Abstract
Mycobacterium smegmatis is a commonly used mycobacterial model system. Here, we show that M. smegmatis protects itself against elevated salinity by synthesizing ectoine and hydroxyectoine and characterize the phenotype of a nonproducing mutant. This is the first analysis of M. smegmatis halotolerance and of the molecular mechanism that supports it.
TEXT
Mycobacterium smegmatis, an actinobacterial species, is commonly found in soil, marine, and freshwater environments (20). Due to its nonpathogenic nature and relatively high growth rate, M. smegmatis is used as a model mycobacterial system, especially for the study of pathogenic mycobacteria, including Mycobacterium tuberculosis. The natural habitat of M. smegmatis, however, is very different from that of obligatory pathogens; to thrive in nature, M. smegmatis must be capable of adapting to fluctuations in various environmental conditions. This report focuses on M. smegmatis adaptation to high salinity.
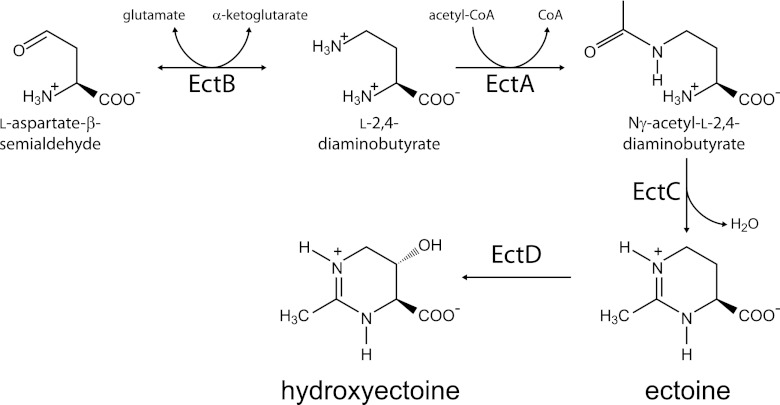
Two different strategies for survival in salty environments are known. Confined mainly to extreme halophiles, one strategy is the intracellular accumulation of inorganic salts to counterbalance the high extracellular salt concentrations (19, 25). A second strategy, typical in moderate halophiles and halotolerants, is the synthesis of low-molecular-weight, organic osmolytes that counterbalance the extracellular osmotic pressure but do not increase the ionic strength of the cytoplasm (19, 26). These molecules, termed compatible solutes, are usually zwitterionic or uncharged and are highly soluble in water (26). Commonly found compatible solutes in bacteria are ectoine and its hydroxylated derivative, hydroxyectoine (7, 10, 11, 21, 24). The ectoine biosynthetic enzymes are encoded by three genes: ectA, ectB, and ectC (22) (Fig. 1). An additional enzyme, encoded by ectD, is often found in many ectoine-producing species and is responsible for the conversion of ectoine to hydroxyectoine (11). These genes are highly conserved in diverse bacterial species residing in environments of high or changing salinity, and it was shown in several cases that ectoine and hydroxyectoine biosyntheses were strongly induced under conditions of high salt concentrations (2, 3, 4, 6, 8, 15, 16) and that ectoine-deficient mutants were salt sensitive (5, 9, 23, 29). There is also evidence for induction of the ect genes at elevated temperatures (2, 8) and for the ability of ectoine and, to a greater extent, of hydroxyectoine to protect proteins against thermal denaturation (1, 12, 13, 14, 17). In addition, ectoine can effectively protect the skin of humans against dehydration and against the carcinogenic effect of long-term exposure to sunlight. Ectoine is, therefore, commonly found in sunscreens and body lotions, which results in an immense industrial importance of this chemical (21).
Fig 1.
The biosynthetic pathway of ectoine and hydroxyectoine.
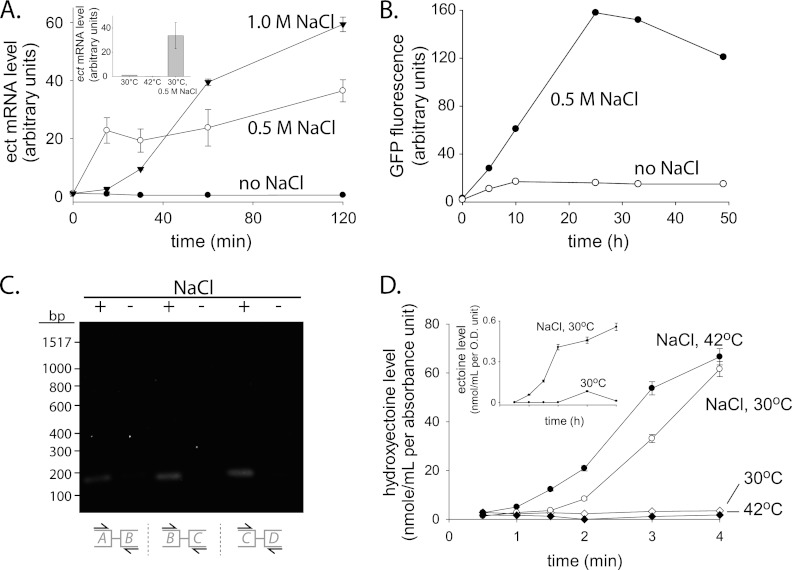
The annotated genome sequence of M. smegmatis strain MC2155 indicates the presence of a putative hydroxyectoine biosynthesis gene cluster (18, 27, 28). To examine whether this cluster is transcribed, a quantitative reverse transcription-PCR (qRT-PCR) analysis was carried out. NaCl was added to exponentially growing M. smegmatis cultures (30°C; 7H9 media containing 0.4% glycerol and 0.05% Tween 80), and samples were collected over the course of 2 h. For each sample, total RNA was purified, and qRT-PCR was carried out using specific ectB primers (see Table S1 in the supplemental material). Control cultures were kept at 30°C in the original growth medium without NaCl addition. An approximately 40- to 60-fold increase in the ectB mRNA level was detected in cultures containing 0.5 and 1 M NaCl, suggesting a strong induction in response to an increase in salinity (Fig. 2A). In contrast, no induction was observed in control cultures and in cultures that were grown at 42°C in the absence of added salt. Therefore, high salinity, but not high temperatures, leads to induction of the M. smegmatis ectB gene. Induction in response to an increase in salinity could also be observed using green fluorescent protein (GFP) as a reporter protein (Fig. 2B). To test whether the ect gene cluster comprises an operon, cDNA was purified and analyzed by PCR using primers specific for the DNA junctions between ectA and -B, ectB and -C, and ectC and -D. Specific bands were clearly detected in samples taken from cultures incubated with NaCl, indicating that the ect gene cluster does indeed form an operon (Fig. 2C).
Fig 2.
The ect biosynthetic pathway is induced at high salt concentrations. (A) Quantitative RT-PCR (qRT-PCR) analysis of the ectB gene was carried out (primers 1 and 2; see Table S1 in the supplemental material) for M. smegmatis cultures (optical density at 600 nm [OD600] = 0.6) that were grown for 2 h at 30°C with 0, 0.5, and 1 M NaCl or without salt addition. M. smegmatis sigA was used as an internal reference gene (primers 3 and 4; see Table S1 in the supplemental material). A similar analysis was carried out for cultures that were incubated at 42°C without salt (inset). (B) M. smegmatis cultures (OD600 = 0.5) that express GFP under the transcriptional control of a putative ectA promoter (237 bp upstream of the ectA translation start site) were grown at 30°C with or without NaCl (0.5 M), and samples were collected at the indicated time points. Following cell lysis, GFP fluorescence was measured in lysates of equal protein concentrations. (C) Exponentially growing M. smegmatis cultures (30°C, OD600 = 0.5) were incubated for 2 h with or without NaCl (0.5 M). cDNA was purified and used as a template for PCR amplification reactions with primer pairs 5 and 6, 7 and 8, and 9 and 10 (see Table S1 in the supplemental material). (D) Hydroxyectoine levels as measured by LCMS analysis of samples collected at the indicated time points from cultures that were grown in 7H9 media with and without NaCl (0.5 M) at 30 or 42°C. Ectoine levels are plotted in the inset. The solute levels were determined using a standard curve of commercially available ectoine and hydroxyectoine (Sigma).
To test whether ectoine and hydroxyectoine are actually synthesized following an increase in salt concentration, M. smegmatis cultures were grown with and without salt at either 30 or 42°C, and samples were collected over the course of 4 h. From each sample, small molecules were extracted as described previously (2), and the amounts of ectoine and hydroxyectoine were analyzed by liquid chromatography-mass spectrometry (LCMS) using a Clarity Oligo-WAX column (100 by 4.6 mm, 300 Å). The results show that after the addition of salt, but not in its absence, hydroxyectoine gradually accumulated (Fig. 2D). Ectoine also accumulated in salt-containing cultures but to a level ∼100-fold lower than that of hydroxyectoine (Fig. 2D, inset). Incubation at 42°C in the absence of salt did not lead to hydroxyectoine synthesis, further indicating that M. smegmatis produces ectoine in response to an increase in salinity but not in response to a temperature upshift.
To phenotypically analyze an ectoine-nonproducing mutant, the ect operon was deleted from the M. smegmatis chromosome using a recently developed gene knockout system. This system is based on the use of plasmids pML2424 and pML2714 for the construction and selection of desired deletion mutants (see Fig. S1 in the supplemental material). Plasmid pML2424 allows directed insertion of a gfp-hyg cassette instead of a gene of interest in the M. smegmatis chromosome. Plasmid pML2714 enables excision of the gfp-hyg cassette from the chromosome via site-specific recombination (see Fig. S2 in the supplemental material). To delete the ect operon from the M. smegmatis chromosome, flanking regions of the M. smegmatis ect operon were cloned before and after the gfp-hyg cluster (see Fig. S1 and S2A in the supplemental material), and isolation of the desired mutants was confirmed by PCR analysis and Southern blotting (see Fig. S2B and C in the supplemental material).
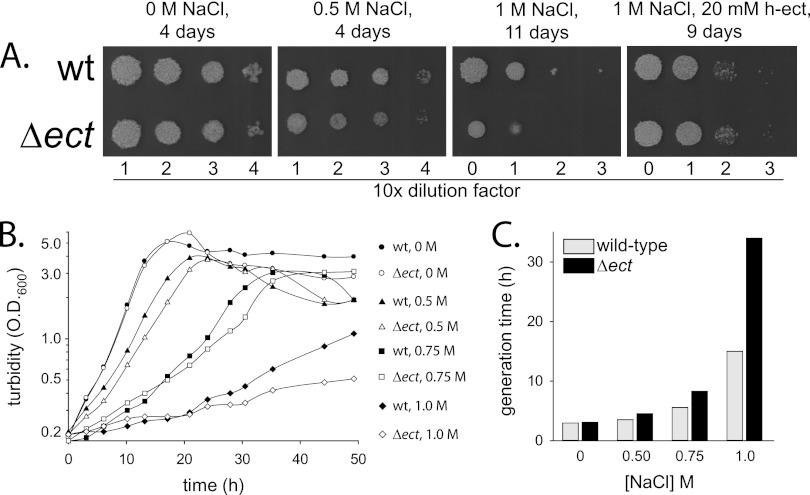
Halotolerance of a Δect mutant was examined initially by spot tests on plates containing 0, 0.5, or 1 M sodium chloride (Fig. 3A). No growth differences between the mutant and the wild type were observed in the absence of added salt. At 0.5 M salt, however, the mutant grew somewhat slower than the wild type, and at 1 M, it grew considerably slower. Addition of hydroxyectoine (20 mM) to the plates completely cured the growth defects of the mutant, indicating that despite its inability to synthesize ectoine, the mutant can accumulate the solute. The Δect growth phenotype could also be complemented by a plasmid expressing the ect operon (see Fig. S2 in the supplemental material). To better assess the phenotype of the Δect strain, its growth in liquid media containing 0, 0.5, 0.75, or 1 M NaCl was compared to that of the wild type (Fig. 3B). The Δect strain and the wild-type strain grew equally well in the absence of added salt, with generation times of 3.0 and 3.1 h, respectively (Fig. 3C). In comparison to the growth seen with the wild type, however, the growth of the mutant strain was suppressed to a greater extent in a salt-concentration-dependent manner (Fig. 3C). For example, at 0.5 M sodium chloride, the wild type multiplied with a generation time of 3.5 h whereas the generation time of the mutant was 4.5 h. The difference between the strains was much higher at 1 M sodium chloride, as the mutant multiplied about 2-fold more slowly than the wild type and had a generation time of 34 h.
Fig 3.
Osmosensitivity of a Δect mutant. (A) Spot tests of the wild-type (wt) strain and of the Δect mutant on 7H10 plates containing 0.4% glycerol and sodium chloride as indicated. In the right-hand panel, a plate containing both NaCl (1 M) and hydroxyectoine (h-ect) (20 mM) was used. (B) Growth curves of the wild-type strain (closed symbols) and of the Δect strain (open symbols) grown at 37°C in 7H9 liquid media with the addition of 0.5 (triangles), 0.75 (squares), and 1.0 M (diamonds) NaCl. No NaCl was added to control cultures (circles). (C) Generation times of the wild-type strain and the Δect mutant strain as calculated from the growth curves presented in panel B.
Conclusively, our results show that M. smegmatis is a halotolerant mycobacterial species that synthesizes ectoine and hydroxyectoine to protect itself against the deleterious effects of high salt concentrations. These mechanisms are of little significance when M. smegmatis is grown in isotonic growth media in the laboratory. In nature, however, osmoprotection likely makes a significant contribution to the ability of M. smegmatis to thrive in its habitat.
Supplementary Material
ACKNOWLEDGMENTS
We thank Frank Wolschendorf for help with the initial design of the gene deletion vector and Torin Weisbrod from Albert Einstein College of Medicine (USA) for generously providing us with plasmid pMV206.
This work was supported by German-Israeli Foundation (GIF) grant no. I-2230-2055.13/2009.
Footnotes
Published ahead of print 10 August 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Borges N, Ramos A, Raven ND, Sharp RJ, Santos H. 2002. Comparative study of the thermostabilizing properties of mannosylglycerate and other compatible solutes on model enzymes. Extremophiles 6:209–216 [DOI] [PubMed] [Google Scholar]
- 2. Bursy J, et al. 2008. Synthesis and uptake of the compatible solutes ectoine and 5-hydroxyectoine by Streptomyces coelicolor A3(2) in response to salt and heat stresses. Appl. Environ. Microbiol. 74:7286–7296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bursy J, Pierik AJ, Pica N, Bremer E. 2007. Osmotically induced synthesis of the compatible solute hydroxyectoine is mediated by an evolutionarily conserved ectoine hydroxylase. J. Biol. Chem. 282:31147–31155 [DOI] [PubMed] [Google Scholar]
- 4. Calderón MI, et al. 2004. Complex regulation of the synthesis of the compatible solute ectoine in the halophilic bacterium Chromohalobacter salexigens DSM 3043T. Microbiology 150:3051–3063 [DOI] [PubMed] [Google Scholar]
- 5. Cánovas D, et al. 1997. Isolation and characterization of salt-sensitive mutants of the moderate halophile Halomonas elongata and cloning of the ectoine synthesis genes. J. Biol. Chem. 272:25794–25801 [DOI] [PubMed] [Google Scholar]
- 6. da Costa MS, Santos H, Galinski EA. 1998. An overview of the role and diversity of compatible solutes in Bacteria and Archaea. Adv. Biochem. Eng. Biotechnol. 61:117–153 [DOI] [PubMed] [Google Scholar]
- 7. Galinski EA, Pfeiffer HP, Trüper HG. 1985. 1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid. A novel cyclic amino acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur. J. Biochem. 149:135–139 [DOI] [PubMed] [Google Scholar]
- 8. García-Estepa R, et al. 2006. The ectD gene, which is involved in the synthesis of the compatible solute hydroxyectoine, is essential for thermoprotection of the halophilic bacterium Chromohalobacter salexigens. J. Bacteriol. 188:3774–3784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Göller K, Ofer A, Galinski EA. 1998. Construction and characterization of an NaCl-sensitive mutant of Halomonas elongata impaired in ectoine biosynthesis. FEMS Microbiol. Lett. 161:293–300 [DOI] [PubMed] [Google Scholar]
- 10. Held C, Neuhaus T, Sadowski G. 2010. Compatible solutes: thermodynamic properties and biological impact of ectoines and prolines. Biophys. Chem. 152:28–39 [DOI] [PubMed] [Google Scholar]
- 11. Inbar L, Lapidot A. 1998. The structure and biosynthesis of new tetrahydropyrimidine derivatives in actinomycin D producer Streptomyces parvulus. Use of 13C- and 15N-labeled L-glutamate and 13C and 15N NMR spectroscopy. J. Biol. Chem. 263:16014–16022 [PubMed] [Google Scholar]
- 12. Kanapathipillai M, Ku SH, Girigoswami K, Park CB. 2008. Small stress molecules inhibit aggregation and neurotoxicity of prion peptide 106–126. Biochem. Biophys. Res. Commun. 365:808–813 [DOI] [PubMed] [Google Scholar]
- 13. Kanapathipillai M, Lentzen G, Sierks M, Park CB. 2005. Ectoine and hydroxyectoine inhibit aggregation and neurotoxicity of Alzheimer's beta-amyloid. FEBS Lett. 579:4775–4780 [DOI] [PubMed] [Google Scholar]
- 14. Knapp S, Ladenstein R, Galinski EA. 1999. Extrinsic protein stabilization by the naturally occurring osmolytes beta-hydroxyectoine and betaine. Extremophiles 3:191–198 [DOI] [PubMed] [Google Scholar]
- 15. Kuhlmann AU, Bremer E. 2002. Osmotically regulated synthesis of the compatible solute ectoine in Bacillus pasteurii and related Bacillus spp. Appl. Environ. Microbiol. 68:772–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuhlmann AU, Bursy J, Gimpel S, Hoffmann T, Bremer E. 2008. Synthesis of the compatible solute ectoine in Virgibacillus pantothenticus is triggered by high salinity and low growth temperature. Appl. Environ. Microbiol. 74:4560–4563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lippert K, Galinski EA. 1992. Enzyme stabilization by ectoine-type compatible solutes: protection against heating, freezing and drying. Appl. Microbiol. Biotechnol. 37:61–65 [Google Scholar]
- 18. Lo CC, Bonner CA, Xie G, D'Souza M, Jensen RA. 2009. Cohesion group approach for evolutionary analysis of aspartokinase, an enzyme that feeds a branched network of many biochemical pathways. Microbiol. Mol. Biol. Rev. 73:594–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oren A, Heldal M, Norland S, Galinski EA. 2002. Intracellular ion and organic solute concentrations of the extremely halophilic bacterium Salinibacter ruber. Extremophiles 6:491–498 [DOI] [PubMed] [Google Scholar]
- 20. Parashar D, et al. 2009. Identification of environmental mycobacteria isolated from Agra, north India by conventional & molecular approaches. Indian J. Med. Res. 129:424–431 [PubMed] [Google Scholar]
- 21. Pastor JM, et al. 2010. Ectoines in cell stress protection: uses and biotechnological production. Biotechnol. Adv. 28:782–801 [DOI] [PubMed] [Google Scholar]
- 22. Peters P, Galinski EA, Trüper HG. 1990. The biosynthesis of ectoin. FEMS Microbiol. Lett. 71:157–162 [Google Scholar]
- 23. Pflughoeft KJ, Kierek K, Watnick PI. 2003. Role of ectoine in Vibrio cholerae osmoadaptation. Appl. Environ. Microbiol. 69:5919–5927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reshetnikov AS, et al. 2011. Diversity and phylogeny of the ectoine biosynthesis genes in aerobic, moderately halophilic methylotrophic bacteria. Extremophiles 15:653–663 [DOI] [PubMed] [Google Scholar]
- 25. Roberts MF. 2004. Osmoadaptation and osmoregulation in archaea: update 2004. Front. Biosci. 9:1999–2019 [DOI] [PubMed] [Google Scholar]
- 26. Roesser M, Müller V. 2001. Osmoadaptation in bacteria and archaea: common principles and differences. Environ. Microbiol. 3:743–754 [DOI] [PubMed] [Google Scholar]
- 27. Schwibbert K, et al. 2011. A blueprint of ectoine metabolism from the genome of the industrial producer Halomonas elongata DSM 2581 T. Environ. Microbiol. 13:1973–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vargas C, et al. 2008. Unravelling the adaptation responses to osmotic and temperature stress in Chromohalobacter salexigens, a bacterium with broad salinity tolerance. Saline Systems 4:14 doi:10.1186/1746-1448-4-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhu D, Cui S, Nagata S. 2008. Isolation and characterization of salt-sensitive mutants of the moderately halophilic bacterium Salinivibrio costicola subsp. yaniae. Biosci. Biotechnol. Biochem. 72:1977–1982 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.