Abstract
The freshwater benthic pearl clam, Hyriopsis schlegeli, was experimentally exposed to Cryptosporidium parvum oocysts, and it was verified that the oocysts were eliminated predominantly via the fecal route, retaining their ability to infect cultured cells (HCT-8). The total fecal oocyst elimination rate was more than 90% within 5 days after exposure to the oocysts. H. schlegeli was able to survive in the final settling pond of a sewage plant for long periods, as confirmed by its pearl production. In the light of these findings, the clam was placed in the final settling pond in a trial to test its long-term efficacy in depleting oocysts contaminating the pond water. The number of clams placed was set to ensure a theoretical oocyst removal rate of around 50%, and the turbidity and the density of feed microbes in the overflow trough water of the pond were about 35% and 40 to 60% lower, respectively, than in the control water throughout the year. It was found that the clam feces containing oocysts were sufficiently heavy for them to settle to the bottom of the pond, despite the upward water flow. From these results, we concluded that efficient depletion of oocysts in the sewage water of small or midscale sewage treatment plants can be achieved by appropriate placement of H. schlegeli clams.
INTRODUCTION
A large number of studies have investigated the presence of Cryptosporidium oocysts in clams, and biomonitoring of this protozoan has been attempted in field tests with various clam species (3, 5, 6, 9–15, 17, 26–30, 34, 41, 43). There have also been many basic research studies on the biological functions of clams (1, 2, 7, 17, 21, 22, 25, 31–33, 36, 37, 39, 40, 49, 51, 52). However, the issue of accumulation and/or filtration of Cryptosporidium oocysts from water has received little attention, perhaps because of the difficulties in maintaining clams for long periods under artificial conditions in the laboratory. We have already established that Corbicula japonica, a brackish water benthic clam, might be applicable for removal of Cryptosporidium oocysts from contaminated river water (23, 24). Here, we report a study in which we attempted to develop an aquatic biological filtration system that could be practically applied for depletion of oocysts from contaminated water. Our study utilized a large Japanese freshwater benthic clam, Hyriopsis schlegeli, which has a water filtration capacity potentially superior to that of C. japonica. H. schlegeli belongs to the Unionidae and is the largest native freshwater pearl clam with a long life span. It was originally endemic to the area around Lake Biwa, Shiga, Japan, and has since been widely transplanted around Japan as a pearl clam. It is a gonochorismal, oviparous clam with a unique reproductive ecology: its larvae, known as glochidia, hatch on the gills of the clam and are released from the exhalant siphon. They then develop benthically after some weeks of parasitic life on the epidermis of fish. H. schlegeli was used effectively in the present study because of the ease with which its reproduction can be controlled, owing to its unique ecology, long life span, and the well-established nursery techniques that have been developed for it as a pearl clam. However, there have been very few previous reports on the basic biological potential and functions of freshwater clams, including H. schlegeli, under laboratory conditions. Before conducting long-term trials of this clam in a sewage treatment plant, it was necessary to examine its biological potential, including its water filtration rates and other parameters, in the laboratory. This is because there has been a lack of fundamental ecological data on H. schlegeli, such as its choice of food, the appropriate amount of food to supply, how to supply it, and the optimum laboratory cistern conditions for ensuring low clam mortality, without the clam ejecting pseudofeces or lapsing into a fasting state (23, 24). Based on the data obtained in preliminary tests, long-term trials were carried out to evaluate the efficacy of oocyst removal from water when using H. schlegeli clams as a biological filter in the final settling pond of a sewage treatment plant.
This first report details the basic potential of H. schlegeli clams maintained under artificial laboratory conditions and provides valuable data for the application of the clams to depletion of Cryptosporidium oocysts in the final settling pond of a sewage plant.
MATERIALS AND METHODS
Preliminary investigations of H. schlegeli. (i) Facilities for housing H. schlegeli.
H. schlegeli clams with body weights of 50 to 500 g, including the shells, were purchased from the Yanase Pearl Company, Ltd., Ibaraki, Japan. The unit for housing the clams comprised a styrene foam outer cistern (15 liters) for temperature retention and a resinous inner cistern (8 liters) as an environment for the clams (see Fig. S1 in the supplemental material). The outer cistern was filled with tap water and contained a stirring magnet, a water temperature sensor, and the cooling pipe of a cooling unit (RZ-90; REI-SEA, Japan), and the whole arrangement was set on a magnetic stirrer. The inner cistern contained dechlorinated tap water, a stirring magnet, and a pumice stone for aeration and circulation. The water in the outer cistern was usually kept at 20°C, and the clams were oriented horizontally in a multistory stainless steel cage placed in the inner cistern. All of the water in the inner cistern was changed once a day, as no water filtration apparatus was installed.
(ii) Feeding.
Powder-type chow (BV-01; DIC-Lifetech, Japan) consisting of Spirulina (Arthrospira platensis) for the Japanese short-necked clam (Ruditapes philippinarum) mixed with about 5% powdered chlorella (Chlorella pyrenoidosa; Sun Chlorella, Japan) was used as chow. Chow equivalent to 0.2 to 0.3% of the total clam body weight was suspended in a small volume of water, and following 5 min of ultrasonic treatment to ensure uniform distribution this was supplied to the clams in the inner cistern, six times a day at 2-h intervals during the daytime (total chow volume, 1.2 to 1.8% of the clam total body weight/day). Liquid chow (Micro-Vert, Kent Marine) for invertebrates and “green water,” consisting of phytoplankton cultivated in a tank with appropriate amounts of chemical fertilizer, under adequate sunlight and aeration, were added several times a week as supplementary chow.
(iii) Nitrogen-oxidizing bacteria.
To oxidize and detoxify the ammonia and nitrites generated by the clam excreta, which are toxic to the clam, 0.3 ml/liter of nitrogen-oxidizing bacteria (Power Bacter PG; Ecological Laboratories Inc.) was added to the water in the inner cistern after every water change.
To estimate the suitability of the laboratory conditions for maintaining H. schlegeli clams, the mortality [as a percentage; 100 × (number of dead clams/number of total clams)] during 3 years (from July 2007 to June 2010) was calculated using 60 clams (50 to 70 g) kept under the conditions described above.
Balance study using C. parvum oocysts with H. schlegeli clams.
To understand the internal accumulation of C. parvum oocysts in the clams and the pattern of oocyst excretion from the clam body after exposure to oocysts, an internal oocyst distribution and excretion study (balance study) was carried out as follows.
C. parvum oocysts (H8 strain) were provided by the Protozoa Laboratory, Department of Parasitology, National Institute of Infectious Diseases, Tokyo, Japan, and stored at 4°C (50). A total of 24 H. schlegeli clams (50 to 70 g) were used in groups of three. After 6 weeks of acclimation at 20°C, three randomly selected control clams were removed from the inner cistern to confirm the absence of any nonendogenous protozoans in them. After 12-h starvation of the clams to collect fresh feces, 1.99 × 106 oocysts (2.49 × 105 oocysts/liter; 9.48 × 104 oocysts/clam), within 3 weeks after preparation from mouse feces, were added to the inner cistern housing the remaining clams. After addition of the oocysts, three randomly selected H. schlegeli clams were sacrificed at 24-h intervals for 7 days.
The water in the inner cistern was sampled, and the clam feces were collected daily. The gastrointestinal (GI) tract, gills, and mantle excised from the removed clams were ground in a mixer (IKA Labor Technik, Switzerland) with a buffer (B100-20; Waterborne, Inc.), followed by oocyst separation with a magnetic bead kit (GC-Combo; Dynabeads). After preheating treatment (100°C, 5 min) to increase stainability with DAPI (4′,6-diamidino-2-phenylindole dihydrochloride; Sigma, St. Louis, MO) (20), the oocysts were applied to a 4-well glass slide (C.A. Hendley Ltd., United Kingdom) and then stained using a direct immunofluorescence assay kit (Aqua-Glo G/C Direct FL; Waterborne, Inc.) in an incubator at 37°C, followed by DAPI treatment for nuclear staining. The oocysts, stained with fluorescent reagents, were dried at 42°C in a drying oven, followed by methanol fixation. DABCO (1,4-diazabicyclo-2,2,2-octane; Sigma)-glycerin (1:63) was then added dropwise, and a coverglass was placed on the wells, which were then sealed with nail enamel. Identification and enumeration of C. parvum oocysts were carried out with a fluorescence microscope (DM LB2; Leica, Germany) under the conditions described elsewhere (23).
The feces were collected by centrifugation (3,000 rpm, 10 min) and treated three times with acetone to remove the high-viscosity component, followed by application to a direct immunofluorescence assay kit and DAPI staining as described above. The cistern water was processed using a mixed cellulose ester membrane filter (A100A090C; Advantec, Japan) dissolution method, followed by treatment with acetone removal of contaminants on a Percoll (Pharmacia Biotech)-sucrose density gradient and staining with fluorescence dyes in a manner similar to that described above (23).
DNA extraction and PCR.
Together with the balance study described above, we conducted PCR on the H. schlegeli clams exposed to C. parvum oocysts to screen for the presence of oocysts based on detection of C. parvum DNA. Feces and GI tract samples from the clams were collected at 1, 3, and 5 days after exposure to the oocysts and examined for C. parvum DNA by PCR using the TRAP-C2-F and TRAP-C2-R primer pair, corresponding to positions 848 to 867 on the coding strand and 1180 to 1199 on the negative strand of GenBank sequence X77586, as reported elsewhere (23), while an internal control (IC; 542 bp), to ensure sufficient progress of the PCR, was applied to only the negative control. The IC was prepared from the PCR product (369 bp), the BssHII-digested fragment, and the Klenow-blunted multiple cloning site from pBluescript KS− by using a rapid DNA ligation kit (Roche, Switzerland) (23).
In vitro infection test for fecal C. parvum oocysts using cultured cells.
In order to determine whether C. parvum oocysts are digested by H. schlegeli clams, or whether they remain infectious after ingestion in vitro, qualitative detection of C. parvum oocysts obtained from clam feces was carried out using cultured human colon tumor (HCT-8) cells, as reported elsewhere (19, 45, 48) and described briefly below.
Feces collected from clams exposed to C. parvum oocysts at a rate of 9.48 × 104 oocysts/clam and pooled in phosphate-buffered saline were prepared for purification of the oocysts in a sucrose density gradient. The purified oocysts were then processed with acid followed by treatment with trypsin (Difco) for 2 h and then subjected to in vitro cell culture for detection of infectiousness during 7 days. Meronts in the HCT-8 cells were stained with an immunofluorescent agent (Sporo-Glo; Waterborne, Inc.) and observed using a fluorescence microscope at 200× magnification. A detailed assessment of HCT-8 cell infection with oocysts was then conducted by counting the number of foci per visual field on the culture plate.
Water and oocyst filtration abilities of H. schlegeli clams.
To determine the water filtration and oocyst filtration capacities of H. schlegeli, a multistory stainless steel cage containing two clams (each weighing 500 g) was set in the inner cistern, and a trial was carried out using 6.6 × 105 C. parvum oocysts/liter (1.7 × 106 oocysts/clam) and 28 mg/liter feed at 20°C. After the start of the trial, water samples (10 ml) were collected every 15 min for 60 min, and the turbidity, expressed in nephelometric turbidity units (NTU), was measured with a water analyzer (Water Analyzer-2000; Nippon, Denshoku Kogyo, Japan). The water samples were also set in a hemacytometer, and the number of oocysts was counted under a microscope at ×200. A similar experiment was conducted without feed as a control. To ascertain whether H. schlegeli clams were able to filter and ingest the oocysts in water of the final settling pond water, a test of oocyst filtration by clams was carried out using water from the final settling pond containing microbes and oocysts at 6.6 × 105 C. parvum oocysts/liter (1.7 × 106 oocysts/clam) in a manner similar to that described above (see also Fig. S2 and S3 in the supplemental material).
In addition, the following experiments were carried out at three water temperatures (15, 20, and 25°C) using each of seven concentrations (9, 14, 28, 42, 84, 168, and 336 mg/liter) of powder-type chow together with 6.6 × 105 C. parvum oocysts/liter (1.7 × 106 oocysts/clam). The water turbidity was determined and plotted versus time, and the linear coefficient and the value of the y intercept of the linear function (y = ax + b, where y is the turbidity, x is time, a is the linear coefficient, and b is the y intercept value) were calculated. The time (x0) to zero turbidity was calculated by extrapolating the linear function obtained, and the water filtration rate (in liters/h) per clam was calculated using the following formula: (cistern water volume)/[(x0)(number of clams)].
Field survey of the sewage plant using H. schlegeli clams.
For the effective removal of Cryptosporidium oocysts from contaminated water by addition of H. schlegeli clams, the following field surveys were carried out with the clams in the final settling pond of the Sosei River wastewater treatment plant, Sapporo, Japan.
Confirmation of water quality in the final settling pond by cultivation of fish and pearls and chemical analysis.
Tests of the biological suitability of the pond water for survival of H. schlegeli clams were carried out by cultivation of fish and pearls in the pond and by chemical water analysis of the water.
We utilized ubiquitous freshwater fish that wide inhabiting the same water systems as H. schlegeli clams in Japan, the slender bitterling (Tanakia lanceolata), known to lay eggs in Unionidae clams, and the silver crucian carp (Carassius gibelio langsdorfi), known to be the most common fish in the lower reaches and ponds in Japan. Fish were kept for 2 weeks in 1-liter glass beakers filled with water from the final settling pond at room temperature. The water in the beakers was exchanged daily, and the suitability of the water for rearing of the clams was determined by observing the behaviors of the fish, including any evident breathing difficulty, changes of body color, and feeding status (fasting).
After these observations, a further test to verify the suitability of the pond water for long-term survival of the clams in the water of the final settling pond was conducted by observing pearl production by H. schlegeli clams over a 3-year period (from September 2007 to October 2010). Twelve clams (500 g), with pearl cores inserted, were placed horizontally in two nylon net holders (40-cm width by 60-cm length), each with six compartments of 20 by 20 cm, with one clam per compartment. The nets were placed horizontally at a vertical interval of 20 cm in a specially made 2-story stainless steel cage (40-cm width by 60-cm length) covered with a resin-treated dust-proof double-folded agricultural polyester sunscreen net (mesh size, 1 mm; Toyobo, Japan) at the sides. This cage was set along the wall of the overflow trough in the final settling pond at a water depth of 15 to 35 cm. After 3 years, the quality of the pond water for survival of the clam was estimated in terms of both clam mortality and clam pearl production, as the latter would clearly reflect the long-term water quality.
To conduct chemical analysis of the water in the final settling pond, the pond water was analyzed monthly for more than 2 years, from August 2006 to December 2008, prior to and during the trial with the H. schlegeli clams described immediately above. The basic analytical procedures used for metals (Al, Ba, Ca, Cd, Cr, Cu, Fe, Mg, Mn, Pb, and Zn) and determination of nitrogen (N) from ammonia and nitrites in the water have been described elsewhere (23). Determination of total phosphorus (TP) and phosphorus derived from soluble phosphate ions (P-PO43−) in the water was conducted using the ascorbic acid method (16). Dissolved oxygen (DO) at a water depth of 50 cm was measured by the azide modification method (16), electrical conductivity (EC) was determined with a conductivity meter (ES-51; Horiba Ltd., Japan), and pH was measured with a D-52 pH meter (Horiba Ltd., Japan).
Measurement of water volume passing through the final settling pond and placement of H. schlegeli clams in the pond.
The final settling pond for separating sediment from the pretreated sewage (see Fig. S2 in the supplemental material) measured 4.3 m (width) by 32.2 m (length) by 2.0 m (depth) and had two overflow troughs (0.5-m width by 20.0-m length by 0.3-m depth) along the sides. After biological treatment of the sewage by using the activated sludge, the treated water was introduced into the pond, and any sediment in the water settled there. The overflow entered the overflow troughs, and from there it was chlorinated for disinfection and then released into a river. Meanwhile, the precipitated sediment was gathered with caterpillar-type spatulas set in the bottom of the pond. The residue evacuated from the pond was then disinfected by automated heat treatment.
The overflow water volume (in L/h) of the pond was calculated from the total drained water volume and the surface area of the pond.
The clams were placed in the final settling pond of the sewage plant because the water further downstream of the final settling pond is disinfected with sodium hypochlorite at around 2.0 mg/liter, which would make it impossible for clams to survive.
The clams were arranged to provide a practical filter of the overflow water. Twenty-four clams (total body weight, 500 g) were placed horizontally in two nylon net holders, each with six compartments, two clams per compartment, in the same manner as that described for clam pearl production, thus creating a substantial density of 1 clam per 100 cm2. Fourteen cages with a total of 168 clams were placed two deep, along the overflow trough at a water depth of 15 to 35 cm, covering overall about 40% of the water surface area along the overflow trough. Fourteen cages without clams were set in the same manner along the side of the opposite overflow trough of the settling pond as a control. (See Fig. S3 in the supplemental material.)
After placing the clams in the final settling pond, any suspended dirt adhering to the nets around the cages was washed off once a week by spraying with water.
Fecal precipitation of H. schlegeli clams in the final settling pond.
Measurements of the rate of fecal precipitation by H. schlegeli clams were conducted to establish whether clam excreta would precipitate to the bottom of the final settling pond, where there is a constant slow upward water flow from the lower to the higher water layers. This test ensured that feces containing Cryptosporidium oocysts would precipitate to the bottom of the settling pond, where heat treatment could be carried out.
The test was conducted in the laboratory, and to simulate the upward water flow, a desiccator (25-cm diameter, 25-cm height) with a soft resinous tube muzzle introducing tap water at the bottom of the vessel was used. The muzzle of the tube was set to point upward in the center at the bottom of the desiccator to generate the upward water flow. The rate of upward flow was set at 2.0 cm/s, as the corresponding rate in the final settling pond was lower than this, and the flow was measured by a current meter (Hiroi type; Rigou, Japan). Measurements of the rate of fecal precipitation by H. schlegeli clams were carried out by dropping mucous feces from the clams in the center of the surface of the desiccator and observing their precipitation.
Estimation of removal of C. parvum oocysts from water by H. schlegeli clams in the final settling pond.
To estimate of the efficacy of oocyst removal by the clams in the pond, changes in turbidity and microbe density in the overflow trough water were determined.
As protozoa, such as Cryptosporidium, were not always detected in the final settling pond, we used substitute parameters for Cryptosporidium oocysts in order to estimate oocyst removal by the clams: the reduction of water turbidity and the reduction in concentrations of the specific microbes Heterochromulina sp., Gymnamoebia sp., Testacealobosia sp., and Vorticella sp.
A 1-year experiment, conducted from October 2007 to December 2008, measured these parameters monthly to verify the ability of the clam to remove Cryptosporidium oocysts in the final settling pond. Overflow trough water from the region of the cages was collected and examined. The turbidity was measured using a water analyzer as described above, and the turbidity removal rate (as a percentage) was calculated as follows: 100 × [1 − (turbidity with the clams)/(turbidity without the clams)]. On the other hand, the density of microorganisms was measured by adding of 2.0 ml of Lugol's iodine solution to 1,000 ml of the overflow trough water and allowing it to settle overnight at room temperature to precipitate the microorganisms. The supernatant was then drained to a final volume of 20 ml by aspiration, and this water sample was added dropwise to the blood counting chamber of a hemacytometer, followed by microscopic observations at 400× magnification to count phytoplankton and zooplankton. Meanwhile, the microbe removal rate (as a percentage) was calculated as follows: 100 × [1 − (microbe density with the clams)/(microbe density without the clams)].
RESULTS
Preliminary investigations using H. schlegeli clams.
Only two dead H. schlegeli clams were noted in the laboratory cistern during the 3-year period, and the clam mortality rate was calculated to be 3.3%. In view of this low mortality, it was considered that there would be no particular problem in maintaining clams in the laboratory.
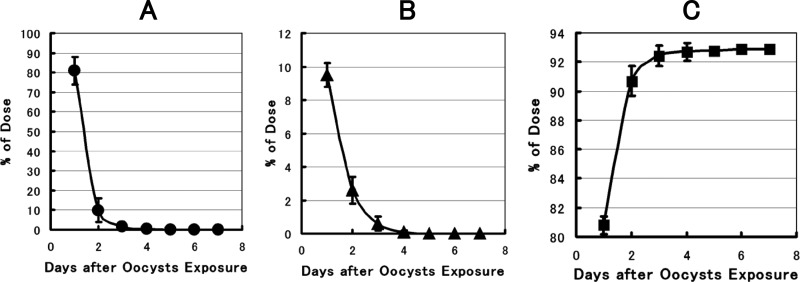
The results of the balance study of the C. parvum oocysts determined in the feces and GI tract contents of the clams after a single exposure to the oocysts showed that oocysts were rapidly ingested by the clams and excreted predominantly via the fecal route, as confirmed both microscopically (Fig. 1) and by PCR (Fig. 2). The pattern of oocyst excretion into the feces indicated two phases: a more rapid initial phase followed by a less rapid later phase, and 5 days after exposure, very few oocysts were detected in the feces. It was verified that the rate of oocyst intake by clams is rapid, and no oocysts were detected microscopically in the cistern water within 1 h after the start of exposure (data not shown). More than 80% of the oocysts were recovered in feces within 1 day after the start of exposure, and the total recovery of oocysts was around 93% within 4 to 5 days (Fig. 1A and C). The biological half-life (t1/2) of the C. parvum oocysts in H. schlegeli clams was estimated to be about 12 h, shorter than that for C. japonica clams, as reported elsewhere (23). The pattern of decrease of oocysts in the GI tract contents was similar to that in the feces, as no oocysts were detected in the GI tract 4 days after the start of oocyst exposure (Fig. 1B).
Fig 1.
Relative amounts of C. parvum oocysts detected in the excreta and GI tracts of H. schlegeli clams after a single dose of oocysts at 6.67 × 104/clam. The data are means ± standard deviations for five replicates. The absence of bars indicates that the error was smaller than the symbol. (A) Percentage of C. parvum oocysts detected in feces. (B) Percentage of C. parvum oocysts in the GI tract. (C) Integrated recovery of oocysts in feces.
Fig 2.
Ethidium bromide-stained 2.0% agarose gel showing amplification products obtained with TRAP-C2 primers from feces and GI tract samples from H. schlegeli. Lane 1, 100-bp molecular marker; lane 2, negative control (with IC); lane 4, fecal sample 1 day after exposure to oocysts; lane 6, fecal sample 3 days after exposure to oocysts; lane 8, fecal sample 5 days after exposure to oocysts; lane 10, GI tract sample 1 day after exposure to oocysts; lane 12, GI tract sample 3 days after exposure to oocysts; lane 14, GI tract sample 5 days after exposure to oocysts; lane 16, positive control; lane 17, 100-bp molecular marker.
The infectious activity of oocysts in the fecal samples, in terms of the rate of excystation, was around 75%, and there were no statistically significant differences between the excystation activities of fecal oocysts from clams and those of freshly prepared oocysts from mouse feces (t test, α = 5% [data not shown]). Meronts were detected in HCT-8 cells 2 days after inoculation (Fig. 3), and there were no significant differences (t test, α = 5%) in the number of foci in infected HCT-8 cells between the clam fecal oocysts and those from mouse feces (data not shown). These results showed that oocysts were not digested by the clams and that oocysts in the feces retained their infectivity to host cells.
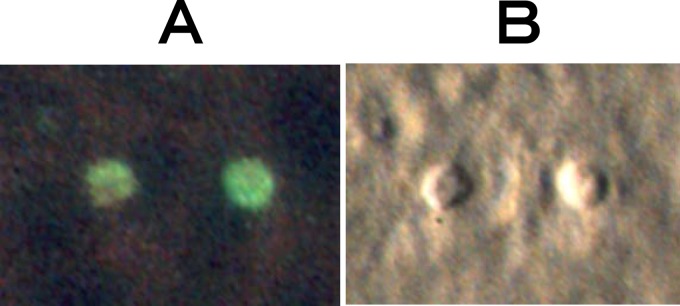
Fig 3.
Photographs of developmental stages of C. parvum prepared from fecal samples of H. schlegeli clams in HCT-8 cells 2 days after inoculation. (A) Fluorescence micrograph of a field of meronts 2 days after inoculation with sporozoites prepared from a fecal sample. (B) Normarski interference-contrast photomicrograph of the corresponding field in panel A.
The changes in C. parvum oocyst concentration and cistern water turbidity with or without the clams or the feed (28 mg/liter) at 20°C are shown in Fig. 4A. The oocyst concentration and water turbidity without the clams showed very moderate reductions due to precipitation of the chow. On the other hand, the oocyst concentration in the presence of the clams, in the absence of chow, showed a slower reduction than when both the clams and chow were present. This suggested that the clams would hardly ingest plain oocysts as a natural food and that the observed slow reduction of oocyst numbers would have been due to ordinary gill breathing by the clam when not ingesting the chow.
Fig 4.
Reductions of C. parvum oocyst concentrations and water turbidity caused by the clams and rates of water filtration by the clams at various water temperatures and feed densities. Means and standard deviations are shown based on results from seven replicates. The absence of bars indicates that the error was smaller than the symbol. (A) Results obtained using laboratory water at 20°C. ●, oocyst density with both clams and feed (28 mg/liter); ○, oocyst density with clams but without feed;  , oocyst density without clams or feed; ▲, turbidity with both clams and feed;
, oocyst density without clams or feed; ▲, turbidity with both clams and feed;  , turbidity without clams but with feed. (B) Results obtained using final settling pond water at 20°C. ●, oocyst density with clams;
, turbidity without clams but with feed. (B) Results obtained using final settling pond water at 20°C. ●, oocyst density with clams;  , oocyst density without clams; ▲, turbidity with clams;
, oocyst density without clams; ▲, turbidity with clams;  , turbidity without clams. (C) Water filtration by clams at various water temperatures and feed densities. See Materials and Methods for details of calculation of the water filtration rate (in L/h).
, turbidity without clams. (C) Water filtration by clams at various water temperatures and feed densities. See Materials and Methods for details of calculation of the water filtration rate (in L/h).
On the other hand, for both the clams and the feed, the reductions in chow turbidity and oocyst concentration were significantly faster than in the former two cases (i.e., without the clam or the feed) (t test, α = 5%) and were very similar, showing synchronized reduction patterns. The faster gill breathing in the presence of the chow was considered attributable to an increased water filtration volume via the inhalant siphon when the clam sensed the presence of feed. We concluded that H. schlegeli would ingest C. parvum oocysts with the chow and that water filtration by the clams would translate into depletion of oocysts from the water.
The similar reductions of both the oocyst concentration and water turbidity caused by the clams in the water of the final settling pond are illustrated in Fig. 4B. It was clear that there were synchronized reductions in both oocyst density and the turbidity derived from impurities comprising the clam feed and various microorganisms. From these results, it was estimated that the clams would ingest any oocysts in the pond water and that neither the oocyst concentration nor turbidity would decrease in the absence of the clams.
On the other hand, the maximum water filtration rate by H. schlegeli (500 g) was between 10 liters/h (ca. 200 liters/day, at 15°C) and 16 liters/h (ca. 300 liters/day, at 20°C) at a chow density of 14 to 28 mg/liter, determined by measuring the initial turbidity reduction rates (Fig. 4C). These data clearly suggest that the clams would perform their filtration function at a moderate water temperature.
Clam fecal precipitation was observed in the presence of an artificial upward flow of 2.0 cm/s, the minimum realizable artificial flow rate, and about 100 times faster than that in the final settling pond. It was found that the feces of H. schlegeli clams settled to the bottom at a velocity of 1.0 to 1.5 cm/s against the upward flow. From this result, it was estimated that clam feces containing C. parvum oocysts would settle in the final settling pond, allowing them to be subjected to automated heat treatment.
Field survey in the sewage plant with H. schlegeli clams.
The tests using fish revealed no unusual behaviors of the fish during the observation period (data not shown) and showed that 12 H. schlegeli clams with pearl cores were able to survive in the final settling pond with a mortality of 0% over a period of more than 3 years; 4 clams produced a total of six pearls during this period. These findings suggested that it was highly likely that H. schlegeli clams would survive in the pond for long periods, as pearls can only be produced in an environment that is stable and appropriate for the clam.
The results of analysis of the settling pond water showed that the temperature of the pond ranged from 15 to 24°C throughout the year and that the DO around the overflow trough ranged between 1.5 and 7.7 mg/liter, which is appropriate for the clams. However, the DO values at other points in the pond were often <1.0 mg/liter. The nitrogen values for ammonia and nitrites, which are harmful to gill-breathing organisms, reached high levels of 1.45 mg/liter and 2.30 mg/liter for a short period in August 2007, which were about 70 and 50 times higher, respectively, than those for the water in the laboratory. The total phosphorus was 0.20 to 0.50 mg/liter, 2 to 5 times higher than in the laboratory water, while the phosphorus in the form of soluble phosphate ions was maintained at a level of around 0.40 mg/liter, 20 times higher than the laboratory values. On the other hand, there were no remarkable differences in the concentrations of metals between the final settling pond water and the cistern water in the laboratory throughout the experimental period.
From these data, the biological and chemical qualities of the final settling pond water were considered to have no effects on the long-term survival of H. schlegeli clams.
The rise in the water level in the final settling pond was calculated as 18.6 m/day on the basis of the total drained water volume (1,600 m3/day) and the water surface area (86 m2) of the pond: this value would correspond to a water volume increase rate of 8.0 liters/h (0.02 cm/s) in a 100-cm2 square column. Accordingly, it was estimated that if clams were placed at a density of 1 per 100 cm2, they would theoretically filter the upward flow of the final settling pond with a water filtering ability of 10 to 16 liters/h throughout the year.
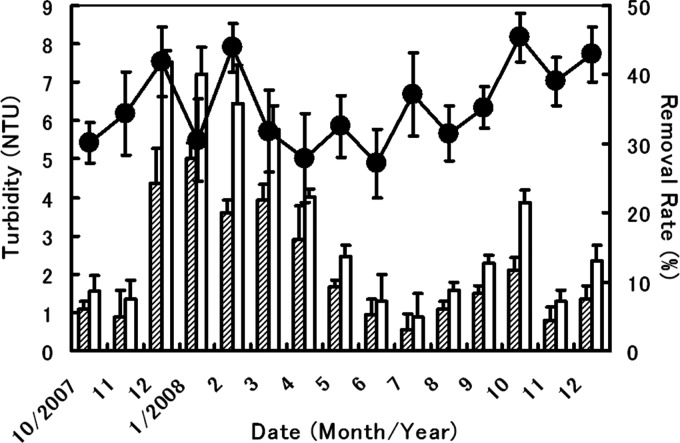
The data related to the reduction of water turbidity when using the clams are shown in Fig. 5, which presents the seasonal changes in the turbidity of water in the overflow trough. The water turbidity in the presence of H. schlegeli clams was shown to be around 35% lower than that of the control throughout the observation period. Significant differences in the rate of turbidity removal were found at 87% of the total data points relative to the controls (t test, α = 5%), proving that H. schlegeli clams filtered out the turbid components along with feed microbes in the pond water.
Fig 5.
Time course of water turbidity and its rate of removal in the overflow trough water by H. schlegeli clams. The rate of turbidity (as a percentage) was calculated as described in the text. Means and standard deviations are shown based on results from five replicates. ▨, turbidity with clams; □, turbidity without clams (control); ●, rate of turbidity removal by clams.
To study the filtration ability of H. schlegeli clams in more detail, in addition to the turbid components, the decreases in the densities of microorganisms trapped by the clams were monitored for 1 year. Classification and determination of microorganisms in the water of the final settling pond were carried out mainly for four phyla: Sarcomastigophora (classes Phytomastigophora, Zoomastigophorea, Lobosea, Filosea, Granuloreticulosea, and Heliozoea), Ciliophora (classes Peritrichia, Oligohymenophora, and Polyhymenophora), Aschelminthes (class Eurotatorea), and Protozoa (class Ciliatea). However, not all phyla were detected during the whole of the experimental period.
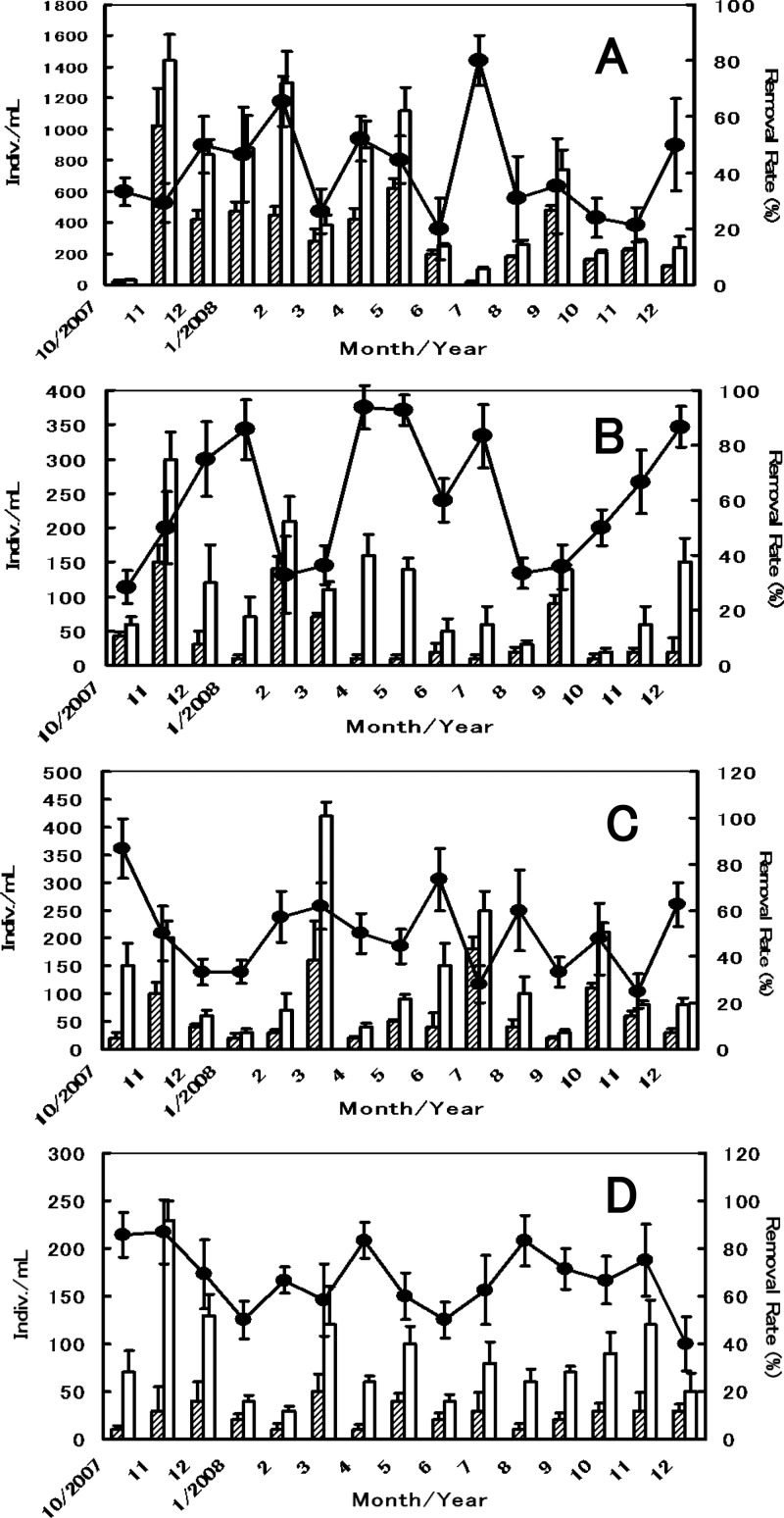
Data for both the seasonal densities of microorganisms stably observed throughout the year in the trough water and the rate of microbe removal by the clams are shown in Fig. 6. The microorganisms listed here were fed to the clams as natural chow and were also detected in the GI tracts of the clams. The rates of decrease of Heterochromulina sp. (class Phytomastigophora), Gymnamoebia sp. (class Lobosea), Testacealobosia sp. (class Lobosea), and Vorticella sp. (class Ciliatea) were around 41%, 61%, 50%, and 62%, respectively, and significant differences were detected in 73%, 80%, 80%, and 87% of total data points for each microbe, respectively (t test, α = 5%). Accordingly, these data confirmed that H. schlegeli clams are able to actively prey on microbes with C. parvum oocysts in the final settling pond water throughout the year, and the clams would consequently deplete 40 to 60% of the oocysts if practically applied to the sewage plant under the present conditions.
Fig 6.
Time course of the density of microbes and their rates of removal from the overflow trough water by H. schlegeli clams. The rate of microbe removal (as a percentage) was calculated as described in the text. Means and standard deviations are shown based on results from five replicates. The absence of bars indicates that the error was smaller than the symbol. (A) Heterochromulina sp. (class Phytomastigophora); (B) Gymnamoebia sp. (class Lobesea); (C) Testacealobosia sp. (class Lobesea); (D) Vorticella sp. (class Ciliatea). ▨, density of microbes with clams present; □, density of microbes without clam present (control); ●, rate of microbe removal by the clams.
DISCUSSION
This study was conducted in order to develop a biological filtration system using the freshwater benthic pearl clam Hyriopsis schlegeli to deplete Cryptosporidium oocysts from contaminated sewage water. The biological trial was conducted with H. schlegeli clams placed in the final settling pond of a sewage plant, and we hypothesized that efficient removal of oocysts was possible based on data collected in preliminary investigations.
The preliminary trials involved maintaining the clams in the laboratory to examine their potential, including preservation of oocysts in the clam GI tracts and in feces, rates of water filtration, and the pattern of oocyst intake by the clams. The present trials produced results that accorded well with those obtained in the laboratory, and it was confirmed that the clams had considerable potential for filtration of a large water volume in the final settling pond throughout the year. It was established that oocysts trapped by the clams were not digested in the GI tract and were excreted predominantly in feces while retaining their ability to infect HCT-8 cells.
The results of the field survey showed that the level of turbidity in the overflow trough water in which the clams were placed was 35% lower than in control water and that the levels of microbes were around 40 to 60%, depending on the microbe species ingested by the clam. From these results, it was expected that oocysts would be depleted by H. schlegeli clams in the pond. The efficiency of oocyst removal might be improved further by increasing the number of clams in the pond water. However, to enable easy maintenance of the clams in the pond for a year, the most appropriate and practical cage occupation area was estimated to be around 40% of the water surface area along the overflow trough.
Clams generally capture a variety of plankton and detritus in water by filter feeding (suspension feeding), and the water containing feed entering via the inhalant siphon passes across the surface of the gills; feed is then trapped by the mucus on the gills during passage of the water to the exhalant siphon. The present field study revealed some variation in rates of microbe removal, which was considered due to variations in the affinities of feed surfaces for the viscous membrane covering the clam gills.
Cryptosporidium hominis is a species morphologically identical to C. parvum, which also infects humans (4). In order to collect detailed ecological data on Cryptosporidium species, studies similar to the present trials should be carried out using C. hominis, although similar data might be obtained, as the genomes of these two species are 97% identical (47).
On the other hand, we have also carried out some experimental studies to investigate the depletion of Giardia cysts by H. schlegeri clams, and we obtained valuable results (data not shown). These data indicated that cysts were taken up by the clam and excreted predominantly (more than 90%) via the fecal route within 5 days. It was also proved that fecal cysts could not be digested in the GI tract of the clam, as was the case for C. oocysts. Accordingly, there is a high possibility that, like C. oocysts, Giardia cysts would be effectively depleted from the water of the final settling pond by H. schlegeli clams.
Norovirus is a well-known pathogen that causes acute nonbacterial gastroenteritis, and it is ordinarily detected at high frequencies in sewage plant water contaminated with excreta. Although we have not yet fully investigated the removal of this virus by H. schlegeri clams, it seems likely that this would be possible, as it has been demonstrated that norovirus has an obvious tendency to accumulate in the digestive diverticula of some bivalves, such as oysters, while retaining its infectivity (8, 18, 35, 38, 42, 44, 46).
Previously, it was shown that the regular-sized brackish water benthic clam, Corbicula japonica (total body weight, 10 to 20 g) (23, 24), has a potential water filtration rate of 300 to 400 ml/h/clam at 15°C. This suggests that C. japonica clams with the same body size as H. schlegeli clams (500 g) could have a water filtration rate of 3 to 4 liters/h/clam at 15 to 20°C, considering that the water filtration rate is roughly proportional to the gill area. These data indicate that H. schlegeli clams have a water filtration potential that is at least three times higher than that of C. japonica clams of the same size. Thus, it is estimated that in order to maintain the same water filtration efficacy as 500-g H. schlegeli clams, the quantity of regular-sized C. japonica clams (10 to 20 g) required would be at least 100 times larger.
The oocyst preservation time in the GI tract was found to be 7 to 8 days for C. japonica clams and 4 to 5 days for H. schlegeli clams, and both had similar oocyst excretion patterns. This suggested that H. schlegeli clams gather the oocysts into feces more efficiently (around 40% faster) than C. japonica clams. Moreover, C. japonica is basically a brackish water clam, and no reports so far have indicated the possibility of maintaining C. japonica clams in fresh water for long periods without creating biologically adverse conditions.
From these data, it is considered that H. schlegeli clams are, from a practical perspective, more suitable and effective than C. japonica clams as biological filters for depletion of oocysts in sewage plant water.
Taking into account the present experimental data and assuming that the environmental conditions in the final settling pond were suitable for long-term maintenance of H. schlegeri clams, we think that H. schlegeri could be utilized as a practical and effective biological filter for depletion of oocysts in the water of the final settling pond in small- and medium-sized wastewater treatment plants if the clams are properly placed. Moreover, there would be little or no undesirable biological proliferation of the clam, owing to both its unique ecology and the disinfection by sodium hypochlorite in the plant before the treated water is released into rivers. Furthermore, H. schlegeli is generally known to be a long-living clam with a life span of at least several decades, much longer than that of C. japonica; therefore, there would be little need to replace dead clams with new clams at frequent intervals after placement in the final settling pond. Moreover, the breeding techniques for the clam as a freshwater pearl-producing clams would be expected to afford not only an environment-friendly biological filter but also an indicator of certain protozoan species contaminating freshwater areas, in addition to its value as a pearl clam.
Here, in order to reduce the level of contamination by oocysts in raw sewage as far as possible, various methods for utilizing the natural and familiar ecological cycle as efficiently as possible should be further investigated. Such ecological methods should be applied to water purification before any conventional physical methods, such as rapid sand filtration, slow sand filtration, or ultramembrane filtration, as the ecological methods would be environmentally friendly and not require a large financial outlay.
Although, theoretically, the oocyst removal efficacy of any ecological method would not be powerful, water purification using H. schlegeli clams could certainly be helpful for the preservation of aquatic environments, together with the conventional purification methods described above. Furthermore, the fact that water purification by the clam would not require substantial changes to the equipment of sewage plants would be a considerable advantage. Furthermore, the new demand for H. schlegeli clams as a biological filter in sewage water would be a secondary benefit to those involved in the fisheries affiliated with the freshwater pearl industry.
However, when employing nonnative (or nondomestic) aquatic organisms, such as H. schlegeli, for reduction or control of protozoans, cautious and careful investigations of any biological impact on surrounding aquatic ecosystems must be conducted to ensure that there is no ecological contamination of the original ecosystem.
Supplementary Material
ACKNOWLEDGMENTS
We express our appreciation to the staff of the Protozoa Laboratory of the National Institute of Infectious Diseases for technical support and assistance, and we also express our gratitude to Keishi Takano of the Hokkaido Institute of Public Health for the classification and counting of microbes in water.
Footnotes
Published ahead of print 17 August 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Aizaki M, Morioka M, Kohata K. 1998. The Water quality control of brackish water using bivalve, Corbicula japonica. J. Water Waste 40:34–39 [Google Scholar]
- 2. Bayne BL, Bayne CG, Carefoot TC, Thompson RJ. 1976. The physiological ecology of Mytilus californianus Conrad. 1. Metabolism and energy balance. Oecologia 22:211–228 [DOI] [PubMed] [Google Scholar]
- 3. Downey AS, Graczyk TK. 2007. Maximizing recovery and detection of Cryptosporidium parvum oocysts from spiked eastern oyster (Crassostrea virginica) tissue samples. Appl. Environ. Microbiol. 73:6910–6915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fayer R. 2010. Taxonomy and species delimitation in Cryptosporidium. Exp. Parasitol. 124:90–97 [DOI] [PubMed] [Google Scholar]
- 5. Fayer R, et al. 2002. Temporal variability of Cryptosporidium in the Chesapeake Bay. Parasitol. Res. 88:998–1003 [DOI] [PubMed] [Google Scholar]
- 6. Freire-Santos F, et al. 2002. Survival of Cryptosporidium parvum oocysts recovered from experimentally contaminated oysters (Ostrea edulis) and clams (Tapes decussatus). Parasitol. Res. 88:130–133 [DOI] [PubMed] [Google Scholar]
- 7. Fujioka K, Toda K, Mori T, Yamaguchi K, Aizaki M. 2006. Seasonal change in filtration rate of Corbicula japonica in shallow experimental pond. J. Jpn. Soc. Water Environ. 29:319–326 [Google Scholar]
- 8. Furuta T, Akiyama M, Kato Y, Nishio O. 2003. A food poisoning out break caused by purple Washington clam contaminated with norovirus (Norwalk-like virus) and hepatitis A virus. Kansenshogaku Zasshi 77:89–94 [DOI] [PubMed] [Google Scholar]
- 9. Giangaspero A, et al. 2009. Giardia and Cryptosporidium in inflowing water and harvested shellfish in a lagoon in southern Italy. Parasitol. Int. 58:12–17 [DOI] [PubMed] [Google Scholar]
- 10. Gomez-Bautista M, Ortega-Mora LM, Tabares E, Lopez-Rodas V, Costas E. 2000. Detection of infectious Cryptosporidium parvum oocysts in mussels (Mytilus galloprovincialis) and cockles (Cerastoderma edule). Appl. Environ. Microbiol. 66:1866–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gómez-Couso H, et al. 2004. Detection of Cryptosporidium and Giardia in molluscan shellfish by multiplexed nested-PCR. Int. J. Food Microbiol. 91:279–288 [DOI] [PubMed] [Google Scholar]
- 12. Gómez-Couso H, Freire-Santos F, Hernández-Córdova GA, Ares-Mazás ME. 2005. A histological study of the transit of Cryptosporidium parvum oocysts through clams (Tapes decussatus). Int. J. Food Microbiol. 102:57–62 [DOI] [PubMed] [Google Scholar]
- 13. Gómez-Couso H, Méndez-Hermida F, Castro-Hermida JA, Ares-Mazás E. 2006. Cryptosporidium contamination in harvesting areas of bivalve mollusks. J. Food Prot. 69:185–190 [DOI] [PubMed] [Google Scholar]
- 14. Gómez-Couso H, Méndez-Hermida F, Ares-Mazás E. 2006. Levels of detection of Cryptosporidium oocysts in mussels (Mytilus galloprovincialis) by IFA and PCR methods. Vet. Parasitol. 141:60–65 [DOI] [PubMed] [Google Scholar]
- 15. Graczyk TK, Girouard AS, Tamang L, Nappier SP, Schwab KJ. 2006. Recovery, bioaccumulation, and inactivation of human waterborne pathogens by the Chesapeake Bay non-native oyster, Crassostrea ariakensis. Appl. Environ. Microbiol. 72:3390–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greenberg AE, Clesceri LS, Eaton AD. 1992. Standard methods for the examination of water and wastewaster, 18th ed American Public Health Association, Washington, DC, American Water Works Association, Denver, CO, and Water Environment Federation, Alexandria, VA [Google Scholar]
- 17. Guiguet Leal DA, Pereira MA, Bueno Franco RM, Branco N, Neto RC. 2008. First report of Cryptosporidium spp. oocysts in oysters (Crassostrea rhizophorae) and cockles (Tivela mactroides) in Brazil. J. Water Health 6:527–532 [DOI] [PubMed] [Google Scholar]
- 18. Hansman GS, et al. 2008. Detection of human enteric viruses in Japanese clams. J. Food Prot. 71:1689–1695 [DOI] [PubMed] [Google Scholar]
- 19. Hirata T, et al. 2001. The effect of temperature on the efficacy of ozonation for inactivating Cryptosporidium parvum oocysts. Water Sci. Technol. 43:163–166 [PubMed] [Google Scholar]
- 20. Inomata A, Oshimi A, Tanaka S. 1999. Investigation of DAPI staining method of Cryptospordium parvum oocysts. J. Jpn. Water Work Assoc. 68:32–36 [Google Scholar]
- 21. Isono R. 1998. Estimation of ingestion rate as nitrogen and factors creating its seasonal changes in Ruditapes philippinarum population in Banzu Tidal Flat, Tokyo Bay. J. Jpn. Soc. Water Environ. 29:319–326 [Google Scholar]
- 22. Isono R, Nakamura Y. 2000. Comparative studies of the water filtering rate in marine bivalves, with particular reference to thermal effects. J. Jpn. Soc. Water Environ. 23:319–326 [Google Scholar]
- 23. Izumi T, Itoh Y, Yagita K, Endo T, Ohyama T. 2004. Brackish water benthic shellfish (Corbicula japonica) as a biological indicator for Cryptosporidium parvum oocysts in river water. Bull. Environ. Contam. Toxicol. 72:29–37 [DOI] [PubMed] [Google Scholar]
- 24. Izumi T, Yagita K, Endo T, Ohyama T. 2006. Detection system of Cryptosporidium parvum oocysts by brackish water benthic shellfish (Corbicula japonica) as a biological indicator in river water. Arch. Environ. Contam. Toxicol. 51:559–566 [DOI] [PubMed] [Google Scholar]
- 25. Kohata K, Hiwatari T, Hagiwara T. 2003. Natural water-purification system observed in a shallow coastal lagoon: Matsukawa-ura, Japan. Mar. Pollut. Bull. 47:148–154 [DOI] [PubMed] [Google Scholar]
- 26. Leoni F, Gómez-Couso H, Ares-Mazás ME, McLauchlin J. 2007. Multilocus genetic analysis of Cryptosporidium in naturally contaminated bivalve mollusks. Appl. Microbiol. 103:2430–2437 [DOI] [PubMed] [Google Scholar]
- 27. Lévesque B, et al. 2006. A study to assess the microbial contamination of Mya arenaria clams from the north shore of the St. Laurence River estuary (Québec, Canada). Can. J. Microbiol. 52:984–991 [DOI] [PubMed] [Google Scholar]
- 28. Li X, et al. 2006. Cryptosporidium oocysts in mussels (Mytilus edulis) from Normandy (France). Int. J. Food Microbiol. 108:321–325 [DOI] [PubMed] [Google Scholar]
- 29. Lucy FE, Graczyk TK, Tamang L, Miraflor A, Minchin D. 2008. Biomonitoring of surface and coastal water for Cryptosporidium, Giardia, and human-virulent microsporidia using molluscan shellfish. Parasitol. Res. 103:1369–1375 [DOI] [PubMed] [Google Scholar]
- 30. MacRae M, Hamilton C, Strachan NJ, Wright S, Ogden ID. 2005. The detection of Cryptosporidium parvum and Escherichia coli O157 in UK bivalve shellfish. J. Microbiol. Methods 60:395–401 [DOI] [PubMed] [Google Scholar]
- 31. Maeda I, Aizaki M, Yamaguchi K, Fujita N. 2000. A study on water purification by the bivalve, Corbicula japonica, using outdoor experimental tanks with continuous flow systems. J. Jpn. Soc. Water Environ. 23:716–720 [Google Scholar]
- 32. McLusky D. 1973. The effect of temperature on the oxygen consumption and filtration rates of Chlamys (Aequipecten) opercularis (L.) (Bivalvia). Ophelia 10:141–154 [Google Scholar]
- 33. Meyhofer E. 1985. Comparative pumping rates in suspension-feeding bivalves. Mar. Biol. 85:137–142 [Google Scholar]
- 34. Molini U, et al. 2007. Temporal occurrence of Cryptosporidium in the Manila clam Ruditapes philippinarum in Northern Adriatic Italian lagoons. J. Food Prot. 70:494–499 [DOI] [PubMed] [Google Scholar]
- 35. Morse DL, et al. 1986. Widespread outbreaks of clam- and oyster-associated gastroenteritis: role of Norwalk virus. N. Engl. J. Med. 314:678–681 [DOI] [PubMed] [Google Scholar]
- 36. Nakamura M, Yamamuro M, Ishikawa M, Nishimura H. 1988. Role of the bivalve Corbicula japonica in the nitrogen cycle in a mesohaline lagoon. Mar. Biol. 99:369–374 [Google Scholar]
- 37. Nakamura Y, Kerciku F. 2000. Effects of filter-feeding bivalves on the distribution of water quality and nutrient cycling in a eutrophic coastal lagoon. J. Mar. Systems 26:209–221 [Google Scholar]
- 38. Nishida T, et al. 2003. Detection, quantitation, and phylogenetic analysis of noroviruses in Japanese oysters. Appl. Environ. Microbiol. 69:5782–5786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oshima K, Suzuki N, Nakamura M, Sakuramoto K. 2004. Shell growth and age determination of the brackish water bivalve Corbicula japonica in Lake Shinji, Japan. Fish. Sci. 70:601–610 [Google Scholar]
- 40. Owen G. 1974. Feeding and digestion in bivalvia. Adv. Comp. Physiol. Biochem. 5:1–35 [DOI] [PubMed] [Google Scholar]
- 41. Robertson LJ. 2007. The potential for marine bivalve shellfish to act as transmission vehicles for outbreaks of protozoan infections in humans: a review. Int. J. Food Microbiol. 120:201–216 [DOI] [PubMed] [Google Scholar]
- 42. Savini G, Casaccia C, Barile NB, Paoletti M, Pinoni C. 2009. Norovirus in bivalve mollusks: a study of the efficacy of the depuration system. Vet. Ital. 45:535–539 [PubMed] [Google Scholar]
- 43. Schets FM, van den Berg HH, Engels GB, Lodder WJ, de Roda Husman AM. 2007. Cryptosporidium and Giardia in commercial and non-commercial oysters (Crassostrea gigas) and water from the Oosterschelde, The Netherlands. Int. J. Food Microbiol. 113:189–194 [DOI] [PubMed] [Google Scholar]
- 44. Schwab KJ, Neill FH, Estes MK, Metcalf TG, Atmar RL. 1998. Distribution of Norwalk virus within shellfish following bioaccumulation and subsequent depuration by detection using RT-PCR. J. Food Prot. 61:1674–1680 [DOI] [PubMed] [Google Scholar]
- 45. Slifko TR, Friedman D, Rose JB, Jakubowski W. 1997. An in vitro method for detecting infectious Cryptosporidium oocysts with cell culture. Appl. Environ. Microbiol. 63:3669–3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sunen E, Sobsey MD. 1999. Recovery and detection of enterovirus, hepatitis A virus and Norwalk virus in hardshell clams (Mercenaria mercenaria) by RT-PCR methods. J. Virol. Methods 77:179–187 [DOI] [PubMed] [Google Scholar]
- 47. Tanriverdi S, Widmer G. 2006. Differential evolution of repetitive sequences in Cryptosporidium parvum and Cryptosporidium hominis. Infect. Genet. Evol. 6:113–122 [DOI] [PubMed] [Google Scholar]
- 48. Upton SJ, Tilley M, Brillhart DB. 1995. Effect of select medium supplements on in vitro development of Cryptosporidium parvum in HCT-8 cells. J. Clin. Microbiol. 33:371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vahl O. 1973. Pumping and oxygen consumption rates of Mytilus edulis L. of different size. Ophelia 12:45–52 [Google Scholar]
- 50. Yagita K, et al. 2001. Molecular characterization of Cryptosporidium isolates obtained from human and bovine infections in Japan. Parasitol. Res. 87:905–955 [DOI] [PubMed] [Google Scholar]
- 51. Yamamuro Y, Koike I. 1993. Nitrogen metabolism of the filter-feeding bivalve Corbicula japonica and its significance in primary production of a brackish lake in Japan. Limnol. Oceanogr. 38:997–1007 [Google Scholar]
- 52. Yamamuro Y, Koike I. 1994. Diel changes of nitrogen species in surface and overlying water of an estuarine lake in summer: evidence for benthic-pelagic coupling. Limnol. Oceanogr. 39:1716–1733 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.