Abstract
A novel (+)-γ-lactamase used for the resolution of racemic γ-lactam from Bradyrhizobium japonicum USDA 6 was found as a result of sequence-structure guided genome mining. It consists of 409 amino acids, only 49% of which are identical to the amino acid sequences of the known (+)-γ-lactamase from Sulfolobus solfataricus. This is only the third (+)-γ-lactamase gene to be reported.
TEXT
Over the past few decades, in the search for new antiviral drugs such as abacavir and peramivir, the synthesis and bioactivity screening of carbocyclic nucleosides have received increased attention. Abacavir is a carbocyclic 2′-deoxyguanosine nucleoside analogue that competitively inhibits HIV reverse transcriptase and terminates proviral DNA chain extension (5, 8). It is an important chemotherapeutic agent in the treatment of human HIV and hepatitis B viruses (3, 21). Traditionally, these nucleoside compounds are synthesized by chemical methods, but enzymatic synthesis of nucleosides can be simpler and quicker (7, 17, 19). In the biosynthesis of abacavir, the resolution of the bicyclic synthon (rac)-γ-lactam (2-azabicyclo [2.2.1] hept-5-en-3-one) is the most important step. A biocatalytic process was developed for the resolution of racemic γ-lactam using (+)-γ-lactamase-containing organisms, as shown in Fig. 1 (14).
Fig 1.
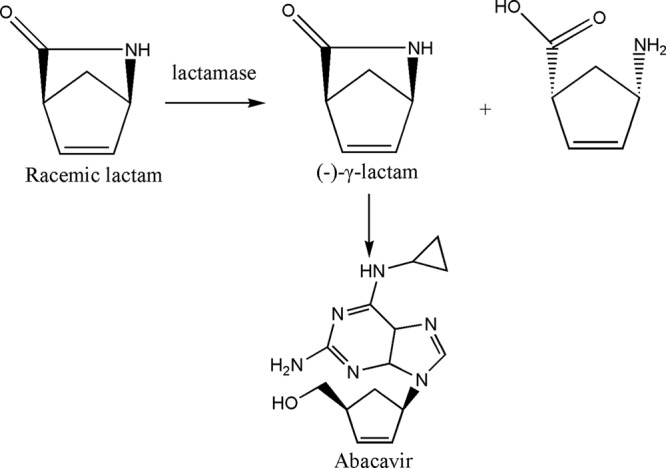
Enzymatic preparation of (−)-γ-lactam for the synthesis of abacavir.
Amidases (EC 3.5.1.4) are an interesting member of the nitrilase superfamily; they are used to cleave carbon-nitrogen bonds by transferring an acyl group to water to form free acids and ammonia (26). In recent years, amidases have turned out to be versatile biocatalysts in white and green chemistry. They have been the subject of extensive research due to their enantioselective and stereoselective properties (20). (+)-γ-Lactamase is a type of amidase that can cleave the [1(S), 4(R)]-(+)-enantiomers of γ-lactam. After the enzymatic reaction, the desired [1(R), 4(S)]-(−)-enantiomer can further be used to synthesize abacavir. There are six organisms with (+)-γ-lactamase in the resolution of (rac)-γ-lactam that have been reported: Microbacterium hydrocarbonoxydans (18), Sulfolobus solfataricus (9, 25, 26), Comamonas acidovorans (12, 22), Pseudomonas cepacia (10, 22), Pseudomonas solanacearum (23, 24), and Pseudomonas fluorescens (1, 23).
Traditionally, when there are no commercial (+)-γ-lactamases available for the biosynthesis of optically pure lactam, researchers usually try to find them from the environment, such as from a microbial source. All six previously mentioned organisms have been found and used as whole-cell biocatalysts. In this process, novel screening methods play a key role in finding the successful biocatalysts (22). But the disadvantage of using whole cells is that wild strains usually have a small amount of protein and are unstable during the reaction. A combination of classical molecular biology techniques, e.g., isolating the enzyme from the wild strain and then cloning the enzyme for overexpression, is a better strategy. The recombinant cloned enzyme with high (+)-γ-lactamase activity would be the best biocatalyst for optically pure lactam in the industrial processes because of its high efficiency and low cost (10). Moreover, when we obtain the gene of (+)-γ-lactamase, it will be easy to obtain the crystal structure of the recombinant enzyme that will give us more information to better understand the mechanism of the catalytic process.
Until now, only two (+)-γ-lactamases have been sequenced, expressed in Escherichia coli, and used in the industry process for (−)-γ-lactam: those from C. acidovorans and S. solfataricus (2, 10, 15, 22, 25, 26). Both enzymes have been crystallized, but structure determination by multiple isomorphous replacement is still in progress (6, 15). It is expected that structural information from these amidases will increase our knowledge of molecular catalytic mechanisms. The sequence of the (+)-γ-lactamase from C. acidovorans showed 80% identity to a formamidase from Bradyrhizobium japonicum USDA 6 and 63% identity to a formamidase from Methylophilus methylotropus (27). The sequence of the (+)-γ-lactamase from S. solfataricus showed about 40% identity to an amidase from Rhodococcus rhodochrous J1 (11) and Rhodococcus sp. N-771 (16). It is interesting that no identity was found between the (+)-γ-lactamases from C. acidovorans and S. solfataricus, which means that two different kinds of amidases showed similar (+)-γ-lactamase activity. Novel (+)-γ-lactamases need to be identified and examined to better understand this useful industrial catalyst.
Although novel screening methods for finding new organisms with (+)-γ-lactamase activity have been developed, it is widely believed that biotechnology has missed up to 99% of existing microbial resources by using traditional screening techniques (13). The isolation of enzymes from wild strains is usually time-consuming and requires a heavy workload. During purification, different methods such as ammonium sulfate fractionation, ion exchange, and hydrophobic and gel filtration chromatography are used, and the protein loses activity during purification and storage (1). This means that it is difficult to obtain the gene of (+)-γ-lactamase using these methods. Current advances in genome sequencing have revolutionized research in the field of biotechnology, not only giving us a glimpse into the uncultured microbial population but also enabling the high-throughput discovery of new enzymes for industrial bioconversions (4). A genomics-inspired strategy has been successful in unveiling a new (+)-γ-lactamase that was overlooked using standard screening methods. We believe that as more genome sequences become available, more novel (+)-γ-lactamases will be found in underexplored or neglected organisms.
In this article, we describe the identification of a novel (+)-γ-lactamase from B. japonicum USDA 6 based on sequence-structure guided genome mining for the synthesis of optically pure lactam. This is the third (+)-γ-lactamase gene to be reported, and it is the first (+)-γ-lactamase found by a genome-mining method.
In silico screening of DNA sequence databases for the putative (+)-γ-lactamase genes in microorganisms was performed. A BLAST search using (+)-γ-lactamase from S. solfataricus as a template was performed in NCBI, and an unnamed protein product (GenBank accession no. BJ6T_02120) was found from B. japonicum USDA 6 that showed 49% identity to the template (Fig. 2). B. japonicum USDA 6 is a Gram-negative, rod-shaped, nitrogen-fixing bacterium that forms a symbiotic relationship with Glycine max. It is a slow-growing bacterium and therefore not suitable for application in biotechnology. However, neglected organisms might contain effective (+)-γ-lactamase. In order to confirm our prediction, the whole cell of B. japonicum USDA 6 (purchased from China General Microbiological Culture Collection Center) was cultured to catalyze the racemic γ-lactam. High (+)-γ-lactamase activity was detected. Based on the gene of the unnamed protein product, the gene was amplified from B. japonicum USDA 6 genomic DNA by the use of forward primer 5′-GGAATTCCATATGGTGACAGTTGTCCTTCC-3′ and reverse primer 5′-CCCAAGCTTTCACATCTTCTTCCAGTCGCC-3′ (the NdeI restriction site is underlined in the former, and the HindIII restriction site is underlined in the latter). Then, the gene was cloned into plasmid pET-30a(+) and transformed into E. coli BL21(DE3) for expression, which was induced by 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at 37°C for 4 h. The cells were harvested by centrifugation (4,000 × g for 10 min) and then analyzed with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Fig 2.
Multiple alignment of (+) γ-lactamase sequence and homologous enzymes. Abbreviations: S. solfataricus, amidase from Sulfolobus solfataricus (accession number NP_343511); R.spN771, amidase from Rhodococcus sp. N771 (Protein Data Bank [PDB] 3A1K_A); P. fluorescens, amidase from Pseudomonas fluorescens (accession number ZP_07774754); B. japonicum, amidase from Bradyrhizobium japonicum (accession number YP_005605100). Helices are represented by large dark-gray cylinders, β-strands by arrows, and coils by lines. A dashed line represents gaps in the amino acid sequence alignment.
The recombinant (+)-γ-lactamase ran as a single band on an SDS-PAGE gel (Fig. 3), corresponding to the predicted molecular mass of 50 kDa. The (+)-γ-lactamase activity was assayed by high-performance liquid chromatography (HPLC). Assay conditions for enzyme activity were as follows: wet recombinant cells (7 mg) were added to 500 μl of the 100 mM substrate solution (pH 7.0, 50 mM phosphate buffer) with an E. coli wild-type strain as a negative control. The reaction solution was incubated at 37°C for 24 h. The reaction solution was extracted with 200 μl ethyl acetate. The ethyl acetate extract (10 μl) was applied to a Daicel Chiralpak AS-H column (Daicel Corp., Tokyo, Japan) and eluted with a mobile phase consisting of 90% acetonitrile and 10% isopropanol (volume ratio) at a flow rate of 0.6 ml/min. The UV absorbance of eluted γ-lactam was measured at 230 nm. The (+)-γ-lactam and (−)-γ-lactam had retention times of 11 and 12.7 min, respectively. As shown in Fig. 4, the (+)-γ-lactamase from B. japonicum USDA 6 appears to have potential for the production of chirally pure γ-lactam by hydrolyzing the (+)-γ-lactam specifically with no action against the (−) isomer. This high specificity and activity resulted in a yield of 49% and an enantiomeric excess of >99%.
Fig 3.

Coomassie-stained gel after SDS-PAGE analysis of expressed proteins from transformed bacteria using gentle lysis. M, protein marker; lane 1, E. coli BL21(DE3) cells transformed with empty pET-30a vector; lane 2, E. coli BL21(DE3) cells transformed with pET-30a-(+)-γ-lactamase vector.
Fig 4.

Chiral HPLC analysis of the resolution.
As predicted by rational genome mining, a novel (+)-γ-lactamase was found and cloned from B. japonicum USDA 6. While only two (+)-γ-lactamases without similarity have been reported in previous literature, this third novel (+)-γ-lactamase will give us more information about this kind of enzyme. Also, while thousands of other industrial biocatalysts have been studied (e.g., esterase), research on (+)-γ-lactamase is relatively scarce. Genome-mining methods provide an opportunity to examine these useful industrial biocatalysts in nature. The (+)-γ-lactamase from B. japonicum USDA 6 was expressed in E. coli, and its industrial applications could include the production of chirally pure (−)-γ-lactam for the synthesis of carbocyclic nucleosides such as the anti-HIV agent abacavir.
Footnotes
Published ahead of print 10 August 2012
REFERENCES
- 1. Brabban AD, Littlechild J, Wisdom R. 1996. Stereospecific γ-lactamase activity in Pseudomonas fluorescens species. J. Ind. Microbiol. Biotechnol. 16:8–14 [Google Scholar]
- 2. Cilia E, Fabbri A, Uriani M, Scialdone GG, Ammendola S. 2005. The signature amidase from Sulfolobus solfataricus belongs to the CX3C subgroup of enzymes cleaving both amides and nitriles. FEBS J. 272:4716–4724 [DOI] [PubMed] [Google Scholar]
- 3. Evans CT, Roberts SM, Shoberu KA, Sutherland AG. 1992. Potential use of carbocyclic nucleosides for the treatment of AIDS: chemo-enzymatic syntheses of the enantiomers of carbovir. J. Chem. Soc. Perkin Transact. 1 1:589–592 [Google Scholar]
- 4. Ferrer M, Golyshina O, Beloqui A, Golyshin PN. 2007. Mining enzymes from extreme environments. Curr. Opin. Microbiol. 10:207–214 [DOI] [PubMed] [Google Scholar]
- 5. Foster RH, Faulds D. 1998. Abacavir. Drugs 55:729–738 [DOI] [PubMed] [Google Scholar]
- 6. Gonsalvez IS, Isupov MN, Littlechild JA. 2001. Crystallization and preliminary X-ray analysis of a [gamma]-lactamase. Acta Crystallogr. D Biol. Crystallogr. 57(Pt 2):284–286 [DOI] [PubMed] [Google Scholar]
- 7. Hanrahan JR, Hutchinson DW. 1992. The enzymatic synthesis of antiviral agents. J. Biotechnol. 23:193–210 [DOI] [PubMed] [Google Scholar]
- 8. Hervey PS, Perry CM. 2000. Abacavir: a review of its clinical potential in patients with HIV infection. Drugs 60:447–479 [DOI] [PubMed] [Google Scholar]
- 9. Hickey AM, et al. 2009. A microreactor for the study of biotransformations by a cross-linked γ-lactamase enzyme. Biotechnol. J. 4:510–516 [DOI] [PubMed] [Google Scholar]
- 10. Holt-Tiffin KE. 2009. (+)- and (−)-2-azabicyclo [2.2.1]hept-5-en-3-one: extremely useful synthons. Chim. Oggi/Chem. Today 27:23–25 [Google Scholar]
- 11. Kobayashi M, et al. 1993. Amidase coupled with low-molecular-mass nitrile hydratase from Rhodococcus rhodochrous J1. Sequencing and expression of the gene and purification and characterization of the gene product. Eur. J. Biochem. 217:327–336 [DOI] [PubMed] [Google Scholar]
- 12. Line K, Isupov MN, Littlechild JA. 2004. The crystal structure of a (−) γ-lactamase from an Aureobacterium species reveals a tetrahedral intermediate in the active site. J. Mol. Biol. 338:519–532 [DOI] [PubMed] [Google Scholar]
- 13. Lorenz P, Liebeton K, Niehaus F, Eck J. 2002. Screening for novel enzymes for biocatalytic processes: accessing the metagenome as a resource of novel functional sequence space. Curr. Opin. Biotechnol. 13:572–577 [DOI] [PubMed] [Google Scholar]
- 14. Mahmoudian M, Lowdon A, Jones M, Dawson M, Wallis C. 1999. A practical enzymatic procedure for the resolution of N-substituted 2-azabicyclo[2.2.1]hept-5-en-3-one. Tetrahedron Asymmetry 10:1201–1206 [Google Scholar]
- 15. Nastopoulos V, et al. 2001. Crystallization and X-ray diffraction measurements of a thermophilic archaeal recombinant amidase from Sulfolobus solfataricus MT4. Acta Crystallogr. D Biol. Crystallogr. 57:1036–1037 [DOI] [PubMed] [Google Scholar]
- 16. Ohtaki A, et al. 2010. Structure and characterization of amidase from Rhodococcus sp. N-771: insight into the molecular mechanism of substrate recognition. Biochim. Biophys. Acta 1804:184–192 [DOI] [PubMed] [Google Scholar]
- 17. Patel RN. 2008. Synthesis of chiral pharmaceutical intermediates by biocatalysis. Coordination Chemistry Rev. 252:659–701 [Google Scholar]
- 18. Qin X, Wang J, Zheng G. 2010. Enantioselective resolution of γ-lactam by a whole cell of microbacterium hydrocarbonoxydans (L29-9) immobilized in polymer of PVA–alginate–boric acid. Appl. Biochem. Biotechnol. 162:2345–2354 [DOI] [PubMed] [Google Scholar]
- 19. Rasor JP, Voss E. 2001. Enzyme-catalyzed processes in pharmaceutical industry. Appl. Catal. A Gen. 221:145–158 [Google Scholar]
- 20. Sharma M, Sharma N, Bhalla T. 2009. Amidases: versatile enzymes in nature. Rev. Environ. Sci. Biotechnol. 8:343–366 [Google Scholar]
- 21. Staszewski S, et al. 2001. Abacavir-lamivudine-zidovudine vs indinavir-lamivudine-zidovudine in antiretroviral-naive HIV-infected adults: a randomized equivalence trial. JAMA 285:1155–1163 [DOI] [PubMed] [Google Scholar]
- 22. Taylor SJC, Brown RC, Keene PA, Taylor IN. 1999. Novel screening methods—the key to cloning commercially successful biocatalysts. Bioorg. Med. Chem. 7:2163–2168 [DOI] [PubMed] [Google Scholar]
- 23. Taylor SJC, et al. 1993. Development of the biocatalytic resolution of 2-azabicyclo[2.2.1]hept-5-en-3-one as an entry to single-enantiomer carbocyclic nucleosides. Tetrahedron Asymmetry 4:1117–1128 [Google Scholar]
- 24. Taylor SJC, et al. 1990. Chemoenzymatic synthesis of (−)-carbovir utilizing a whole cell catalysed resolution of 2-azabicyclo[2.2.1]hept-5-en-3-one. J. Chem. Soc. Chem. Commun. 1990:1120–1121 [Google Scholar]
- 25. Toogood HS, et al. 2004. The use of a thermostable signature amidase in the resolution of the bicyclic synthon (rac)-γ-lactam. Tetrahedron 60:711–716 [Google Scholar]
- 26. Wang J, Zhang X, Min C, Wu S, Zheng G. 2011. Single-step purification and immobilization of γ-lactamase and on-column transformation of 2-azabicyclo [2.2.1] hept-5-en-3-one. Process Biochem. 46:81–87 [Google Scholar]
- 27. Wyborn NR, Scherr DJ, Jones CW. 1994. Purification, properties and heterologous expression of formamidase from Methylophilus methylotrophus. Microbiology 140:191–195 [Google Scholar]



