Abstract
Myosin was isolated from the main pulmonary artery of swine and was phosphorylated or dephosphorylated by utilizing the endogenous kinase or phosphatase, respectively. The myosins, phosphorylated to various degrees, were purified free of kinase and phosphatase activities by gel filtration on Sepharose CL-4B agarose columns. The level of actin-activated ATPase activity was dependent upon the degree of myosin light chain phosphorylation. Fully phosphorylated myosin reconstituted with actin and tropomyosin (actin/tropomyosin = 61:1) had the highest ATPase activity (0.1 mumol of Pi/mg . min). The actin-activated ATPase activity showed maximal (60--65%) Ca2+ sensitivity at 2 mol of Ca2+ bound per mol of myosin. The actin-activated ATPase activity, Ca2+ binding, and Ca2+ sensitivity of arterial myosin were also dependent upon Mg2+ concentration. The ATPase activity was maximal at 2--3 mM Mg2+ and, at low (0.5 mM) Mg2+ concentration, the activity was only one-third of the maximal activity. Increasing the Mg2+ above 3 mM was not associated with a further increase in ATPase activity, but the Ca2+ binding and Ca2+ sensitivity decreased with increasing Mg2+ concentration. The maximal Ca2+ sensitivity was observed at 2--3 mM Mg2+, a concentration at which the myosin bound 2 mol of Ca2+/mol. Both the ATPase activity and the Ca2+ sensitivity were more remarkable when actin that contained tropomyosin was used to activate the ATPase activity. The data indicate that calcium regulates the actin-activated ATP hydrolysis not only by its effects on the phosphorylation system but also by direct binding to the myosin.
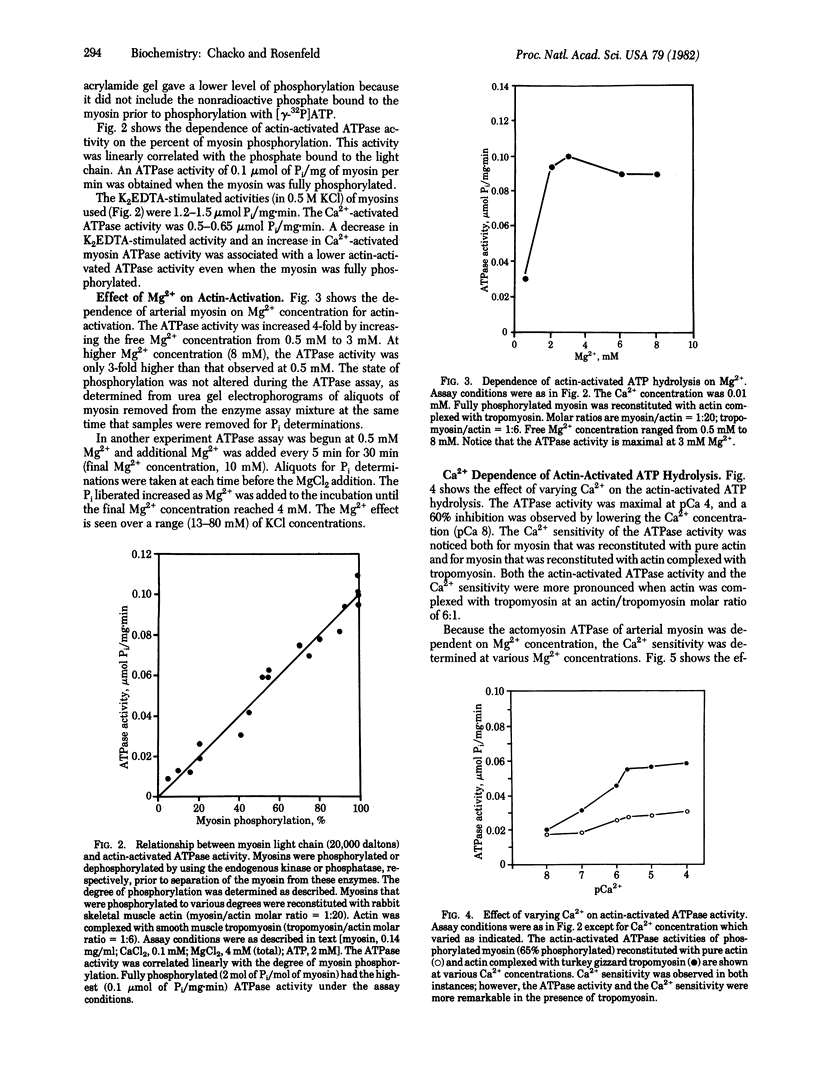
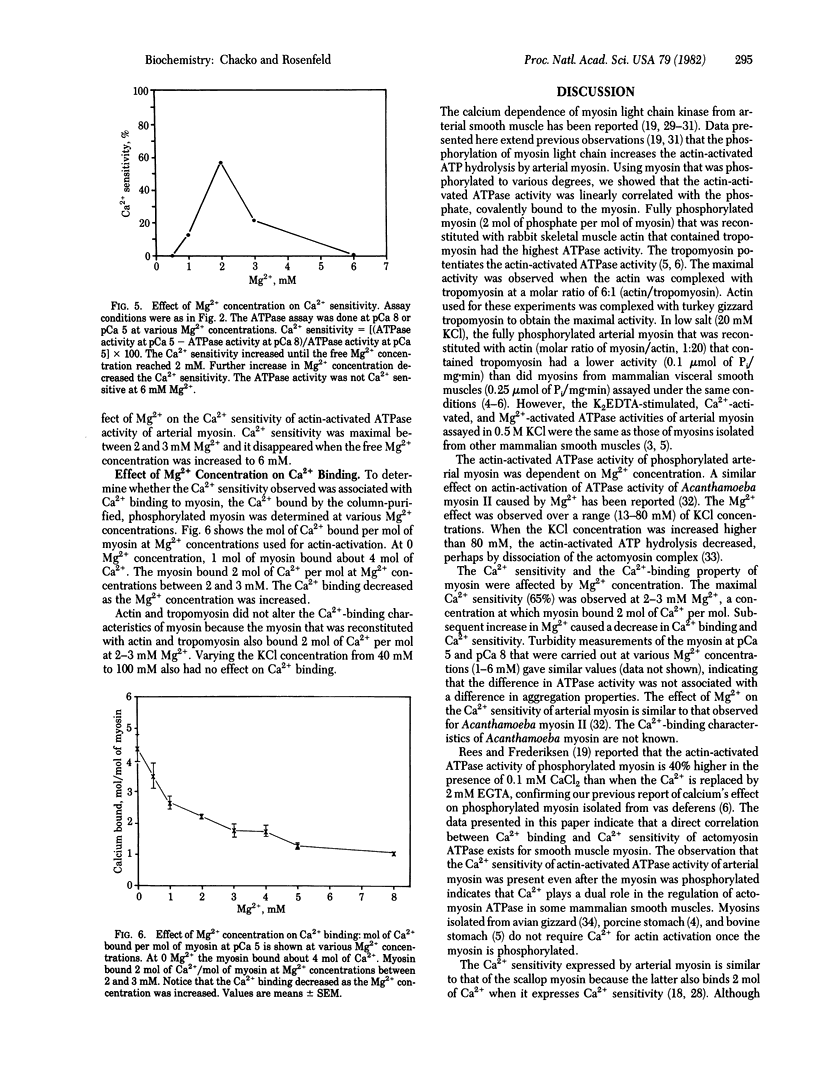
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barron J. T., Bárány M., Bárány K. Phosphorylation of the 20,000-dalton light chain of myosin of intact arterial smooth muscle in rest and in contraction. J Biol Chem. 1979 Jun 25;254(12):4954–4956. [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Calcium binding to rabbit skeletal myosin under physiological conditions. Biochim Biophys Acta. 1975 Feb 17;376(2):366–374. doi: 10.1016/0005-2728(75)90028-6. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Scarpa A., Tiffert T. The concentration of ionized magnesium in barnacle muscle fibres. J Physiol. 1977 Apr;266(3):545–565. doi: 10.1113/jphysiol.1977.sp011781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko S., Conti M. A., Adelstein R. S. Effect of phosphorylation of smooth muscle myosin on actin activation and Ca2+ regulation. Proc Natl Acad Sci U S A. 1977 Jan;74(1):129–133. doi: 10.1073/pnas.74.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko S. Effects of phosphorylation, calcium ion, and tropomyosin on actin-activated adenosine 5'-triphosphatase activity of mammalian smooth muscle myosin. Biochemistry. 1981 Feb 17;20(4):702–707. doi: 10.1021/bi00507a005. [DOI] [PubMed] [Google Scholar]
- Chantler P. D., Sellers J. R., Szent-Györgyi A. G. Cooperativity in scallop myosin. Biochemistry. 1981 Jan 6;20(1):210–216. doi: 10.1021/bi00504a035. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Burt C. T. 31P nuclear magnetic relaxation studies of phosphocreatine in intact muscle: determination of intracellular free magnesium. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4271–4275. doi: 10.1073/pnas.74.10.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. H., Korn E. D. Purification and characterization of actin-activatable, Ca2+-sensitive myosin II from Acanthamoeba. J Biol Chem. 1981 Mar 10;256(5):2586–2595. [PubMed] [Google Scholar]
- DiSalvo J., Gruenstein E., Schmidt C. Relationships between Ca2+, myosin light chains, and ATPase in bovine aortic actomyosin: presence of Ca2+-requiring inactivation factor. Proc Soc Exp Biol Med. 1979 Nov;162(2):337–341. doi: 10.3181/00379727-162-40677. [DOI] [PubMed] [Google Scholar]
- Dillon P. F., Aksoy M. O., Driska S. P., Murphy R. A. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science. 1981 Jan 30;211(4481):495–497. doi: 10.1126/science.6893872. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Kielley W. W. Troponin-tropomyosin complex. Column chromatographic separation and activity of the three, active troponin components with and without tropomyosin present. J Biol Chem. 1974 Aug 10;249(15):4742–4748. [PubMed] [Google Scholar]
- Eisenberg E., Moos C. The interaction of actin with myosin and heavy meromyosin in solution at low ionic strength. J Biol Chem. 1967 Jun 25;242(12):2945–2951. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Frederiksen D. W. Myosin-mediated Ca++-regulation of actomyosin-adenosinetriphosphatase from porcine aorta. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2706–2710. doi: 10.1073/pnas.73.8.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górecka A., Aksoy M. O., Hartshorne D. J. The effect of phosphorylation of gizzard myosin on actin activation. Biochem Biophys Res Commun. 1976 Jul 12;71(1):325–331. doi: 10.1016/0006-291x(76)90286-2. [DOI] [PubMed] [Google Scholar]
- Hirata M., Mikawa T., Nonomura Y., Ebashi S. Ca2+ regulation in vascular smooth muscle. II. Ca2+ binding of aorta leiotonin. J Biochem. 1980 Feb;87(2):369–378. doi: 10.1093/oxfordjournals.jbchem.a132757. [DOI] [PubMed] [Google Scholar]
- Hoar P. E., Kerrick W. G., Cassidy P. S. Chicken gizzard: relation between calcium-activated phosphorylation and contraction. Science. 1979 May 4;204(4392):503–506. doi: 10.1126/science.432654. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J., Lehman W., Szent-Györgyi A. G. Regulation in molluscan muscles. J Mol Biol. 1970 Dec 14;54(2):313–326. doi: 10.1016/0022-2836(70)90432-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mikawa T., Nonomura Y., Hirata M., Ebashi S., Kakiuchi S. Involvement of an acidic protein in regulation of smooth muscle contraction by the tropomyosin-leiotonin system. J Biochem. 1978 Dec;84(6):1633–1636. doi: 10.1093/oxfordjournals.jbchem.a132290. [DOI] [PubMed] [Google Scholar]
- Mrwa U., Rüegg J. C. Myosin-linked calcium regulation in vascular smooth muscle. FEBS Lett. 1975 Dec 1;60(1):81–84. doi: 10.1016/0014-5793(75)80423-6. [DOI] [PubMed] [Google Scholar]
- Murphy R. A. Contractile system function in mammalian smooth muscle. Blood Vessels. 1976;13(1-2):1–23. doi: 10.1159/000158076. [DOI] [PubMed] [Google Scholar]
- Perrie W. T., Smillie L. B., Perry S. B. A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J. 1973 Sep;135(1):151–164. doi: 10.1042/bj1350151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persechini A., Mrwa U., Hartshorne D. J. Effect of phosphorylation on the actin-activated ATPase activity of myosin. Biochem Biophys Res Commun. 1981 Feb 12;98(3):800–805. doi: 10.1016/0006-291x(81)91182-7. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Frederiksen D. W. Calcium regulation of porcine aortic myosin. J Biol Chem. 1981 Jan 10;256(1):357–364. [PubMed] [Google Scholar]
- Saida K., Nonomura Y. Characteristics of Ca2+- and Mg2+-induced tension development in chemically skinned smooth muscle fibers. J Gen Physiol. 1978 Jul;72(1):1–14. doi: 10.1085/jgp.72.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry J. M., Górecka A., Aksoy M. O., Dabrowska R., Hartshorne D. J. Roles of calcium and phosphorylation in the regulation of the activity of gizzard myosin. Biochemistry. 1978 Oct 17;17(21):4411–4418. doi: 10.1021/bi00614a009. [DOI] [PubMed] [Google Scholar]
- Small J. V., Sobieszek A. Ca-regulation of mammalian smooth muscle actomyosin via a kinase-phosphatase-dependent phosphorylation and dephosphorylation of the 20 000-Mr light chain of myosin. Eur J Biochem. 1977 Jun 15;76(2):521–530. doi: 10.1111/j.1432-1033.1977.tb11622.x. [DOI] [PubMed] [Google Scholar]
- Sobieszek A., Small J. V. Regulation of the actin-myosin interaction in vertebrate smooth muscle: activation via a myosin light-chain kinase and the effect of tropomyosin. J Mol Biol. 1977 Jun 5;112(4):559–576. doi: 10.1016/s0022-2836(77)80164-2. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- de Lanerolle P., Stull J. T. Myosin phosphorylation during contraction and relaxation of tracheal smooth muscle. J Biol Chem. 1980 Oct 25;255(20):9993–10000. [PubMed] [Google Scholar]