Abstract
Complex microbiomes reside in marine sponges and consist of diverse microbial taxa, including functional guilds that may contribute to host metabolism and coastal marine nutrient cycles. Our understanding of these symbiotic systems is based primarily on static accounts of sponge microbiota, while their temporal dynamics across seasonal cycles remain largely unknown. Here, we investigated temporal variation in bacterial symbionts of three sympatric sponges (Ircinia spp.) over 1.5 years in the northwestern (NW) Mediterranean Sea, using replicated terminal restriction fragment length polymorphism (T-RFLP) and clone library analyses of bacterial 16S rRNA gene sequences. Bacterial symbionts in Ircinia spp. exhibited host species-specific structure and remarkable stability throughout the monitoring period, despite large fluctuations in temperature and irradiance. In contrast, seawater bacteria exhibited clear seasonal shifts in community structure, indicating that different ecological constraints act on free-living and on symbiotic marine bacteria. Symbiont profiles were dominated by persistent, sponge-specific bacterial taxa, notably affiliated with phylogenetic lineages capable of photosynthesis, nitrite oxidation, and sulfate reduction. Variability in the sponge microbiota was restricted to rare symbionts and occurred most prominently in warmer seasons, coincident with elevated thermal regimes. Seasonal stability of the sponge microbiota supports the hypothesis of host-specific, stable associations between bacteria and sponges. Further, the core symbiont profiles revealed in this study provide an empirical baseline for diagnosing abnormal shifts in symbiont communities. Considering that these sponges have suffered recent, episodic mass mortalities related to thermal stresses, this study contributes to the development of model sponge-microbe symbioses for assessing the link between symbiont fluctuations and host health.
INTRODUCTION
Sponges are sessile invertebrates that form a species-rich phylum at the base of the metazoan tree of life (>8,500 valid species [65]). Renowned for their efficient filter-feeding capabilities and bioactive secondary metabolite production, sponges have important ecological and biotechnological relevance as major players in marine nutrient cycles (11, 12, 26) and the most prolific producers of marine natural products (>6,600 secondary metabolites [16]). The discovery and characterization of diverse microbial symbionts inhabiting the sponge body have prompted the adoption of the holobiont concept, thereby incorporating microbial symbionts in the study of sponge ecology and evolution (55). The resulting field of sponge microbiology has grown rapidly in the past 2 decades (59) and revealed a tight ecological link between host health and symbiont composition. Indeed, sponge-associated microbes have been implicated in host metabolism and growth (20, 22, 75), chemical defense production (21), and susceptibility to biotic (e.g., disease) and abiotic (e.g., temperature stress) stressors (33, 66, 72).
The remarkable diversity of the sponge microbiota has presented a formidable challenge to understanding the structure and function of microbial guilds in sponge hosts (24, 59, 70). The sponge microbiota includes diverse phylogenetic lineages of Archaea and Bacteria, as well as fungi and viruses (52, 56). Among bacterial symbionts alone, thousands of taxa have been reported, spanning 17 described phyla and 12 candidate phyla (50), and hundreds of bacterial taxa can occur in a single host individual (32, 71). Accordingly, considerable effort has focused on describing the vast diversity of the sponge microbiota, while more applied studies of symbiont functioning have targeted specific components (e.g., Cyanobacteria [59]) or functional gene pathways (e.g., ammonia oxidation [35]) in these communities. As a result, most studies of sponge microbiology have been limited in scope to one or a few host species collected at a single time point, and thus, much of our knowledge concerning the sponge microbiota is based on a static representation of these potentially dynamic communities (59).
Understanding the complex sponge microbiota requires a basic knowledge of how these communities change over time. The general consensus is that sponge-microbe associations are largely stable over temporal scales (56), including epibionts (31), cultivatable symbionts (68), and entire bacterial communities (57, 60, 61, 73). Other studies have reported higher levels of variability across seasons (74) and when repeatedly sampling the same individuals over time (3), indicating some degree of symbiont fluctuation over time and individual variation among hosts. The prospect of sponge aquaculture for the production of bioactive metabolites has prompted investigations of host-symbiont stability under ex situ aquarium conditions, revealing high symbiont stability over short-term time scales (11 days to 12 weeks [23, 67]), while longer-term maintenance (6 months to 2 years) can result in substantial shifts in symbiont composition (39, 40, 67). Additional studies of temporal variation in sponge-associated bacteria under natural conditions will aid future aquaculture efforts by determining natural variation in the sponge microbiota and its consequences for host-symbiont dynamics. Further, such studies establish the baseline levels of symbiont variability required to define abnormal shifts and ascribe symbiont fluctuations to specific abiotic and biotic factors.
In this study, we investigated temporal variation in the microbiota of three congeneric sponge hosts from the Mediterranean Sea: Ircinia fasciculata, I. variabilis, and I. oros. These sponges are common members of coastal benthic communities in the Mediterranean Sea and harbor diverse, host-specific communities of bacterial and cyanobacterial symbionts (15, 17, 46, 63). Replicate individuals of each sponge species were tagged in situ and sampled quarterly for 1.5 years to monitor their bacterial symbiont communities, using terminal restriction fragment length polymorphism (T-RFLP) and clone library analyses of bacterial 16S rRNA gene sequences. In addition, photosynthetic pigments were monitored in the tissues of the cyanobacterium-rich sponges I. fasciculata and I. variabilis, using chlorophyll a (chl a) quantification. The specific objectives of the study were (i) to determine the temporal stability of host-symbiont specificity, (ii) to identify permanent and transient symbiont taxa in association with sponge hosts, and (iii) to document natural variability in symbiont communities over time. Collectively, these objectives contribute to the broader goal of establishing the empirical baselines required to diagnose abnormal symbiont shifts and develop these symbiotic systems as an impact assessment tool in coastal ecosystems.
METHODS
Sample collection.
The sponge species Ircinia fasciculata (Pallas, 1766), I. variabilis (Schmidt, 1862), and I. oros (Schmidt, 1864) were monitored in shallow (<20 m) littoral zones at two neighboring sites (<12 km apart) along the Catalan Coast (Spain) in the northwestern (NW) Mediterranean Sea. I. fasciculata colonies were studied in Punta de S'Agulla (Blanes; 41°40′54.87″N, 2°49′00.01″E) and I. variabilis and I. oros in Mar Menuda (Tossa de Mar; 41°43′13.62″N, 2°56′26.90″E) from March 2010 to June 2011. Initial sampling of I. oros (March 2010) was performed in the nearby Punta Santa Anna (Blanes; 41°40′21.48″N, 2°48′13.55″E); however, from June 2010 to June 2011, sampling was conducted in Tossa de Mar, due to the onset of heavy construction in the adjacent Blanes Port (<300 m from the Punta Santa Anna sampling site) in May 2010.
Individual sponges were marked in situ and sampled quarterly for genetic analyses and chlorophyll a concentrations by scuba diving, using a scalpel blade and forceps. At each site, ambient seawater samples (500 ml) were collected simultaneously and in close proximity (<1 m) to sampled sponges. Sponge and seawater samples were transported in an insulated cooler to the laboratory (ca. 2 h of transit time), where sponge samples for genetic analyses were preserved in 100% ethanol and stored at −20°C and seawater samples were concentrated on 0.2-μm filters and stored at −80°C. Tissue samples for chlorophyll a quantification were processed immediately (see below).
Temperature and light measurements.
Hourly temperature and light intensity levels were recorded in situ at Punta de S'Agulla and Tossa de Mar by Hobo Pendant Temperature/Light Data Loggers (UA-002-64; Onset Computer Corporation) deployed in close proximity (<2 m) to sampled sponges. Consistent with the distribution of the studied sponge taxa (15), data loggers were deployed at Punta de S'Agulla on the horizontal (exposed) substrate, the typical habitat of I. fasciculata, and at Tossa de Mar on the vertical wall (cryptic) substrate, the typical habitat of I. variabilis and I. oros. Submarine in situ light measurements are complicated by light sensor orientation and the occurrence of sensor encasement fouling. To minimize orientation error, data loggers were attached parallel to the substrate in stable epoxy molds for consistent orientation of light sensors. To minimize fouling error, data loggers were replaced monthly and only the first 7 days of light measurements (70 to 105 data points per month) were used in subsequent analyses. Light measurements were recorded as lux (lumen m−2), the SI-derived unit for luminous flux density, across a broad spectrum of wavelengths (200 to 1,200 nm) and used to compare relative changes in light intensity across sites and seasons. Light duration was calculated as the number of hourly light readings per day greater than 0. Missing data from Tossa de Mar (March 2010 to May 2010) resulted from the loss of data loggers. For comparative analyses, seasons were defined as winter (January, February, and March), spring (April, May, and June), summer (July, August, and September), and fall (October, November, and December).
DNA extraction.
DNA extracts were prepared from sponge samples containing both ectosome and choanosome for six individuals per host species and time point (n = 108) and three replicates of filtered seawater per time point (n = 18), using the DNeasy Blood & Tissue kit (Qiagen). Dilutions (1:10) of DNA extracts were used as the templates in subsequent PCR amplifications.
T-RFLP analysis.
PCR amplification of 16S rRNA gene sequences (ca. 1,500 bp) for T-RFLP analysis was conducted using the universal bacterial forward primer 8F (44) and reverse primer 1509R (38), with a 5′-end 6-carboxyfluorescein (6-FAM) label attached to the forward primer. The total PCR volume was 50 μl, and each reaction mixture contained 15 pmol of the labeled forward primer, 10 pmol of the reverse primer, 10 nmol of each deoxynucleoside triphosphate (dNTP), 1× reaction buffer (Ecogen), and five units of Biotaq polymerase (Ecogen). Thermocycler reaction conditions were an initial denaturing time of 2 min at 94°C, followed by 30 cycles of 1 min at 94°C, 0.5 min at 50°C, and 1.5 min at 72°C, and a final extension time of 2 min at 72°C. To minimize PCR amplification biases, a low annealing temperature and low cycle number were used and three separate reactions were conducted for each sample. Triplicate PCR products were gel purified and cleaned using the QIAquick gel extraction kit (Qiagen) and then combined and quantified using a Qubit fluorometer and the Quant-iT dsDNA assay kit (Invitrogen).
Purified PCR products (ca. 100 ng) were digested separately with the restriction endonucleases HaeIII and MspI (Promega) at 37°C for 8 h and ethanol precipitated to remove residual salts from enzyme buffers. Samples were eluted in 10 μl formamide and 0.5 μl GeneScan 600-LIZ size standard, heated for 2 min at 94°C, cooled on ice, and analyzed by capillary electrophoresis on an automated sequencer (ABI 3730 Genetic Analyzer; Applied Biosystems) at the Scientific and Technical Services of the University of Barcelona (Spain). The lengths of individual terminal restriction fragments (T-RF) were determined by comparison with internal size standards using the program GeneScan (PE; Applied Biosystems). T-RFs beyond the resolution of internal size standards (50 to 600 bp) or with peak areas of less than 50 fluorescence units were removed, and peak profiles were imported into the program T-REX (10). Prior to T-RF alignment in T-REX, the objective filtering algorithm of Abdo et al. (1) based on peak area and a cutoff value of 2 standard deviations (SD) was applied to denoise the data set by eliminating background peaks. Following noise reduction, T-RFs were aligned across samples using a 1-bp clustering threshold, and peak profiles were standardized using relative abundance (percentage total fluorescence).
To compare the similarity of bacterial community profiles, Bray-Curtis similarity matrices were constructed using square root transformations of relative T-RF abundance data and visualized in nonmetric multidimensional scaling (nMDS) plots and heat maps. Permutational multivariate analyses of variance (PERMANOVA) were used to determine significant differences in bacterial community structure across sources (sponge species and seawater) and across seasons within sources (nested analysis). Permutational multivariate analyses of dispersion (PERMDISP) were conducted for all significant PERMANOVA outcomes to test for differences in homogeneity (dispersion) among groups. A significant PERMDISP outcome indicates that differences in community structure detected by PERMANOVA may result from unequal structural variability among groups (i.e., heterogeneity of dispersion) rather than consistent structural shifts. Multiple pairwise comparisons of symbiont structure and dispersion were corrected based on the Benjamini-Yekutieli (B-Y) false discovery rate control (7) and an experiment-wise error rate of 0.05. nMDS, PERMANOVA, and PERMDISP calculations were performed using Primer v6 and Permanova+ (Plymouth Marine Laboratory, United Kingdom). Heat maps were constructed using JColorGrid v1.869 (28).
Clone library construction and sequence analysis.
In a previous study, we provided an initial characterization of bacterial communities in I. fasciculata, I. variabilis, and I. oros collected in the winter season (March 2010) by 16S rRNA gene sequence clone libraries (15). In the current study, we resampled the same host individuals in the summer season (September 2010) and constructed clone libraries following the same methodology to (i) monitor changes in symbiont communities across seasons and (ii) identify T-RFLP profile peaks not represented in the winter clone library. In total, 320 clones from the summer clone libraries were bidirectionally sequenced using vector primers at Macrogen, Inc., to recover near-full-length 16S rRNA gene sequences (range, 1,399 to 1,525 bp; average length, 1,478 bp). Raw sequence reads were processed in Geneious (13) by aligning high-quality forward and reverse reads to yield a final consensus sequence for each clone. Consensus sequences were screened for sequencing anomalies (e.g., chimeras) using Mallard (6) and a reference 16S rRNA gene sequence from Escherichia coli (GenBank accession no. U00096) and confirmed or refuted using Pintail (5) and two related reference sequences.
To determine seasonal overlap and divergence in symbiont communities, sequences were ascribed to operational taxonomic units (OTUs) (99% sequence identity, nearest-neighbor algorithm), as implemented in the mothur software package (49), and compared to 99% OTUs from the winter clone library (see Table S1 in the supplemental material). Representative sequences from each 99% OTU were analyzed using the Ribosomal Database Project II (9) sequence classifier to assess taxonomic affiliations. In addition, OTU-independent statistical tests were conducted to determine seasonal differences in the genetic diversity (homogeneity of molecular variance [HOMOVA]), genetic differentiation (analysis of molecular variance [AMOVA]) (54) and phylogenetic structure (unweighted UNIFRAC [36]) of bacterial communities within each source. HOMOVA, AMOVA, and UNIFRAC analyses were performed as implemented in the mothur software package (49).
To match clone library sequences with T-RFLP profile peaks, a reference database (IRC) was created by in silico digestions of 16S rRNA gene sequences and consisted of 5′-terminal restriction fragment lengths (reference T-RFs) for each 99% OTU (n = 190) and restriction endonuclease (HaeIII or MspI) combination. Following correction of T-RF drift (see below), the IRC reference database was used to match empirical T-RFs from T-RFLP profiles with known 16S rRNA gene sequences from clone libraries, using the phylogenetic assignment tool (PAT) (30) with 1.5-bp bins. Discrepancies between the predicted length of reference T-RFs and actual length of empirical T-RFs can occur due to the phenomenon of T-RF drift (29), where small differences in the molecular weight of fluorescent labels attached to samples (e.g., FAM) and size standards (e.g., LIZ) result in differential capillary migration rates and underestimation of DNA fragment sizes (41). To correct for T-RF drift associated with the fluorescent labels used here, the empirical lengths of T-RFs were determined for eight dominant bacterial OTUs (IRC001, IRC002, IRC003, IRC004, IRC006, IRC007, IRC0012, and IRC0015) using monocultures of each clone as the templates for PCR amplification and T-RFLP analyses, as described above. Regression analysis of the empirical versus predicted lengths of T-RFs from these clones produced a standard curve (R2 > 0.99; see Fig. S1 in the supplemental material) used to correct for the discrepancies of T-RF drift and more accurately match DNA sequences with T-RFLP profile peaks.
Chlorophyll a concentrations.
Tissue samples for chl a quantification were collected from ectosomal regions of I. fasciculata (n = 48) and I. variabilis (n = 47) and processed following previously described methods (17). Due to the absence of photosymbionts in I. oros (15), this species was not included in chlorophyll analysis. For I. fasciculata, the same eight marked individuals were repeatedly sampled, due to the large size and rapid healing processes of this species. For I. variabilis, three to 11 nonmarked individuals were randomly sampled each month from the same population, as the smaller size and slower healing rate of this species prevented repeated sampling of the same colonies. Accordingly, a one-way repeated-measures analysis of variance (ANOVA) for I. fasciculata and a one-way ANOVA for I. variabilis were conducted to compare chl a concentrations within each species across sampling months. Multiple pairwise comparisons of chl a concentrations between species within each month were conducted using Student's t tests with Bonferroni corrections. Statistical analyses were performed using the software Sigmaplot v11.
Nucleotide sequence accession numbers.
The sequences determined in this study have been quality checked and are archived in GenBank under accession numbers JX206477 to JX206796.
RESULTS
Seasonal variation in temperature and light intensity.
Both monitoring sites exhibited clear seasonal trends in temperature (Fig. 1). Annual temperature minima occurred during the winter season, with the lowest average monthly values recorded in March 2010 (12.7°C in S'Agulla and 12.3°C in Tossa) and lowest average daily values in February 2011 (12.4°C in S'Agulla and 12.1°C in Tossa). Annual temperature maxima occurred during the summer season, with the highest average monthly and daily values recorded in August 2010 (23.8°C and 25.3°C in S'Agulla; 22.2°C and 24.8°C in Tossa). Annual temperature fluctuations were accordingly high at both sites (>12.7°C). Small differences in seawater temperatures between the monitoring sites likely resulted from slightly deeper data logger deployment in Tossa (7 m) than in S'Agulla (5 m). The summer season was also characterized by large fluctuations in daily temperatures, averaging 2.2°C (±1.3 SD) in S'Agulla and 1.8°C (±1.2 SD) in Tossa, with >3°C daily fluctuations recorded on 15 and 12 days in S'Agulla and Tossa, respectively. In contrast, the winter season exhibited minor fluctuations in daily temperatures, averaging 0.4°C (±0.2 SD) in S'Agulla and 0.3°C (±0.2 SD) in Tossa and never exceeding 0.8°C at either site. A notable upwelling event occurred in August 2010, causing drastic temperature decreases at both sites and resulting in weekly temperature fluctuations of 7.7°C and 9.4°C and daily fluctuations of 6.9°C and 5.4°C in S'Agulla and Tossa, respectively.
Fig 1.
Seasonal variation in seawater temperature from March 2010 to June 2011 at two monitoring sites in the NW Mediterranean Sea. Monthly averages (±SD) for Punta de S'Agulla (black circles) and Tossa de Mar (gray diamonds) (A) and daily averages for Punta de S'Agulla (B) and Tossa de Mar (C). Gray triangles highlight sampling times, and black dots indicate discrete measurements prior to successful data logger deployment at Tossa de Mar.
Both monitoring sites also exhibited clear trends in irradiance conditions across seasons (Fig. 2). Light duration (i.e., day length) was longer in spring and summer than in the fall and winter seasons, which experienced up to 6 h less of light exposure per day. Maximum and average light intensity values were higher during the spring and summer seasons than in the fall and winter. Light intensity levels in S'Agulla averaged 1,569 to 10,240 lx per month, with maximum values reaching over 38,000 lx. Lower levels were observed in Tossa, averaging 264 to 1,198 lx per month, and maximum values never exceeded 3,700 lx. The large differences in irradiance between sites were consistent with the deployment of data loggers in photophilic (S'Agulla) and semisciophilous (Tossa) communities and correspond to the distinct habitats of the host sponge species investigated.
Fig 2.
Seasonal variation in light duration (day length) and intensity from March 2010 to June 2011 at two monitoring sites in the NW Mediterranean Sea. Monthly averages (±SD) for day length at Punta de S'Agulla (black circles) and Tossa de Mar (gray diamonds) (A). Monthly averages (black diamonds) and maximum light intensity (gray bars) at Punta de S'Agulla (B) and Tossa de Mar (C).
Host specificity of bacterial communities.
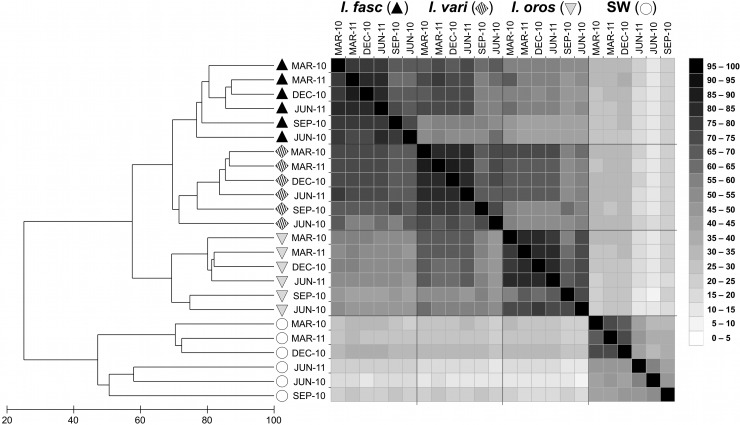
A total of 213 unique microbial symbiont T-RFs were identified using the restriction enzyme HaeIII (151 in I. fasciculata, 149 in I. variabilis, 147 in I. oros, and 144 in seawater) and 237 unique T-RFs with MspI (185 in I. fasciculata, 164 in I. variabilis, 156 in I. oros, and 159 in seawater). Binary data analysis of individual T-RFs (presence/absence) revealed highly congruent specificity patterns between the two restriction enzymes used to construct T-RFLP profiles (see Fig. S2 in the supplemental material). One-third of the unique T-RFs (32.4% for HaeIII and 33.0% for MspI) were sponge specific, present in one or more host species and absent from seawater, while <1/10 (8.9%, HaeIII; 5.9%, MspI) were recovered exclusively from seawater (see Fig. S2 in the supplemental material). The majority of T-RFs were shared among sponges and seawater, present in at least 1 sponge host and seawater (23.0%, HaeIII; 26.6%, MspI) or among all 3 host sponges and seawater (35.7%, HaeIII; 34.6%, MspI) (see Fig. S2). Among the sponge-specific T-RFs, the highest number of unique (host species-specific) T-RFs was detected in I. fasciculata (n = 14, HaeIII; n = 11, MspI), and I. fasciculata and I. variabilis shared more T-RFs than any other pair (n = 12, HaeIII; n = 14, MspI). Similarly, community-level analysis based on the relative abundance of microbial T-RFs revealed clear differentiation of sponge and seawater communities and more similar symbiont communities in I. fasciculata and I. variabilis than in I. oros (Fig. 3).
Fig 3.
Average similarity of bacterial communities in I. fasciculata (black triangles), I. variabilis (barred diamonds), I. oros (gray triangles), and ambient seawater (white circles) over the 1.5-year monitoring period. Dendrogram (left) based on Bray-Curtis (BC) similarity values from T-RFLP profiles with HaeIII. Heat map (right) shows all pairwise BC similarity values for both HaeIII (upper diagonal) and MspI (lower diagonal) data sets.
Statistical analyses of community structure (PERMANOVA) revealed significant differences between sponge and seawater microbial fingerprints and among all pairwise comparisons of host sponge species (Table 1). Nonmetric multidimensional scaling (nMDS) plots exhibited clear spatial segregation of sponge and seawater-derived microbial communities, while among host sponges, symbiont communities consistently clustered by host species across all seasons, with no overlap between I. fasciculata and I. oros and higher variability in the symbiont profiles of I. variabilis (Fig. 4A and C). Dispersion analysis revealed higher variability within seawater communities than among sponge-associated bacteria, as pairwise comparisons between sponges and seawater were significant for at least one enzyme while no significant differences in dispersion were found in pairwise comparisons among sponge species (Table 1).
Table 1.
Permutational statistical analyses of T-RFLP dataa
| Analysis | Pairwise comparison | HaeIII |
MspI |
||
|---|---|---|---|---|---|
| t | P (perm) | t | P (perm) | ||
| PERMANOVA | I. fasciculata/I. variabilis | 3.683 | 0.001* | 3.682 | 0.001* |
| I. variabilis/I. oros | 5.164 | 0.001* | 4.508 | 0.001* | |
| I. oros/I. fasciculata | 6.988 | 0.001* | 6.637 | 0.001* | |
| I. fasciculata/seawater | 10.408 | 0.001* | 9.500 | 0.001* | |
| I. variabilis/seawater | 9.258 | 0.001* | 8.082 | 0.001* | |
| I. oros/seawater | 9.136 | 0.001* | 10.028 | 0.001* | |
| PERMDISP | I. fasciculata/I. variabilis | 1.615 | 0.177 | 0.848 | 0.475 |
| I. variabilis/I. oros | 0.516 | 0.639 | 1.350 | 0.235 | |
| I. oros/I. fasciculata | 2.152 | 0.071 | 0.456 | 0.683 | |
| I. fasciculata/seawater | 4.016 | 0.001* | 3.575 | 0.002* | |
| I. variabilis/seawater | 2.451 | 0.046 | 2.933 | 0.015* | |
| I. oros/seawater | 1.997 | 0.093 | 4.424 | 0.002* | |
Analyses included bacterial community structure (PERMANOVA) and dispersion (PERMDISP) among sponges and seawater.
, comparison was found to be significant following B-Y correction (7). P (perm), permutation P value.
Fig 4.
Nonmetric multidimensional scaling (nMDS) plots of bacterial community structure from replicate individuals of I. fasciculata, I. variabilis, and I. oros and ambient seawater over the 1.5-year monitoring period. nMDS ordination based on Bray-Curtis similarity of T-RFLP profiles for HaeIII (A, B) and MspI (C, D) data sets. Stress values for two-dimensional ordination are shown in parentheses for each enzyme. Data points are coded by source (A, C), with circles encompassing all samples from each source, and by season (B, D), with shaded circles denoting core bacterial symbiont profiles and nonshaded circles highlighting deviations from core profiles in spring/summer 2010 (B, D).
Seasonal variation in bacterial communities.
Symbiont communities within each host sponge species exhibited high stability throughout the monitoring period, averaging 69.9% (I. fasciculata), 64.0% (I. variabilis), and 63.2% (I. oros) community similarity in T-RFLP profiles. nMDS plots revealed two tight spatial clusters for I. oros and I. fasciculata plus I. variabilis samples, particularly when considering HaeIII profiles (Fig. 4B and D). Each cluster consisted of all samples from the 2010-2011 fall and winter and from spring of 2011, as well as some individuals from spring and summer of 2010. However, most samples from spring and summer of 2010 were displaced from these central clusters, indicating some change in bacterial profiles during these seasons. In contrast, seawater bacterial communities exhibited clear and consistent seasonal shifts in composition, resulting in spatially segregated clusters in nMDS plots that corresponded to distinct bacterioplankton communities in the fall/winter, spring, and summer seasons (Fig. 4).
Statistical analyses of community structure (PERMANOVA) and dispersion (PERMDISP) revealed significant differences in structure and homogeneity of dispersion among all pairwise comparisons of seawater bacteria (see Table S2 in the supplemental material), confirming the seasonal shifts in seawater bacteria visualized in nMDS plots. Among host sponges, significant differences in community structure were observed in the transition from winter to spring and summer to fall of 2010 for at least one enzyme (see Table S2), due to high variability in bacterial community profiles among individuals of each host sponge in spring and summer of 2010. Indeed, PERMDISP analyses revealed significant differences in dispersion during these transitional periods, indicating that heterogeneity was the main driver of structural differences in symbiont communities. Within the fall/winter and spring/summer seasons, structural differences in sponge-associated bacteria were generally not significant (see Table S2).
Clone library analysis of 16S rRNA gene sequences confirmed the stability of sponge-associated microbial communities over time and the seasonal variability of seawater communities. Comparisons of clone libraries constructed from the same individuals sampled in winter (March) and summer (September) 2010 seasons revealed that a large portion of sponge symbiont communities (57 to 80% of clones) were stable across seasons, with no significant differences in the genetic differentiation and community structure (Table 2; see Fig. S3 in the supplemental material). Bacterial communities in I. variabilis and I. oros also exhibited no significant differences in genetic diversity between sampling times, while I. fasciculata symbionts showed significantly lower diversity in September 2010, due to increased representation of the dominant cyanobacterium, “Candidatus Synechococcus spongiarum,” in the summer library compared to the winter one (51% and 26% of clones, respectively). Seawater clone libraries from winter and summer shared few sequences (16 to 22% of clones) and exhibited significant differences in community structure, genetic diversity, and genetic differentiation (Table 2; see Fig. S3).
Table 2.
Statistical comparisons of genetic diversity and community structure of bacterial communities in sponges (Ircinia spp.) and seawater between winter and summer seasonsa
| Community | Statistical result for: |
|||||
|---|---|---|---|---|---|---|
| AMOVA |
HOMOVA |
UNIFRAC |
||||
| Fs | P | B | P | U | P | |
| I. fasciculata | 4.434 | 0.065 | 1.834 | <0.001 | 0.670 | 0.147 |
| I. variabilis | 2.241 | 0.634 | 0.043 | 0.248 | 0.593 | 0.073 |
| I. oros | 3.365 | 0.397 | 0.024 | 0.502 | 0.584 | 0.054 |
| Seawater | 4.408 | <0.001 | 0.620 | 0.007 | 0.734 | 0.006 |
Fs, F statistic; B, Bartlett's statistic; U, unweighted UniFrac value.
Seasonal variation in bacterial OTUs.
Combined analysis of the winter and summer clone libraries revealed 190 bacterial OTUs (99% sequence identity) in sponges and seawater, corresponding to 13 microbial phyla. Within each host sponge species, similar phylogenetic compositions of bacteria were observed between seasons (Table 3), with differences between seasons typically resulting from shifts in rare bacterial OTUs. For example, I. fasciculata hosted a single rare OTU (2.6% of clones) affiliated with Nitrospira in winter that was absent from summer clone libraries. In contrast, seawater bacteria exhibited large fluctuations in specific lineages and OTUs. For example, cyanobacterial OTUs accounted for only 1.4% of seawater clones in winter and over one-fourth of clones (27.6%) in summer (Table 3). Similarly, rank-abundance plots of bacterial OTUs revealed that dominant sponge symbionts were stable across seasons and rare OTUs were more variable, whereas shifts in dominant and rare seawater bacteria were observed between the winter and summer seasons (see Fig. S3 in the supplemental material).
Table 3.
Composition of bacterial communities in Ircinia spp. and ambient seawater sampled in winter (March) and summer (September) seasons
| Bacterial phylum | % of total clones (no. of 99% OTUs) |
|||||||
|---|---|---|---|---|---|---|---|---|
|
I. fasciculata |
I. variabilis |
I. oros |
Seawater |
|||||
| Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | |
| Proteobacteria | 45.5 (16) | 30.0 (11) | 61.3 (22) | 50.7 (18) | 56.1 (17) | 47.7 (19) | 56.2 (28) | 43.7 (21) |
| Alphaproteobacteria | 6.5 (4) | 2.9 (2) | 11.3 (6) | 12.2 (4) | 6.8 (5) | 30.1 (15) | 32.2 (11) | |
| Betaproteobacteria | 1.3 (1) | 2.7 (2) | ||||||
| Gammaproteobacteria | 20.8 (10) | 12.9 (6) | 33.8 (11) | 32.0 (13) | 28.0 (10) | 25.0 (10) | 23.3 (11) | 11.5 (10) |
| Deltaproteobacteria | 18.2 (2) | 14.3 (3) | 15.0 (4) | 18.7 (5) | 15.9 (3) | 15.9 (4) | ||
| Cyanobacteria | 32.5 (2) | 61.4 (2) | 7.5 (1) | 21.3 (1) | 1.1 (1) | 1.4 (1) | 27.6 (4) | |
| Acidobacteria | 5.2 (3) | 1.4 (1) | 5.0 (3) | 10.7 (1) | 18.3 (3) | 25.0 (5) | 1.1 (1) | |
| Bacteroidetes | 9.1 (3) | 2.9 (1) | 3.8 (2) | 5.3 (2) | 3.7 (2) | 9.6 (7) | 8.0 (4) | |
| Chloroflexi | 3.9 (3) | 1.4 (1) | 4.0 (2) | 4.9 (2) | 14.8 (7) | |||
| Actinobacteria | 3.8 (2) | 1.3 (1) | 3.7 (2) | 1.1 (1) | 12.3 (3) | 5.7 (3) | ||
| Nitrospira | 2.6 (1) | 15.0 (1) | 4.0 (1) | 2.4 (1) | 2.3 (2) | |||
| Bacillariophyta | 2.5 (2) | 9.8 (5) | 8.2 (4) | 1.1 (1) | ||||
| Verrucomicrobia | 1.4 (1) | 12.6 (2) | ||||||
| Firmicutes | 1.4 (1) | 5.7 (2) | 1.4 (1) | |||||
| Gemmatimonadetes | 1.3 (1) | 1.4 (1) | 1.3 (1) | 1.3 (1) | 1.2 (1) | 2.3 (2) | ||
| Chlorophyta | 4.1 (3) | |||||||
| Planctomycetes | 1.3 (1) | 1.4 (1) | ||||||
| Uncertain | 4.1 (2) | |||||||
Clone libraries also revealed the presence of dominant symbiont OTUs in the three sponge species. Overall, eight symbiont OTUs comprised over one-half of all Ircinia-associated bacterial clones (51.7%) and were absent from ambient seawater (Table 4). Seven of the eight dominant OTUs were recovered from both winter and summer seasons and matched closely (>98% sequence identity) other sponge-associated bacteria. The exception was a member of Gammaproteobacteria (IRC012) present only in the winter season and whose closest sequence match was a sediment-derived bacterium (Table 4). The most dominant Ircinia-associated OTU (IRC002) matched the sponge-specific cyanobacterium “Candidatus Synechococcus spongiarum” (64) and represented the most common symbiont in I. fasciculata and I. variabilis. The second most dominant OTU (IRC001) matched a member of Deltaproteobacteria in the order Desulfovibrionales and represented the second most common symbiont in all Ircinia hosts. An Acidobacterium (IRC003) was the third most dominant OTU and represented the most common symbiont in I. oros, while also present in I. variabilis yet absent in I. fasciculata. The remaining four dominant, sponge-specific OTUs were less abundant (<5% of clones) and corresponded to symbiont taxa affiliated with Gammaproteobacteria, Nitrospira, and Cyanobacteria (Table 4).
Table 4.
Characteristics of dominant symbiont OTUs in Ircinia spp.
| OTU | No. (%) of total clonesa |
Source of closest BLAST match (% sequence identity, accession no.) | Taxonomic classification (Bayesian probability) |
Putative function | |||||
|---|---|---|---|---|---|---|---|---|---|
| IF | IV | IO | All Ircinia spp. | SW | Taxonb | Lowest taxonomic rank | |||
| IRC001 | 18 (12.2) | 16 (10.3) | 22 (13.0) | 56 (11.9) | 0 | Sponge associated (99.2, EU495967) | Deltaproteobacteria (79) | Order Desulfovibrionales (70) | Sulfate reduction |
| IRC002 | 56 (38.1) | 22 (14.2) | 0 (0.0) | 78 (16.5) | 0 | Sponge associated (98.8, GU981862) | Cyanobacteria (100) | Genus Synechococcus (100) | Carbon fixation |
| IRC003 | 0 (0.0) | 10 (6.5) | 26 (15.3) | 36 (7.6) | 0 | Sponge associated (98.7, AJ347029) | Acidobacteria (100) | Gp10 (100) | NAc |
| IRC004 | 2 (1.4) | 15 (9.7) | 0 (0.0) | 17 (3.6) | 0 | Sponge associated (99.3, EU183762) | Nitrospira (100) | Genus Nitrospira (100) | Nitrite oxidation |
| IRC006 | 2 (1.4) | 11 (7.1) | 1 (0.6) | 14 (3.0) | 0 | Sponge associated (98.8, EU495951) | Gammaproteobacteria (100) | Incertae sedis (68) | NA |
| IRC007 | 0(0.0) | 6(3.9) | 10(5.9) | 16(3.4) | 0 | Sponge associated (98.7, GQ163729) | Gammaproteobacteria (100) | Order Oceanospirillales (46) | NA |
| IRC012 | 4 (2.7) | 6 (3.9) | 5 (2.9) | 15 (3.2) | 0 | Sediment bacterium (97.4, GQ143791) | Proteobacteria (100) | Incertae sedis (84) | NA |
| IRC015 | 12 (8.2) | 0 (0.0) | 0 (0.0) | 12 (2.5) | 0 | Sponge associated (99.3, JN655231) | Cyanobacteria (100) | GpIIa (100) | Carbon fixation |
IF, I. fasciculata; IV, I. variabilis; IO, I. oros; SW, seawater.
All taxa are phyla except Deltaproteobacteria and Gammaproteobacteria, which are classes.
NA, not available.
Comparison of clone library and T-RFLP data revealed high congruency between these techniques and allowed for the identification of most symbiont taxa in the T-RFLP profiles. In silico restriction enzyme digestion of clone libraries predicted 71.6% and 95.8% of all peaks in T-RFLP profiles (HaeIII and MspI data, respectively). Empirical T-RFs of the eight dominant OTUs were well represented in sponge symbiont profiles and accounted for 53.0% (±2.2% standard error [SE], HaeIII data) and 34.2% (±1.2%, MspI data) of total profile peak areas, while the same T-RF peaks comprised only a small portion of seawater bacteria profiles (7.5% ± 0.8% and 6.9% ± 1.1%, HaeIII and MspI data, respectively). Further, these eight dominant symbionts were present in their respective hosts throughout the seasonal cycle (see Table S3 in the supplemental material), confirming the stability of these symbionts over annual temporal scales and seasonal environmental conditions.
Seasonal variation in chlorophyll a content.
The photosymbiont-harboring sponges I. fasciculata and I. variabilis exhibited different average concentrations and temporal variability in chlorophyll a content. Chlorophyll a levels were higher in I. fasciculata than in I. variabilis, consistent with the habitat preferences of I. fasciculata (higher irradiance zones) and I. variabilis (lower irradiance zones). Differences between species were significant for all months except June (2010 and 2011 [Fig. 5]), which is, notably, the month with the highest average irradiance levels (Fig. 2). For both host sponges, significant variation (P < 0.001) in chl a content was observed across the monitoring period. In I. variabilis, this variation was due to a significant decrease in average chl a content in September 2010 (83.3 μg/g), whereas the remaining months exhibited similar average values (131.0 to 162.4 μg/g). Seasonal changes in chl a content were more pronounced in I. fasciculata and inversely related to daylight hours and light intensity (Fig. 5), as lower values occurred during the spring and summer months (149.8 to 210.7 μg/g) and higher values during fall and winter (235.1 to 330.2 μg/g).
Fig 5.
Chlorophyll a content of the photosymbiont-bearing sponges I. fasciculata (black bars) and I. variabilis (gray bars) over the 1.5-year monitoring period. Asterisks denote significant differences (P < 0.05) between host sponge species by month; letters indicate significant differences among months within each host species (uppercase letters for I. fasciculata and lowercase letters for I. variabilis). Error bars represent ±1 SD.
DISCUSSION
Seasonal stability and specificity of sponge microbiota.
Temporal monitoring of three Ircinia spp. and ambient seawater over 1.5 years revealed remarkable stability and specificity of sponge-associated bacterial symbiont communities, despite large fluctuations in ambient environmental conditions. Across all seasons, each Ircinia host maintained a specific bacterial symbiont community, more similar within each host species over time than among hosts. Further, higher symbiont similarity occurred between the microbiotas of I. fasciculata and I. variabilis than with that of I. oros, consistent with previous analyses of host specificity among these species (15). Host specificity patterns in Ircinia-associated bacteria are complex, due to variable levels of symbiont overlap among hosts. Despite the prevalence of generalist symbionts in Ircinia microbiotas (i.e., taxa occurring in multiple, unrelated sponge hosts), community level analyses revealed host species-specific symbiont assemblages in each host (15). Here, we show that this phenomenon, termed “a specific mix of generalists,” is maintained over time and across seasons, with little evidence for symbiont restructuring or specificity shifts in response to different environmental conditions. The seasonal stability of host specificity patterns in the Ircinia microbiota supports the hypothesis of host species-specific, stable associations between bacteria and marine sponges (32, 56, 59, 71, 73).
In contrast, seawater bacterial communities exhibited clear temporal shifts in diversity and composition according to a seasonal cycle. Previous studies of surface bacterioplankton in the coastal NW Mediterranean Sea have revealed a similar seasonal succession of seawater bacterial communities (47, 48), including a greater community similarity in the fall and winter seasons as observed here (2). Regional stratification of the water column is a seasonal phenomenon in the NW Mediterranean Sea, where restricted upwelling and vertical mixing of nutrient-rich, cold water results in nutrient depletion of surface waters during the summer months (14). The summer stratification period and its effects on nutrient availability are primary drivers of seasonal microbial dynamics in the Mediterranean Sea (43). Comparatively low seasonal dynamics of sponge-associated bacterial community structure suggest that different ecological constraints act on free-living versus symbiotic marine bacteria. The effects of nutrient-poor conditions during summer stratification on bacterial communities in the sponge microbiota appear to be limited, supporting the hypothesis of a unique and comparatively stable microbial habitat within the sponge body.
Persistent components of the sponge microbiota.
The observed stability of bacterial communities associated with Ircinia hosts was driven by the persistent presence of dominant symbiont OTUs. Despite the high diversity of the Ircinia microbiota, a small number of symbiont OTUs accounted for the majority of bacteria represented in clone libraries and T-RFLP profiles, similar to what was seen in previous studies of sponge-associated bacteria (18, 71). Selective pressures that maintain specific symbiont taxa in the sponge host may result from microbial adaptations to these unique niche microenvironments, as suggested by the presence of unique, vertically transmitted (51) sponge-specific bacterial lineages (52, 56), and/or the fulfillment of functional roles by particular symbiont guilds that enhance sponge-bacteria holobiont fitness (20, 22, 58). In the latter context, it is noteworthy that several of the dominant symbiont OTUs recovered in Ircinia hosts were classified into bacterial lineages with known physiological capabilities, such as photosynthesis (IRC002 and IRC015, Cyanobacteria), sulfate reduction (IRC001, Desulfovibrionales), and nitrite oxidation (IRC004, Nitrospira). The metabolic profile of the sponge microbiome, assessed by both metagenomic (34, 62) and nutrient flux (45) approaches, has shown diverse and active functional guilds involved in the nutrient cycles of carbon (59), nitrogen (26), and sulfur (25) that may boost host sponge metabolism and contribute significantly to coastal marine nutrient cycles (4, 12, 19, 27). As such, symbiont functionality and its ecological consequences may represent key factors for the selective mechanisms that establish and maintain specific guilds of sponge-associated bacterial symbionts.
Temporal analyses of photosynthetic pigments in I. fasciculata and I. variabilis provided further insight into symbiont functionality and evidence for seasonal variation in the activity of persistent photosymbiont taxa. Cyanobacteria are a key functional guild in the sponge microbiota, capable of photosynthetic carbon assimilation and the transfer of surplus carbon stores to their hosts (59). A recent study reported higher photosynthetic activity of cyanobacterial symbionts in I. fasciculata than in I. variabilis, with differences in symbiont functionality related to ambient irradiance levels in preferred host habitats rather than symbiont composition (17). Here, we show that I. variabilis exhibited minimal seasonal fluctuations in chl a content, consistent with reduced irradiance levels in the shaded habitats where this species thrives. In contrast, the chl a content of photosymbionts in I. fasciculata followed a seasonal pattern, with annual minima in summer and peak values in winter, similar to those reported in surface seawater from the NW Mediterranean (14, 43, 47). Thus, while the factors that determine microbial structure may differ between the sponge niche and open seawater environments (e.g., nutrient levels), some seasonal physiological constraints that dictate microbial function (e.g., irradiance exposure) may be conserved between symbiotic and free-living microbes. Structurally, a single cyanobacterial taxon dominated the symbiotic microbiota in I. fasciculata and I. variabilis across all seasons; yet functionally, their photosynthetic activity differed among hosts and appears to have a seasonal component in I. fasciculata, with potential consequences for host metabolism and growth. The critical ecological link between symbiont structure and function is not well resolved in the sponge microbiota and requires further study, including the potential for seasonal variability in the physiology and functioning of permanent sponge symbionts and its consequence for host metabolism and marine nutrient cycles.
Variable components of the sponge microbiota.
Similar to previous studies of temporal variation in the sponge microbiota (3), some variability was observed in symbiont communities over time and among individual hosts, though primarily restricted to rare symbiont taxa. Transient components of the sponge microbiota are not unexpected, as microbes recovered from sponge tissue may represent food source bacteria (42), invasive (69) or fouling (31) microbes, or simply environmental bacteria present in the sponge filtration system during collection. For example, a common and relatively abundant bacterial OTU (IRC012, 3.2% of sponge clones) was present in the microbiotas of all sponge hosts in winter and absent in the summer. Unlike the majority of sponge-associated bacteria in Ircinia, this Gammaproteobacterium was not phylogenetically related to other sponge symbionts but rather matched most closely a sediment-derived sequence. Considering such possible sources of transient microbes in the sponge microbiota, the high degree of bacterial community similarity observed throughout the monitoring period here is even more extraordinary.
Variability in the composition of bacterial symbionts among conspecific hosts was also detected here by monitoring the same individuals over time, a sampling design rarely utilized to date in the field of sponge microbiology (3). Although this variability was minimal compared to differences among host species, some symbionts were consistently recovered from particular individuals and not others. The most notable example is a Synechocystis-related cyanobacterium in Ircinia fasciculata. A previous report has shown that this cyanobacterium represented a distinct clade of sponge symbionts specific to I. fasciculata yet occurred in only one of three I. fasciculata individuals studied (17). Here, we report similar findings, with the same Synechocystis phylotype recovered in only one of six host individuals, and we showed that this association was stable over time, as the cyanobacterium was recovered in winter and summer clone libraries and present in all symbiont profiles for this particular sponge host. These results show that interindividual variation in the sponge microbiota, often ascribed to the nonspecific or transient bacterial associates discussed above, can result from persistent symbionts that occur sporadically among a host population. The implications of interindividual variability in symbiont composition on host ecology and symbiont evolution are unknown for sponge-microbe associations but have the potential to affect symbiont community function (e.g., photosynthetic activity) and host-symbiont metabolic interactions.
Symbiont fluctuations and thermal thresholds.
Recent reports of widespread disease and mass mortality events in Ircinia spp. have raised concerns about the future of these sponge populations in the warming Mediterranean Sea. Elevated seawater temperatures are hypothesized to trigger such episodic mortality events, as recurrent disease outbreaks in I. fasciculata and I. variabilis occurred annually following peak seawater temperatures in summer (37, 53) and greater disease prevalence has been correlated with the length of exposure to temperatures exceeding threshold values (8). In addition to tissue necrosis, affected sponges also exhibit characteristic changes in their associated microbiota, including the loss of stable symbionts (8) and/or their replacement by pathogenic microbes (37, 53). Similar symbiont disruption and proliferation of putatively pathogenic bacteria were reported in a tropical sponge, Rhopaloeides odorabile, when exposed to elevated seawater temperatures (66), suggesting that symbiont community collapse and host sponge mortality may become widespread as thermal tolerances are exceeded.
A critical question is whether symbiont disruption precedes and precipitates host mortality (e.g., symbiont evacuation followed by colonization of infectious microbes) or simply results from declining host health. In the current study, no sponge mortality events occurred during the monitoring period, consistent with previous surveys of the study area (8), yet deviations from core symbiont communities (i.e., increased heterogeneity) were reported in warmer months, due to fluctuations in rare symbiont taxa within some host individuals. At our monitoring sites, lower temperatures (daily averages of >25°C during only 3 days) were recorded than those that preceded sponge mortality events in other Mediterranean regions (daily averages of 26 to 27°C). Accordingly, no pathogenic lineages (e.g., Vibrio spp.) were detected in sponge hosts, and the symbiont community shifts observed in our study were minor (i.e., restricted to heterogeneity in rare symbionts, while dominant symbionts were present throughout) and temporary (i.e., symbiont structure in all sponge hosts reverted to homogeneous core profiles following the 2010 summer season). However, considering the warming trends in the Mediterranean Sea and the proximity of temperature maxima in our study area (25°C) to those preceding sponge mortality events (26 to 27°C), the observed shifts in rare symbiont taxa may represent a precursor to larger symbiont declines and indicate approaching thermal thresholds for Mediterranean sponge-microbe symbioses. Additional monitoring studies and controlled experimentation are required to assess whether elevated seawater temperatures induce shifts in rare symbiont taxa, how these symbiont fluctuations affect host health, and the utility of symbiont monitoring for predicting sponge mortality events.
Supplementary Material
ACKNOWLEDGMENTS
We thank F. Crespo (CEAB) for field assistance.
This research was supported by the Spanish Government projects CTM2010-17755 and CTM2010-22218, by the Catalan Government grant 2009SGR-484 for Consolidated Research Groups, and by the U.S. National Science Foundation under grant 0853089.
Footnotes
Published ahead of print 10 August 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Abdo Z, et al. 2006. Statistical methods for characterizing diversity of microbial communities by analysis of terminal restriction fragment length polymorphisms of 16S rRNA genes. Environ. Microbiol. 8:929–938 [DOI] [PubMed] [Google Scholar]
- 2. Alonso-Sáez L, et al. 2007. Seasonality in bacterial diversity in north-west Mediterranean coastal waters: assessment through clone libraries, fingerprinting and FISH. FEMS Microbiol. Ecol. 60:98–112 [DOI] [PubMed] [Google Scholar]
- 3. Anderson SA, Northcote PT, Page MJ. 2010. Spatial and temporal variability of the bacterial community in different chemotypes of the New Zealand marine sponge Mycale hentscheli. FEMS Microbiol. Ecol. 72:328–342 [DOI] [PubMed] [Google Scholar]
- 4. Arillo A, Bavestrello G, Burlando B, Sará M. 1993. Metabolic integration between symbiotic cyanobacteria and sponges: a possible mechanism. Mar. Biol. 117:159–162 [Google Scholar]
- 5. Ashelford KE, Chuzhanova NA, Fry JC, Jones AJ, Weightman AJ. 2005. At least 1 in 20 16S rRNA sequence records currently held in public repositories is estimated to contain substantial anomalies. Appl. Environ. Microbiol. 71:7724–7736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ashelford KE, Chuzhanova NA, Fry JC, Jones AJ, Weightman AJ. 2006. New screening software shows that most recent large 16S rRNA gene clone libraries contain chimeras. Appl. Environ. Microbiol. 72:5734–5741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benjamini Y, Yekutieli D. 2001. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 29:1165–1188 [Google Scholar]
- 8. Cebrian E, Uriz MJ, Garrabou J, Ballesteros E. 2011. Sponge mass mortalities in a warming Mediterranean Sea: are cyanobacteria-harboring species worse off? PLoS One 6:e20211 doi:10.1371/journal.pone.0020211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cole JR, et al. 2007. The ribosomal database project (RDP-II): introducing myRDP space and quality controlled public data. Nucleic Acids Res. 35:D169–D172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Culman SW, Bukowski R, Gauch HG, Cadillo-Quiroz H, Buckley DH. 2009. T-REX: software for the processing and analysis of T-RFLP data. BMC Bioinform. 10:171 doi:10.1186/1471-2105-10-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diaz MC, Rützler K. 2001. Sponges: an essential component of Caribbean coral reefs. Bull. Mar. Sci. 69:535–546 [Google Scholar]
- 12. Diaz MC, Ward BB. 1997. Sponge-mediated nitrification in tropical benthic communities. Mar. Ecol. Prog. Ser. 156:97–107 [Google Scholar]
- 13. Drummond AJ, et al. 2010. Geneious v5.3. http://www.geneious.com
- 14. Duarte CM, Agustí S, Kennedy H, Vaqué D. 1999. The Mediterranean climate as a template for Mediterranean marine ecosystems: the example of the northeast Spanish littoral. Prog. Oceanogr. 44:245–270 [Google Scholar]
- 15. Erwin PM, López-Legentil S, González-Pech R, Turon X. 2012. A specific mix of generalists: bacterial symbionts in Mediterranean Ircinia spp. FEMS Microbiol. Ecol. 79:619–637 [DOI] [PubMed] [Google Scholar]
- 16. Erwin PM, López-Legentil S, Schuhmann PW. 2010. The pharmaceutical value of marine biodiversity for anti-cancer drug discovery. Ecol. Econ. 70:445–451 [Google Scholar]
- 17. Erwin PM, López-Legentil S, Turon X. 2012. Ultrastructure, molecular phylogenetics, and chlorophyll a content of novel cyanobacterial symbionts in temperate sponges. Microb. Ecol. doi:10.1007/s00248-012-0047-5 [DOI] [PubMed] [Google Scholar]
- 18. Erwin PM, Olson JB, Thacker RW. 2011. Phylogenetic diversity, host-specificity and community structure of sponge-associated bacteria in the northern Gulf of Mexico. PLoS One 6:e26806 doi:10.1371/journal.pone.0026806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Erwin PM, Thacker RW. 2007. Incidence and identity of photosynthetic symbionts in Caribbean coral reef sponge assemblages. J. Mar. Biol. Assoc. U.K. 87:1683–1692 [Google Scholar]
- 20. Erwin PM, Thacker RW. 2008. Phototrophic nutrition and symbiont diversity of two Caribbean sponge-cyanobacteria symbioses. Mar. Ecol. Prog. Ser. 362:139–147 [Google Scholar]
- 21. Flatt P, et al. 2005. Identification of the cellular site of polychlorinated peptide biosynthesis in the marine sponge Dysidea (Lamellodysidea) herbacea and symbiotic cyanobacterium Oscillatoria spongeliae by CARD-FISH analysis. Mar. Biol. 147:761–774 [Google Scholar]
- 22. Freeman CJ, Thacker RW. 2011. Complex interactions between marine sponges and their symbiotic microbial communities. Limnol. Oceanogr. 56:1577–1586 [Google Scholar]
- 23. Friedrich AB, Fischer I, Proksch P, Hacker J, Hentschel U. 2001. Temporal variation of the microbial community associated with the mediterranean sponge Aplysina aerophoba. FEMS Microbiol. Ecol. 38:105–113 [Google Scholar]
- 24. Hentschel U, et al. 2002. Molecular evidence for a uniform microbial community in sponges from different oceans. Appl. Environ. Microbiol. 68:4431–4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoffmann F, et al. 2005. An anaerobic world in sponges. Geomicrobiol. J. 22:1–10 [Google Scholar]
- 26. Hoffmann F, et al. 2009. Complex nitrogen cycling in the sponge Geodia barretti. Environ. Microbiol. 11:2228–2243 [DOI] [PubMed] [Google Scholar]
- 27. Jiménez E, Ribes M. 2007. Sponges as a source of dissolved inorganic nitrogen: nitrification mediated by temperate sponges. Limnol. Oceanogr. 52:948–958 [Google Scholar]
- 28. Joachimiak MP, Weisman JL, May BCH. 2006. JColorGrid: software for the visualization of biological measurement. BMC Bioinform. 7:225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaplan CW, Kitts CL. 2003. Variation between observed and true terminal restriction fragment length is dependent on true TRF length and purine content. J. Microbiol. Methods 54:121–125 [DOI] [PubMed] [Google Scholar]
- 30. Kent AD, Smith DJ, Benson BJ, Triplett EW. 2003. Web-based phylogenetic assignment tool for analysis of terminal restriction fragment length polymorphism profiles of microbial communities. Appl. Environ. Microbiol. 69:6768–6776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee OO, Lau SCK, Qian PY. 2006. Consistent bacterial community structure associated with the surface of the sponge Mycale adhaerens Bowerbank. Microb. Ecol. 52:693–707 [DOI] [PubMed] [Google Scholar]
- 32. Lee OO, et al. 2011. Pyrosequencing reveals highly diverse and species-specific microbial communities in sponges from the Red Sea. ISME J. 5:650–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lemoine N, Buell N, Hill A, Hill M. 2007. Assessing the utility of sponge microbial symbiont communities as models to study global climate change: a case study with Halichondria bowerbanki, p 239–246 In Custódio MR, Lôbo-Hajdu G, Hajdu E, Muricy G. (ed), Porifera research: biodiversity, innovation, and sustainability. Série livros 28. Museu Nacional, Rio de Janeiro, Brazil [Google Scholar]
- 34. Liu M, Fan L, Zhong L, Kjelleberg S, Thomas T. 2012. Metaproteogenomic analysis of a community of sponge symbionts. ISME J. doi:10.1038/ismej.2012.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. López-Legentil S, Erwin PM, Pawlik JR, Song B. 2010. Effects of sponge bleaching on ammonia-oxidizing Archaea: distribution and relative expression of ammonia monooxygenase genes associated with the barrel sponge Xestospongia muta. Microb. Ecol. 60:561–571 [DOI] [PubMed] [Google Scholar]
- 36. Lozupone CA, Hamady M, Kelley ST, Knight R. 2006. Quantitative and qualitative β diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 73:1576–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maldonado M, Sánchez-Tocino L, Navarro C. 2010. Recurrent disease outbreaks in the corneous demosponges of the genus Ircinia: epidemic incidence and defense mechanisms. Mar. Biol. 157:1577–1590 [Google Scholar]
- 38. Martínez-Murcia AJ, Acinas SG, Rodriguez-Valera F. 1995. Evaluation of prokaryotic diversity by restrictase digestion of 16S rDNA directly amplified from hypersaline environments. FEMS Microbiol. Ecol. 17:247–255 [Google Scholar]
- 39. Mohamed NM, Enticknap JJ, Lohr JE, McIntosh SM, Hill RT. 2008. Changes in bacterial communities of the marine sponge Mycale laxissima on transfer into aquaculture. Appl. Environ. Microbiol. 74:1209–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mohamed NM, Rao V, Hamann MT, Kelly M, Hill RT. 2008. Monitoring bacterial diversity of the marine sponge Ircinia strobilina upon transfer into aquaculture. Appl. Environ. Microbiol. 74:4133–4143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pandey J, Ganesan K, Jain RK. 2007. Variations in T-RFLP profiles with differing chemistries of fluorescent dyes used for labeling the PCR primers. J. Microbiol. Methods 68:633–638 [DOI] [PubMed] [Google Scholar]
- 42. Pile AJ, Patterson MR, Witman JD. 1996. In situ grazing on plankton <10 μm by the boreal sponge Mycale lingua. Mar. Ecol. Prog. Ser. 141:95–102 [Google Scholar]
- 43. Pinhassi J, et al. 2006. Seasonal changes in bacterioplankton nutrient limitation and their effects on bacterial community composition in the NW Mediterranean Sea. Aquat. Microb. Ecol. 44:241–252 [Google Scholar]
- 44. Reysenbach AL, Wickham GS, Pace NR. 1994. Phylogenetic analysis of the hyperthermophilic pink filament community in Octopus Spring, Yellowstone National Park. Appl. Environ. Microbiol. 60:2113–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ribes M, et al. 2012. Functional convergence of microbes associated with temperate marine sponges. Environ. Microbiol. 14:1224–1239 [DOI] [PubMed] [Google Scholar]
- 46. Sarà M. 1971. Ultrastructural aspects of the symbiosis between two species of the genus Aphanocapsa (Cyanophyceae) and Ircinia variabilis (Demospongiae). Mar. Biol. 11:214–221 [Google Scholar]
- 47. Schauer M, Balagué V, Pedrós-Alió C, Massana R. 2003. Seasonal changes in the taxonomic composition of bacterioplankton in a coastal oligotrophic system. Aquat. Microb. Ecol. 31:163–174 [Google Scholar]
- 48. Schauer M, Massana R, Pedrós-Alió C. 2000. Spatial differences in bacterioplankton composition along the Catalan coast (NW Mediterranean) assessed by molecular fingerprinting. FEMS Microb. Ecol. 33:51–59 [DOI] [PubMed] [Google Scholar]
- 49. Schloss PD, et al. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75:7537–7541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schmitt S, et al. 2012. Assessing the complex sponge microbiota: core, variable and species-specific bacterial communities in marine sponges. ISME J. 6:564–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schmitt S, Weisz JB, Lindquist N, Hentschel U. 2007. Vertical transmission of a phylogenetically complex microbial consortium in the viviparous sponge Ircinia felix. Appl. Environ. Microbiol. 73:2067–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Simister RL, Deines P, Botté ES, Webster NS, Taylor MW. 2012. Sponge-specific clusters revisited: a comprehensive phylogeny of sponge-associated microorganisms. Environ. Microbiol. 14:517–524 [DOI] [PubMed] [Google Scholar]
- 53. Stabili L, et al. 2012. Epidemic mortality of the sponge Ircinia variabilis (Schmidt, 1862) associated to proliferation of a Vibrio bacterium. Microb. Ecol. doi:10.1007/s00248-012-0068-0 [DOI] [PubMed] [Google Scholar]
- 54. Stewart CN, Excoffier L. 1996. Assessing population genetic structure and variability with RAPD data: application to Vaccinium macrocarpon (American Cranberry). J. Evol. Biol. 9:153–171 [Google Scholar]
- 55. Taylor MW, Hill RT, Hentschel U. 2011. Meeting report: 1st International Symposium on Sponge Microbiology. Mar. Biotechnol. 6:1057–1061 [DOI] [PubMed] [Google Scholar]
- 56. Taylor MW, Radax R, Steger D, Wagner M. 2007. Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol. Mol. Biol. Rev. 71:295–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taylor MW, Schupp PJ, Dahllof I, Kjelleberg S, Steinberg PD. 2004. Host specificity in marine sponge-associated bacteria, and potential implications for marine microbial diversity. Environ. Microbiol. 6:121–130 [DOI] [PubMed] [Google Scholar]
- 58. Thacker RW. 2005. Impacts of shading on sponge-cyanobacteria symbioses: a comparison between host-specific and generalist associations. Integr. Comp. Biol. 45:369–376 [DOI] [PubMed] [Google Scholar]
- 59. Thacker RW, Freeman CJ. 2012. Sponge-microbe symbioses: recent advances and new directions. Adv. Mar. Biol. 62:57–111 doi:10.1016/B978-0-12-394283-8.00002-3 [DOI] [PubMed] [Google Scholar]
- 60. Thiel V, Leininger S, Schmaljohann R, Brümmer F, Imhoff JF. 2007. Sponge-specific bacterial associations of the Mediterranean sponge Chondrilla nucula (Demospongiae, Tetractinomorpha). Microb. Ecol. 54:101–111 [DOI] [PubMed] [Google Scholar]
- 61. Thiel V, Neulinger SC, Staufenberger T, Schmaljohann R, Imhoff JF. 2007. Spatial distribution of sponge-associated bacteria in the Mediterranean sponge Tethya aurantium. FEMS Microbiol. Ecol. 59:47–63 [DOI] [PubMed] [Google Scholar]
- 62. Thomas T, et al. 2010. Functional genomic signatures of sponge bacteria reveal unique and shared features of symbiosis. ISME J. 4:1557–1567 [DOI] [PubMed] [Google Scholar]
- 63. Usher KM. 2008. The ecology and phylogeny of cyanobacterial symbionts in sponges. Mar. Ecol. 29:178–192 [DOI] [PubMed] [Google Scholar]
- 64. Usher KM, Toze S, Fromont J, Kuo J, Sutton DC. 2004. A new species of cyanobacterial symbiont from the marine sponge Chondrilla nucula. Symbiosis 36:183–192 [Google Scholar]
- 65. Van Soest RWM, et al. 2012. Global diversity of sponges (Porifera). PLoS One 7:e35105 doi:10.1371/journal.pone.0035105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Webster NS, Cobb RE, Negri AP. 2008. Temperature thresholds for bacterial symbiosis with a sponge. ISME J. 2:830–842 [DOI] [PubMed] [Google Scholar]
- 67. Webster NS, et al. 2011. Bacterial community dynamics in the marine sponge Rhopaloeides odorabile under in situ and ex situ cultivation. Mar. Biotechnol. 13:296–304 [DOI] [PubMed] [Google Scholar]
- 68. Webster NS, Hill RT. 2001. The culturable microbial community of the Great Barrier Reef sponge Rhopaloeides odorabile is dominated by an α-Proteobacterium. Mar. Biol. 138:843–851 [Google Scholar]
- 69. Webster NS, Negri AP, Webb RI, Hill RT. 2002. A spongin-boring alpha-proteobacterium is the etiological agent of disease in the Great Barrier Reef sponge Rhopaloeides odorabile. Mar. Ecol. Prog. Ser. 232:305–309 [Google Scholar]
- 70. Webster NS, Taylor MW. 2011. Marine sponges and their microbial symbionts: love and other relationships. Environ. Microbiol. 14:335–346 [DOI] [PubMed] [Google Scholar]
- 71. Webster NS, et al. 2010. Deep sequencing reveals exceptional diversity and modes of transmission for bacterial sponge symbionts. Environ. Microbiol. 12:2070–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Webster NS, Xavier JR, Freckelton M, Motti CA, Cobb R. 2008. Shifts in microbial and chemical patterns within the marine sponge Aplysina aerophoba during a disease outbreak. Environ. Microbiol. 10:3366–3376 [DOI] [PubMed] [Google Scholar]
- 73. White JR, et al. 2012. Pyrosequencing of bacterial symbionts within Axinella corrugate sponges: diversity and seasonal variability. PLoS One 7:e38204 doi:10.1371/journal.pone.0038204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wichels A, Würtz S, Döpke H, Schütt C, Gerdts G. 2006. Bacterial diversity in the breadcrumb sponge Halichondria panicea. FEMS Microb. Ecol. 56:102–118 [DOI] [PubMed] [Google Scholar]
- 75. Wilkinson CR. 1983. Net primary productivity in coral reef sponges. Science 219:410–412 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.