank accession no.) | Category | % similarity | Ability to degrade polyestera |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P(3HO) | P(3HP) | P(3HB) | P(HB-HV[12%]) | PCL | PLA | PES | ||||
| SL1 | Pseudomonas alcaligenes (Z76653) | Gammaproteobacteria | 99.6 | +++ | ND | − | − | + | − | − |
| SL2 | Streptomyces atratus (DQ026638) | Actinobacteria | 99.6 | + | + | + | + | − | − | − |
| SL3 | Streptomyces roseolus (AB184168) | Actinobacteria | 99.8 | + | ++ | +++ | +++ | + | − | − |
| SL6 | Stenotrophomonas maltophilia (HQ406762.1) | Gammaproteobacteria | 99.2 | + | ND | − | ND | − | − | − |
| SL11 | Streptomyces anulatus (AB184875) | Actinobacteria | 99.5 | + | + | + | + | ++ | − | − |
| SL15 | Streptomyces beijiangensis (AB249973) | Actinobacteria | 99.4 | +++ | ND | ++ | ++ | + | − | − |
| SO2 | Streptomyces omiyaensis (AB184411) | Actinobacteria | 99.5 | ++ | + | ++ | ++ | +++ | − | − |
| W1 | Pseudomonas beteli (DQ299947.1) | Gammaproteobacteria | 99.0 | + | ND | − | ND | − | − | − |
| W2 | Rhodococcus equi (X80614) | Actinobacteria | 99.6 | + | ND | − | − | − | − | − |
| W3 | Streptomyces pulveraceus (AB184808) | Actinobacteria | 99.8 | + | ND | + | + | + | − | − |
| GK13 | Pseudomonas fluorescens | Gammaproteobacteria | +++ | − | − | − | ND | − | − | |
The ability to degrade different polyesters was determined by clear-zone formation around the colony on the opaque plates after 2 to 3 days of growth at 30°C. Symbols and abbreviations: −, no clearing zone; +, small clearing zone; ++, medium clearing zone; +++, large clearing zone; P(3HP), poly(3-hydroxypropionate); P(3HB), poly(3-hydroxybutyrate); P(HB-HV[12%]), poly(3-hydroxybutyrate-co-3-hydroxyvalerate); PCL, poly-ε-caprolactone; PLA, poly(l-lactide); PES, poly(ethylene succinate); ND, not determined.
For the isolation of genomic DNA, the bacteria were grown for 3 days at 30°C in 250-ml Erlenmeyer flasks containing 100 ml of S-YEME medium (19) in an orbital incubator shaker at 250 rpm. Cultures were harvested at 4°C by centrifugation (10,000 × g for 20 min). The resulting pellet was used for DNA extraction. Genomic DNAs of Streptomyces strains were isolated as described by Kieser et al. (19).
Purification of mcl PHA depolymerases.
S. roseolus SL3 and S. venezuelae SO1 cells were grown in 2-liter Erlenmeyer flasks containing 800 ml of mineral medium (23) supplemented with a film (1.2 g) of P(3HO). Flasks were inoculated with 100 ml of a culture of mineral medium supplemented with glucose (0.4%, wt/vol) that had been grown for 72 h, and the cultures were grown for 3 days in an orbital incubator shaker at 250 rpm and 30°C. Cells were harvested by filtration, and the enzyme present in the supernatant was purified by adsorption onto porous polypropylene (Accurel MP-1000) as reported by Gangoiti et al. (8).
Enzyme assays.
Esterase activity was assayed using several pNP-alkanoates as the substrate (8). Blanks without enzyme were performed to determine spontaneous hydrolysis not due to enzymatic activity. One unit of esterase activity was the amount of enzyme that released 1 μmol of p-nitrophenol per min under standard conditions. The extinction coefficient (ε) for pNP at pH 9.5 was determined to be 16.635 mM−1 · cm−1.
Qualitative estimation of the hydrolytic activity of mcl PHA depolymerase from S. roseolus SL3 toward different polymers was performed by a drop test on indicator plates (14). Briefly, 5 ml of a 1% (wt/vol) polymer emulsion was mixed with 5 ml of 1% (wt/vol) agarose in 200 mM Tris-HCl buffer, pH 8.5, and poured on a glass plate. Samples (20 μl) were loaded in 5-mm-diameter holes made in the gel and incubated at 30°C for 24 h. Similarly, qualitative determination of esterase activity was performed on agarose plates using tributyrin as the substrate, as described by Gandolfi et al. (7). The diameters of the resulting clearing zones were semiquantitatively correlated with the enzyme activity.
Identification of hydrolysis products of P(3HO).
The hydrolysis products from the P(3HO) substrate catalyzed by PhaZSroSL3 were identified. For this purpose, reaction mixtures containing 250 μg of P(3HO) latex in 20 mM Tris-HCl buffer, pH 8.0, and 50 μg of the purified enzyme were incubated (in 2-ml tubes) at 30°C and in an orbital shaker at 160 rpm for various time intervals (3 h, 24 h, 48 h, and 72 h). The enzymatic reaction was stopped by incubating the tubes for 5 min at 100°C, and then the tubes were centrifuged at 4°C for 60 min at 14,000 × g. The degradation products were isolated from supernatants and derivatized using bromophenacyl bromide (BPB), as described by Gebauer and Jendrossek (11). The detection and quantification of the hydrolysis products were performed by high-pressure liquid chromatography (HPLC)-photo diode array (PDA), and the identity of the 3-hydroxyoctanoic acid (3-HO) oligomer peaks detected at 254 nm were determined by HPLC-mass spectrometry (MS) (8). The peak of 26.1 min corresponds to unreacted BPB.
Determination of the N-terminal protein sequences.
The pure PhaZSroSL3 and PhaZSveSO1 were electroblotted from an SDS-polyacrylamide gel to a polyvinylidene difluoride (PVDF) membrane (Biotrace; Pall Corporation). Edman degradation analysis was carried out in the Proteomics and Bioinformatics facility from UAB, a member of the ProteoRed network.
Identification of mcl PHA depolymerases genes.
In order to determine the mcl PHA depolymerase sequences from Streptomyces, PhaZSveSO1 was subjected to de novo peptide sequence analysis by mass spectrometry. For this purpose, a Coomassie blue-stained gel spot corresponding to the enzyme was excised, washed, reduced with dithiothreitol (DTT), and alkylated with iodoacetamide. The in-gel digest with trypsin was carried out at 37°C. The resultant peptides were analyzed by matrix-assisted laser desorption ionization (MALDI)–tandem time of flight (TOF/TOF) mass spectrometry (4700 proteomics analyzer; Applied Biosystems) in MS and MS/MS modes. To enhance the quality of the tandem MS/MS spectra for the de novo-sequencing, N-terminal chemical modification using 4-sulfophenyl isothiocyanate (SPITC) was carried out at 55°C for 30 min (10). The N-terminal derivatized peptides were desalted and concentrated using μZip-Tips C18 (Millipore), as described by the manufacturer. The sample was spotted onto the MALDI target plate prespotted with alpha-cyano-4-hydroxycinnamic acid matrix.
Peptide de novo sequencing was carried out manually using the program mMass (http://www.mmass.org/). De novo-derived peptide sequences were combined in one search query and analyzed by MS-BLAST (53). Searches were performed against nonredundant proteins with PAM30MS as the search matrix. The sequences obtained were subjected to multiple alignments employing CLUSTALW (28).
The phaZSroSL3, phaZSveSO1, and S. omiyaensis SO2 phaZ (phaZSomSO2) genes were partially amplified by PCR using chromosomal DNA as the template. The degenerated PCR primers were designed in accordance with the N-terminal protein sequences, considering the codon usage in Streptomyces (19), as well as on the basis of the sequence of the hypothetical protein SVEN_7345 from S. venezuelae ATCC 10712 (GenBank accession no. CCA60631.1). PCR amplifications were performed in a Px2 thermal cycler (Thermo Hybaid, United Kingdom) using the TDPfu program, adjusted for the high G+C content of Streptomyces genomes, and employing Pfu DNA polymerase (Promega) (9). The phaZSveSO1 and phaZSomSO2 genes were partially amplified using primers VN1 (5′-CGAGGTGGACGTCGACATCGAGG-3′) and A4R (5′-GCGCAGCCACGCCGTGGTCGG-3′), whereas in the case of the phaZSroSL3 gene, primers NSL3 (5′-GTSGGSACSGACTGGGACCG-3′) and A4R were used.
DNA fragments (∼600 bp) amplified in each PCR were purified from the agarose bands. DNA sequences were determined by the dideoxy chain termination method (46) with an automated sequencer, DNA analyzer 3730 (Applied Biosystems).
Enzyme analysis.
SDS-PAGE was performed as described by Laemmli (27). Two-dimensional electrophoresis was performed by isoelectric focusing using immobilized pH gradient strips (pH 3 to 10) (first dimension) and SDS-polyacrylamide gel electrophoresis (second dimension). The protein concentration was determined by the method of Peterson (39), using bovine serum albumin as the standard.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences from isolates obtained in this study were deposited in GenBank under the accession numbers JX305978 to JX305987. The partial sequences of phaZSroSL3, phaZSveSO1, and phaZSomSO2 have been deposited in GenBank under the accession numbers JX305988, JX305989, and JX305990, respectively.
RESULTS
Screening of mcl PHA-degrading bacteria and their ability to hydrolyze other aliphatic polyesters.
Nineteen bacteria able to grow on P(3HO) as the sole source of carbon and energy were isolated from samples of soil, sludge, and water [the first letter(s) in the isolate designations, SO, SL, and W, respectively]. All the isolates produced a clearing zone surrounding the colony within 2 to 3 days of incubation on opaque P(3HO) agar at 30°C. Ten different bacteria were identified from their 16S rRNA gene sequences (Table 1), including three Gram-negative and seven Gram-positive bacteria. The closest relative strain to each isolate has also been included in Table 1. Interestingly, six of these bacteria belonged to the genus Streptomyces.
The isolated bacteria were screened for polymer-degrading capacity using the clear-zone method. None of the Gram-negative bacteria were able to hydrolyze scl PHA. In contrast, all Streptomyces strains showed rapid growth and degradation of scl PHA, as well as PCL (Table 1). However, none of the isolated bacteria were able to hydrolyze PES and PLA.
Characterization of strains SO2 and SL3.
On the basis of their great ability to degrade different polyesters, the mcl PHA-degrading SL3 and SO2 strains, isolated from sludge and soil, respectively, were selected to study the degradation of P(3HO) in detail. SL3 and SO2 are Gram-positive, aerobic, and nonmotile filamentous bacteria with branching vegetative hyphae embedded in the substrate and aerial hyphae bearing spores. The spores of both bacteria show a smooth surface and occur in rectiflexible chains containing more than 10 spores per chain (see Fig. S1 in the supplemental material). Strain SL3 developed an aerial mycelium in the red color series and a yellow-brownish substrate mycelium. In contrast, the color of the aerial mycelium of strain SO2 on ISP3 was gray, while that of the substrate mycelium was yellow-brownish. These bacteria did not produce diffusible pigments in any of the media tested. SL3 and SO2 strains utilized d-glucose, d-xylose, and rhamnose but were unable to use myo-inositol, d-mannitol, sucrose, and raffinose. SL3 utilized l-arabinose and d-fructose, whereas only a trace of growth was observed in the case of SO2 in the presence of these sugars. On the basis of phylogenetic analyses of the sequence of the 16S rRNA gene and morphological and physiological characteristics, strains SL3 and SO2 were identified as Streptomyces roseolus and Streptomyces omiyaensis, respectively (see Materials and Methods for details). The results shown in Table 1 suggest that S. roseolus SL3 and S. omiyaensis SO2 may synthesize at least two different PHA depolymerases specific for scl or mcl PHAs, as it has been suggested for S. exfoliatus (25).
Biochemical properties of mcl PHA depolymerase from S. roseolus SL3.
The molecular mass of the purified enzyme from S. roseolus SL3, determined by SDS-PAGE analysis, was approximately 28 kDa (Fig. 1). Nondenaturing (ND)-PAGE analyses showed only one enzyme form with an estimated native molecular mass of 28 kDa, indicating that this native enzyme consists of a single polypeptide chain. Besides, the isoelectric point of PhaZSroSL3 was about 5.2. The effect of pH on PhaZSroSL3 activity was examined at pH values ranging from 6.0 to 12.0, using pNP-octanoate (pNPO) as the substrate. This enzyme exhibited its maximum activity at pH 9.5 and retained more than 60% of this activity over a pH range from 8.0 to 10.5. The N-terminal amino acid sequence of the mature PhaZSroSL3 was determined by Edman degradation to be AIPPVGTDWDRP (Fig. 1). This sequence showed at least 50% identity only to that of PhaZSspKJ-72 (23). However, it showed low identity to the sequences corresponding to Pseudomonas species (22), indicating that the mcl PHA depolymerases produced by Streptomyces strains may be encoded by a different type of gene.
Fig 1.
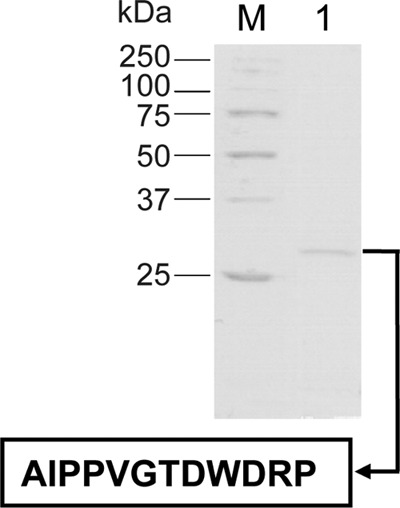
SDS-PAGE analysis of the purified mcl PHA depolymerase from S. roseolus SL3. Proteins were separated in a homogeneous 12% (wt/vol) acrylamide gel and revealed by Coomassie brilliant blue R-250. Lane M, molecular mass markers; lane 1, purified enzyme. The N-terminal amino acid sequence of the enzyme, in one-letter code, was determined by Edman degradation.
Substrate specificity of PhaZSroSL3 depolymerase.
PhaZSroSL3 hydrolyzes mcl PHA and PCL (see Fig. S2 in the supplemental material), forming large clearing zones after 24 h of incubation at 30°C. These results suggest that the depolymerase is able to hydrolyze ester bonds of β- and ω-polyhydroxyalkanoates with a relatively long side chain. However, as expected, no hydrolytic activity was detected with scl PHA, such as P(3HB), P(3HP), and P(3HB-HV[12%]). Moreover, the enzyme was unable to hydrolyze PES and PLA, a poly(alkenedicarboxylate) and a polyester consisting of α-hydroxyalkanoate repeating units, respectively. Besides, PhaZSroSL3 showed slight activity toward tributyrin, which is a typical substrate for esterases. However, after 3 days of reaction at 30°C, the enzyme was unable to hydrolyze olive oil, which is a suitable substrate for lipases, indicating that this depolymerase does not show lipase activity (data not shown). Similar substrate specificity was observed with PhaZSveSO1 (47) and PhaZSomSO2 (unpublished data). As described before (14), the prototype PhaZPflGK13 did not hydrolyze scl PHA or PLA. In addition, similar to Streptomyces enzymes, in this study no hydrolytic activity was observed in the presence of PES or olive oil using PhaZPflGK13 as the catalyst. However, PhaZPflGK13 was not able to hydrolyze tributyrin, and only a small clearing zone was observed in PCL-agarose plates after 24 h at 30°C (data not shown).
Moreover, the esterase activity of PhaZSroSL3 was assayed using several pNP-alkanoates as the substrates (Table 2). The enzyme showed the highest esterase activity with pNPO (4.1 U/mg protein), whereas it was unable to hydrolyze pNP-hexadecanoate. On the other hand, its activity with scl pNP-alkanoates was significantly lower. Similar substrate specificities for pNP-alkanoates were described for PhaZSspKJ-72 (23) and PhaZSveSO1 (47). In contrast, PhaZPflGK13 showed maximum esterase activity when pNP-tetradecanoate was used as the substrate (8).
Table 2.
Relative activity of mcl PHA depolymerase of S. roseolus SL3a
| Substrate | Relative activity (%) |
|---|---|
| pNP-acetate | 0.5 |
| pNP-butyrate | 3 |
| pNP-valerate | 30 |
| pNP-octanoate | 100 |
| pNP-decanoate | 93 |
| pNP-dodecanoate | 87 |
| pNP-hexadecanoate | 13 |
| pNP-octadecanoate | 4 |
The pure enzyme was assayed with the indicated chromogenic substrates at a final concentration of 0.3 mM, in all cases. One hundred percent activity corresponded to 4.1 U/mg protein.
Products of extracellular mcl PHA depolymerase from S. roseolus SL3 reaction.
Enzymatic degradation of P(3HO) latex catalyzed by PhaZSroSL3 was followed by HPLC-PDA, and the identity of the resulting peaks was determined by HPLC-MS. The composition and relative amounts of the hydrolysis products identified were significantly dependent on the time of hydrolysis used (Fig. 2). Thus, during the early enzymatic period (3 h), trimer 3-HO-HO-HO (∼41%) was the main hydrolysis product detected. However, longer periods of incubation yielded higher concentrations of 3-HO monomers, whereas those of trimers markedly decreased. In fact, after 72 h of enzymatic hydrolysis, 3-HO monomers were the main degradation products (∼57%) obtained, while the trimers were almost absent (Fig. 2). The trimer 3-HO-HO-HX and the dimer 3-HO-HX could also be detected. However, it was difficult to determine the relative amount of the monomer 3-hydroxyhexanoic acid (3-HX) since it showed the same retention time as the unreacted BPB (26.1 min). When P(3HO) was incubated at 30°C for 72 h in the absence of the enzyme, no degradation occurred (data not shown).
Fig 2.
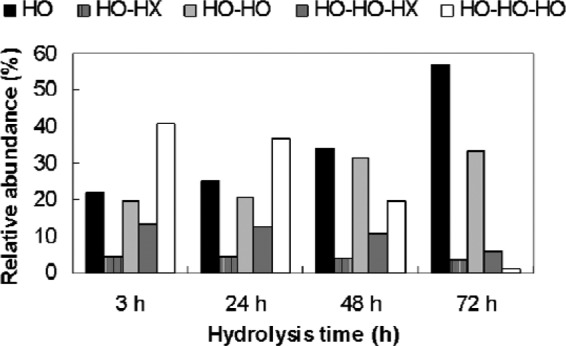
Evolution with the hydrolysis time of the abundance of P(3HO) products catalyzed by the S. roseolus SL3 mcl PHA depolymerase. The products are indicated as follows: HO, HO-HO, and HO-HO-HO, monomer, dimer, and trimer of 3-hydroxyoctanoic acid, respectively; HO-HX and HO-HO-HX, dimer and trimer of 3-hydroxyoctanoic acid and 3-hydroxyhexanoic acid, respectively. The P(3HO) used as a substrate was a copolymer composed of 89% 3-HO and 11% 3-HX.
Identification of mcl PHA depolymerases from Actinobacteria.
To identify the depolymerase-encoding genes from Actinobacteria, the amino acid sequences of four peptides of the purified PhaZSveSO1 (47) were determined by de novo sequencing analysis as VDLEHIGSAGHSQGGAAAVNAAIDAR, DSSHLPAVYGEVR, APTTAWIR, and RNWHNGDENAR. MS-BLAST analysis of these peptide sequences revealed a best match with a hypothetical protein from Streptosporangium roseum DSM43021 (34) (GenBank accession no. YP_003340976). The mcl PHA-degrading ability of this bacterium was confirmed by the clear-zone formation method (data not shown). Furthermore, this protein exhibited high amino acid similarity (more than 69%) with the hypothetical proteins of other Actinobacteria species, including two sequences from Rhodococcus erythropolis strains (Table 3).
Table 3.
Similarity between amino acid sequences of Sros_5476 and hypothetical proteins identified from sequenced microbial genomes
| Protein | Source/microorganism | GenBank accession no. | % identity/% similarity | Reference |
|---|---|---|---|---|
| Hypothetical protein Sros_5476 | Streptosporangium roseum DSM 43021 | YP_003340976 | Nolan et al. (34) | |
| Hypothetical acetyl xylan esterase SPW_6174 | Streptomyces sp. W007 | ZP_09405870 | 78/85 | Unpublished |
| Hypothetical acetyl xylan esterase SACT1_2252 | Streptomyces griseus Xyleb KG-1 | ZP_08235685 | 81/85 | Grubbs et al. (12) |
| Hypothetical protein SGR_2003 | Streptomyces griseus subsp. griseus NCBR 13350 | YP_001823515 | 80/84 | Ohnishi et al. (35) |
| Hypothetical protein SVEN_7345 | Streptomyces venezuelae ATCC 10712 | CCA60631 | 59/70 | Pullan et al. (41) |
| Hypothetical protein RHOER0001_1689 | Rhodococcus erythropolis SK121 | ZP_04385744 | 57/69 | Unpublished |
| Putative hydrolase RER_58150 | Rhodococcus erythropolis PR4 | YP_002769262 | 56/69 | Sekine et al. (52) |
In parallel with this work, the complete genome of S. venezuelae ATCC 10712 was elucidated (41). Although a putative P(3HB) depolymerase (GenBank accession no. CCA60573.1) was annotated, no open reading frame (ORF) encoding a mcl PHA depolymerase was identified. Interestingly, among the amino acid sequences obtained by BLAST analysis, the hypothetical protein SVEN_7345 (GenBank accession no. CCA60631.1) from this bacterium was found (Table 3). On the basis of the DNA sequence of this protein, as well as on the N-terminal sequences determined by Edman degradation (see Materials and Methods for details), DNA fragments of ∼600 bp of the phaZSroSL3, phaZSveSO1, and phaZSomSO2 genes were amplified using their corresponding isolated chromosomal DNAs as the template (Fig. 3). The deduced amino acid sequences shared significantly high similarity (71 to 94%) with all the hypothetical mcl PHA depolymerase proteins identified by de novo sequencing and homology search.
Fig 3.
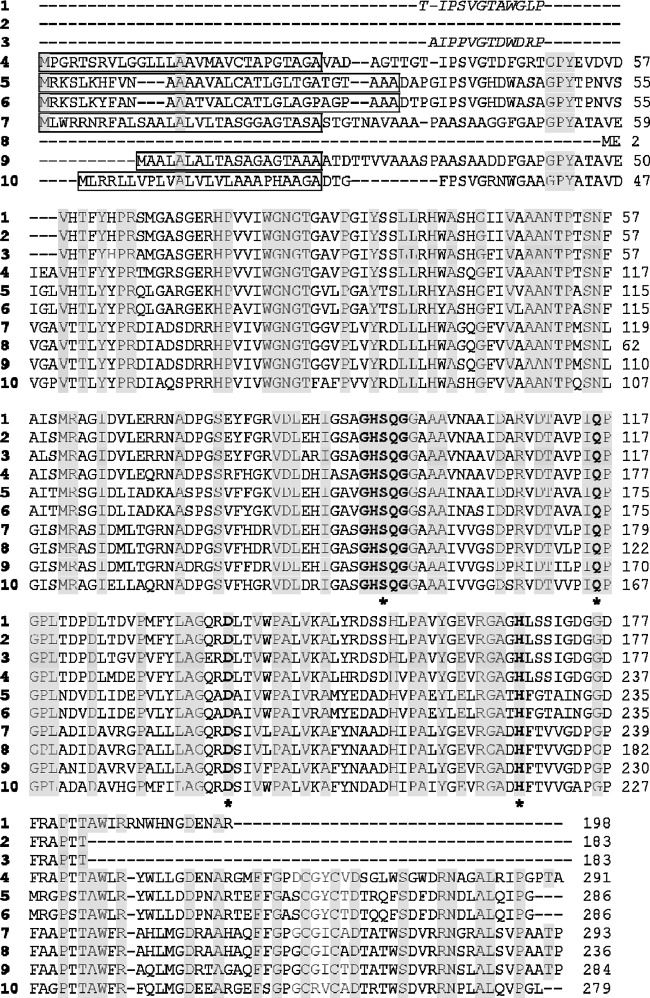
Alignment of amino acid sequences of mcl PHA depolymerases. Identical amino acids are shaded in gray. The lipase consensus sequence is marked in bold. Amino acids that might constitute a catalytic triad and the possible oxyanion are indicated in bold and by asterisks. The signal peptides predicted by the SignalP (version 4.0) program are boxed. The N-terminal amino acid sequences determined by Edman degradation are indicated in italics. 1, S. venezuelae SO1; 2, S. omiyaensis SO2; 3, S. roseolus SL3; 4, S. venezuelae ATCC 10712 (GenBank accession no. CCA60631); 5, R. erythropolis SK121 (GenBank accession no. ZP_04385744); 6, R. erythropolis PR4 (GenBank accession no. YP_002769262); 7, S. griseus Xyleb KG-1 (GenBank accession no. ZP_08235685); 8, S. griseus subsp. griseus NCBR 13350 (GenBank accession no. YP_001823515); 9, Streptomyces sp. strain W007 (GenBank accession no. ZP_09405870); 10, S. roseum DSM 43021 (GenBank accession no. YP_003340976).
The identified mcl PHA depolymerase gene sequences (overall G+C content range, 65 to 74% mol) encoded proteins consisting of ∼279 to 293 amino acids (29.4 to 30.5 kDa). All these sequences included a classical N-terminal signal peptide of 25 to 35 amino acids, as predicted by the SignalP (version 4.0) program (38) (Fig. 3). The calculated molecular masses of the mature proteins ranged from 26.1 to 27.8 kDa. In addition, the high content of aromatic (7.2 to 9.4%) and uncharged aliphatic (44.3 to 50.8%) side chain residues in these amino acid sequences suggested that these enzymes were strongly hydrophobic. In general, these proteins showed a larger number of charged amino acids (17.8 to 21.8% for E, D, R, K, and H) than the mature enzyme of P. fluorescens GK13 (15%). On the other hand, these sequences did not show significant similarity to any of the already known extracellular mcl PHA depolymerases. In fact, no more than 32.5% and 22.1% similarities were observed between these proteins and PhaZPflGK13 and PhaZTthHB8, respectively (see Table S3 in the supplemental material). However, similar to all extracellular PHA depolymerases, the primary structure corresponding to Actinobacteria strains contained strictly conserved amino acids (Ser-Asp-His) that comprise a catalytic triad in the active center (Table 4). Moreover, the catalytic domain of these proteins contained the consensus lipase box pentapeptide of serine hydrolases G-X1-S-X2-G, in which X1 is a His residue and X2 is a Gln residue. Additionally, Table 4 shows those residues identified to be possible oxyanion hole amino acids, on the basis of the homology modeling of the mcl PHA depolymerase from S. venezuelae ATCC 10712 (see Fig. S4 in the supplemental material).
Table 4.
Alignment of amino acid sequences of mcl PHA depolymerases in neighborhood of putative active sitesa
| Strain | Sequence at the following active-site positionb |
Reference or source | |||
|---|---|---|---|---|---|
| Ser (S) | Asp (D) | His (H) | Oxyanion hole | ||
| S. venezuelae ATCC 10712 | 154 VDLDHIASAGHSQGGAAA | 198 YLAGQRDLTVW | 228 RGAGHLSSIGDG | 176 DTAVPIQPGPLTDPD | Pullan et al. (41) |
| R. erythropolis PR4 | 152 VDLEHIGASGHSQGGAAA | 196 YLAGQADAIVW | 226 RGATHFGTAING | 174 DTAVAIQPGPLNDVD | Sekine et al. (52) |
| R. erythropolis SK112 | 152 VDLEHIGASGHSQGGAAA | 196 YLAGQADAIVW | 226 RGATHFGTAING | 174 DTAVAIQPGPLNDVD | Unpublished |
| Streptosporangium roseum DSM 43021 | 144 VDLDRIGASGHSQGGAAA | 188 ILAGQRDSIVW | 218 RGADHFTVVGAP | 166 DTVVPIQPGPLADAD | Nolan et al. (34) |
| S. griseus subsp. griseus NCBR 13350 | 99 VDLEHIGAVGHSQGGSAA | 149 LLAGQRDSIVL | 173 RGADHFTVVGDP | 121 DTVLPIQPGPLADID | Ohnishi et al. (35) |
| S. griseus Xyleb KG-1 | 156 VDLEHIGAVGHSQGGSAA | 200 LLAGQRDSIVL | 230 RGADHFTVVGDP | 105 DTVLPIQPGPLADID | Grubbs et al. (12) |
| Streptomyces sp. W007 | 147 VDLEHIGASGHSQGGAAA | 191 LLAGQRDSIVF | 221 RGADHFTVVGDP | 169 DTILPIQPGPLANID | Unpublished |
| S. roseolus SL3 | VDLARIGSAGHSQGGAAA | YLAGERDLTVW | RGAGHLSSIGDG | DTAVPIQPGPLTDPD | This study |
| S. venezuelae SO1 | VDLEHIGSAGHSQGGAAA | YLAGQRDLTVW | RGAGHLSSIGDG | DTAVPIQPGPLTDPD | This study |
| S. omiyaensis SO2 | VDLEHIGSAGHSQGGAAA | YLAGQRDLTVW | RGAGHLSSIGDG | DTAVPIQPGPLTDPD | This study |
| Consensus | VDL-:I+--GHSQGG-AA | -LAG--D--V* | RGA-H*--*-- | DT***IQPGPL--*D | This study |
| P. fluorescens GK13 | 172 LNAQRQYATGISSGGYNT | 228 FLHGFVDAVVP | 260 PLGGHEWFAASP | 111 VQNLLDHGYAVIAP | Schirmer and Jendrossek (50) |
| Consensus of lipases | -V-**GhS-G+--- | -----D-*v | ---H*------ | --***HG*----- | Jendrossek (16) |
The positions of Ser, Asp, His, and the putative oxyanion hole in the premature depolymerase proteins are indicated. Consensus sequences are presented, in which amino acids conserved in all the analyzed sequences are indicated by capital letters and in which symbols are as follows: *, amino acids with hydrophobic side chains; +, amino acids with a small side chain; :, amino acids with charged side chains. The corresponding sequences of the P(3HO) depolymerase of P. fluorescens GK13 are given. The consensus sequences of lipases are also shown, in which amino acids conserved in all the analyzed sequences are indicated by capital letters, those which have been conserved in 10 or more proteins are marked by lowercase letters, and the symbols are as defined for the consensus sequences.
Amino acids that constitute the catalytic triad, the possible oxyanion, and the conserved amino acids of the lipase consensus sequence are indicated in bold.
DISCUSSION
In this work, 10 mcl PHA-degrading depolymerase producer bacteria were isolated from natural samples and their abilities to degrade different aliphatic biodegradable polyesters were evaluated. Among our identified bacteria, only three of them, Pseudomonas alcaligenes, Stenotrophomonas maltophilia, and Rhodococcus equi, have already been described to be extracellular mcl PHA-degrading bacteria (20, 29, 42). Previous work (22, 33) demonstrated that Gram-negative bacteria belonging to Pseudomonas and Stenotrophomonas species are the predominant mcl PHA degraders in soil and marine environments. However, six Streptomyces strains were identified among our isolates. Additionally, only those strains belonging to the Streptomyces genus showed the ability to degrade not only mcl PHA but also scl PHA and PCL. These results indicated that streptomycetes may play an important role in the degradation of polyesters. However, none of the isolates can degrade PLA and PES.
The majority of the PHA-degrading microorganisms are known to express only one type of PHA depolymerase that acts upon either scl PHA or mcl PHA (17). However, the ability to degrade scl PHA and mcl PHA by producing two types of depolymerases is rare and has been reported in only a few bacteria (5, 23, 25, 36, 47). In this work, the mcl PHA degraders S. roseolus SL3 and S. omiyaensis SO2 were also found to express scl PHA depolymerase in the presence of P(3HB). Additionally, when S. roseolus SL3 was grown in the presence of P(3HO) as the sole carbon source, it produced one polypeptide chain of mcl PHA depolymerase with a mass of ∼28 kDa and a pI of ∼5.2. These results are similar to those for several mcl PHA depolymerases characterized from other sources (47) but significantly different from those for the P(3HO) depolymerase from Pseudomonas fluorescens GK13 (dimer; 48 kDa; pI ∼7).
As previously reported by Santos et al. (47), it is likely that the mcl PHA depolymerases produced from Streptomyces strains have a wider range of substrate specificity. In this work, the substrate specificity of PhaZSroSL3 confirms this hypothesis. In fact, in contrast to the mcl PHA depolymerases from Pseudomonas, the enzyme degrades PCL and tributyrin but not olive oil. However, none of the mcl PHA depolymerases reported so far exhibited detectable activities against PLA (14, 20, 23, 47) and PES (47).
The pure PhaZSroSL3 mainly hydrolyzed P(3HO) to the monomeric unit of 3-hydroxyoctanoate (3-HO) after 72 h of reaction. In this sense, PhaZSroSL3 behaves like the extracellular mcl PHA depolymerases of P. alcaligenes LB19 (20) and S. venezuelae SO1 (47). On the other hand, PhaZPflGK13 (49) and PhaZSspKJ-72 (23) mainly hydrolyzed P(3HO) to the dimeric form of 3-hydroxyoctanoate. Thus, PhaZSroSL3 appears to have a promising potential for biotechnological application in the production of enantiomerically pure (R)-3-HO monomers. Several mcl PHA depolymerases have been biochemically characterized. However, only the PhaZPflGK13-coding gene and a few other homologous genes have been cloned and sequenced (21, 29, 37, 50), including an mcl PHA depolymerase from the predator Bdellovibrio bacteriovorus (31). Additionally, in a recent work, a significant different gene from a thermophilic bacterium, T. thermophilus HB8, has been identified (36). However, no gene sequence of the genus Streptomyces has been reported so far.
In this work, de novo sequencing of PhaZSveSO1 allowed the identification of a novel subgroup of mcl PHA depolymerases from Actinobacteria. These new types of mcl PHA depolymerases showed high sequence similarity (more than 60%) to each other (see Table S3 in the supplemental material), as well as to the deduced amino acid sequences of PhaZSroSL3, PhaZSveSO1, and PhaZSomSO2. Inspection of the amino acid sequences revealed no significant similarity to previously characterized mcl PHA depolymerases (less than 33%). The primary structure of these enzymes showed the signal peptide domain typical of mcl PHA depolymerases. Besides, like most serine hydrolases, these enzymes showed the catalytic triad amino acids (Ser, Asp, His) and the lipase consensus pentapeptide Gly-X1-Ser-X2-Gly. In all the enzymes identified in this work, X1 was a His residue and X2 was a Gln residue. Similarly, in true lipases, the X1 residue is generally occupied by His or Tyr, whereas X2 is variable (50). However, in all mcl PHA depolymerases of Pseudomonas strains analyzed so far, X1 was an Ile residue and X2 was a Ser residue. Interestingly, contrary to Pseudomonas enzymes, PhaZSroSL3, PhaZSomSO2, and PhaZSveSO1 can degrade PCL and tributyrin as bacterial lipases. The presence of a His residue instead of a hydrophobic one in the X1 position would be a possible explanation for the differences in substrate specificities observed between mcl PHA depolymerases from Pseudomonas and Streptomyces origins. Moreover, the mcl PHA depolymerases described in this study contained a larger number of charged amino acids (∼18 to 22%) than the mature enzyme from P. fluorescens GK13 (15%). An increased number of intramolecular ion bonds by charged amino acids is known to contribute to the thermal stability of enzymes by conferring rigidity on them (36, 43). This fact is in accordance with previous results that demonstrated that PhaZSveSO1 showed higher thermostability than its PhaZPflGK13 counterpart (8, 47).
The three-dimensional (3D) model structure of the mcl PHA depolymerase of S. venezuelae ATCC 10712 was deduced by homology modeling using Pseudomonas mendocina lipase as the template (see Fig. S4 in the supplemental material). This model revealed that the enzyme consisted of an α/β hydrolase core with the catalytic triad (Ser125-Asp169-His199) at its surface, being very exposed to the solvent, and Gln147 as the oxyanion hole amino acid that stabilized the tetrahedral transition estate. Therefore, it is assumed that this enzyme does not undergo the typical phenomenon known as interfacial activation described for several lipases and for the intracellular mcl PHA depolymerase from Pseudomonas putida KT2442. Similar conclusions were deduced by de Eugenio et al. (4) on the basis of the 3D model of PhaZPflGK13. Similar to PhaZPflGK13, S. venezuelae ATCC 10712 PhaZ does not have a lid domain, and the two enzymes show a similar architecture and catalytic mechanism of ester hydrolysis. Moreover, a disulfide bridge was predicted by the model, explaining the previously observed inhibition of PhaZSveSO1 in the presence of DTT (47).
mcl PHA depolymerases are excellent candidate biocatalysts for environmental, industrial, and medical applications. This study provides novel information on mcl PHA depolymerases from Actinobacteria, in terms of molecular structure, revealing significant differences from Pseudomonas enzymes. Additionally, these results offer the possibility of cloning and expression of these distinct enzymes for their possible exploitation in biotechnological processes.
Supplementary Material
ACKNOWLEDGMENTS
This work was carried out in the framework of the IP project Sustainable Microbial and Biocatalytic Production of Advanced Functional Materials (BIOPRODUCTION/NMP-2-CT-2007-026515), funded by the European Commission and by the Spanish Ministry of Education and Science (BIO2007-28707-E and CTQ2011-25052), and UPV/EHU (GIU07/55 and GIU11/25). M.S. and J.G. were the recipients of scholarships from the Spanish Ministry of Education.
P(3HO) was kindly supplied by Biopolis, S.A. (Valencia, Spain), and CPI (Newcastle, United Kingdom). P(3HP) was kindly donated by CIBA (Manchester, United Kingdom).
Footnotes
Published ahead of print 3 August 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Chen GQ, Wu Q. 2005. Microbial production and applications of chiral hydroxyalkanoates. Appl. Microbiol. Biotechnol. 67:592–599 [DOI] [PubMed] [Google Scholar]
- 2. Chen GQ. 2011. Biofunctionalization of polymers and their applications. Adv. Biochem. Eng. Biotechnol. 125:29–45 [DOI] [PubMed] [Google Scholar]
- 3. de Eugenio LI, et al. 2010. The turnover of medium-chain-length polyhydroxyalkanoates in Pseudomonas putida KT2442 and the fundamental role of PhaZ depolymerase for the metabolic balance. Environ. Microbiol. 12:207–221 [DOI] [PubMed] [Google Scholar]
- 4. de Eugenio LI, García JL, García PL, Prieto MA, Sanz JM. 2008. Comparative analysis of the physiological and structural properties of a medium chain length polyhydroxyalkanoate depolymerase from Pseudomonas putida KT2442. Eng. Life Sci. 8:260–267 [Google Scholar]
- 5. Elbanna K, Lutke-Eversloh T, Jendrossek D, Luftmann H, Steinbüchel A. 2004. Studies on the biodegradability of polythioester copolymers and homopolymers by polyhydroxyalkanoate (PHA)-degrading bacteria and PHA depolymerases. Arch. Microbiol. 182:212–225 [DOI] [PubMed] [Google Scholar]
- 6. Escapa IF, et al. 2011. Disruption of β-oxidation pathway in Pseudomonas putida KT2442 to produce new functionalized PHAs with thioester groups. Appl. Microbiol. Biotechnol. 89:1583–1598 [DOI] [PubMed] [Google Scholar]
- 7. Gandolfi R, Gaspari F, Franzetti L, Molinari F. 2000. Hydrolytic and synthetic activities of esterases and lipases of non-starter bacteria isolated from cheese surface. Ann. Microbiol. 50:183–189 [Google Scholar]
- 8. Gangoiti J, Santos M, Llama MJ, Serra JL. 2010. Production of chiral (R)-3-hydroxyoctanoic acid monomers catalyzed by Pseudomonas fluorescens GK13 poly(3-hydroxyoctanoic acid) depolymerase. Appl. Environ. Microbiol. 76:3554–3560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. García-Hidalgo J, Hormigo D, Prieto MA, Arroyo M, de la Mata I. 2012. Extracellular production of Streptomyces exfoliatus poly(3-hydroxybutyrate) depolymerase in Rhodococcus sp. T104: determination of optimal biocatalyst conditions. Appl. Microbiol. Biotechnol. 93:1975–1988 [DOI] [PubMed] [Google Scholar]
- 10. García-Murria MJ, Valero ML, Sánchez del Pino MM. 2011. Simple chemical tools to expand the range of proteomics applications J. Proteomics 74:137–150 [DOI] [PubMed] [Google Scholar]
- 11. Gebauer B, Jendrossek D. 2006. Assay of poly(3-hydroxybutyrate) depolymerase activity and product determination. Appl. Environ. Microbiol. 72:6094–6100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grubbs KJ, et al. 2011. Genome sequence of Streptomyces griseus strain XylebKG-1, an ambrosia beetle-associated actinomycete. J. Bacteriol. 193:2890–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ihssen J, Magnani D, Thöny-Meyer L, Ren Q. 2009. Use of extracellular medium chain length polyhydroxyalkanoate depolymerase for targeted binding of proteins to artificial poly[(3-hydroxyoctanoate)-co-(3-hydroxyhexanoate)] granules. Biomacromolecules 10:1854–1864 [DOI] [PubMed] [Google Scholar]
- 14. Jaeger KE, Steinbüchel A, Jendrossek D. 1995. Substrate specificities of bacterial polyhydroxyalkanoate depolymerases and lipases: bacterial lipases hydrolyze poly(omega-hydroxyalkanoates). Appl. Environ. Microbiol. 61:3113–3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jendrossek D, Schirmer A, Handrick R. 1997. Recent advances in characterization of bacterial PHA depolymerases, p 89–101 In Eggink G, Steinb̈uchel A, Poirier Y, Witholt B. (ed), 1996 International Symposium on Bacterial Polyhydroxyalkanoates NRC Research Press, Ottawa, ON, Canada [Google Scholar]
- 16. Jendrossek D. 2001. Extracellular polyhydroxyalkanoate depolymerases: the key enzymes of PHA degradation, p 41–83 In Doi Y, Steinb̈uchel A. (ed), Biopolymers, vol 386 3b. Polyesters II. Wiley-VCH, Weinheim, Germany [Google Scholar]
- 17. Jendrossek D, Handrick R. 2002. Microbial degradation of polyhydroxyalkanoates. Annu. Rev. Microbiol. 56:403–432 [DOI] [PubMed] [Google Scholar]
- 18. Kelly KL. 1964. Color-name charts illustrated with centroid colors. Inter-Society Color Council, National Bureau of Standards, Chicago, IL [Google Scholar]
- 19. Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. 2000. Practical Streptomyces genetics. John Innes Foundation, Norwich, United Kingdom [Google Scholar]
- 20. Kim DY, Nam JS, Rhee YH. 2002. Characterization of an extracellular medium-chain-length poly(3-hydroxyalkanoate) depolymerase from Pseudomonas alcaligenes LB19. Biomacromolecules 3:291–296 [DOI] [PubMed] [Google Scholar]
- 21. Kim DY, Kim HC, Kim SY, Rhee YH. 2005. Molecular characterization of extracellular medium-chain-length poly(3-hydroxyalkanoate) depolymerase genes from Pseudomonas alcaligenes strains. J. Microbiol. 43:285–294 [PubMed] [Google Scholar]
- 22. Kim DY, Kim HW, Chung MG, Rhee YH. 2007. Biosynthesis, modification, and biodegradation of bacterial medium-chain-length polyhydroxyalkanoates. J. Microbiol. 45:87–97 [PubMed] [Google Scholar]
- 23. Kim HJ, Kim DY, Nam JS, Bae KS, Rhee YH. 2003. Characterization of an extracellular medium-chain-length poly(3-hydroxyalkanoate) depolymerase from Streptomyces sp. KJ-72. Antonie Van Leeuwenhoek 83:183–189 [DOI] [PubMed] [Google Scholar]
- 24. Kim O, Gross RA, Hammar WJ, Newmark RA. 1996. Microbial synthesis of poly(beta-hydroxyalkanoates) containing fluorinated side-chains substituents. Macromolecules 29:4572–4581 [Google Scholar]
- 25. Klingbeil B, Kroppenstedt R, Jendrossek D. 1996. Taxonomical identification of Streptomyces exfoliatus K10 and characterization of its poly(3-hydroxybutyrate) depolymerase gene. FEMS Microbiol. Lett. 142:215–221 [DOI] [PubMed] [Google Scholar]
- 26. Knoll M, Hamm T, Wagner F, Martinez V, Pleiss J. 2009. The PHA depolymerase engineering database: a systematic analysis tool for the diverse family of polyhydroxyalkanoate (PHA) depolymerases. BMC Bioinformatics 10:89–90 doi:10.1186/1471-2105-10-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 28. Larkin MA, et al. 2007. ClustalW and ClustalX version 2. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 29. Lim JH. 2006. Expression, purification and characterization of Rhodococcus equi P2 MCL-PHA depolymerase in Escherichia coli. MS thesis Chungnam National University, Daejeon, South Korea [Google Scholar]
- 30. Madison LL, Huisman GW. 1999. Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol. Mol. Biol. Rev. 63:21–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martínez V, et al. 2012. Identification and biochemical evidence of a medium-chain-length polyhydroxyalkanoate depolymerase in the Bdellovibrio bacteriovorus predatory hydrolytic arsenal. Appl. Environ. Microbiol. 78:6017–6026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mergaert J, Swings J. 1996. Biodiversity of microorganisms that degrade bacterial and synthetic polyesters. J. Ind. Microbiol. 17:463–469 [Google Scholar]
- 33. Nam JS, Kim HC, Kim DY, Rhee YH. 2002. Distribution and diversity of microbial communities relating to biodegradation of medium-chain-length poly(3-hydroxyalkanoates) in soils, p 192 Proceedings of the 9th International Symposium on the Genetics of Industrial Microorganisms, Gyeongju, South Korea [Google Scholar]
- 34. Nolan M, et al. 2010. Complete genome sequence of Streptosporangium roseum type strain (NI 9100). Stand. Genomic Sci. 2:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ohnishi Y, et al. 2008. Genome sequence of the streptomycin-producing microorganism Streptomyces griseus IFO 13350 J. Bacteriol. 190:4050–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Papaneophytou CP, Velalia EE, Pantazaki AA. 2011. Purification and characterization of an extracellular medium-chain length polyhydroxyalkanoate depolymerase from Thermus thermophilus HB8. Polym. Degrad. Stab. 96:670–678 [Google Scholar]
- 37. Park IJ, Rhee YH, Cho NY, Shin KS. 2006. Cloning and analysis of medium-chain-length poly(3-hydroxyalkanoate) depolymerase gene of Pseudomonas luteola M13-4. J. Microbiol. Biotechnol. 16:1935–1939 [Google Scholar]
- 38. Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8:785–786 [DOI] [PubMed] [Google Scholar]
- 39. Peterson GL. 1983. Determination of total protein. Methods Enzymol. 91:95–119 [DOI] [PubMed] [Google Scholar]
- 40. Prieto MA, et al. 1999. Engineering of stable recombinant bacteria for production of chiral medium-chain-length poly-3-hydroxyalkanoates. Appl. Environ. Microbiol. 65:3265–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pullan ST, Chandra G, Bibb MJ, Merrick M. 2011. Genome-wide analysis of the role of GlnR in Streptomyces venezuelae provides new insights into global nitrogen regulation in actinomycetes. BMC Genomics 12:175 doi:10.1186/1471-2164-12-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ramsay BA, Saracovan I, Ramsay JA, Marchessault RH. 1994. A method for the isolation of microorganisms producing extracellular long-side-chain poly (β-hydroxyalkanoate) depolymerase. J. Environ. Polym. Deg. 2:1–7 [Google Scholar]
- 43. Romen F, Reinhardt S, Jendrossek D. 2004. Thermotolerant poly(3-hydroxybutyrate)-degrading bacteria from hot compost and characterization of the PHB-depolymerase of Schlegelella sp. KB1a. Arch. Microbiol. 182:157–164 [DOI] [PubMed] [Google Scholar]
- 44. Ruth K, et al. 2007. Efficient production of (R)-3-hydroxycarboxylic acids by biotechnological conversion of polyhydroxyalkanoates and their purification. Biomacromolecules 8:279–286 [DOI] [PubMed] [Google Scholar]
- 45. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 46. Sanger F, Nicklen S, Coulson AR. 1977. DNA sequencing with chain terminating inhibitors. Proc. Natl. Acad. Sci. U. S. A. 74:5463–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Santos M, et al. Polyester hydrolytic and synthetic activity catalysed by the medium-chain-length poly(3-hydroxyalkanoate) depolymerase from Streptomyces venezuelae SO1. Appl. Microbiol. Biotechnol., in press doi:10.1007/s00253-012-4210-1 [DOI] [PubMed] [Google Scholar]
- 48. Santos M, et al. 2012. Poly(3-hydroxyoctanoate) depolymerase from Pseudomonas fluorescens GK13: catalysis of ester-forming reactions in non-aqueous media. J. Mol. Catal. B Enzym. 77:81–88 [Google Scholar]
- 49. Schirmer A, Jendrossek D, Schlegel HG. 1993. Degradation of poly(3-hydroxyoctanoic acid) [P(3HO)] by bacteria: purification and properties of a P(3HO) depolymerase from Pseudomonas fluorescens GK13. Appl. Environ. Microbiol. 59:1220–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schirmer A, Jendrossek D. 1994. Molecular characterization of the extracellular poly(3-hydroxyoctanoic acid) [P(3HO)] depolymerase gene of Pseudomonas fluorescens GK13 and of its gene product. J. Bacteriol. 176:7065–7073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schirmer A, Matz C, Jendrossek D. 1995. Substrate specificities of poly(hydroxyalkanoate)-degrading bacteria and active site studies on the extracellular poly(3-hydroxyoctanoic acid) depolymerase of Pseudomonas fluorescens GK13. Can. J. Microbiol. 41(Suppl 1):170–179 [DOI] [PubMed] [Google Scholar]
- 52. Sekine M, et al. 2006. Sequence analysis of three plasmids harboured in Rhodococcus erythropolis strain PR4. Environ. Microbiol. 8:334–346 [DOI] [PubMed] [Google Scholar]
- 53. Shevchenko A, et al. 2001. Charting the proteomes of organisms with unsequenced genomes by MALDI-quadrupole time-of-flight mass spectrometry and BLAST homology searching. Anal. Chem. 73:1917–1926 [DOI] [PubMed] [Google Scholar]
- 54. Shirling EB, Gottlieb D. 1966. Method for classification of Streptomyces species. Int. J. Syst. Bacteriol. 16:313–340 [Google Scholar]
- 55. Shirling EB, Gottlieb D. 1968. Cooperative description of type cultures of Streptomyces. 11. Species descriptions from first study. Additional descriptions. Int. J. Syst. Bacteriol. 18:69–189 [Google Scholar]
- 56. Shirling EB, Gottlieb D. 1972. Cooperative description of type strains of Streptomyces V. Additional descriptions. Int. J. Syst. Bacteriol. 22:265–394 [Google Scholar]
- 57. Steinbüchel A, Valentin HE. 1995. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 128:219–228 [Google Scholar]
- 58. Vroman I, Tighzert L. 2009. Biodegradable polymers. Materials 2:307–334 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.