Abstract
Salmonella enterica subsp. enterica serovar Typhimurium is responsible for the majority of salmonellosis cases worldwide. This Salmonella serovar is also responsible for die-offs in songbird populations. In 2009, there was an S. Typhimurium epizootic reported in pine siskins in the eastern United States. At the time, there was also a human outbreak with this serovar that was associated with contaminated peanuts. As peanuts are also used in wild-bird food, it was hypothesized that the pine siskin epizootic was related to this human outbreak. A comparison of songbird and human S. Typhimurium pulsed-field gel electrophoresis (PFGE) patterns revealed that the epizootic was attributed not to the peanut-associated strain but, rather, to a songbird strain first characterized from an American goldfinch in 1998. This same S. Typhimurium strain (PFGE type A3) was also identified in the PulseNet USA database, accounting for 137 of 77,941 total S. Typhimurium PFGE entries. A second molecular typing method, multiple-locus variable-number tandem-repeat analysis (MLVA), confirmed that the same strain was responsible for the pine siskin epizootic in the eastern United States but was distinct from a genetically related strain isolated from pine siskins in Minnesota. The pine siskin A3 strain was first encountered in May 2008 in an American goldfinch and later in a northern cardinal at the start of the pine siskin epizootic. MLVA also confirmed the clonal nature of S. Typhimurium in songbirds and established that the pine siskin epizootic strain was unique to the finch family. For 2009, the distribution of PFGE type A3 in passerines and humans mirrored the highest population density of pine siskins for the East Coast.
INTRODUCTION
The genus Salmonella has a worldwide distribution and is one of the most common causes of diarrheal diseases of people and animals. It includes more than 2,400 distinct serotypes, most of which show little specificity for their host species. However, certain S. enterica subsp. enterica serovars are preferentially found in a particular host in which they cause disease. Examples include S. enterica subsp. enterica serovar Dublin in cattle and S. enterica subsp. enterica serovar Pullorum and S. enterica subsp. enterica serovar Gallinarum in chickens. Other Salmonella serovars, such as S. enterica subsp. enterica serovar Typhimurium, cause enteric disease in a wide range of hosts (7). Salmonella Typhimurium is also the causative agent of avian salmonellosis in wild birds, a nonenteric disease (41). Salmonella Typhimurium causes the majority of avian salmonellosis outbreaks and can cause high mortality rates in affected songbirds and aquatic birds (11, 15). Among songbirds, avian salmonellosis is a widespread and annual problem in the United States, Canada, and the United Kingdom (41). The repeated and frequent isolation of specific strains suggests that these S. Typhimurium strains have become adapted to some species of passerines, may be endemic in those populations, and cause epizootics under specific conditions (11). Although S. Typhimurium is typically thought to have a wide host range, strain-host relationships in pine siskins, American goldfinches, and other members of the finch family have been identified. There is still much to learn about the epidemiology of S. Typhimurium and the population impacts of salmonellosis for this group of birds; however, reports indicate that incidents of avian salmonellosis are on the rise (11, 12, 19). Of songbirds submitted to the National Wildlife Health Center (NWHC) from 1985 to 2004, 21.5% of mortality events were due to salmonellosis, and the annual trend for all avian Salmonella-related mortality events exhibited a 12% increase in proportional mortality (the number of birds that died from salmonellosis divided by the total number of birds presented to the diagnostic lab for necropsy) (19). The increase in outbreaks over the last 25 years has caused some to consider avian salmonellosis an emergent disease that may be directly related to anthropogenic activities such as backyard feeding (15, 41).
Salmonellosis is also a significant source of enteric disease for people in the United States (5) and elsewhere (32), with the majority of cases originating from contaminated food (18). Infections with S. Typhimurium are one of the most frequent causes of salmonellosis in humans (22). In some cases, direct transmission of S. Typhimurium between birds and people has been reported (33). However, most reports rely on indirect associations, whereby Salmonella strain types associated with outbreaks of human cases were concurrent with avian mortality in the same region or the risk of infection was directly related to contact with a wild bird (1, 36, 39). A better understanding of ecological interactions between S. Typhimurium, avian hosts, the environment, and humans will be essential to reduce the burden of human illnesses due to Salmonella in the United States.
In 2009, an exceptionally severe and widespread outbreak of avian salmonellosis occurred throughout the United States. This epizootic was coincidental with a human outbreak of S. Typhimurium linked to contaminated peanut products (10), and both the general public and the scientific community became concerned that peanut products in wild-bird food were a possible source of the avian infections. This concern led to recalls of products by multiple bird seed manufacturers and to a temporary cessation of feeding activities (35). The objectives of this study were to investigate the geographic, spatial, and genetic relationships of the avian salmonellosis cases submitted to wildlife diagnostic laboratories in the United States during this outbreak, to utilize pulsed-field gel electrophoresis (PFGE) to elucidate important epidemiological details about this outbreak, and to investigate the genetic relationship between this outbreak and non-food-borne human salmonellosis cases. The potential for transmission of S. Typhimurium from wild birds to people (1, 21, 26, 33, 40), domestic pets (39), and livestock (1, 17, 31), its dissemination to other wildlife (1, 20), and the potential that backyard feeding may increase the potential for outbreaks (34) illustrate the complexity of the epidemiology of this disease.
MATERIALS AND METHODS
Sample collection.
Beginning in January and ending in June 2009, several reports from the public and state agencies of moribund and dead backyard songbirds were received at the Southeastern Cooperative Wildlife Disease Study (SCWDS) and NWHC. Birds were described as lethargic, puffed in appearance, or unaware of human presence or were found dead. Birds were submitted fresh or frozen and were examined by necropsy. Sections of representative organs were fixed in 10% buffered formalin for histopathology. Unfixed samples of liver, esophagus, and crop were submitted to diagnostic laboratories for microbiology analyses. Segments of esophagus and crop with lesions were preferentially selected.
Bacteriology.
Tissue samples were macerated, homogenized, and inoculated in dulcitol selenite broth (BD, Franklin Lakes, NJ) overnight at 42°C. Broths were subcultured to selective media, including XLT4 agar, brilliant green novobiocin (BGN) agar, and MacConkey agar (Remel, Lenexa, KS), which were incubated overnight at 37°C. Suspect Salmonella colonies from the selective media were picked and streaked again for isolation on tryptic soy agar with 5% sheep blood. Final identification was determined based on biochemical results for citrate, motility-indole-ornithine, and triple-sugar iron media and a whole-cell agglutination test using Salmonella-specific poly A-I and Vi antiserum (Fisher Scientific, Pittsburgh, PA) (14). Salmonella isolates were submitted to the National Veterinary Services Laboratory (Ames, IA) for serotyping.
Molecular typing of S. Typhimurium isolates.
PFGE was used to determine the genetic relatedness (25, 28, 37) of S. Typhimurium isolates from songbirds to archived songbird isolates, to isolates from other animal sources, and to human isolates represented in the PulseNet national database (16). A master database of S. Typhimurium PFGE patterns was generated in BioNumerics (Applied Maths, Austin, TX). This database consisted of 204 total PFGE patterns generated for S. Typhimurium isolates obtained from various avian species (n = 162), including passerines (n = 96), galliformes (n = 35), psittacines (n = 9), water fowl (n = 14), shore birds (n = 8), and nonavian species (n = 42). Included in this data set were S. Typhimurium isolates from the Salmonella Reference Collection (SARA isolates 1 to 20) (8) and isolates from past studies (13, 23, 30, 42). Comparisons were made between PFGE patterns using Dice coefficient (42) and unweighted-pair group method using average linkage (UPGMA) clustering. A subset of isolates were characterized using a sequence-based subtyping method, multiple-locus variable-number tandem-repeat (VNTR) analysis (MLVA), by following the standardized procedure established by PulseNet (3). Comparisons between MLVA profiles were made with BioNumerics software using categorical coefficient and UPGMA clustering (2).
Geographic information system (GIS) analysis.
A U.S. map showing the distribution of Salmonella bird and human isolates was produced using ArcGIS 10 software (ESRI, Redlands, CA) and a base U.S. physical map. Using ArcGIS 10, we selected the affected counties and exported from the original data set, generating layers of geographical information (mountains, rivers, etc.) for each state on this map. Each county depicted was linked to the type of Salmonella isolate (human, bird, or both). Human population density for large urban centers was layered onto this map using information provided by the 2010 U.S. Census (http://2010.census.gov/2010census/).
RESULTS
2009 S. Typhimurium outbreak in pine siskins.
From January 2009 to November 2009, SCWDS diagnosed Salmonella infection as the primary cause of death for 109 birds. Simultaneously, across the country, other labs reported high avian mortalities due to Salmonella. For example, the NWHC received 57 cases from 16 states and the Pennsylvania Game Commission received 6 cases. The peak of total case submissions was February for the SCWDS and April for the NWHC. Northern cardinals were the first species to be diagnosed (9 January 2009), but by 30 January 2009, pine siskins became the most frequently diagnosed species.
Nearly all birds diagnosed with salmonellosis had round, firm, yellow to tan, thickened segments of the esophagus and, less commonly, the crop. These foci corresponded to dense infiltrates of mostly degenerate heterophils which expanded and often obliterated the wall of the esophagus. The overlying epithelium was ulcerated, and large colonies of Gram-negative rods were admixed with the necrotic debris and inflammatory infiltrates. In many cases, the spleen was enlarged and mottled due to multifocal infiltrates of heterophils with necrosis of associated tissue and bacterial colonies as previously described. Other tissues, such as liver, testes, and skeletal muscle, were variably affected, consistent with septicemia.
Identification of a single S. Typhimurium strain associated with the 2009 pine siskin outbreak in the eastern United States by PFGE.
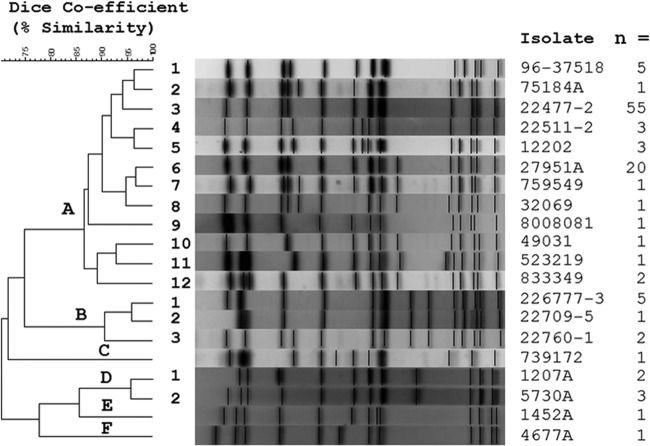
Fifty-seven S. Typhimurium isolates retained from the SCWDS cases, 24 isolates from the NWHC, and 6 isolates from the Pennsylvania Game Commission from the 2009 songbird epizootic were compared to determine by PFGE the genetic relatedness of songbird isolates from the recent epizootic to isolates from past cases. Ninety-three passerine isolates were examined by PFGE, and 99% of the isolates clustered tightly in one clonal group (clonal group A) (Fig. 1 and Table 1). Within this clonal group, most subtypes differed from each other by only 1 to 4 DNA fragments (A1 to A12). Salmonella Typhimurium PFGE type A3 was responsible for most 2009 cases in pine siskins and American goldfinches (94%). This PFGE type was associated primarily with birds belonging to the finch family Fringillidae that were collected as far back as 1998 (Table 1) (23). Salmonella Typhimurium PFGE type A3 was frequently isolated from pine siskins and American goldfinches collected or sampled in the eastern United States (Georgia, South Carolina, North Carolina, Tennessee, Virginia, West Virginia, and Pennsylvania), but it was different from the PFGE type A4 isolated from pine siskins and the common redpoll from the central and western United States (Minnesota and Washington). Salmonella Typhimurium PFGE subtype A6 was also isolated in 2009 from several songbird species other than finches, most notably the northern cardinal. This same PFGE subtype has been encountered in past years (Table 1). The two dominant S. Typhimurium PFGE subtypes identified from songbirds in 2009 were distinctly different from the S. Typhimurium subtypes isolated in the same year from water fowl (Fig. 1 and Table 1).
Fig 1.
Cluster analysis of avian S. Typhimurium pulsed-field gel electrophoresis (PFGE) patterns generated with the restriction enzyme XbaI. Tiff images of S. Typhimurium PFGE patterns were compared using DNA pattern recognition software (BioNumerics; Applied Maths, Austin, TX). Levels of similarity were calculated using the band-based Dice similarity coefficient, and clustering of samples was performed using the unweighted-pair group method using average linkages (UPGMA). Six major PFGE clusters, A to F (>85% similarity), were identified among 162 avian S. Typhimurium isolates examined. Within PFGE clusters A, B, and D, additional PFGE subtypes, in which patterns differed by only 1 to 4 DNA fragments, were identified. The majority of songbird S. Typhimurium isolates produced PFGE A (subtypes 1 to 12) patterns with the restriction enzyme XbaI.
Table 1.
Salmonella isolates used in this studyi
| Isolate name | No. of isolates | Familya | Speciesa | Mo | Yr | State | County | PFGE type | PulseNet matchd | No. with matching PFGE patterne | No. of matches in yr of isolationf | MLVA clusterg | MLVA type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 22709-5 | 1 | Charadriidae | Piping plover | August | 2009 | MA | Barnstable | B2 | No matches | ||||
| 22677-3 | 1 | Laridae | Ring-billed gull | July | 2009 | ND | Mountrail | B1 | JPXX01.1261 | 91 | 4 | ||
| 22710-1 | 1 | Laridae | Ring-billed gull | August | 2009 | ND | Foster | B1 | JPXX01.1261 | 91 | 4 | II | I |
| 22728-2 | 1 | Laridae | Ring-billed gull | August | 2009 | ND | Mountrail | B1 | JPXX01.1261 | 91 | 4 | ||
| 22728-4 | 1 | Laridae | Ring-billed gull | August | 2009 | ND | Mountrail | B1 | JPXX01.1261 | 91 | 4 | ||
| 22791-1 | 1 | Laridae | Herring gull | October | 2009 | ME | Adroscoggin | B1 | JPXX01.1261 | 91 | 4 | ||
| 22716-2 | 1 | Laridae | Franklin's gull | August | 2009 | SD | Kingsbury | B3 | JPXX01.1378 | 4 | 0 | I | A |
| 22760-1 | 1 | Laridae | Franklin's gull | September | 2009 | ND | Burleigh | B3 | JPXX01.1378 | 4 | 0 | I | B |
| 192432 | 1 | Gallininae | Chicken | NA | 2006 | GA | NA | A1 | JPXX01.0179 | 592 | 40 | I | C |
| 1207A | 1 | Gallininae | Chicken | June | 2006 | GA | NA | D1 | JXXP01.0111 | 987 | 140 | II | E |
| 1208A | 1 | Gallininae | Chicken | June | 2006 | GA | NA | D1 | JXXP01.0111 | 987 | 140 | II | E |
| 5730A | 1 | Gallininae | Chicken | April | 2007 | GA | NA | D2 | JPXX01.0038 | 1,368 | 157 | II | F |
| 1452A | 1 | Gallininae | Chicken | July | 2006 | GA | NA | E | ND | II | H | ||
| 4677A | 1 | Gallininae | Chicken | March | 2007 | GA | NA | F | JPXX01.0621 | 4,165 | 529 | I | D |
| 751384A | 1 | Cardinalidae | Northern cardinal | April | 2007 | GA | Catoosa | A2 | No matches | IIIa | N | ||
| 751384B | 1 | Cardinalidae | Northern cardinal | April | 2007 | GA | Catoosa | A1 | JPXX01.0179 | 592 | 32 | ||
| 30052B | 1 | Cardinalidae | Northern cardinal | January | 2009 | GA | Spaulding | A3 | JPXX01.1043 | 137 | 33 | ||
| 6005419 | 1 | Cardinalidae | Northern cardinal | April | 2006 | GA | Hall | A6 | JPXX01.0150 | 603 | 42 | IIIa | K2 |
| 742217 | 1 | Cardinalidae | Northern cardinal | March | 2007 | GA | Richmond | A6 | JPXX01.0150 | 603 | 44 | ||
| 848065 | 1 | Cardinalidae | Northern cardinal | May | 2008 | TN | Coffey | A6 | JPXX01.0150 | 603 | 70 | IIIa | O |
| 27437B | 6c | Cardinalidae | Northern cardinal | January | 2009 | GA | Spaulding | A6 | JPXX01.0150 | 603 | 50 | IIIa | O |
| 930052 | 1 | Cardinalidae | Northern cardinal | July | 2009 | GA | Spaulding | A6 | JPXX01.0150 | 603 | 50 | ||
| 27457E | 1 | Cardinalidae | Northern cardinal | February | 2009 | GA | Spaulding | A6 | JPXX01.0150 | 603 | 50 | ||
| 523219 | 1 | Fringillidae | Finch | November | 2004 | NA | NA | A11 | JPXX01.1297 | 16 | 1 | ||
| 98A-33516 | 1 | Fringillidae | American goldfinch | March | 1998 | KY | McCreary | A3 | JPXX01.1043 | 137 | 1 | IIIb | U |
| 851809 | 1 | Fringillidae | American goldfinch | May | 2008 | NC | Wilkes | A3 | JPXX01.1043 | 137 | 13 | IIIb | T1h |
| 30453 | 1 | Fringillidae | American goldfinch | February | 2009 | GA | Dawson | A3 | JPXX01.1043 | 137 | 33 | ||
| 33463 | 1 | Fringillidae | American goldfinch | February | 2009 | VA | Floyd | A3 | JPXX01.1043 | 137 | 33 | ||
| 31876A | 1 | Fringillidae | American goldfinch | February | 2009 | TN | Sevier | A3 | JPXX01.1043 | 137 | 33 | IIIb | T1h |
| 31877A | 1 | Fringillidae | American goldfinch | February | 2009 | TN | Blount | A3 | JPXX01.1043 | 137 | 33 | ||
| 33461A | 4c | Fringillidae | American goldfinch | February | 2009 | VA | Rockbridge | A3 | JPXX01.1043 | 137 | 33 | ||
| 22477-5 | 1 | Fringillidae | American goldfinch | March | 2009 | NC | Moore | A3 | JPXX01.1043 | 137 | 33 | IIIb | T1h |
| 34332 (liver) | 1 | Fringillidae | House finch | February | 2009 | GA | Whitfield | A3 | JPXX01.1043 | 137 | 33 | ||
| 731877B | 1 | Fringillidae | Purple finch | February | 2009 | TN | Blount | A3 | JPXX01.1043 | 137 | 33 | ||
| 22471-1 | 1 | Fringillidae | Purple finch | March | 2009 | NC | Caldwell | A3 | JPXX01.1043 | 137 | 33 | IIIb | T1h |
| 28251B | 3c | Fringillidae | Pine siskin | January | 2009 | GA | Rabun | A3 | JPXX01.1043 | 137 | 33 | IIIb | T1h |
| 31879 | 1 | Fringillidae | Pine siskin | February | 2009 | GA | Cherokee | A3 | JPXX01.1043 | 137 | 33 | ||
| 31880 | 1 | Fringillidae | Pine siskin | February | 2009 | GA | Gilmer | A3 | JPXX01.1043 | 137 | 33 | ||
| 34331 | 1 | Fringillidae | Pine siskin | February | 2009 | GA | Whitfield | A3 | JPXX01.1043 | 137 | 33 | ||
| 22459-2 | 1 | Fringillidae | Pine siskin | February | 2009 | MD | Garrett | A3 | JPXX01.1043 | 137 | 33 | ||
| 30452C | 1 | Fringillidae | Pine siskin | February | 2009 | GA | Dawson | A3 | JPXX01.1043 | 137 | 33 | ||
| 31876B | 1 | Fringillidae | Pine siskin | February | 2009 | TN | Sevier | A3 | JPXX01.1043 | 137 | 33 | IIIb | T1h |
| 31878A | 2c | Fringillidae | Pine siskin | February | 2009 | TN | Knox | A3 | JPXX01.1043 | 137 | 33 | ||
| 32532A | 4c | Fringillidae | Pine siskin | February | 2009 | GA | Oconee | A3 | JPXX01.1043 | 137 | 33 | IIIb | T1h |
| 33146C (liver) | 2c | Fringillidae | Pine siskin | February | 2009 | SC | Spartanburg | A3 | JPXX01.1043 | 137 | 33 | IIIb | T1h |
| 33462A | 3c | Fringillidae | Pine siskin | February | 2009 | VA | Rockbridge | A3 | JPXX01.1043 | 137 | 33 | ||
| 33464B | 3c | Fringillidae | Pine siskin | February | 2009 | VA | Floyd | A3 | JPXX01.1043 | 137 | 33 | IIIb | T1h |
| 34113 (liver) | 1 | Fringillidae | Pine siskin | February | 2009 | GA | Whitfield | A3 | JPXX01.1043 | 137 | 33 | ||
| P0905345 | 1 | Fringillidae | Pine siskin | February | 2009 | PA | Huntingdon | A3 | JPXX01.1043 | 137 | 33 | IIIb | T1h |
| 22471-9 | 1 | Fringillidae | Pine siskin | March | 2009 | NC | Caldwell | A3 | JPXX01.1043 | 137 | 33 | IIIb | T2 |
| 22477-2 | 1 | Fringillidae | Pine siskin | March | 2009 | NC | Moore | A3 | JPXX01.1043 | 137 | 33 | IIIb | T1h |
| 22487-2 | 1 | Fringillidae | Pine siskin | March | 2009 | AL | Calhoun | A3 | JPXX01.1043 | 137 | 33 | ||
| 34333 (liver) | 1 | Fringillidae | Pine siskin | March | 2009 | WV | Randolph | A3 | JPXX01.1043 | 137 | 33 | IIIb | T1h |
| P0906327 | 1 | Fringillidae | Pine siskin | March | 2009 | PA | Huntingdon | A3 | JPXX01.1043 | 137 | 33 | IIIb | T1h |
| P0907343 | 1 | Fringillidae | Pine siskin | March | 2009 | PA | Bedford | A3 | JPXX01.1043 | 137 | 33 | ||
| P0907344 | 1 | Fringillidae | Pine siskin | March | 2009 | PA | Huntingdon | A3 | JPXX01.1043 | 137 | 33 | ||
| P0909859 | 1 | Fringillidae | Pine siskin | March | 2009 | PA | Franklin | A3 | JPXX01.1043 | 137 | 33 | ||
| 22526-4 | 1 | Fringillidae | Pine siskin | April | 2009 | VT | Orange | A3 | JPXX01.1043 | 137 | 33 | ||
| 22528-1 | 1 | Fringillidae | Pine siskin | April | 2009 | WV | Upshur | A3 | JPXX01.1043 | 137 | 33 | ||
| P0909858 | 1 | Fringillidae | Pine siskin | April | 2009 | PA | Huntingdon | A3 | JPXX01.1043 | 137 | 33 | ||
| P0912925 | 1 | Fringillidae | Pine siskin | April | 2009 | PA | Snyder | A3 | JPXX01.1043 | 137 | 33 | ||
| 29988 | 1 | Fringillidae | Pine siskin | February | 2009 | GA | Habersham | A3 | JPXX01.1043 | 137 | 33 | ||
| 34504 | 1 | Fringillidae | Pine siskin | March | 2009 | WV | Randolph | A3 | JPXX01.1043 | 137 | 33 | ||
| P0917357 | 1 | Fringillidae | Pine siskin | June | 2009 | PA | Somerset | A3 | JPXX01.1043 | 137 | 33 | ||
| 22413-1 | 1 | Fringillidae | Pine siskin | January | 2009 | WA | Thurston | A4 | JPXX01.0199 | 159 | 22 | ||
| 22511-2 | 1 | Fringillidae | Pine siskin | April | 2009 | MN | Carlton | A4 | JPXX01.0199 | 159 | 22 | IIIb | V |
| 22511-4 | 1 | Fringillidae | Common redpoll | April | 2009 | MN | Carlton | A4 | JPXX01.0199 | 159 | 22 | IIIb | T3h |
| 739172 | 1 | Fringillidae | Canary | NA | NA | NA | NA | C | No matches | ||||
| 96-37518 | 1 | Icteridae | Brown-headed cowbird | March | 1996 | GA | Clarke | A1 | JPXX01.0179 | 592 | 5 | IIIa | L |
| 98A-24966 | 1 | Icteridae | Brown-headed cowbird | January | 1998 | GA | Camden | A1 | JPXX01.0179 | 592 | 5 | IIIa | Q |
| 98A-28238 | 1 | Icteridae | Brown-headed cowbird | February | 1998 | GA | Camden | A1 | JPXX01.0179 | 592 | 5 | IIIa | R |
| 833349 | 1 | Icteridae | Brown-headed cowbird | January | 2008 | GA | Houston | A12 | JPXX01.1054 | 230 | 26 | ||
| 11203 | 1 | Icteridae | Rusty blackbird | NA | NA | NA | NA | A12 | JPXX01.1054 | 230 | 26 | ||
| 48796 | 1 | Icteridae | Red-winged blackbird | August | 2003 | GA | Glynn | A6 | JPXX01.0150 | 603 | 71 | ||
| 49052 | 1 | Icteridae | Common grackle | August | 2003 | GA | Cherokee | A6 | JPXX01.0150 | 603 | 71 | ||
| 728131 | 1 | Icteridae | Red-winged blackbird | December | 2006 | GA | Glynn | A6 | JPXX01.0150 | 603 | 42 | IIIa | O |
| 732619 | 1 | Icteridae | Red-winged blackbird | January | 2007 | GA | Richmond | A6 | JPXX01.0150 | 603 | 44 | IIIa | M2 |
| 833347 | 1 | Icteridae | Red-winged blackbird | January | 2008 | GA | Houston | A6 | JPXX01.0150 | 603 | 70 | IIIa | K4 |
| 27951A | 1 | Icteridae | Red-winged blackbird | February | 2009 | GA | Burke | A6 | JPXX01.0150 | 603 | 50 | IIIa | K1 |
| 27951C | 1 | Icteridae | Red-winged blackbird | February | 2009 | GA | Burke | A6 | JPXX01.0150 | 603 | 50 | IIIa | M3 |
| 759549 | 1 | Icteridae | Brown-headed cowbird | June | 2007 | GA | Meriwether | A7 | No matches | IIIa | K3 | ||
| 32069 | 1 | Icteridae | Brown-headed cowbird | July | 2002 | GA | Greene | A8 | No matches | IIIa | K2 | ||
| 12202 | 1 | Passeridae | English sparrow | NA | 1998 | WY | NA | A5 | JPXX01.0002 | 200 | 2 | IIIa | S |
| A7852 | 1 | Passeridae | English sparrow | NA | 1998 | WY | NA | A5 | JPXX01.0002 | 200 | 2 | ||
| W12198 | 1 | Passeridae | English sparrow | NA | 1998 | WY | NA | A5 | JPXX01.0002 | 200 | 2 | ||
| P0907752 | 1 | Passeridae | House sparrow | March | 2009 | PA | Clearfield | A3 | JPXX01.1043 | 137 | 33 | ||
| 22827-1 | 1 | Passeridae | House sparrow | October | 2009 | NJ | Warren | A6 | JPXX01.0150 | 603 | 50 | IIIa | K1 |
| 49051E | 1 | Cardinalidae | Northern cardinal | August | 2003 | GA | Bibb | A10 | ND | IIIa | M1 | ||
| 231449 | 1 | Icteridae | Red-winged blackbird | January | 2002 | GA | Richmond | A6 | JPXX01.0150 | 603 | 50 | IIIa | P |
| 8008081 | 1 | Psittacidae | Parakeet | NA | 2009 | NA | NA | A9 | ND | II | J | ||
| 1448Ab | 1 | Muridae | Mouse | June | 2006 | GA | NA | D2 | JPXX01.0038 | 1,368 | 112 | II | G |
| 1449Ab | 1 | Muridae | Mouse | June | 2006 | GA | NA | D2 | JPXX01.0038 | 1,368 | 112 | II | G |
S. Typhimurium isolates were organized in this table according to family and species.
Mice were caught in a chicken house.
S. Typhimurium was isolated from multiple birds of the same species. All isolates had the same PFGE pattern.
There were a total of 77,941 S. Typhimurium entries in the PulseNet database as of 8 March 2012 (accession date).
Total number of entries with a matching PFGE pattern in PulseNet.
Number of matches for that year of isolation.
Major MLVA cluster.
Matches with a human isolate.
NA, not available; ND, not done.
The same S. Typhimurium pine siskin strain (A3) is indistinguishable by PFGE and MLVA from human isolates.
None of these songbird Salmonella PFGE patterns matched the S. Typhimurium PFGE pattern associated with the 2009 outbreak in humans, tied to contaminated peanut products (data not shown). However, several passerine PFGE patterns were indistinguishable from (100% similarity) S. Typhimurium PFGE patterns in the PulseNet USA database. The songbird PFGE patterns A3 and A6 matched PFGE patterns associated with 137 and 603 S. Typhimurium PFGE entries, respectively, of a total of 77,941 S. Typhimurium PFGE entries in the PulseNet database (as of 8 March 2012). A minor songbird PFGE type, A5, first described in 1998 (23), matched the S. Typhimurium PFGE pattern for 200 entries in the PulseNet database. Another minor PFGE type, A1, matched a PFGE pattern for 592 entries in the PulseNet database. None of these PFGE patterns had been associated with known outbreaks in humans that were identified by PulseNet (16). The minor songbird PFGE types A2, A7, A8, and C did not match any PFGE patterns in the PulseNet database. In comparing passerine S. Typhimurium to other animal isolates in our PFGE database, we identified only one isolate with the same passerine A1 PFGE pattern (Table 1).
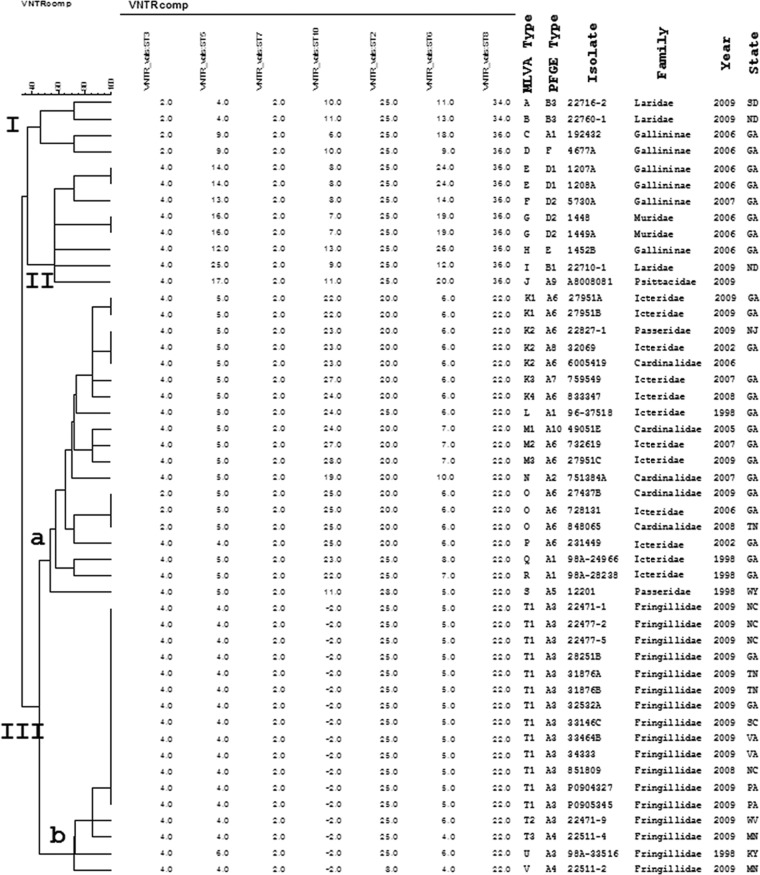
A second molecular typing method, MLVA, was used to further assess the genetic relatedness among a subset of S. Typhimurium songbird isolates, epidemiologically unrelated animal isolates, and human isolates with matching PFGE patterns (Fig. 2). As observed with PFGE, MLVA profiles for passerine isolates clustered together, and within this cluster, these profiles were further segregated into two groups, IIIa and IIIb. MLVA also separated the nonpasserine isolates 192432 and 80008081 with the similar or matching passerine PFGE patterns A1 and A9, respectively, into the separate clusters I and II, respectively. All MLVA profiles for songbirds belonging to the finch family Fringillidae grouped into the IIIb branch. MLVA was also able to discern genetic differences among S. Typhimurium isolates with identical PFGE patterns whose isolations were separated temporally (isolate 27951A [2009] versus isolates 6005419, 732619, and 833347 [2006 to 2008] or isolate 22471-1 [2009] versus isolate 98A-33516 [1998]) or spatially (isolate 27951A [Georgia; 2009] versus isolate 22827-1 [New Jersey; 2009]). MLVA also separated epidemiologically unrelated isolates indistinguishable by PFGE (isolate 5730A versus isolates 1448 and 1449A). However, isolates from the same locale and time had the same MLVA profiles (isolates 1207A and 1208A, 1448 and 1449A, and 27951A and 27951B). Although a diversity of MLVA profiles was observed for songbird cluster IIIa, 76% of isolates in MLVA cluster IIIb produced a single profile, T1, despite their spatial separation. Most genetic differences within MLVA cluster IIIb were at one or, at most, two VNTR loci (ST2 and ST6) and usually consisted of one to two repeat differences. Locus ST2 is a highly conserved locus and has never previously been observed to evolve during an outbreak. Locus ST6 is a highly variable locus, so differences seen in that locus are significant (E. Trees and P. Gerner-Schmidt, personal communication). Hence, patterns U and V cannot be regarded as closely related to pattern T1, whereas patterns T2 and T3 are highly related to pattern T1. Human and songbird S. Typhimurium isolates with the same MLVA type, T1, and PFGE pattern, A3, were identified in this study.
Fig 2.
Cluster analysis of avian S. Typhimurium isolates by MLVA. The dendrogram was generated in BioNumerics (Applied Maths) using the categorical coefficient and unweighted-pair group method using average linkages (UPGMA). Avian S. Typhimurium isolates subtyped by MLVA belong to one of three major clusters (I to III). Of the songbird isolates typed by MLVA, those belonging to the bird families Cardinalidae (cardinals), Fringillidae (American goldfinch, common redpoll, pine siskin, and purple finch), Icteridae (brown-headed cowbird and red-winged blackbird), and Passeridae (English sparrow and house sparrow) grouped into cluster III. Within cluster III, two additional branches were identified. All S. Typhimurium isolates typed by MLVA that belonged to cluster IIIb were isolated from birds belonging to the finch family Fringillidae.
Temporal and spatial distribution of S. Typhimurium strain A3.
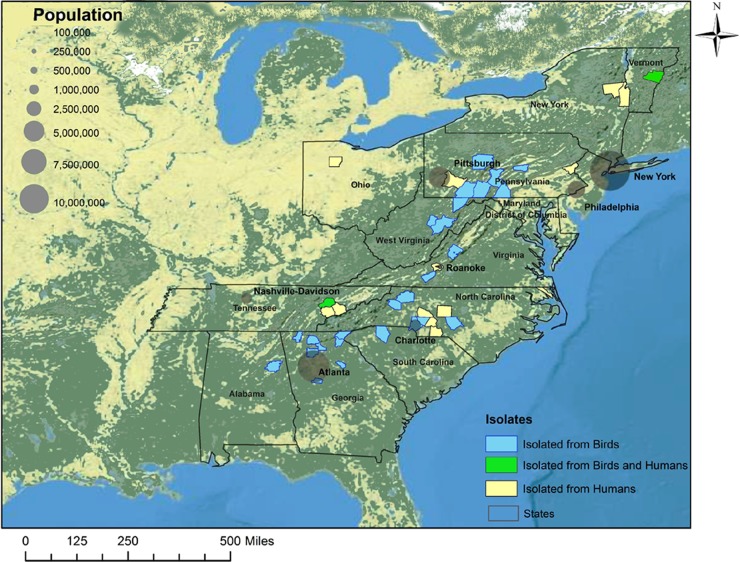
February was the peak of the 2009 epizootic with S. Typhimurium strain A3 in the songbird populations (Fig. 3). Human cases associated with this S. Typhimurium strain were scattered throughout 2008 and 2009. Most human illnesses due to PFGE type A3 were reported for the Atlantic states (76%; n = 34) (Fig. 4) from December 2008 to November 2009. There was spatial (Orange County, VT) and temporal (February 2009, Knox County, TN) overlap in the identification of PFGE type A3 for songbird and human S. Typhimurium isolates (Fig. 4). The distribution of this S. Typhimurium strain mirrored the highest-density distribution of pine siskins for the East Coast (4).
Fig 3.
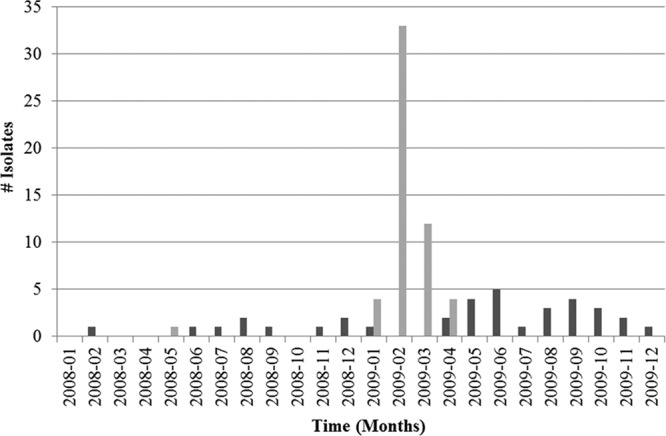
Temporal distribution of S. Typhimurium strains with the PFGE pattern A3 in birds and humans for the years 2008 and 2009. Light gray, birds; dark gray, humans.
Fig 4.
Spatial distribution of S. Typhimurium strains with the PFGE pattern A3 in the eastern United States. Human (yellow) and bird (light blue) isolates of PFGE type A3 that were obtained between May 2008 and November 2009 were mapped to the county level. Counties from which both bird and human isolates were obtained are colored green. Major urban centers on the Atlantic coast are depicted along with their population size, shown in gray. The scale for the human population is shown in the upper left, ranging from 100,000 to 10 million. (Map used by permission. Copyright © 2012 Esri and its data providers. All rights reserved.)
DISCUSSION
In 2009, a single S. Typhimurium strain A3, as defined by PFGE, was observed and accounted for all infections and deaths of pine siskins and American goldfinches along the Atlantic coast. MLVA confirmed that a single Salmonella strain was responsible for this epizootic in pine siskins. This Salmonella strain was distinct from 2009 isolates from other bird families and pine siskin/common redpoll isolates obtained from the central/western United States. MLVA was able to discern differences between epidemiologically unrelated isolates with the same or similar PFGE patterns, illustrating the finer resolving power of sequencing-based subtyping methods for identifying subtler genetic differences than PFGE (6, 9).
An archived American goldfinch isolate from 1998 had the same A3 PFGE pattern (23) and clustered with 2009 pine siskin isolates by MLVA, with the only difference observed in one of the seven VNTR loci. This pine siskin strain appears to have been circulating in songbirds for some time. Songbird S. Typhimurium isolates were distinctly different by MLVA from other avian isolates and belonged to one of two major clades, with finch isolates forming their own clade (IIIb). S. Typhimurium strains infecting passerines appear to be adapted to their avian host (24, 29), but unlike other host-adapted S. enterica serovars (e.g., S. Gallinarum and S. Pullorum [7]), these avian strains appear to be capable of causing human illnesses (27).
There have been several reported human cases of salmonellosis linked to exposure to wild birds. Penfold and coworkers (33) described an outbreak of Salmonella gastroenteritis in a hospital that was linked to sparrows present in the kitchen facility. In New Zealand, after an avian epizootic, S. Typhimurium phage type DT160 was found in domestic livestock and humans. Interviews with patients suffering from salmonellosis (DT160) showed that direct handling of wild birds was a significant risk factor (1). In Norway, sporadic human outbreaks (1966 to 1996) were analyzed and found to be seasonal, with 78% of the cases occurring in January to April, a time corresponding to annual avian salmonellosis outbreaks, and people known to have direct contact with wild birds or their droppings had a statistically significant increased risk of infection (36). Spatial and temporal overlaps were identified between a few avian and human cases in this report; however, the peak of human cases associated with this and other passerine S. Typhimurium strains was in late spring and early summer months, a period in which the epizootics have generally subsided. These sporadic cases in humans may reflect transmission resulting from either human contact with birds actively shedding Salmonella or, more likely, contact with a common, contaminated environmental source.
Since the 1980s, epizootics have caused tens of thousands of mortalities in songbirds in the United States, Europe, and New Zealand. In the United Kingdom, avian salmonellosis appears to be contributing to the decline of some songbird species, particularly in combination with other infectious diseases (38). It is unclear whether the same scenario is likely in the United States, although several large outbreaks, likely totaling more than tens of thousands of birds each, have occurred. Investigations that describe the spatial, temporal, and molecular relationships of Salmonella outbreaks are paramount for piecing together how these outbreaks affect songbird populations. For example, a recent review of salmonellosis of garden birds in England showed that there are currently two host-adapted Salmonella phage types, S. Typhimurium DT40 and DT56v, that are circulating widely in British garden birds and that the reservoir of infection is maintained within wild bird populations (29). Based on these findings, it also appears that host-adapted Salmonella strains circulate among wild birds and are conserved for long periods of time. Additionally, two clonal types (A3 and A4) were circulating among pine siskins from different flyways (eastern and western, respectively), and they could be distinguished from other songbird S. Typhimurium strains. Ideally, coordinated nationwide surveillance and standardization of methods will further our understanding of salmonellosis for wild birds. Ultimately, studies that simultaneously investigate avian, human, and environmental sources of Salmonella and describe potential mechanisms of movement of this pathogen among all three compartments by utilizing both empirical data and predictive modeling will lead to a more complete picture of the epidemiology of Salmonella. In addition, whole-genome sequencing of the avian S. Typhimurium isolates described in this study will provide insight into the evolution of these songbird strains in North America and the adaptations to their songbird hosts.
ACKNOWLEDGMENTS
This work was supported by NIH grant 1R15AI089565-01, USDA NRICGP 2005-01378, cooperative agreement no. 2001-96130032-CA, Veterinary Services, APHIS, USDA, cooperative agreement no. 01ERAG0013, United States Geological Survey, USDOI, and sponsorship of the SCWDS by the fish and wildlife agencies of Alabama, Arkansas, Florida, Georgia, Kentucky, Kansas, Louisiana, Maryland, Mississippi, Missouri, North Carolina, Ohio, Puerto Rico, South Carolina, Tennessee, Virginia, and West Virginia. Support from the states to the SCWDS was provided in part by the Federal Aid to Wildlife Restoration Act (50 Stat. 917).
We thank Kirk Smith with the Minnesota Department of Health, St. Paul, MN, for performing PFGE comparisons of the songbird S. Typhimurium isolates to human isolates associated with the 2009 outbreak linked to peanut butter and Patricia LaFon from the Centers for Disease Control and Prevention for technical assistance in MLVA typing.
Use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Footnotes
Published ahead of print 10 August 2012
REFERENCES
- 1. Alley MR, et al. 2002. An epidemic of salmonellosis caused by Salmonella Typhimurium DT160 in wild birds and humans in New Zealand. N. Z. Vet. J. 50:170–176 [DOI] [PubMed] [Google Scholar]
- 2. Anonymous. 2007. Laboratory standard operating procedure for analysis of MLVA data of Salmonella enterica serotype Typhimurium in BioNumerics—Beckman Coulter 8000 data. http://www.pulsenetinternational.org/SiteCollectionDocuments/mlva/PND15_MLVA_20Analysis_20Salm_20T_20Beckman.pdf
- 3. Anonymous 2007. Laboratory standard operating procedure for PulseNet MLVA of Salmonella enterica serotype Typhimurium—Beckman Coulter CEQ 8000 platform. http://www.pulsenetinternational.org/SiteCollectionDocuments/mlva/PNL21_MLVA_20Salm_20T_20Beckman_20Protocol.pdf
- 4. Anonymous 2009. 2009 results: map of Pine Siskin. The Great Backyard Bird Count. http://gbbc.birdsource.org/gbbcApps/maproom?cmd=OneMapDisplay&sppOrder=alpha&species=pinsis&year=2009®ion=NAm&submit.x=37&submit.y=9&submit=View+the+Map%21
- 5. Anonymous 2011. Vital signs: incidence and trends of infection with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 1996-2010. MMWR Morb. Mortal. Wkly. Rep. 60:749–755 [PubMed] [Google Scholar]
- 6. Bakker HC, et al. 2011. A whole-genome single nucleotide polymorphism-based approach to trace and identify outbreaks linked to a common Salmonella enterica subsp. enterica serovar Montevideo pulsed-field gel electrophoresis type. Appl. Environ. Microbiol. 77:8648–8655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baumler AJ, Tsolis RM, Ficht TA, Adams LG. 1998. Evolution of host adaptation in Salmonella enterica. Infect. Immun. 66:4579–4587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beltran P, et al. 1991. Reference collection of strains of the Salmonella typhimurium complex from natural populations. J. Gen. Microbiol. 137:601–606 [DOI] [PubMed] [Google Scholar]
- 9. Bergamini F, Iori A, Massi P, Pongolini S. 2011. Multilocus variable-number of tandem-repeats analysis of Salmonella enterica serotype Gallinarum and comparison with pulsed-field gel electrophoresis genotyping. Vet. Microbiol. 149:430–436 [DOI] [PubMed] [Google Scholar]
- 10. Cavallaro E, et al. 2011. Salmonella typhimurium infections associated with peanut products. N. Engl. J. Med. 365:601–610 [DOI] [PubMed] [Google Scholar]
- 11. Daoust P, Prescott JF. 2007. Salmonellosis, p 270–288 In Thomas N, Hunter DB, Atkinson CT. (ed), Infectious disease of wild birds. Blackwell Publishing, Ames, IA [Google Scholar]
- 12. Daoust PY, et al. 2000. Salmonellosis in songbirds in the Canadian Atlantic provinces during winter-summer 1997-98. Can. Vet. J. 41:54–59 [PMC free article] [PubMed] [Google Scholar]
- 13. Dorea FC, et al. 2010. Effect of Salmonella vaccination of breeder chickens on contamination of broiler chicken carcasses in integrated poultry operations. Appl. Environ. Microbiol. 76:7820–7825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farmer JJ, III, Kelly MT. 1991. Enterobacteriaceae, p 360–383 In Ballows A, Hausler WJ, Jr, Herrmann KL, Isenberg HD, Shadomy HJ. (ed), Manual of clinical microbiology, 5th ed ASM Press, Washington, DC [Google Scholar]
- 15. Friend M, Franson JC. (ed). 1999. Field manual of wildlife diseases: general field procedures and diseases of birds. Information and Technology Report 1999-001. U.S. Geological Survey, Biological Resources Division, National Wildlife Health Center, Madison, WI [Google Scholar]
- 16. Gerner-Smidt P, et al. 2006. PulseNet USA: a five-year update. Foodborne Pathog. Dis. 3:9–19 [DOI] [PubMed] [Google Scholar]
- 17. Goodchild WM, Tucker JF. 1968. Salmonellae in British wild birds and their transfer to domestic fowl. Br. Vet. J. 124:95–101 [DOI] [PubMed] [Google Scholar]
- 18. Guo C, et al. 2011. Application of Bayesian techniques to model the burden of human salmonellosis attributable to U.S. food commodities at the point of processing: adaptation of a Danish model. Foodborne Pathog. Dis. 8:509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hall AJ, Saito EK. 2008. Avian wildlife mortality events due to salmonellosis in the United States, 1985-2004. J. Wildl. Dis. 44:585–593 [DOI] [PubMed] [Google Scholar]
- 20. Handeland K, et al. 2008. Natural and experimental Salmonella Typhimurium infections in foxes (Vulpes vulpes). Vet. Microbiol. 132:129–134 [DOI] [PubMed] [Google Scholar]
- 21. Hauser E, et al. 2009. Characterisation of a phenotypic monophasic variant belonging to Salmonella enterica subsp. enterica serovar Typhimurium from wild birds and its possible transmission to cats and humans. Berl. Munch. Tierarztl. Wochenschr. 122:169–177 (In German.) [PubMed] [Google Scholar]
- 22. Hendriksen RS, et al. 2011. Global monitoring of Salmonella serovar distribution from the World Health Organization Global Foodborne Infections Network Country Data Bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog. Dis. 8:887–900 [DOI] [PubMed] [Google Scholar]
- 23. Hudson CR, et al. 2000. Genetic relatedness of Salmonella isolates from nondomestic birds in Southeastern United States. J. Clin. Microbiol. 38:1860–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hughes LA, Wigley P, Bennett M, Chantrey J, Williams N. 2010. Multi-locus sequence typing of Salmonella enterica serovar Typhimurium isolates from wild birds in northern England suggests host-adapted strain. Lett. Appl. Microbiol. 51:477–479 [DOI] [PubMed] [Google Scholar]
- 25. Hunter SB, et al. 2005. Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. J. Clin. Microbiol. 43:1045–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kapperud G, Lassen J, Hasseltvedt V. 1998. Salmonella infections in Norway: descriptive epidemiology and a case-control study. Epidemiol. Infect. 121:569–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kapperud G, Stenwig H, Lassen J. 1998. Epidemiology of Salmonella typhimurium O:4-12 infection in Norway: evidence of transmission from an avian wildlife reservoir. Am. J. Epidemiol. 147:774–782 [DOI] [PubMed] [Google Scholar]
- 28. Koort JM, Lukinmaa S, Rantala M, Unkila E, Siitonen A. 2002. Technical improvement to prevent DNA degradation of enteric pathogens in pulsed-field gel electrophoresis. J. Clin. Microbiol. 40:3497–3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lawson B, et al. 2011. Pulsed-field gel electrophoresis supports the presence of host-adapted Salmonella enterica subsp. enterica serovar Typhimurium strains in the British garden bird population. Appl. Environ. Microbiol. 77:8139–8144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liljebjelke KA, et al. 2005. Vertical and horizontal transmission of Salmonella within integrated broiler production system. Foodborne Pathog. Dis. 2:90–102 [DOI] [PubMed] [Google Scholar]
- 31. Macdonald JW, Bell JC. 1980. Salmonellosis in horses and wild birds. Vet. Rec. 107:46–47 [DOI] [PubMed] [Google Scholar]
- 32. Majowicz SE, et al. 2010. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 50:882–889 [DOI] [PubMed] [Google Scholar]
- 33. Penfold JB, Amery HC, Peet PJ. 1979. Gastroenteritis associated with wild birds in a hospital kitchen. Br. Med. J. 2:802. [PMC free article] [PubMed] [Google Scholar]
- 34. Pennycott TW, Cinderey RN, Park A, Mather HA, Foster G. 2002. Salmonella enterica subspecies enterica serotype Typhimurium and Escherichia coli O86 in wild birds at two garden sites in south-west Scotland. Vet. Rec. 151:563–567 [DOI] [PubMed] [Google Scholar]
- 35. Reardon J. 2009. Bird food recalled due to Salmonella contamination: dead birds found in N.C. initiates testing of bird food. North Carolina Department of Agriculture and Consumer Services, Raleigh, NC [Google Scholar]
- 36. Refsum T, Heir E, Kapperud G, Vardund T, Holstad G. 2002. Molecular epidemiology of Salmonella enterica serovar Typhimurium isolates determined by pulsed-field gel electrophoresis: comparison of isolates from avian wildlife, domestic animals, and the environment in Norway. Appl. Environ. Microbiol. 68:5600–5606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ribot EM, et al. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 3:59–67 [DOI] [PubMed] [Google Scholar]
- 38. Robinson RA, et al. 2010. Emerging infectious disease leads to rapid population declines of common British birds. PLoS One 5:e12215 doi:10.1371/journal.pone.0012215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tauni MA, Osterlund A. 2000. Outbreak of Salmonella typhimurium in cats and humans associated with infection in wild birds. J. Small Anim. Pract. 41:339–341 [DOI] [PubMed] [Google Scholar]
- 40. Thornley CN, et al. 2003. First incursion of Salmonella enterica serotype typhimurium DT160 into New Zealand. Emerg. Infect. Dis. 9:493–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tizard I. 2004. Salmonellosis in wild birds. Semin. Avian Exot. Pet Med. 13:50–66 [Google Scholar]
- 42. Zamperini K, et al. 2007. Molecular characterization reveals Salmonella enterica serovar 4,[5],12:i:− from poultry is a variant Typhimurium serovar. Avian Dis. 51:958–964 [DOI] [PubMed] [Google Scholar]