Abstract
Aerobic anoxygenic phototrophs contain photosynthetic reaction centers composed of bacteriochlorophyll. These organisms are photoheterotrophs, as they require organic carbon substrates for their growth whereas light-derived energy has only an auxiliary function. To establish the contribution of light energy to their metabolism, we grew the phototrophic strain Erythrobacter sp. NAP1 in a carbon-limited chemostat regimen on defined carbon sources (glutamate, pyruvate, acetate, and glucose) under conditions of different light intensities. When grown in a light-dark cycle, these bacteria accumulated 25% to 110% more biomass in terms of carbon than cultures grown in the dark. Cultures grown on glutamate accumulated the most biomass at moderate light intensities of 50 to 150 μmol m−2 s−1 but were inhibited at higher light intensities. In the case of pyruvate, we did not find any inhibition of growth by high irradiance. The extent of anaplerotic carbon fixation was detemined by radioactive bicarbonate incorporation assays. While the carboxylation activity provided 4% to 11% of the cellular carbon in the pyruvate-grown culture, in the glutamate-grown cells it provided only approximately 1% of the carbon. Additionally, we tested the effect of light on respiration and photosynthetic electron flow. With increasing light intensity, respiration decreased to approximately 25% of its dark value and was replaced by photophosphorylation. The additional energy from light allows the aerobic anoxygenic phototrophs to accumulate the supplied organic carbon which would otherwise be respired. The higher efficiency of organic carbon utilization may provide an important competitive advantage during growth under carbon-limited conditions.
INTRODUCTION
Aerobic anoxygenic phototrophs (AAPs) are photoheterotrophic organisms which harvest light using BChl a-containing reaction centers (35). This group was discovered in the coastal waters of Japan in the late 1970s (8, 29) and was later recognized as an important part of marine microbial communities (12, 17, 21, 37). The presence of AAPs was also reported in a number of other environments: freshwater (23, 28), soda and saline lakes (7, 22, 24), soils (4, 13), and acid hot springs (11).
AAPs contain light-harvesting complexes, but little is known about the transformation of light energy in their cellular metabolism. The photosynthetic competence of the AAPs has been demonstrated by spectroscopic analyses (9, 27, 32) and infrared (IR) kinetic fluorescence measurements (16, 21). It was shown that light exposure of AAP cells inhibits respiration (10, 16) and increases their cellular ATP concentration (2, 25). This indicates that AAPs are able to partially replace oxidative phosphorylation with photophosphorylation.
Little is known about the impact of light on AAP carbon metabolism. These organisms are unable to grow photoautotrophically, as they require organic substrates (15, 31, 34, 35). The lack of growth in the absence of organic substrates indicates that AAPs utilize light energy as an additional source of energy for their mostly heterotrophic metabolism (10). AAPs display light-mediated CO2 incorporation, although this activity is rather low (14, 15, 30). They lack RubisCO (18, 33), which suggests that the registered activity reflects anaplerotic carboxylation reactions (34). It has been repeatedly demonstrated that the exposure of AAP cultures to light increases biomass yields (1, 31, 32, 36); however, the extent of the light stimulation was estimated from optical density measurements or roughly quantified on a dry weight or protein basis only.
Therefore, the aim of this study was to rigorously quantify the accumulated biomass on a carbon basis (using a CN elemental analyzer) in continuous cultures grown under well-defined light and nutrient conditions. The obtained organic carbon data and the known amount of organic substrate in the medium were used to calculate the bacterial growth efficiencies (BGE) under each individual set of growth conditions. In addition, the contribution of carbon fixed through carboxylation to the total biomass was determined using radiolabeled bicarbonate radioassays. The obtained data should clearly establish the contribution of light energy in the carbon metabolism of AAPs.
MATERIALS AND METHODS
Chemostat setup.
Erythrobacter sp. strain NAP1 was grown in thermostated (23°C) glass vessels (volume, 0.75 liters) and exposed to 12-h/12-h light-dark cycles. Illumination was provided by a bank of fluorescent tubes (Lumilux Cool White L36W/840; OSRAM AG, Germany) providing white light (spectral temperature, 4,000 K) at 50, 150, 400, or 2,000 μmol quanta m−2 s−1. The growth medium was composed of artificial seawater (in the case of glucose media, natural seawater) enriched with 5 × 10−3 M (NH4)2SO4, 3 × 10−4 M Na2HPO4, vitamins, and a trace metal mix, as described earlier (15), and supplemented with 106 M nicotinic acid. Glutamate, pyruvate, glucose, or acetate (selected as the best growth substrates) was added as a sole source of organic carbon. The concentration of the organic carbon substrates was kept low (15 mM carbon equivalent) to ensure the carbon-limited growth conditions. pH was adjusted to 8.0 to 8.05 before autoclaving. The chemostat was inoculated with several milliliters of thick batch-grown culture. The chemostat culture was thoroughly stirred at a rate of 200 rpm and aerated (airflow of 120 ml min−1) to ensure fully aerobic and homogenous conditions. The growth medium was pumped in and out of the vessel in 30-min intervals. A continuous culture was operated with a dilution rate equal to 0.33, 0.5, or 1.0 day−1. The set dilution rate determined the culture growth rate. The steady state was considered to have been reached when the cell density (determined as optical density [OD] at 650 nm) and BChl a concentration remained constant for three consecutive days. The purity of the bacterial culture was routinely checked using IR epifluorescence microscopy. Due to very low growth rates on glucose, the dark-adapted culture was cultivated only in Erlenmeyer flasks.
Analytical methods.
To determine the total organic carbon content in the biomass, 20 to 40 ml of bacterial culture was collected by centrifugation at 10,000 × g for 10 min. The pelleted cells were resuspended in deionized water, transferred to a 1.5-ml Eppendorf tube, and spun down to remove excess salts. The bacterial cells were then transferred to tin capsules, dried at 60°C for 30 min, and stored in a freezer (−20°C). Finally, the organic carbon content was determined by an elemental CN analyzer. Protein concentrations were assayed in cells using the modified Lowry method (Sigma, Saint Louis, MO). For pigment analyses, chemostat samples were collected by centrifugation and extracted in 100% methanol. The BChl a concentration was determined spectroscopically using the absorption coefficient ε771 = 54.8 mM−1 cm−1 (26). The residual glutamate concentration was determined using ninhydrin. Collected supernatant (1 ml) was mixed with 0.25 ml of 8% ninhydrin solution in acetone. The mixture was kept in 2-ml Eppendorf tubes at 95°C for 15 min and then cooled to room temperature, and 0.25 ml of 50% ethanol was added. The glutamate concentration was determined by optical absorption at 570 nm. All analytical values are presented as means ± standard errors determined from a minimum of four samples.
Carboxylation activity was determined using a radiolabeled bicarbonate incorporation assay. Bacterial cultures (50 ml) grown on different substrates were labeled with 50 μCi NaH14CO3 and divided into 10-ml plastic transparent vials. Vials were placed into a temperature-controlled incubator providing various levels of light exposure (0 to 400 μmol quanta m−2 s−1). After 40 min, the incubation was terminated with the addition of 35% HCl and continuous shaking over 24 h. For the determination of the incorporated bicarbonate, 10 ml scintillation cocktail EcoLite(+) was added and counts were performed using a liquid scintillation analyzer (Perkin Elmer Tri-Carb 2810 TR).
Respiration was measured using a Clark oxygen electrode placed in a temperature-stabilized measuring chamber at 23°C. The electrode signal was recorded using a custom-made potentiostat and nanoammeter.
The photosynthetic electron transport was assessed using an infrared fast-repetition-rate (IR FRR) fluorometer (19, 20). The cells were illuminated in the chamber by a continuous light provided by an array of blue-light-emitting diodes (Nichia) (488 nm). The photosynthetic electron transport for each light intensity level was assessed from the FRR protocol as ET = I × σ470 nm × (FM − FS)/(FM − F0), where I is irradiance, σ470 nm is the effective absorption cross-section, FM is the maximum fluorescence in the dark, F0 is the minimum fluorescence in the dark, and FS is the steady-state fluorescence at a given light intensity (for details, see reference 19).
RESULTS
Impact of light intensity and carbon source on growth in Erythrobacter sp. NAP1.
To assess the contribution of light to carbon metabolism, the AAP cultures were grown in a carbon-limited chemostat at defined dilution rates. From chemostat principles, the dilution rate determines the growth rate of the culture under steady-state conditions. This regimen was chosen to establish conditions where the bacterial cultures utilize the maximum amount of available organic substrates; the other key nutrients such as phosphate, ammonium, and vitamins were provided in excess. The accumulated biomass of AAP cells was quantified in terms of total organic carbon by a CN analyzer. Total protein content was also determined to allow comparison with earlier studies. The bacterial growth efficiency was calculated by dividing the amount of carbon in biomass ([C]biomass) by the total concentration of organic carbon supplied in the medium ([C]medium) as follows:
The stability of the chemostat regimen at various dilution rates was tested using glutamate-grown cultures. The highest BGE values were found at lower dilution rates (0.3 to 0.5 day−1). The residual glutamate content in the medium was below 0.1 mM, which documents the carbon-limiting conditions. At higher dilution rates, the BGE declined (see Fig. S1 in the supplemental material). This may be explained by the fact that the dilution rate of 1.0 day−1 was close to the maximum growth rate and that the culture was washed out of the chemostat faster than the organisms were reproducing.
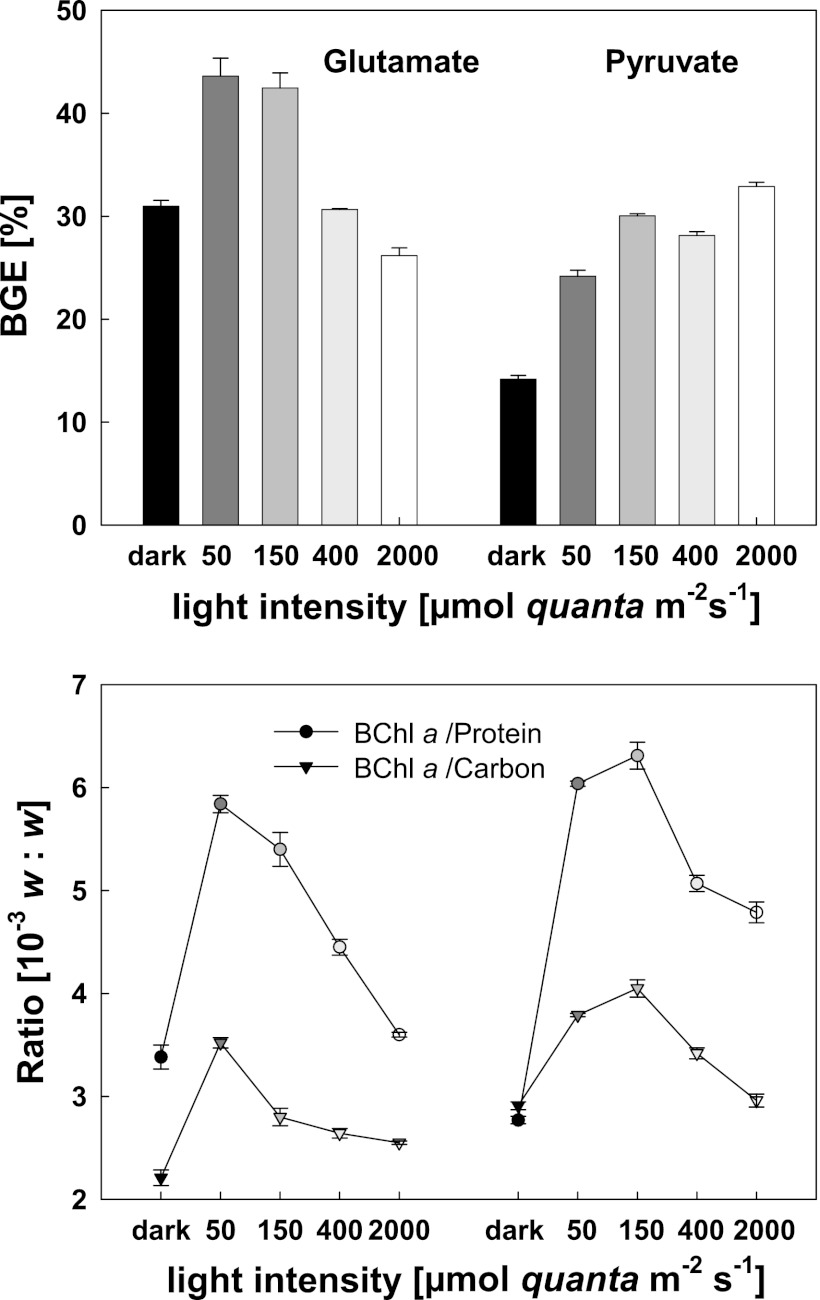
The impact of light on carbon accumulation in Erythobacter sp. NAP1 was tested in cultures grown at various light intensities. Under conditions of growth in the dark, the BGE of the pyruvate culture was 14.2% ± 0.4% (see Fig. S2 in the supplementary material). Using glutamate as the growth substrate, the BGE was more than two times higher (30.9% ± 0.6%). The transfer of the cultures to a light/dark regimen had a stimulatory effect in both cases. In the case of glutamate-grown culture, the BGE increased by approximately 50%, up to 43.62% ± 1.75% at 50 μmol quanta m−2 s−1 or 42.5% ± 1.5% at 150 μmol quanta m−2 s−1. Interestingly, with increasing intensity of illumination, the BGE decreased to 30.7% ± 0.1% at 400 μmol quanta m−2 s−1 and to 26.2% ± 0.7% at 2,000 μmol quanta m−2 s−1 (Fig. 1). For cultures grown on pyruvate at 150 μmol quanta m−2 s−1, the BGE more than doubled (30.4% ± 0.1%). In contrast to glutamate-grown culture results, the BGE for pyruvate remained high even at light intensities of 400 and 2,000 μmol quanta m−2 s−1 (Fig. 1). Along with the BGE, the protein content and BChl a concentrations were all determined simultaneously and their ratios calculated (Fig. 1). The lowest BChl a/carbon (or BChl a/total protein) ratios were observed for cultures grown in full darkness. The highest ratios were registered in cultures grown at medium light intensity (50 to 150 μmol quanta m−2 s−1); at higher intensities, the pigmentation decreased.
Fig 1.
BGE levels (upper panel) and BChl a/carbon and BChl a/total protein ratios (lower panel) in chemostat cultures of Erythrobacter sp. NAP1 under conditions of fully dark and light/dark regimens (12 h/12 h), cultivated on glutamate (left) and on pyruvate (right) with a dilution rate of 0.33 day−1.
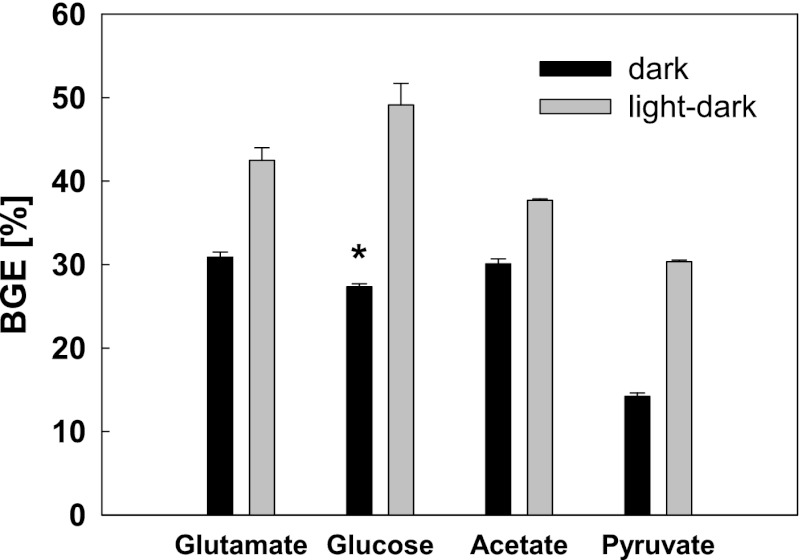
For comparison, the BGE values were also determined for Erythrobacter sp. NAP1 cultures grown on ammonium acetate and glucose (Fig. 2). The cells were grown with a dilution rate of 0.33 day−1, under conditions of fully dark and light/dark regimens (12 h/12 h; 150 μmol quanta m−2 s−1). The determined BGEs for the culture grown on ammonium acetate were 30.1% ± 0.6% in the dark. In the light/dark regimen, the BGE increased to 37.7% ± 0.2%. These values were higher than in the case of pyruvate. When grown on glucose in the dark, the obtained BGE values were somewhat lower (27.36% ± 0.36%). However, when grown in the light/dark regimen, the BGE more than doubled to 49.1% ± 2.6%, which is even higher than the values obtained for glutamate.
Fig 2.
BGE levels in chemostat cultures of Erythrobacter sp. NAP1 under conditions of fully dark and light/dark regimens (12 h/12 h) with a light intensity of 150 μmol quanta m−2 s−1 (dilution rate, 0.33 day−1). *, cells grown on glucose in the dark were cultivated in Erlenmeyer flasks.
Impact of light on respiration and photosynthetic activity.
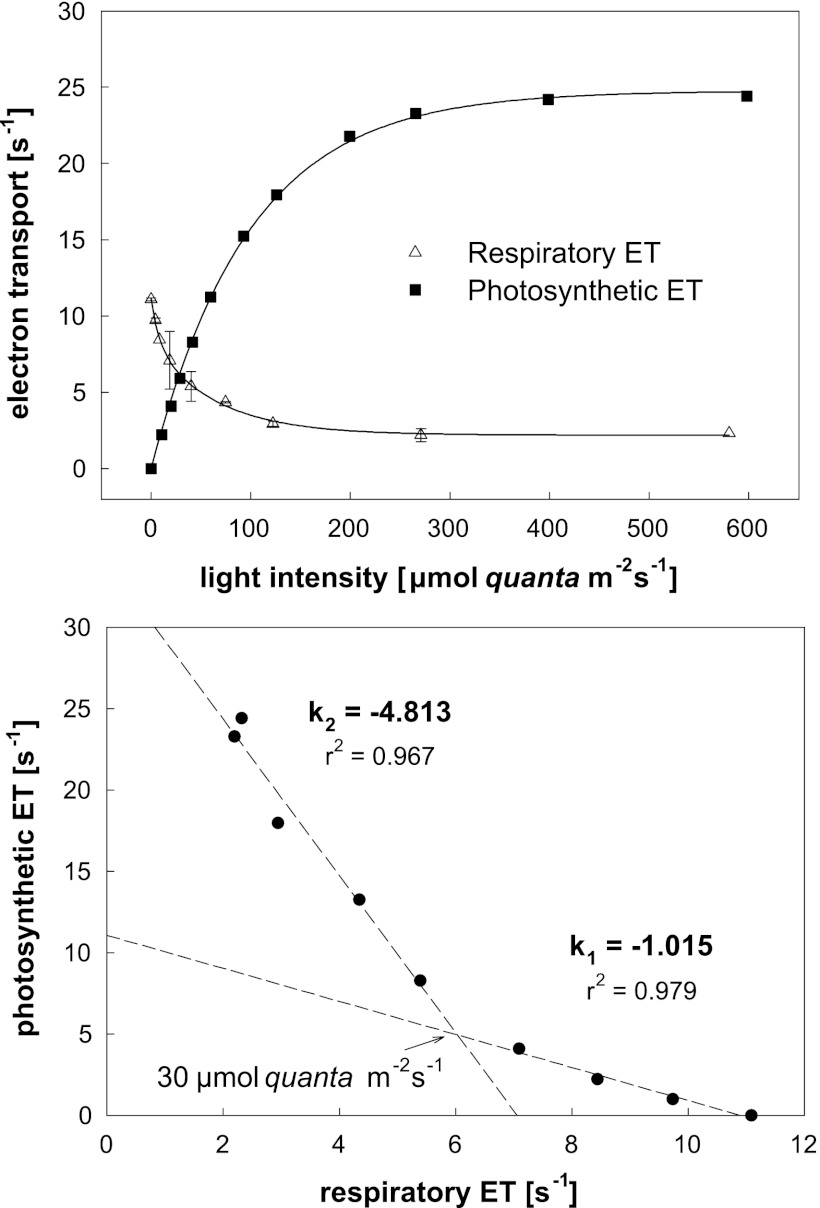
Several earlier studies documented the inhibition of respiration of AAP cultures in light. To test this effect, we performed similar measurements of respiration using oxygen electrode and photosynthetic electron fluxes by using an IR FRR fluorometer. The experiments were performed with a chemostat culture of Erythrobacter sp. NAP1 grown on 3 mM glucose. The respiration of the culture gradually decreased with increasing light intensity to approximately 25% of its initial value. To be able to compare the respiratory and photosynthetic electron flow, the respiration was normalized per reaction center. The number of the reaction centers was determined from the known bacteriochlorophyll concentration, assuming 36 BChl a molecules per reaction center. In parallel, the electron transport measured by fluorometry rose from 0 to 25 electrons per s (Fig. 3). After plotting the photosynthetic electron transport against the respiratory electron transport, we obtained, at the lowest light intensities (<30 μmol m−2 s−1), a linear relationship with a slope of approximately k = −1.015 (Fig. 3, lower panel). This demonstrates that photophosphorylation (photosynthetic electron transport) directly replaces oxidative phosphorylation (respiratory electron transport) in a 1:1 ratio. At higher light intensities, the photophosphorylation largely exceeds the oxidative phosphorylation, which is, however, never completely suppressed.
Fig 3.
The upper panel depicts the dependence of respiratory and photosynthetic electron transport (ET) on irradiance in Erythrobacter sp. NAP1 grown on 3 mM glucose with a dilution rate of 0.1 day−1. Respiratory electron flow was determined from oxygen consumption activity and photosynthetic electron flow from the IR-FRR fluorescence measurements. The electron transport rates are expressed relative to one reaction center. The lower panel shows the same data presented as photosynthetic electron transport plotted against respiratory electron transport.
Anaplerotic carboxylation.
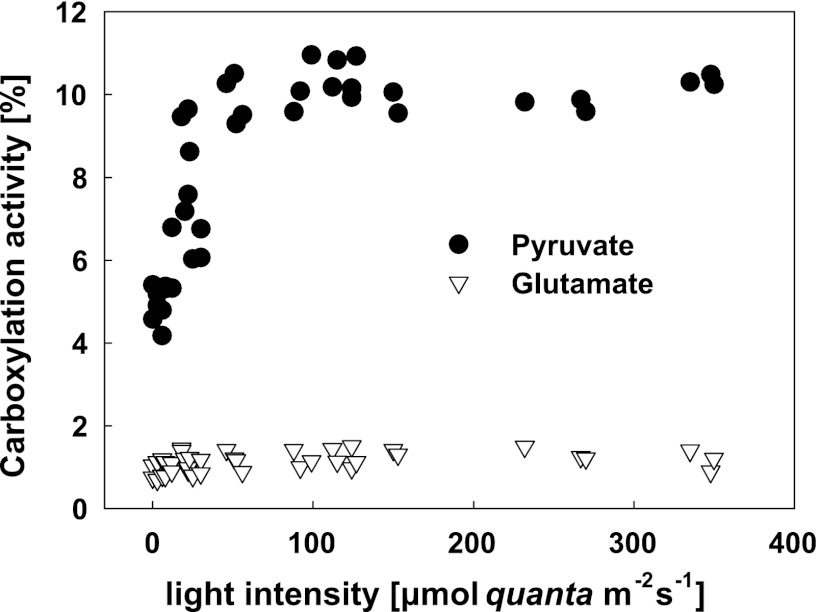
The remaining issue is which portion of AAP biomass originated directly from organic substrates and which portion was acquired through anaplerotic carboxylation. To quantify the amount of carbon gained through anaplerotic carboxylation, we performed radiolabeled bicarbonate assays. The chemostat-grown cultures of Erythrobacter sp. NAP1 were mixed with NaH14CO3 and exposed to different irradiances ranging from 0 to 400 μmol quanta m−2 s−1. The precentage of carbon originating from carboxylation was calculated as follows: carboxylation activity per hour/(24 × growth rate × total carbon content in the biomass).
The carboxylation in cultures grown on glutamate was low (between 0.6% and 1.6%) without any clear light stimulation. For pyruvate-grown cultures, the registered activity was significantly higher; it increased from 4% in the dark, reaching a maximum of 11% at light intensities above 100 μmol quanta m−2 s−1 (Fig. 4). Similar enhancement of HCO3− incorporation has also been observed in cells grown on glucose (not shown).
Fig 4.
The extent of anaplerotic carboxylation in Erythrobacter sp. NAP1 cultures grown on pyruvate or glutamate under conditions of a light/dark regimen (12/12 h) with a light intensity of 400 μmol quanta m−2 s−1 and a dilution rate of 0.5 day−1. The carboxylation activity was determined using radiolabeled bicarbonate incorporation at various light intensities.
DISCUSSION
The presented data clearly demonstrate that light stimulates the biomass accumulation in continuous cultures of AAP bacteria. In contrast to previous studies, we quantified the bacterial biomass in terms of organic carbon and calculated the BGE for each cultivation regimen. BGE is a key parameter for the evaluation of carbon fluxes through the microbial loop, providing information about the efficiency of conversion of organic substrate into bacterial biomass (6). The BGE values found in dark-grown cultures of Erythrobacter sp. NAP1 ranged from 14.2% to 30.9%. Similar numbers were also found in another AAP bacterium, Roseobacter sp. COL2P (see Fig. S3 in the supplemental material). This roughly corresponds to values of 5% to 60% detemined in different natural planktonic systems (mesotrophic freshwater and oligotrophic world ocean) (5). The exposure of the cultures to moderate light levels led to a significant increase in BGE values, from 30.0% in the case of pyruvate up to 49.1% in the case of glucose. Even higher numbers were obtained in the light/dark cultures of Roseobacter sp. COL2P grown on leucine or fumarate (see Fig. S3 in the supplemental material).
The enhanced BGE in light/dark-grown cultures indicates that AAP species utilize light energy to supplement their metabolic demands. The measurement of respiration and photosynthetic electron transport documents that the oxidative respiration can largely be replaced by photophosphorylation, allowing the AAP species to save carbon which would otherwise be respired and used in anabolic reactions and for building cell biomass. This increases the efficiency of utilization of organic carbon sources and leads to a higher BGE.
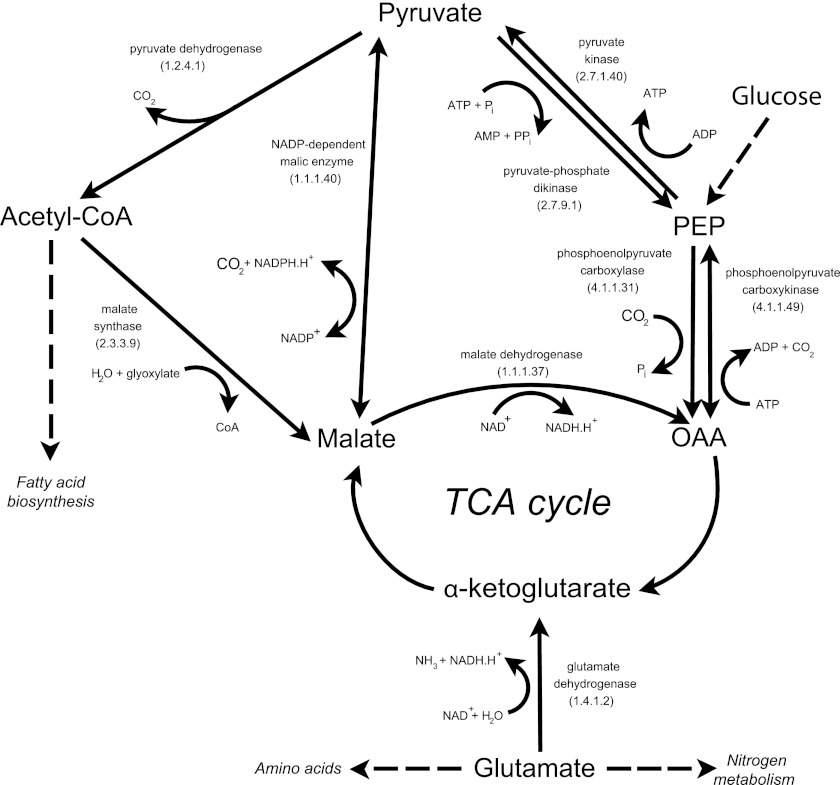
An important issue is the origin of the weak-light-enhanced CO2 incorporation activity registered repeatedly in different AAP species (14, 15, 30). Since AAPs do not contain RubisCO (18, 33), it is generally assumed that the observed activity originates from anaplerotic carboxylation (34). Based on our H14CO3− incorporation measurements, we calculated that Erythrobacter sp. NAP1 grown on glutamate gained approximately 1% of its cellular carbon through carboxylation, whereas in pyruvate-grown culture the contribution was around 4% in the dark and increased to 11% in the light. Our values are somewhat higher than the first estimates of 1% to 3% made by Kolber et al. (21) but lower than the 10% to 15% of protein carbon determined using stable isotope fractionation in Roseobacter denitrificans (34). The striking difference in the levels of carboxylation activity between glutamate- and pyruvate-grown cultures can be explained based on the general enzymatic pathway of bacterial metabolism. Our manual annotation of the Erythrobacter sp. NAP1 genome (18) revealed that it contains genes encoding three carboxylases that could be responsible for the registered activity: phosphoenolpyruvate (PEP) carboxylase (EC 4.1.1.31), NADP-dependent malic enzyme (EC 1.1.1.40), and PEP-carboxykinase (EC 4.1.1.49). Pyruvate carboxylase, reported from the Roseobacter denitrificans genome by Tang et al. (34), was not found in the Erythrobacter sp. NAP1 genome. All of the proposed carboxylases use pyruvate (directly or indirectly) as a substrate to replenish the tricarboxylic acid (TCA) cycle. This fact may explain the higher CO2 incorporation activity in cultures grown on pyruvate or glucose compared to glutamate, as glutamate is converted in one step to α-ketoglutarate, a part of the TCA cycle, without the need of carboxylation (Fig. 5).
Fig 5.
Metabolic pathways connected with pyruvate and glutamate metabolism in Erythrobacter sp. NAP1. The scheme was proposed based on the genes identified in the full-genome sequence (18). PEP, phosphoenolpyruvate; OAA, oxaloacetate; TCA cycle, tricarboxylic acid cycle.
One of the most interesting results of this study is the inhibition of glutamate-grown cultures at higher light intensities. The lower light optimum can be demonstrated from BChl a/protein or BChl a/carbon ratios, which also show a light optimum at moderate light intensities of 50 to 150 μmol quanta m−2 s−1. Remarkably, the lower light optimum observed in Erythrobacter sp. NAP1 is consistent with the fact that AAP bacteria in the ocean form typical subsurface maxima between 20- and 100-m depths (3, 12, 21).
The nature of the inhibition of glutamate cultures under conditions of higher light intensity is not clear. It is not related to UV damage, as the growth suppression was also observed under conditions of intense monochromatic green light (505 nm) provided by light-emitting diodes. Moreover, the inhibition was not observed in pyruvate-grown cultures. We assume that at higher light intensities the energy provided by photophosphorylation exceeds the metabolic demands of the cell. We speculate that the excess energy leads to the downregulation of the cellular metabolism. In the case of cultures grown on pyruvate, this scenario might be avoided, as pyruvate consumes more ATP for its transformation to enter the TCA cycle (Fig. 5).
In summary, we observed a significant enhancement of bacterial growth efficiency in AAP cultures grown in light/dark cycles compared to cultures grown in the dark. The light enhancement of carboxylation activity is relatively small, making the ability of AAPs to replace oxidative respiration with photophosphorylation much more important (see Fig. 3). This allows the AAPs to accumulate organic carbon which would otherwise be respired, making them more economical in terms of carbon utilization than heterotrophic bacteria. Thus, the possession of photosynthetic apparatus can represent a significant ecological advantage under conditions in which bacterial growth is carbon limited and light is available.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by Czech projects GAČR P501/12/G055 and Algatech (CZ.1.05/2.1.00/03.0110).
We thank Paul G. Falkowski for his advice in the initial phases of this project and for the opportunity to use the IR FRR fluorometer, Jarmila Mlčoušková for her contribution in Roseobacter sp. COL2P measurements, and Jason Dean for the language correction.
Footnotes
Published ahead of print 10 August 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Biebl H, Wagner-Dobler I. 2006. Growth and bacteriochlorophyll a formation in taxonomically diverse aerobic anoxygenic phototrophic bacteria in chemostat culture: influence of light regiment and starvation. Proc. Biochem. 41:2153–2159 [Google Scholar]
- 2. Candela M, Zaccherini E, Zannoni D. 2001. Respiratory electron transport and light-induced energy transduction in membranes from the aerobic photosynthetic bacterium Roseobacter denitrificans. Arch. Microbiol. 175:168–177 [DOI] [PubMed] [Google Scholar]
- 3. Cottrell MT, Mannio A, Kirchman DL. 2006. Aerobic anoxygenic phototrophic bacteria in the Mid-Atlantic Bight and North Pacific gyre. Appl. Environ. Microbiol. 72:557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Csotonyi JT, Swiderski J, Stackebrandt E, Yurkov V. 2010. A new environment for aerobic anoxygenic phototrophic bacteria: biological soil crusts. Environ. Microbiol. Rep. 2:651–656 [DOI] [PubMed] [Google Scholar]
- 5. Del Giorgio PA, Cole JJ, Cimbleris A. 1997. Respiration rates in bacteria exceed phytoplankton production in unproductive aquatic systems. Nature 385:148–151 [Google Scholar]
- 6. Del Giorgio PA, Cole JJ. 1998. Bacterial growth efficiency in natural aquatic systems. Annu. Rev. Ecol. Syst. 29:503–541 [Google Scholar]
- 7. Gorlenko VM, et al. 2010. Microbial communities of the stratified soda Lake Doroninskoe (Transbaikal region). Microbiologiya 79:390–401 [Google Scholar]
- 8. Harashima K, Shiba T, Totsuka T, Simidu U, Taga N. 1978. Occurrence of bacteriochlorophyll a in strain of an aerobic heterotrophic bacterium. Agric. Biol. Chem. 42:1627–1628 [Google Scholar]
- 9. Harashima K, Nakagawa M, Murata N. 1982. Photochemical activities of bacteriochlorophyll in aerobically grown cells of heterotrophs, Erythrobacter species (OCh 114) and Erythrobacter longus (OCh 101). Plant Cell Physiol. 23:185–193 [Google Scholar]
- 10. Harashima K, Kawazoe K, Yoshida I, Kamata H. 1987. Light stimulated aerobic growth of Erythrobacter species OCh 114. Plant Cell Physiol. 28:365–374 [Google Scholar]
- 11. Hiraishi A, Matsuzawa Y, Kanbe T, Wakao N. 2000. Acidisphaera rubrifaciens gen. nov., sp. nov., an aerobic bacteriochlorophyll-containing bacterium isolated from acidic environments. Int. J. Syst. Evol. Microbiol. 50:1539–1546 [DOI] [PubMed] [Google Scholar]
- 12. Jiao N, et al. 2007. Distinct distribution pattern of abundance and diversity of aerobic anoxygenic phototrophic bacteria in the global ocean. Environ. Microbiol. 9:3091–3099 [DOI] [PubMed] [Google Scholar]
- 13. Kim K, et al. 2007. Sphingomonas kaistensis sp. nov., a novel alphaproteobacterium containing pufLM genes. Int. J. Syst. Evol. Microbiol. 57:1527–1534 [DOI] [PubMed] [Google Scholar]
- 14. Kishimoto N. 1995. Distribution of bacteriochlorophyll a among aerobic and acidophilic bacteria and light-enhanced CO2-incorporation in Acidiphillium rubrum. FEMS Microbiol. Ecol. 16:291–296 [Google Scholar]
- 15. Koblízek M, et al. 2003. Isolation and characterization of Erythrobacter sp. strains from the upper ocean. Arch. Microbiol. 180:327–338 [DOI] [PubMed] [Google Scholar]
- 16. Koblízek M, Mlčoušková J, Kolber ZS, Kopecký J. 2010. On the photosynthetic properties of marine bacterium COL2P belonging to Roseobacter clade. Arch. Microbiol. 192:41–49 [DOI] [PubMed] [Google Scholar]
- 17. Koblížek M, et al. 2011. Role of photoheterotrophic bacteria in the marine carbon cycle, p 49–51 In Jiao N, Azam F, Sanders S. (ed), Microbial carbon pump in the ocean. Science/AAAS, Washington, DC [Google Scholar]
- 18. Koblížek M, et al. 2011. Genome sequence of the marine photoheterotrophic bacterium Erythrobacter sp. strain NAP1. J. Bacteriol. 193:5881–5882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kolber ZS, Prášil O, Falkowski PG. 1998. Measurements of variable chlorophyll fluorescence using fast repetition rate techniques: defining methodology and experimental protocols. Biochim. Biophys. Acta 1367:88–106 [DOI] [PubMed] [Google Scholar]
- 20. Kolber ZS, Van Dover CL, Niederman RA, Falkowski PG. 2000. Bacterial photosynthesis in surface waters of the open ocean. Nature 407:177–179 [DOI] [PubMed] [Google Scholar]
- 21. Kolber ZS, et al. 2001. Contribution of aerobic photoheterotrophic bacteria to the carbon cycle in the ocean. Science 292:2492–2495 [DOI] [PubMed] [Google Scholar]
- 22. Labrenz M, et al. 1999. Roseovarius tolerans gen. nov., sp. nov., a budding bacterium with variable bacteriochlorophyll a production from hypersaline Ekho Lake. Int. J. Syst. Bacteriol. 49:137–147 [DOI] [PubMed] [Google Scholar]
- 23. Masín M, Nedoma J, Pechar L, Koblížek M. 2008. Distribution of aerobic anoxygenic phototrophs in temperate freshwater systems. Environ. Microbiol. 10:1988–1996 [DOI] [PubMed] [Google Scholar]
- 24. Medová H, et al. 2011. High abundance of aerobic anoxygenic phototrophs in saline steppe lakes. FEMS Microbiol. Ecol. 76:393–400 [DOI] [PubMed] [Google Scholar]
- 25. Okamura K, Mitsumori F, Ito O, Takamiya KI, Nishimura M. 1986. Photophosphorylation and oxidative phosphorylation in intact cells and chromatophores of an aerobic photosynthetic bacterium, Erythrobacter sp. strain OCh 114. J. Bacteriol. 168:1142–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Permentier HP, et al. 2000. Composition and optical properties of reaction centre core complexes from the green sulfur bacteria Prosthecochloris aestuarii and Chlorobium tepidum. Photosynth. Res. 64:27–39 [DOI] [PubMed] [Google Scholar]
- 27. Rathgeber C, Alric J, Hughes E, Vermeglio A, Yurkov V. 2012. The photosynthetic apparatus and photoinduced electron transfer in the aerobic phototrophic bacteria Roseicyclus mahoneyensis and Porphyrobacter meromictius. Photosynth. Res. 110:193–203 [DOI] [PubMed] [Google Scholar]
- 28. Salka I, Cuperova Z, Masin M, Koblizek M, Grossart HP. 2011. Rhodoferax-related pufM gene cluster dominates the aerobic anoxygenic phototrophic communities in German freshwater lakes. Environ. Microbiol. 13:2865–2875 [DOI] [PubMed] [Google Scholar]
- 29. Sato K. 1978. Bacteriochlorophyll formation by facultative methylotrophs, Protaminobacter ruber and Pseudomonas AM1. FEBS Lett. 85:207–210 [DOI] [PubMed] [Google Scholar]
- 30. Shiba T. 1984. Utilization of light energy by the strictly aerobic bacterium Erythrobacter sp. Och 114. J. Gen. Appl. Microbiol. 30:239–244 [Google Scholar]
- 31. Shioi Y. 1986. Growth characteristics and substrate specificity of aerobic photosynthetic bacterium Erythrobacter sp. (OCh 114). Plant Cell Physiol. 27:567–572 [Google Scholar]
- 32. Spring S, Lunsdorf H, Fuchs BM, Tindall BJ. 2009. The photosynthetic apparatus and its regulation in the aerobic gammaproteobacterium Congregibacter litoralis gen. nov., sp. nov. PLoS One 4:e4866 doi:10.1371/journal.pone.0004866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Swingley WD, et al. 2007. The complete genome sequence of Roseobacter denitrificans reveals a mixotrophic rather than photosynthetic metabolism. J. Bacteriol. 189:683–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tang K, Feng X, Tang YJ, Blankenship R. 2009. Carbohydrate metabolism and carbon fixation in Roseobacter denitrificans OCh 114. PLoS One 4:e7233 doi:10.1371/journal.pone.0007233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yurkov V, Csotonyi T. 2009. New light on aerobic anoxygenic phototrophs, p 31–55 In Hunter CN, Daldal F, Thurnauer MC, Beatty JT. (ed), The purple phototrophic bacteria, vol 28 Springer Verlag, Dordrecht, The Netherlands [Google Scholar]
- 36. Yurkov V, van Gemerden H. 1993. Impact of light/dark regimen on growth rate, biomass formation and bacteriochlorophyll synthesis in Erythromicrobium hydrolyticum. Arch. Microbiol. 159:84–89 [Google Scholar]
- 37. Yutin N, et al. 2007. Assessing diversity and biogeography of aerobic anoxygenic phototrophic bacteria in surface waters of the Atlantic and Pacific Oceans using the Global Ocean Sampling expedition metagenomes. Environ. Microbiol. 9:1464–1475 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.