Abstract
In red algae, spermatial binding to female trichogynes is mediated by a lectin-carbohydrate complementary system. Aglaothamnion oosumiense is a microscopic filamentous red alga. The gamete recognition and binding occur at the surface of the hairlike trichogyne on the female carpogonium. Male spermatia are nonmotile. Previous studies suggested the presence of a lectin responsible for gamete recognition on the surface of female trychogynes. A novel N-acetyl-d-galactosamine-specific protein was isolated from female plants of A. oosumiense by affinity chromatography and named AOL1. The lectin was monomeric and did not agglutinate horse blood or human erythrocytes. The N-terminal amino acid sequence of the protein was analyzed, and degenerate primers were designed. A full-length cDNA encoding the lectin was obtained using rapid amplification of cDNA ends-PCR (RACE-PCR). The cDNA was 1,095 bp in length and coded for a protein of 259 amino acids with a deduced molecular mass of 21.4 kDa, which agreed well with the protein data. PCR analysis using genomic DNA showed that both male and female plants have this gene. However, Northern blotting and two-dimensional electrophoresis showed that this protein was expressed 12 to 15 times more in female plants. The lectin inhibited spermatial binding to the trichogynes when preincubated with spermatia, suggesting its involvement in gamete binding.
INTRODUCTION
The precise point of gamete recognition varies along the continuum of reproduction and development, from directional movements that bring the compatible gametes together through many steps of fertilization to the formation of embryonic offspring. Fertilization in red algae, however, begins with direct contact between a male spermatium and a female trichogyne because both male and female gametes are nonmotile (7, 32). As spermatial binding to trichogynes is highly selective, some recognition factors are expected to be present along their surfaces (11, 16, 17, 25). Cell surface glycoconjugates have been reported as important factors for cell-cell recognition in many organisms (24). Such recognition systems depend on complementary binding between carbohydrate moieties on one cell with specific sugar-binding lectins on another cell.
Lectin-carbohydrate complementary systems have been reported in gamete recognition of marine algae for a long time (1, 8–10, 17, 22, 31), but most studies used indirect evidence from inhibition experiments using carbohydrates or foreign lectins (mostly from land plants) as blocking agents of gamete binding. Although several studies have reported on the isolation of marine algal lectins, the number of these proteins that have been purified and characterized is still small (4, 33).
Our previous cytochemical study on the fertilization of Aglaothamnion oosumiense Itono suggested the presence of N-acetyl-d-galactosamine (GalNAc) and/or d-methyl mannose-specific lectin(s) on the surface of female trichogynes (11). Here we report the purification and molecular characterization of a novel GalNAc-binding lectin from this species. The sex-specific expression of the lectin was analyzed, and its role in gamete binding was examined.
MATERIALS AND METHODS
Organism and laboratory culture.
Tetrasporic plants of A. oosumiense were collected from Wando, on the southern coast of Korea, and maintained in unialgal cultures in IMR medium (13). Plants were grown at 15°C in 16:8-h light and dark cycles with >20 μmol photons m−2 s−1 provided by cool-white fluorescent bulbs.
Preparation of algal extract and purification of AOL1.
For preparation of algal extract and purification of AOL1 (Aglaothamnion oosumiense lectin 1), materials were frozen with liquid nitrogen and stored at −80°C before homogenization. Samples were homogenized using a mortar and pestle to fine powder, and then 10 volumes of Tris-buffered saline (TBS) (25 mM Tris-HCl, 150 mM NaCl, 5 mM CaCl2, 1 mM phenylmethylsulfonyl fluoride [PMSF], pH 7.4) was added. Homogenates were centrifuged at 5,000 × g for 20 min to sediment out cell organelles and debris. Then, the supernatant was recentrifuged at 30,000 × g for 30 min and concentrated using an ultrafiltration unit (model 8200, MWCO, 10K; Amicon) at 75 lb/in2 (5.3 kg/cm2). All procedures were performed at 4°C.
Fresh crude extract was applied to a GalNAc-agarose affinity column (Sigma-Aldrich, Seoul, South Korea) equilibrated with TBS buffer. The column was washed with the same solution until the absorbency (at 280 nm) of the washed fraction was lowered to 0.001. The bound proteins were eluted with the same solution containing 0.5 M GalNAc (12). Twenty sequential fractions of 2 ml each were collected and analyzed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (14). The fractions showing a single protein band were pooled, concentrated, and dialyzed against TBS buffer. The protein content was determined by the method of Lowry et al. (15) or Bradford (2).
SDS-PAGE.
SDS-PAGE was performed according to the method described by Laemmli (14) with a 4% stacking gel and a 12% separating gel. Samples were treated with 4% 2-mercaptoethanol to reduce disulfide bonds. Control samples without 4% 2-mercaptoethanol were prepared separately. Proteins were stained with Coomassie brilliant blue R-250 and time-limited silver staining.
MS determination.
The purified lectin was dialyzed extensively against double-distilled water and lyophilized. The exact molecular mass of the protein was determined by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Applied Biosystems, CA) at the Korean Basic Science Institute (KBSI) (Daejeon, South Korea).
Analysis of N-terminal and internal amino acid sequence.
The purified protein was electrophoresed on a 12% SDS-polyacrylamide gel and electroblotted onto a polyvinylidene difluoride (PVDF) membrane. The first 20 amino acids of the N-terminal sequence and seven internal sequences were determined with an Applied Biosystems Precise Sequencer (Applied Biosystems) at KBSI.
Construction of cDNA libraries.
Male and female cDNA libraries were constructed. Double-stranded cDNA was synthesized using 5 μg of poly(A) RNA as a template, directionally cloned into a UniZAP-XR vector phage (ZAP-cDNA synthesis kit; Stratagene, La Jolla, CA), and packaged using the ZAP-cDNA Gigapack III Gold packaging extract. Approximately 1.8 million and 1.5 million recombinants were represented in the male and female cDNA libraries, respectively.
Cloning of cDNA encoding AOL1.
Based on the amino acid sequences derived from the purified protein, degenerate primers of both sense and antisense strands were designed (Table 1). The first round of PCR was performed with a set of primers (AOL1-NDF and T7) with cDNA library as a template using an Ex Taq polymerase (TaKaRa, Tokyo, Japan). For the second round of nested PCR, primer set AOL1-IDF/T7 and AOL1-NDF/AOL1-IDR was used. PCR was performed under the following conditions: cDNA was denatured at 95°C for 3 min followed by 40 cycles of amplification (95°C for 30 s, 53°C for 30 s, 72°C for 1 min) and by 10 min at 72°C. The PCR products were cloned into pDrive Cloning Vector (Qiagen), and their DNA sequences were determined.
Table 1.
Primers used in isolation of AOL1 cDNA
| Primer name | Sequence | Corresponding amino acid sequence |
|---|---|---|
| AOL1-NDF | TAY GTN AAY GTN GAY GA | YVNVDD |
| AOL1-IDF | GCN TTY GGN GGN TTY CC | AFGGFP |
| AOL1-IDR | GGR AAN CCN CCR AA | AFGGFP |
| AOL1-ISR1 | CGG ACG TAC ACG TCA GTT TTC G | |
| AOL1-ISR2 | CAT TGG CAC GCA TCG TAT GCC | |
| AOL1-NSF | GAT ATG TCC GGC TCG TAC ACG CTC | |
| AOL1-CSR | CTA GTT TCC GCA CCA ATG ATA ATC |
Based on the sequences of the 3′ end PCR products, the specific primers were designed. The initial PCR was performed with a gene-specific primer (AOL1-ISR1) and vector primer (M13 reverse). To increase the specificity and identify the desired amplification product, an aliquot of reaction product from the initial PCR was reamplified using the nested gene-specific primer (AOL1-ISR2) and vector primer (SK). The resulting PCR product was subcloned and sequenced as described above, and the sequence was deposited in GenBank.
Screening of the cDNA library.
To isolate a full-length cDNA, the cDNA library was screened by plaque hybridization. A probe for plaque hybridization was generated by PCR using primers AOL1-NSF and AOL1-CSR, which amplified the coding sequence of AOL1. The probe was labeled with digoxigenin (DIG)-dUTP using the PCR DIG probe synthesis kit (Roche, Mannheim, Germany) and purified using the QIAquick gel extraction kit (Qiagen). For cDNA library screening, 3 × 105 plaques were transferred onto Hybond-N+ membranes (Amersham Pharmacia Bioscience). After UV cross-linking, the membranes were prehybridized for 3 h at 42°C in DIG EasyHyb Solution (Roche, USA), followed by hybridization with DIG-labeled DNA probe at 42°C for 16 h. After washing twice with 0.5× SSC (1×SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 0.1% SDS at 68°C for 15 min, colorimetric detection was performed using nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate in the DIG-DNA detection kit as described in the manufacturer's instructions. Five positive plaques were converted to plasmids using the VCSM13 helper phage (Stratagene, La Jolla, CA) and sequenced.
Reverse transcriptase PCR (RT-PCR) and genomic DNA-PCR.
Total RNA and genomic DNA (gDNA) were isolated simultaneously from the same tissue sample. Contaminated DNA in the RNA preparation was removed by RNase-free DNase I treatment, while contaminated RNA in DNA preparations was removed by DNase-free RNase A treatment (Promega, Madison, WI). The total RNA underwent oligo(dT)-primed reverse transcription using StrataScriptase according to the manufacturer's instructions. The cDNA and gDNA (at 0.1 μg/μl) were used as the template for PCRs. PCRs were done with Ex Taq Polymerase (TaKaRa, Tokyo, Japan) using the same primers (AOL1-NSF/AOL1-CSR) and annealing conditions.
2-DE.
For two-dimensional polyacrylamide gel electrophoresis (2-DE), male and female plants were frozen with liquid nitrogen and stored at −80°C before use. Materials were homogenized using a mortar-driven homogenizer (PowerGen125) in a lysis solution consisting of 7 M urea, 2 M thiourea, 4% (wt/vol) 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfate (CHAPS), 1% (wt/vol) dithiothreitol (DTT), 2% (vol/vol) pharmalyte, and 1 mM benzamidine. Freezing and thawing steps for the samples were repeated 5 times in 1 day. After centrifugation at 15,000 × g for 60 min at 18°C, insoluble material was discarded and the soluble fraction was used for 2-DE. Protein loading was normalized using a modified Bradford assay (2, 21).
Isoelectric focusing (IEF) was performed with 200 μg of sample at 20°C using a Multiphor II electrophoresis unit and EPS 3500 XL power supply (Amersham Biosciences) following the manufacturer's instruction, and then SDS-PAGE (20 by 24 cm, 10 to 16% polyacrylamide) was performed using Hoefer DALT 2-DE system (Amersham Biosciences) following the manufacturer's instruction. The gels were stained with CBB G250.
Northern blot analysis.
The DNA probe was directly amplified and labeled with DIG-dUTP by PCR from the cDNA library using a DIG probe synthesis kit (Roche, USA). The PCR product was separated on a 1.2% (wt/vol) agarose gel. Total RNA was extracted from male and female plants developing sexual reproductive structures. Equal amounts of total RNA (5 μg) were loaded and separated on 1.2% (wt/vol) formaldehyde-agarose gels and photographed to confirm RNA quality and to verify equal sample loading. RNA was transferred onto Biodyne Nylon B membranes (Pall Life Science) by capillary transfer with 20× SSC (3 M NaCl, 0.3 M sodium citrate [pH 7.0]), and immobilized to the membranes by UV cross-linking. Membrane was incubated with prehybridization solution (DIG Easy Hyb; Roche) for 2 h and then hybridization solution (DIG Easy Hyb with 25 ng/ml DIG probe) overnight at 50°C. The blots were washed in 2× SSC–0.1% SDS and then 0.5× SSC–0.1% SDS at room temperature. The mRNA was immunologically detected by antidigoxigenin probes. The blots were then exposed to X-ray films (CP-BU; Agfa) for 2 h.
Computer-aided sequence analyses and secondary structure predictions.
Nucleotide and amino acid sequence homology searches and comparisons were carried out using BLAST in GenBank (http://www.ncbi.nlm.nih.gov/GenBank/), EMBL (http://www.ebi.ac.uk/embl/), PDB (http://www.rcsb.org/), and Uniprot (http://www.uniprot.org/). Posttranslational modifications of protein were identified using the CBS prediction service (http://www.cbs.dtu.dk/services/).
Hemagglutinating activity assay.
Fresh crude extract was prepared before the experiment. Horse blood and rabbit blood used in the assay were purchased from Hanil Comed (Seongnam, South Korea), and human blood types O, A, and B were obtained from the Chungnam National University hospital (Daejeon, South Korea). Erythrocytes were first washed from the blood plasma with saline solution (0.9% NaCl in double-distilled water). For the investigation of hemagglutinating activity, we followed protocols described by Hori et al. (5). A serial 2-fold dilution of the crude extract was made in a final volume of 25 μl saline in microtiter plate wells, and 25-μl erythrocyte suspensions were added sequentially to each well. The reciprocal of the highest dilution of the lectin showing complete agglutination was taken as the agglutination titer. The minimum amount of lectin required for complete agglutination was defined as 1 agglutination unit (AU).
Binding assay.
Dense suspensions of spermatia were obtained from male plants with actively developing spermatial clusters as described previously (11). Male plants were incubated in 10 ml of fresh culture medium with mild shaking for 12 h. After male plants were removed, the solution was diluted to various concentrations (102 to 104 spermatia/ml) by adding culture medium. Released spermatia were preincubated for 60 min at 20°C in a solution containing various concentrations of purified proteins or foreign lectins (1 to 25 μg/ml) as well as carbohydrates (10 mM). Spermatial binding to trichogynes was assayed with ca. 100 trichogynes placed in various concentrations of spermatia (10 to 400 spermatia per trichogyne) for 2 h at 20°C with mild rotation. The total number of trichogynes and the percentage of trichogynes with one or more attached spermatia were recorded. The average number of spermatia attached to a trichogyne was also determined. Assays were conducted in triplicate, counting a minimum of 100 trichogynes from each replicate. Variation between replicates was within 5%.
Nucleotide sequence accession number.
The sequence determined in this study has been deposited in GenBank under accession number JX164251.
RESULTS
Isolation and purification of the lectin.
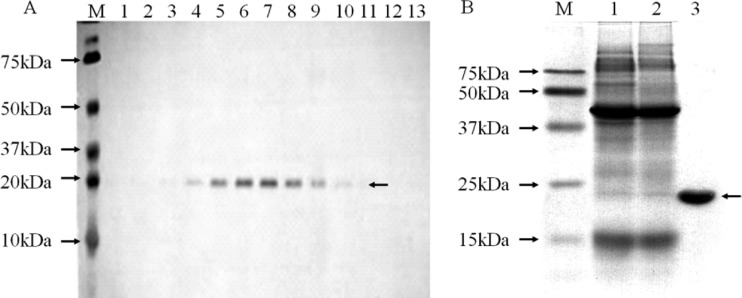
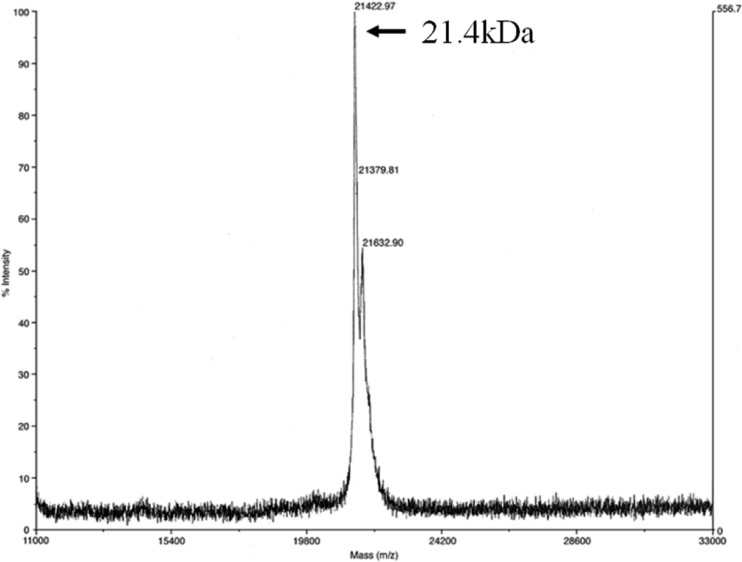
We chose a GalNAc-agarose affinity column to purify the lectin from A. oosumiense because previous cytochemical studies suggested the presence of a complementary lectin for GalNAc on the surface of female trichogynes. Crude extract of female plants was loaded onto the affinity column, and the eluate fractions showed only one protein band (Fig. 1A). SDS-PAGE analyses of the protein contents showed that this one-step column purification was good enough to yield a homogeneous protein (Fig. 1B). The molecular mass of the intact lectin was determined as 21.4 kDa by MALDI-TOF MS (Fig. 2). The lectin showed a single protein band pattern on SDS-PAGE with or without the reducing agent, 2-mercaptoethanol, and the mobility of the protein band was not changed (Fig. 3). The purified lectin did not show agglutinating activity to rabbit and horse blood cells or to human blood groups (data not shown).
Fig 1.
Purification of female-specific lectin AOL1 from Aglaothamnion oosumiense by the use of agarose-bound GalNAc affinity chromatography. (A) SDS-PAGE of eluted proteins collected from GalNAc-agarose column. Lanes 1 to 13, gel electrophoresis according to the order of eluted fractions 1 through 13. The arrow points to the protein bands with a molecular mass of ca. 22 kDa. (B) SDS-PAGE of samples according to purification step. Lane 1, crude extract; lane 2, washing fraction; lane 3, purified AOL1. M, molecular mass markers.
Fig 2.
Molecular mass determination of AOL1 by MALDI-TOF MS.
Fig 3.
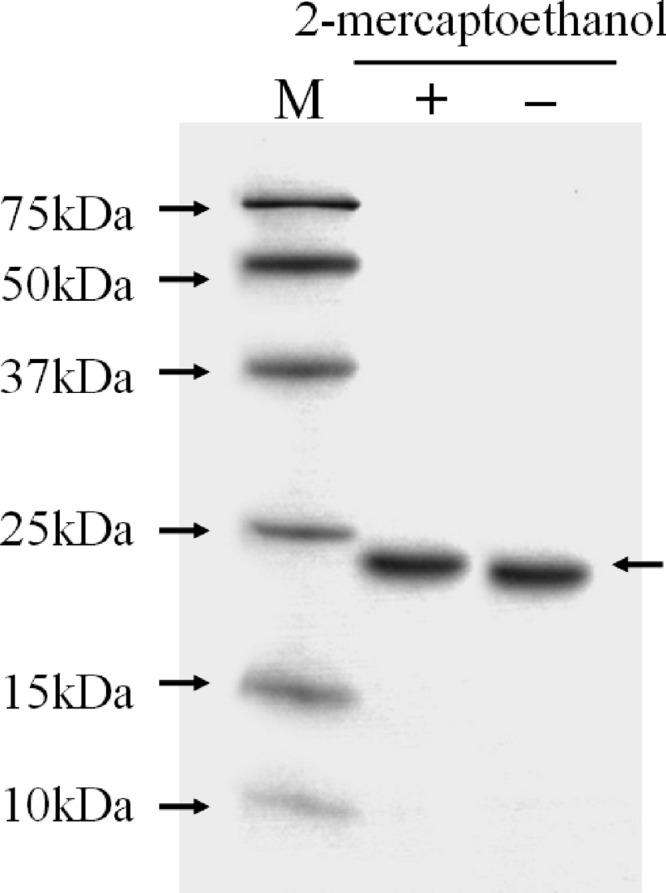
Effect of 2-mercaptoethanol on the structural properties of AOL1. M, molecular mass markers; +, protein treated with 2-mercaptoethanol; −, protein without 2-mercaptoethanol.
Molecular characterization and cloning.
The N-terminal 20 amino acids of the lectin were Asp-Met-Ser-Gly-Ser-Tyr-Thr-Leu-Tyr-Val-Asn-Val-Asp-Asp-Ala-Ala-Asp-Val-Tyr-Leu, which shows no sequence homology with any proteins in public databases (NCBI, PDB, EMBL, etc.). To develop effective degenerate primers, we analyzed an internal sequence (Table 1; Fig. 4). Three degenerate primers were also designed based on the internal amino acid sequences of the protein. A fragment of about 850 bp was amplified using primers AOL1-NDF and T7, and a 3′ untranslated region (UTR) of 240 bp was found downstream from the stop codon in the amplified sequence. The deduced amino acid sequence of the 850-bp fragment contained those of the two known internal tryptic peptide sequences obtained from the protein. Based on the sequence of the 3′ rapid amplification of cDNA ends (RACE) fragment, two reverse specific primers were designed and used for the amplification of the 5′ end sequence of the cDNA. A 550-bp fragment was obtained, in which a 5′ UTR of 80 bp was found upstream of the first ATG codon. Based on the sequences of the 3′ and 5′ RACE products, a full-length cDNA fragment was obtained (Fig. 4).
Fig 4.
Full cDNA sequence and deduced amino acid sequence of AOL1. Solid lines represent N-terminal and internal amino acid sequences used for designing degenerate primers; the dashed line shows the internal amino acid sequence obtained from the protein spots in 2-DE gel. CAF, chemically assisted fragmentation.
cDNA cloning of the lectin was performed using an A. oosumiense cDNA library by plaque hybridization to confirm the sequence obtained by the modified RACE-PCR. After the screening of 120,000 colonies, five positive plaques were obtained. Comparison of these sequences revealed no significant variation. The full-length cDNA of the protein was 1,095 bp.
Analysis of deduced amino acid sequences.
An open reading frame (ORF) of 780 bp within the AOL1 cDNA sequence encoded a polypeptide of 259 amino acids. The N-terminal and tryptic peptide amino acid sequences, which are the characteristics of isolated AOL1, were present in the deduced sequences (Fig. 4). According to the rules of predicting signal peptides (18), a 19-amino-acid-long signal peptide with a signal peptide cleavage site between Thr19 and Ser20 was identified from the deduced protein sequence. The predicted protein sequence did not contain a potential N-linked glycosylation site. From the deduced amino acid sequence, the molecular mass was calculated to be 21.4 kDa and the theoretical isoelectric point (pI) value was 4.17, which was in good agreement with that of AOL1 estimated by SDS-PAGE and MALDI-TOF mass spectrometry.
Alignment and Web-based structure prediction of the deduced amino acid sequence.
BLAST search results indicated that the amino acid sequence of AOL1 has no significant similarity to any known proteins. The subcellular location of the protein was analyzed using Web-based prediction programs (Targetp v 1.1, plant option [http://www.cbs.dtu.dk/services/TargetP]). AOL1 with an N-terminal signal peptide was predicted to be a secretory protein targeted to the cell wall or vacuole (prediction value, 0.818) rather than to mitochondria (prediction value, 0.063) or to chloroplasts (prediction value, 0.090). Another analysis using a molecular structure prediction program, Phyre2 (http://www.sbg.bio.ic.ac.uk/phyre2), showed that AOL1 has a unique molecular structure with no significant similarity with any known protein.
Determination of the genomic DNA encoding AOL1.
Genomic DNA PCR analysis using primers AOL-NSF and AOL-CSR, which span 621 bp of the ORF of AOL1, resulted in a single product of the same size, which indicated that the gene encoding AOL1 contains no intron, and sequences like that obtained by RT-PCR using the same primers (data not shown). The male nuclear genome also contained the AOL1 gene, and the sequence was identical to that of the female.
Sex specificity of the lectin.
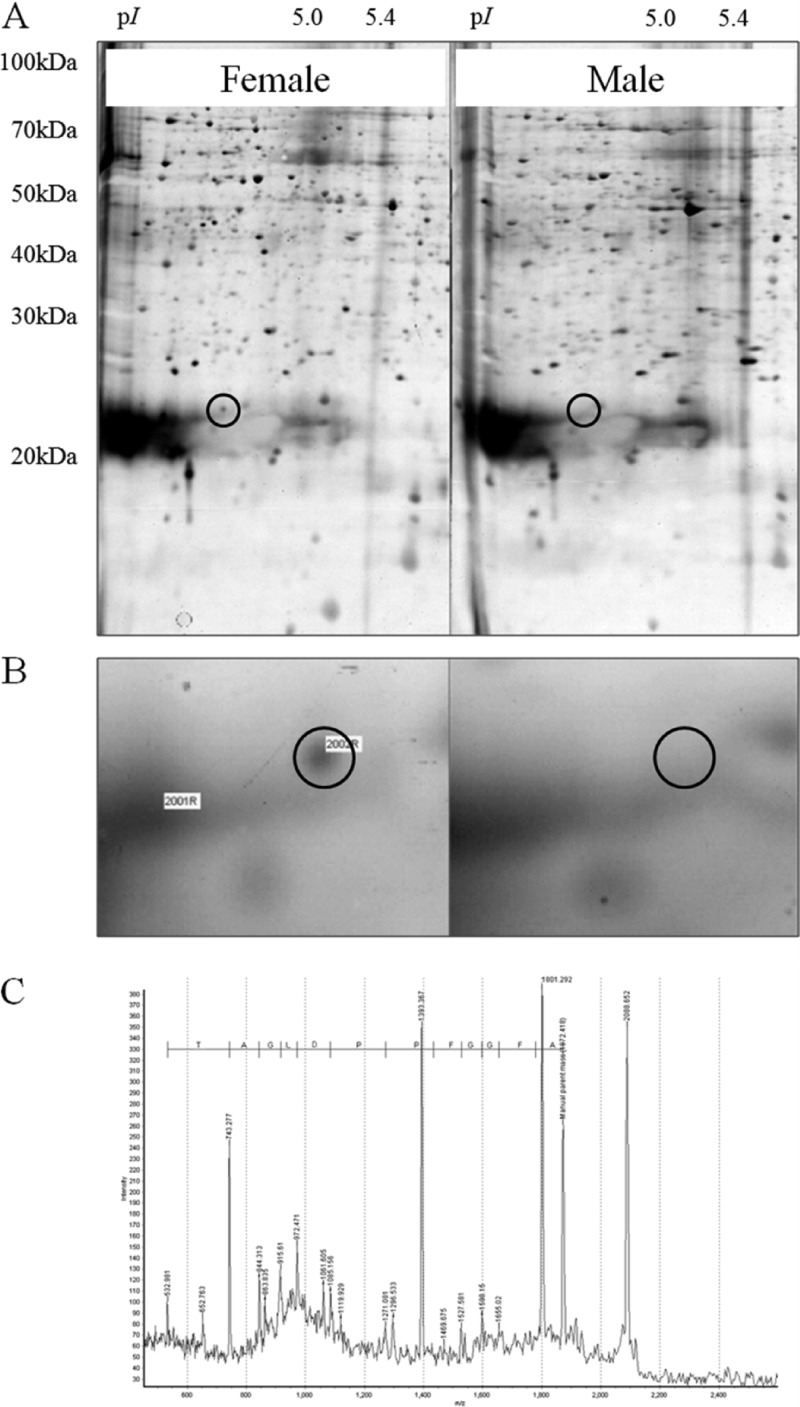
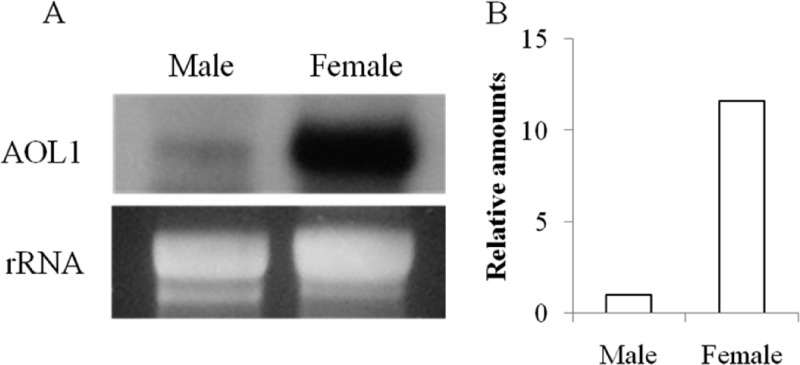
Sex specificity of the lectin has been analyzed using proteomic and Northern blot analyses. When 2-DE images of male and female plants were compared, a female-specific protein was found at the position predicted from the molecular mass (about 22 kDa) and pI (4.82 value) of AOL1 (Fig. 5). MALDI-TOF MS analysis of the internal amino acid sequence of the female-specific protein confirmed it as AOL1 (Fig. 4, dashed line, and 5). Northern blot analysis showed that the expression of the AOL1 gene was 12 times higher in female plants than in male plants (Fig. 6).
Fig 5.
(A) 2-DE gel images of the female and male plants of Aglaothamnion oosumiense. (B) Enlarged 2-DE gel images. Circles point to the protein spot corresponding to AOL1. (C) Internal amino acid sequence obtained by chemically assisted fragmentation (CAF)-MALDI sequencing.
Fig 6.
Northern blot using AOL1 probe showing relative expression level of AOL1 gene in males and females of Aglathamnion oosumiense. (A) Gel image and RNA loading control. (B) Relative amount of mRNA in male and female plants determined by image analysis system.
Inhibition experiment of gamete binding by AOL1.
Spermatial attachment to trichogynes occurred within a few seconds after mixing and reached its maximum value within 30 min at 2 × 104 spermatia/ml (data not shown). The percentage of spermatial attachment to trichogynes was assayed after preincubation of spermatia or trichogynes with GalNAc and purified lectin (Table 2). The binding of spermatia to trichogynes was inhibited by AOL1 and soybean agglutinin (SBA). However, AOL1 showed almost the same inhibitory effect as the same concentration of SBA. When AOL1 was heat denatured or preincubated with the complementary sugar, GalNAc, the inhibitory effect disappeared (Table 2). The average number of spermatia attached to a trichogyne also decreased when they were preincubated with AOL1.
Table 2.
Inhibition of spermatial attachment to trichogynes by lectins and carbohydratesa
| Treatment | % of trichogynes with attached spermatia | Avg no. of attached spermatia per trichogyne |
|---|---|---|
| Control (enriched seawater) | 97.8 ± 1.2 | 7.1 |
| Preincubation of spermatia with: | ||
| AOL1 | 56.2 ± 4.5 | 4.6 |
| Heat-denatured AOL1 | 92.8 ± 4.7 | 6.6 |
| AOL1 + GalNAc | 90.2 ± 5.4 | 5.3 |
| SBAb | 58.7 ± 4.8 | 4.3 |
| SBA + GalNAc | 82.6 ± 5.4 | 6.8 |
Concentrations used were 20 μg/ml for lectins and 10 mM for carbohydrates.
SBA, soybean agglutinin.
DISCUSSION
A novel GalNAc-specific lectin in A. oosumiense was purified and named AOL1. AOL1 was monomeric and had a molecular mass of 21.4 kDa. Full-length cDNA encoding the lectin was obtained. The lectin was expressed in a sex-specific manner, in female plants. The lectin reduced spermatial binding to the trichogyne when the spermatia were preincubated with it.
A lectin-carbohydrate complementary binding was proposed for the gamete recognition in A. oosumiense (11). Fluorescent lectin staining on the spermatial surface showed two types of glycoconjugates, GalNAc and α-methyl-d-mannose, specific to SBA and ConA, respectively. The presence of their complementary lectinlike receptors was shown by inhibition experiment. We chose a GalNAc affinity column to purify AOL1 because the GalNAc moieties were located on spermatial appendages and its complementary lectin, SBA, showed a stronger inhibitory effect on gamete binding, while the ConA receptors were involved in development of the fertilization canal (11). Our results showed that AOL1 was only one of the lectins specific to this sugar in female plants of A. oosumiense. Sex-specific expression of AOL1 also suggests that it might be a female gamete recognition molecule. Although PCR analysis using genomic DNA showed that both male and female plants have the same AOL1 gene, Northern blot analysis showed that mRNA of AOL1 was expressed 12 times more in female plants and 2-DE analysis confirmed the female-specific expression of AOL1 at the protein level. These results support the idea that AOL1 is a strong candidate for a female recognition molecule (GalNAc receptor) in A. oosumiense. Recently, another sex-specific lectin, rhodobindin, was reported from a closely related species, Aglaothamnion callophyllidicola, and was suggested to be involved in gamete binding as well (25). Rhodobindin could block the spermatial binding to female trichogyne when the spermatia were preincubated with this lectin. However, rhodobindin did not show any specificity to the sugar GalNAc. Our previous cytochemical study suggested that the gamete binding in A. oosumiense might be mediated by a double-docking mechanism involving two lectins (11). Purification of both lectins in one species of Aglaothamnion is essential to examine this hypothesis.
Spermatial attachment to trichogynes was inhibited when the spermatia were preincubated with purified AOL1. The inhibitory effect of AOL1 disappeared when the protein was preincubated with the complementary sugar GalNAc. However, AOL1 blocked gamete binding to almost the same level as the positive control, SBA; 20 μg/ml of AOL1 and SBA was necessary to reduce the gamete binding to 57% and 59% of control, respectively. This inhibitory effect seems too low to be a recognition molecule because we expected a more specific and stronger inhibitory effect from AOL1 than from foreign lectins. As gamete binding of A. oosumiense occurs first between the cell walls of trichogynes and the spermatial coverings, the female binding proteins are expected to be embedded in the trichogyne cell wall. The results of primary structure analysis suggest that AOL1 might be a secretory protein targeted to the cell wall because AOL1 has no N-terminal signal peptide and no predicted subcellular location. The molecular size of AOL1 (21.4 kDa), however, seems a bit small to mediate binding between male spermatia and trichogynes. It is still too early to conclude that AOL1 is the female gamete recognition molecule. More direct evidence using antibody labeling to localize the distribution of AOL1 on the trichogyne surface would confirm this.
The purified AOL1 did not show agglutinating activity for rabbit and horse blood cells or for any human blood group. Although the lectins were originally known as agglutinins, which agglutinate cells or precipitate glycoconjugates because of their sugar binding activity, a recent definition of lectin is more focused on their reversible sugar binding activity (19, 23). There are many lectins that have only one noncatalytic domain and hence do not show agglutinating activity. A negative agglutination result does not necessarily mean that a sugar binding activity is absent (30). Furthermore, agglutination assays detect only lectins having multiple binding sites (hololectins), whereas other lectins, although able to interact with specific sugars, do not cause agglutination (20).
Accumulating evidence indicates that the vast majority of known terrestrial plant lectins can be classified into four large and three small families of structurally and evolutionarily related proteins (29), but AOL1 did not belong to any of them based on its primary structure. AOL1 showed no sequence homology with any proteins reported in public protein databases. It is not surprising, because very few gene sequences of algal lectins have been reported so far (3, 6). The sex-specific nature of AOL1 may also explain the unique primary structure of the protein because the genes that mediate sexual reproduction are more divergent and rapidly evolving than the genes that are expressed in nonreproductive tissues (26–28).
As a consequence of their sugar binding properties, lectins have become a useful tool in many fields of biological research and one of the most commercially important groups of proteins (for an example, see reference 23). Although publications on algal lectins increased dramatically during the last several years, their biological roles are still unknown. AOL1 has a characteristic effect in gamete binding, but it is still too early to conclude that it is a female recognition molecule. Further studies including purification of possible membrane-bound lectins and antibody labeling to localize the distribution of AOL1 on the trichogyne surface are necessary to confirm this.
ACKNOWLEDGMENTS
We express our sincere thanks to G. C. Zuccarello for his careful review of and helpful comments on the manuscript.
This study was funded by the National Research Foundation of Korea (grant NRF 20120006718 to G.H.K.). This research was also supported by a grant from the Extreme Genomics Program funded by the Ministry of Land, Transport and Maritime Affairs of the Korean Government to G.H.K.
Footnotes
Published ahead of print 3 August 2012
REFERENCES
- 1. Bolwell GP, Callow JA, Callow ME, Evans LV. 1980. Fertilization in brown algae. III. Preliminary characterization of putative gamete receptors from eggs and sperm of Fucus serratus. J. Cell Sci. 43:209–224 [DOI] [PubMed] [Google Scholar]
- 2. Bradford MM. 1976. A rapid and sensitive method for the quantification of microgram quantities of proteins using the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 3. Han JW, Yoon KS, Klochkova TA, Hwang MS, Kim GH. 2011. Purification and characterization of a lectin, BPL-3, from the marine green alga Bryopsis plumosa. J. Appl. Phycol. 23:745–753 [Google Scholar]
- 4. Han JW, Yoon KS, Jung MG, Chah K-H, Kim GH. 2012. Molecular characterization of a lectin, BPL-4, from the marine green alga Bryopsis plumosa (Chlorophyta). Algae 27:55–62 [Google Scholar]
- 5. Hori K, Miyazawa K, Fusetani N, Hashimoto K, Ito K. 1986. Hypnins, low-molecular weight peptidic agglutinins isolated from a marine red alga, Hypnea japonica. Biochim. Biophys. Acta 873:228–236 [Google Scholar]
- 6. Jung MG, et al. 2010. Characterization of carbohydrate combining sites of Bryohealin, an algal lectin from Bryopsis plumosa. J. Appl. Phycol. 22:793–802 [Google Scholar]
- 7. Kim GH. 1997. Gamete recognition and signal transduction during fertilization in red algae. Algae (Kor. J. Phycol.) 12:263–268 [Google Scholar]
- 8. Kim GH, Fritz L. 1993. A signal glycoprotein with a-D-mannosyl residues is involved in the wound-healing response of Antithamnion sparsum (Ceramiales, Rhodophyta). J. Phycol. 29:85–90 [Google Scholar]
- 9. Kim GH, Fritz L. 1993. Gamete recognition during fertilization in a red alga Antithamnion nipponicum. Protoplasma 174:69–73 [Google Scholar]
- 10. Kim GH, Lee IK, Fritz L. 1996. Cell-cell recognition during the fertilization in a red alga, Antithamnion sparsum (Ceramiaceae, Rhodophyta). Plant Cell Physiol. 37:621–628 [Google Scholar]
- 11. Kim S-H, Kim GH. 1999. Cell-cell recognition during the fertilization in a red alga Aglaothamnion oosumiense (Ceramiaceae, Rhodophyta). Hydrobiologia. 398/399:81–89 [Google Scholar]
- 12. Kim GH, Klochkova TA, Yoon KS, Song Y-S, Lee KP. 2006. Purification and characterization of a lectin, bryohealin, involved in the protoplast formation of a marine green alga Bryopsis plumosa (Chlorophyta). J. Phycol. 42:86–95 [DOI] [PubMed] [Google Scholar]
- 13. Klochkova TA, Han JW, Kim J-H, Kim KW, Kim GH. 2012. Feeding specificity and photosynthetic activity of Korean sacoglossan mollusks. Algae 25:217–227 [Google Scholar]
- 14. Laemmli UK. 1970. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 15. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275 [PubMed] [Google Scholar]
- 16. Maier I, Müller DG. 1986. Sexual pheromones in algae. Biol. Bull. 170:145–175 [Google Scholar]
- 17. Mine I, Tatewaki M. 1994. Gamete surface and attachment during fertilization of Palmaria sp. (Palmariales, Rhodophyta). Jpn. J. Phycol. 42:291–299 [Google Scholar]
- 18. Nielsen H, Engelbrecht J, Brunak S, Heijine GV. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. Des. Sel. 10:1–6 [DOI] [PubMed] [Google Scholar]
- 19. Peumans WJ, Van Damme EJM. 1995. Lectin as plant defense proteins. Plant Physiol. 109:347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peumans WJ, Van Damme EJM. 1996. Prevalence, biological activity and genetic manipulation of lectins in foods. Trends Food Sci. Technol. 7:132–138 [Google Scholar]
- 21. Ramagli LS, Rodriguez LV. 1985. Quantitation of microgram amounts of protein in two-dimensional polyacrylamide gel electrophoresis sample buffer. Electrophoresis 6:559–563 [Google Scholar]
- 22. Schmid C. 1993. Cell-cell recognition during fertilization in Ectocarpus siliculosus (Phaeophyceae). Hydrobiologia 260/261:437–443 [Google Scholar]
- 23. Sharon N. 2007. Lectins: carbohydrate-specific reagents and biological recognition molecules. J. Biol. Chem. 282:2753–2764 [DOI] [PubMed] [Google Scholar]
- 24. Sharon N, Lis H. 2004. Lectins: from hemagglutinins to biological recognition molecules. A historical overview. Glycobiology 14:53R–62R [DOI] [PubMed] [Google Scholar]
- 25. Shim E, Shim J, Klochkova TA, Han JW, Kim GH. 2012. Purification of a sex-specific lectin involved in gamete binding of Aglaothamnion callophyllidicola (Rhodophyta). J. Phycol. 48:916–924 [DOI] [PubMed] [Google Scholar]
- 26. Singh RS, Kulathinal RJ. 2000. Sex gene pool evolution and speciation: a new paradigm. Genes Genet. Syst. 75:119–130 [DOI] [PubMed] [Google Scholar]
- 27. Swanson W, Vacquier VD. 2002. The rapid evolution of reproductive proteins. Nat. Rev. Genet. 3:137–144 [DOI] [PubMed] [Google Scholar]
- 28. Vacquier VD. 1998. Evolution of gamete recognition proteins. Science 281:1995–1998 [DOI] [PubMed] [Google Scholar]
- 29. Van Damme EJM, Peumans WJ, Barre A, Rouge P. 1998. Plant lectins: a composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. CRC Crit. Rev. Plant Sci. 17:575–692 [Google Scholar]
- 30. Vincenzi S, et al. 2002. Quantitative determination of dietary lectin activities by enzyme-linked immunosorbent assay using specific glycoproteins immobilized on microtiter plates. J. Agric. Food Chem. 50:6266–6270 [DOI] [PubMed] [Google Scholar]
- 31. Wiese L, Shoemaker DW. 1970. On sexual agglutination and mating-type substance (gamones) in isogamous heterothalic Chlamydomonas. II. The effect of concanavalin A upon the mating-type reaction. Biol. Bull. 138:88–95 [Google Scholar]
- 32. Wilson S, West JA, Pickett-Heaps JD. 2003. Time-lapse videomicroscopy of fertilization and the actin cytoskeleton in Murrayella periclados (Rhodomelaceae, Rhodophyta). Phycologia 42:638–645 [Google Scholar]
- 33. Yoon K-S, Lee KP, Klochkova TA, Kim GH. 2008. Molecular characterization of the lectin, bryohealin, involved in protoplast regeneration of the marine alga Bryopsis plumosa (Chlorophyta). J. Phycol. 44:103–112 [DOI] [PubMed] [Google Scholar]