Abstract
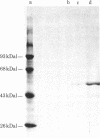
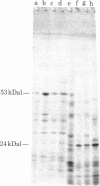
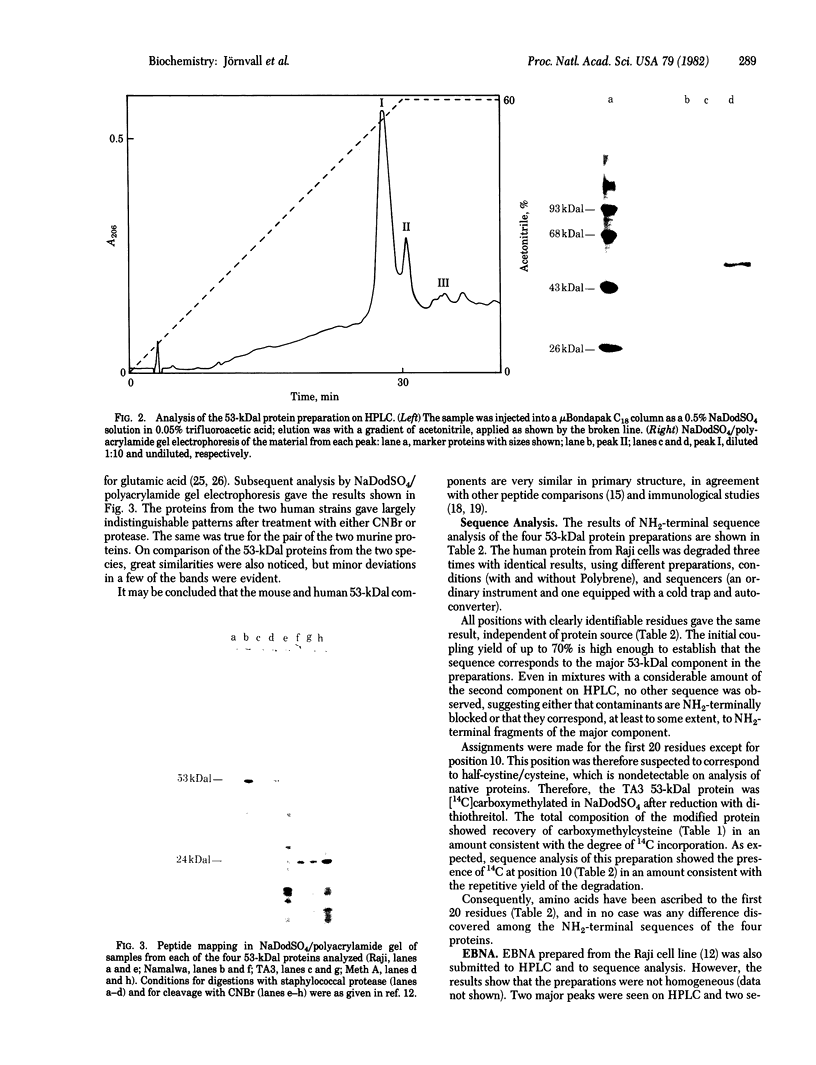
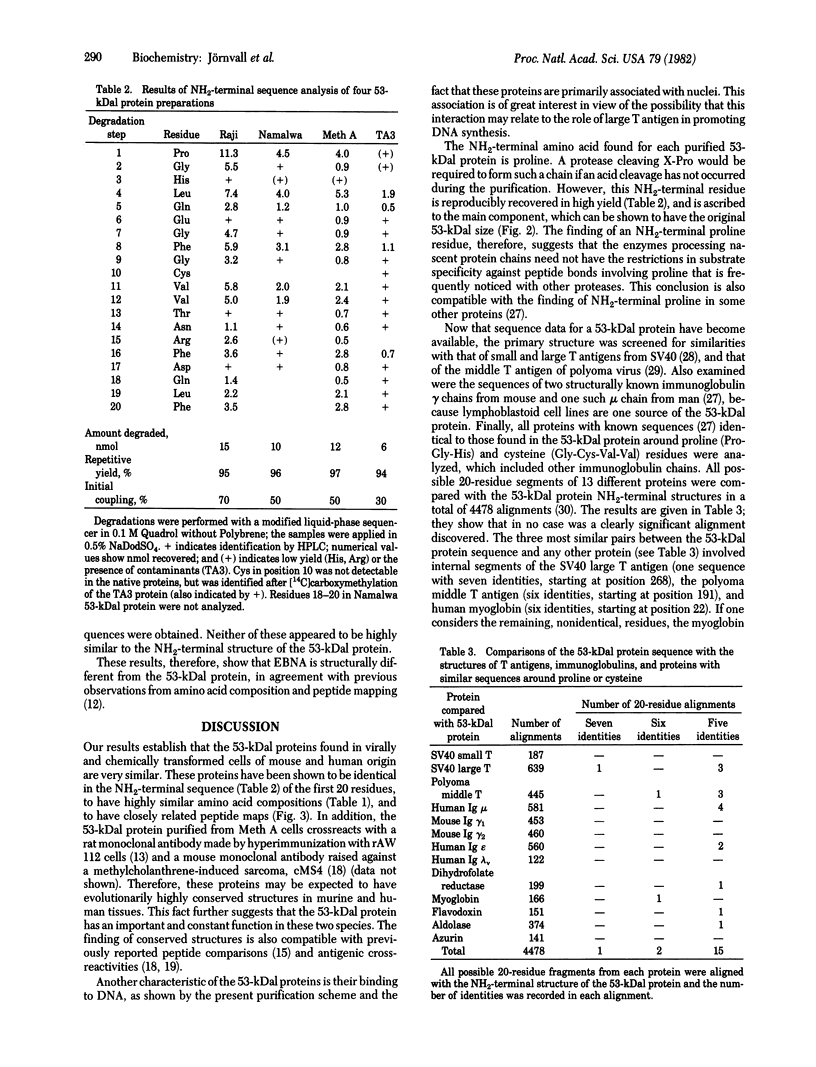
A heat-stable DNA-binding protein with subunits of about 53 kilodaltons (kDal) was purified from two virally transformed human cell lines (Epstein-Barr virus-positive Raji and Namalwa) and two mouse tumor cell lines (methylcholanthrene-induced Meth A sarcoma and TA3 mammary carcinoma). All four 53kDal proteins showed closely related total amino acid compositions, similar peptide maps, and identical NH2-terminal amino acid sequences for 20 residues. These 53-kDal proteins are therefore evolutionarily highly conserved, independent of whether they originate from virally or chemically transformed cells. The NH2-terminal sequence and the protein chain as a whole are not hydrophobic; however, some unexpected residue distributions were observed. Comparisons with other proteins reveal no clear sequence similarity with known tumor antigen structures, homologous immunoglobulins, or some other proteins of known sequence. Epstein-Barr virus-determined nuclear antigen also appears to have a different NH2-terminal sequence. Thus, the results show that the 53-kDal proteins represent a unique protein type with little species variation; this finding suggests that these proteins must perform an important common function in different transformation systems.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjoörklind A., Jörnvall H. Substrate specificity of three different extracellular proteolytic enzymes from Staphylococcus aureus. Biochim Biophys Acta. 1974 Dec 29;370(2):524–529. doi: 10.1016/0005-2744(74)90113-2. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran K., Winterbourne D. J., Luborsky S. W., Mora P. T. Surface proteins of simian-virus-40-transformed cells. Int J Cancer. 1981 Mar 15;27(3):397–407. doi: 10.1002/ijc.2910270320. [DOI] [PubMed] [Google Scholar]
- Chang C., Simmons D. T., Martin M. A., Mora P. T. Identification and partial characterization of new antigens from simian virus 40-transformed mouse cells. J Virol. 1979 Aug;31(2):463–471. doi: 10.1128/jvi.31.2.463-471.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Pim D. C., Gurney E. G., Goodfellow P., Taylor-Papadimitriou J. Detection of a common feature in several human tumor cell lines--a 53,000-dalton protein. Proc Natl Acad Sci U S A. 1981 Jan;78(1):41–45. doi: 10.1073/pnas.78.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo A. B., Jay G., Appella E., Dubois G. C., Law L. W., Old L. J. Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc Natl Acad Sci U S A. 1979 May;76(5):2420–2424. doi: 10.1073/pnas.76.5.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dippold W. G., Jay G., DeLeo A. B., Khoury G., Old L. J. p53 transformation-related protein: detection by monoclonal antibody in mouse and human cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1695–1699. doi: 10.1073/pnas.78.3.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau G. R., Boily Y., Houmard J. Purification and properties of an extracellular protease of Staphylococcus aureus. J Biol Chem. 1972 Oct 25;247(20):6720–6726. [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Protein phosphorylated by the RSV transforming function. Cell. 1980 Dec;22(3):647–648. doi: 10.1016/0092-8674(80)90539-5. [DOI] [PubMed] [Google Scholar]
- Jay G., DeLeo A. B., Appella E., Dubois G. C., Law L. W., Khoury G., Old L. J. A common transformation-related protein in murine sarcomas and leukemias. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):659–664. doi: 10.1101/sqb.1980.044.01.069. [DOI] [PubMed] [Google Scholar]
- Jay G., Khoury G., DeLeo A. B., Dippold W. G., Old L. J. p53 transformation-related protein: detection of an associated phosphotransferase activity. Proc Natl Acad Sci U S A. 1981 May;78(5):2932–2936. doi: 10.1073/pnas.78.5.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H., Carlström A., Pettersson T., Jacobsson B., Persson M., Mutt V. Structural homologies between prealbumin, gastrointestinal prohormones and other proteins. Nature. 1981 May 21;291(5812):261–263. doi: 10.1038/291261a0. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Fish W. W., Björk I. The thrombin cleavage site in bovine antithrombin. FEBS Lett. 1979 Oct 15;106(2):358–362. doi: 10.1016/0014-5793(79)80532-3. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Philipson L. Limited proteolysis and a reactive cysteine residue define accesible regions in the native conformation of the adenovirus hexon protein. Eur J Biochem. 1980 Feb;104(1):237–247. doi: 10.1111/j.1432-1033.1980.tb04421.x. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Lassam N. J., Bayley S. T., Graham F. L., Branton P. E. Immunoprecipitation of protein kinase activity from adenovirus 5-infected cells using antiserum directed against tumour antigens. Nature. 1979 Jan 18;277(5693):241–243. doi: 10.1038/277241a0. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Maltzman W., Levine A. J. The SV40 A gene product is required for the production of a 54,000 MW cellular tumor antigen. Virology. 1979 Oct 30;98(2):308–318. doi: 10.1016/0042-6822(79)90554-3. [DOI] [PubMed] [Google Scholar]
- Luka J., Jörnvall H., Klein G. Purification and biochemical characterization of the Epstein-Barr virus-determined nuclear antigen and an associated protein with a 53,000-dalton subunit. J Virol. 1980 Sep;35(3):592–602. doi: 10.1128/jvi.35.3.592-602.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney W. C., Hermodson M. A. Separation of large denatured peptides by reverse phase high performance liquid chromatography. Trifluoroacetic acid as a peptide solvent. J Biol Chem. 1980 Dec 10;255(23):11199–11203. [PubMed] [Google Scholar]
- McCormick F., Harlow E. Association of a murine 53,000-dalton phosphoprotein with simian virus 40 large-T antigen in transformed cells. J Virol. 1980 Apr;34(1):213–224. doi: 10.1128/jvi.34.1.213-224.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J., McCormick F. Lymphocyte stimulation: concanavalin A induces the expression of a 53K protein. Cell Biol Int Rep. 1980 Jul;4(7):663–667. doi: 10.1016/0309-1651(80)90205-2. [DOI] [PubMed] [Google Scholar]
- Mora P. T., Chandrasekaran K., McFarland V. W. An embryo protein induced by SV40 virus transformation of mouse cells. Nature. 1980 Dec 25;288(5792):722–724. doi: 10.1038/288722a0. [DOI] [PubMed] [Google Scholar]
- Rotter V., Witte O. N., Coffman R., Baltimore D. Abelson murine leukemia virus-induced tumors elicit antibodies against a host cell protein, P50. J Virol. 1980 Nov;36(2):547–555. doi: 10.1128/jvi.36.2.547-555.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T. Characterization of tau antigens isolated from uninfected and simian virus 40-infected monkey cells and papovavirus-transformed cells. J Virol. 1980 Nov;36(2):519–525. doi: 10.1128/jvi.36.2.519-525.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Martin M. A., Mora P. T., Chang C. Relationship among Tau antigens isolated from various lines of simian virus 40-transformed cells. J Virol. 1980 Jun;34(3):650–657. doi: 10.1128/jvi.34.3.650-657.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Griffin B., Fried M. Protein kinase activity associated with polyoma virus middle T antigen in vitro. Cell. 1979 Dec;18(4):915–924. doi: 10.1016/0092-8674(79)90204-6. [DOI] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Griffin B. E. Sequence from early region of polyoma virus DNA containing viral replication origin and encoding small, middle and (part of) large T antigens. Cell. 1979 Jun;17(2):357–370. doi: 10.1016/0092-8674(79)90162-4. [DOI] [PubMed] [Google Scholar]
- Strand M. Transformation-related antigens identified by monoclonal antibodies. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3234–3238. doi: 10.1073/pnas.77.6.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Robbins A. Enzymatic activities associated with a purified simian virus 40 T antigen-related protein. Proc Natl Acad Sci U S A. 1979 Feb;76(2):610–614. doi: 10.1073/pnas.76.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Appella E., Pisano J. J. Rapid analysis of amino acid phenylthiohydantoins by high-performance liquid chromatography. Anal Biochem. 1977 Feb;77(2):569–573. doi: 10.1016/0003-2697(77)90276-7. [DOI] [PubMed] [Google Scholar]