Abstract
Cytomegalovirus (CMV) coinfection is associated with infant HIV-1 disease progression and mortality. In a cohort of Kenyan HIV-infected infants, the frequencies of activated (CD38+ HLA-DR+) and apoptosis-vulnerable (CD95+ Bcl-2−) CD4+ and CD8+ T cells increased substantially during acute CMV infection. The frequency of activated CD4+ T cells was strongly associated with both concurrent CMV coinfection (P = 0.001) and HIV-1 viral load (P = 0.05). The frequency of apoptosis-vulnerable cells was also associated with CMV coinfection in the CD4 (P = 0.02) and CD8 (P < 0.001) T cell subsets. Similar observations were made in HIV-exposed uninfected infants. CMV-induced increases in T cell activation and apoptosis may contribute to the rapid disease progression in coinfected infants.
TEXT
Acute infant HIV-1 infection is characterized by very high HIV-1 viral loads (25, 28), rapid CD4 depletion, and high rates of mortality (1, 22, 24). Cytomegalovirus (CMV) coinfection is associated with more-rapid HIV-1 progression in children (10, 17, 23), and in adults, plasma CMV DNA is associated with survival time (6, 12, 34). In resource-poor settings, in which CMV is often acquired during infancy (21, 32), a large population of children undergo simultaneous primary HIV-1 and CMV infection (32).
CD8+ T cell activation has previously been shown to accompany acute CMV infection in healthy Gambian infants (21). Since T cell activation is a strong predictor of HIV disease progression (7, 13, 14, 27), we hypothesized that the acquisition of CMV during primary HIV-1 infection may accelerate infant disease progression by increasing frequencies of activated cells. In this report, we describe longitudinal changes in activated and apoptosis-vulnerable T cells during acute CMV infection in HIV-infected and HIV-exposed uninfected (HIV-EU) infants.
Study participants and specimens.
The primary cohort involved follow-up of 474 Kenyan infants from 1999 to 2003, detailed elsewhere (15, 19). This study was conducted before antiretroviral therapy (ART) became widely available in Kenya, and women and infants received ART only for the prevention of mother-to-child transmission (PMTCT). Serial infant blood specimens were collected at delivery, at months 1 and 3, and quarterly thereafter; HIV-EU infants exited at 1 year and HIV-infected infants exited at 2 years. Plasma specimens were used for measurement of HIV-1 (11) and CMV (20, 32) viral load. Infant HIV-1 infection was diagnosed as the first detection of HIV-1 using dried blood spot PCR for HIV gag (8) or plasma HIV-1 RNA viral load, whichever appeared first. CD4 counts were performed on freshly isolated blood using TriTest antibodies (BD Biosciences) and flow cytometry.
CMV viral loads were measured in a subset of 64 infants (32, 33); the current report involves a sample of 19 HIV-infected and 6 HIV-exposed uninfected (HIV-EU) infants selected by availability of cryopreserved peripheral blood mononuclear cells (PBMC) (see Table S1 in the supplemental material). CMV DNA was detected in the plasma of all but one infant.
Acute CMV infection is associated with an expansion of activated and apoptosis-vulnerable T cells in HIV-infected infants.
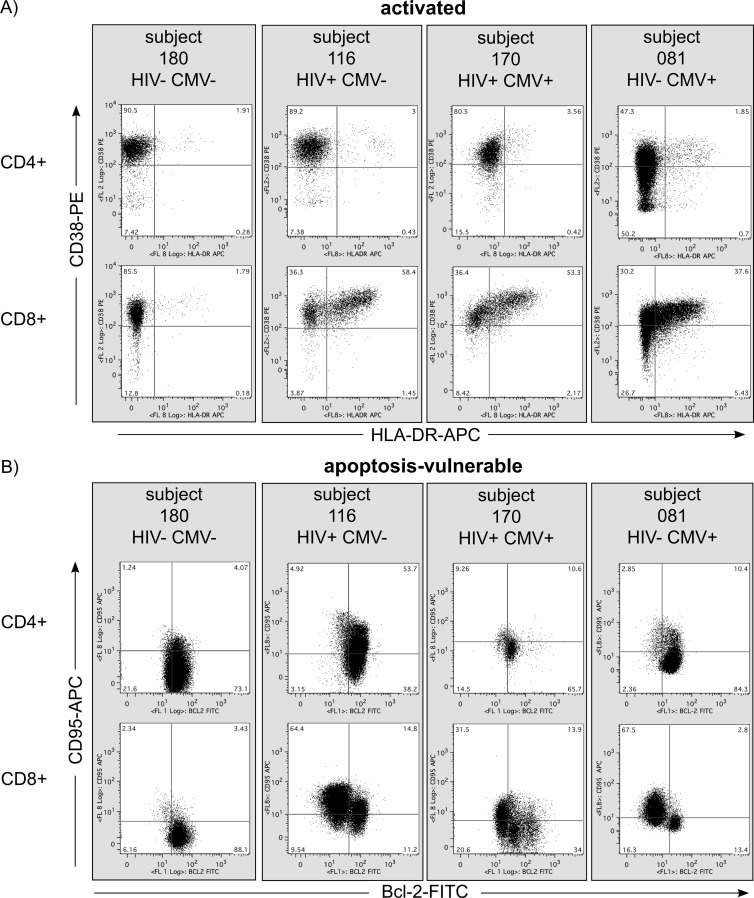
An increase in CD8+ T cell activation has been observed in HIV-negative children and transplant recipients with primary CMV infection (18, 21, 26, 29, 35). Cellular activation contributes to HIV-1 pathogenesis by a number of mechanisms (reviewed in references 9 and 16), including depletion of T cells via activation-induced cell death (AICD). Apoptosis is a hallmark of HIV infection; CD95 (Fas) is upregulated during HIV-1 infection, and its expression increases during disease progression (2–4, 30). We measured frequencies of activated and apoptosis-vulnerable CD4+ and CD8+ T cells. Peripheral blood mononuclear cells (PBMC) were thawed and stained with CD3-Pacific Blue (UCHT1, Dako, United Kingdom), CD4-APC-Cy7 (RPA-T4, Pharmingen, United Kingdom), CD8-PE-Cy7 (RPA-T8, Pharmingen), CD38-PE (AT13/5, Serotec, United Kingdom), and HLA-DR-APC (TU36, Pharmingen) antibodies and analyzed with multicolor flow cytometry, using standard methods described elsewhere (31). Activated CD3+ CD4+ and CD3+ CD8+ T cells were defined as CD38+ HLA-DR+ (Fig. 1A). Cells expressing CD95 which had downregulated expression of Bcl-2 were considered apoptosis-vulnerable cells likely to undergo AICD (5, 36) (CD95+ Bcl-2−) (Fig. 1B).
Fig 1.
Activated and apoptosis-vulnerable T cells in infants with HIV-1 infection, CMV infection, and HIV-1/CMV coinfection. Representative CD38 and HLA-DR (A) and CD95 and Bcl-2 (B) staining from four infants, categorized by HIV-1 and CMV infection status. All plots show data at 1 month of age, with the exception of subject 081, whose data are from month 3 (all HIV-EU infants first tested CMV DNA positive at 3 months).
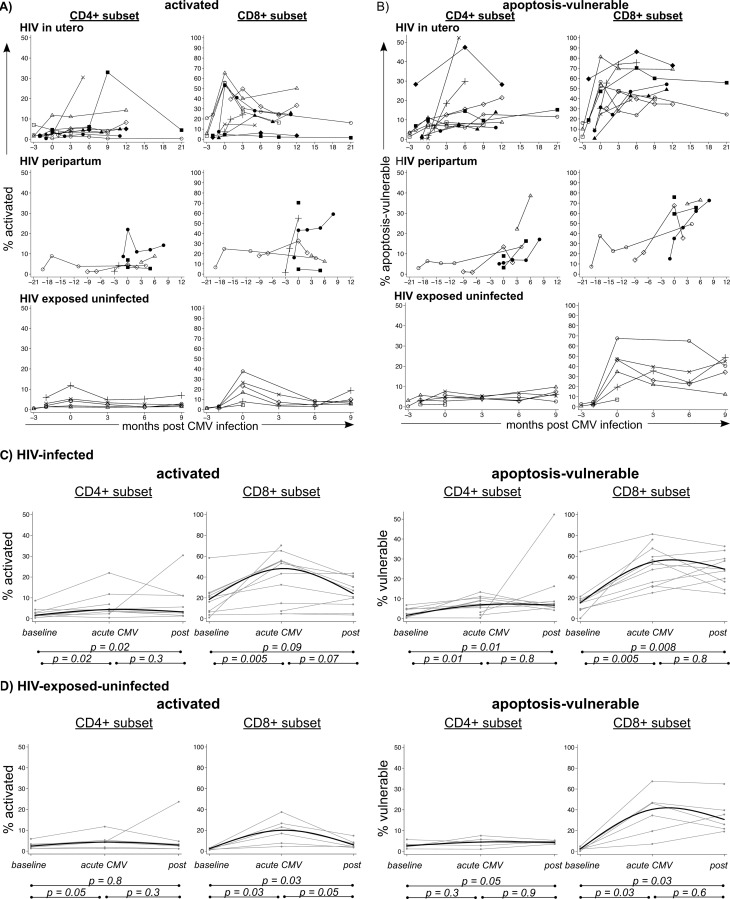
Figure 2A and B show longitudinal frequencies of activated and apoptosis-vulnerable T cells in infants, grouped by the timing of the HIV-1 infection. In HIV-infected infants, the frequencies of activated and apoptosis-vulnerable CD4+ and CD8+ T cells increased concurrently with the first detection of CMV DNA (Fig. 2C) (P value of <0.05 for baseline versus acute infection). This increase was also observed in HIV-EU infants, although it occurred more gradually in apoptosis-vulnerable cells in the CD4 subset (Fig. 2D) (P value of 0.05 for baseline versus postinfection). The frequencies of activated (median, 20%; interquartile range [IQR], 20 to 47) and apoptosis-vulnerable (median, 41%; IQR, 7.7 to 27) CD8+ T cells that we measured in HIV-EU infants are consistent with an earlier study in HIV-unexposed Gambian infants which measured high levels of activated (28% HLA-DR+) and apoptosis-vulnerable (56% Bcl-2−) CD8+ T cells during acute CMV infection (21).
Fig 2.
Changes in frequencies of activated and apoptosis-vulnerable T cells during acute CMV infection. Connected lines show individual infant trajectories of activated (A) and apoptosis-vulnerable (B) CD4+ and CD8+ T cells in three groups of infants (HIV-infected in utero, HIV-infected peripartum, and HIV-exposed uninfected infants). The time point of 0 corresponds to the first detection of CMV DNA. (C and D) Formal statistical comparison of frequencies of activated and apoptosis-vulnerable cells at baseline (last CMV-negative visit), acute CMV infection (first CMV-positive visit), and postacute infection (first visit after acute infection detected) for HIV-infected and HIV-EU infants. Individual infants are shown by gray lines, and the solid black median spline is overlaid. P values are from paired signed-rank tests. Note: y axes are shown on different scales for CD4+ and CD8+ T cell subsets for clarity of the data presentation.
HIV-1 load also increased by an average of 0.52 log10 copies/ml (standard deviation [SD] = 1.1; P = 0.03) between baseline and acute CMV infection. Although CMV coinfection was associated with more-rapid HIV-1 progression and higher mortality in an American cohort, CMV coinfection was not associated with higher HIV-1 viral loads (17). To determine whether HIV-1 viral load was affected by acute CMV infection in this Kenyan cohort, we compared mean HIV-1 viral loads between children who were CMV infected and children who were CMV negative at 1 month of age. Consistent with previous findings, there was no difference in CMV viral load between infants with CMV coinfection (mean ± SD, 6.2 ± 0.91 log10 copies/ml) and those with HIV-1 infection alone (6.5 ± 0.77 log10 copies/ml; P = 0.4). It is thus likely that the increase in HIV-1 viral load that we observed during acute CMV infection was not due to CMV but, rather, was coincidental to acute HIV-1 infection.
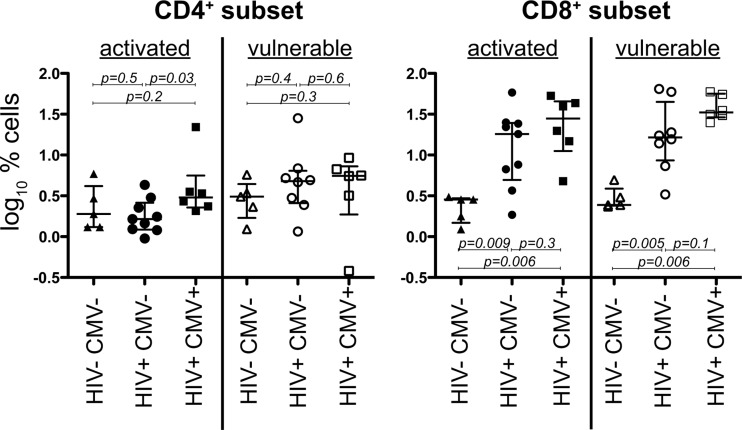
At 1 month of age, HIV-1/CMV-coinfected infants have a higher frequency of activated CD4+ T cells.
Because T cell activation is predictive of long-term risk of HIV-1 disease progression, we compared frequencies of activated and apoptosis-vulnerable cells at 1 month of age (Fig. 3). HIV-positive, CMV-positive (HIV+ CMV+) infants had a higher frequency of activated CD4+ T cells than HIV+ CMV− infants (median, 3.0% versus 1.6%, respectively; Mann-Whitney U test; P = 0.03). In the CD8+ T cell subset, HIV+ CMV+ and HIV+ CMV− infants both had higher frequencies of activated and apoptosis-vulnerable cells at 1 month than HIV− CMV− infants (P < 0.05 for each comparison).
Fig 3.
Comparisons of activated and apoptosis-vulnerable T cells at 1 month of age by HIV/CMV coinfection. Median and interquartile ranges are shown for infants that are grouped by coinfection at 1 month of age into one of the following groups: negative for both viruses (HIV− CMV−), infected with HIV only (HIV+ CMV−), or infected with both viruses (HIV+ CMV+). Because all HIV-exposed uninfected infants first tested positive for CMV at 3 months of age, there is no HIV− CMV+ group to display at month 1.
CMV coinfection is associated with frequencies of activated T cells.
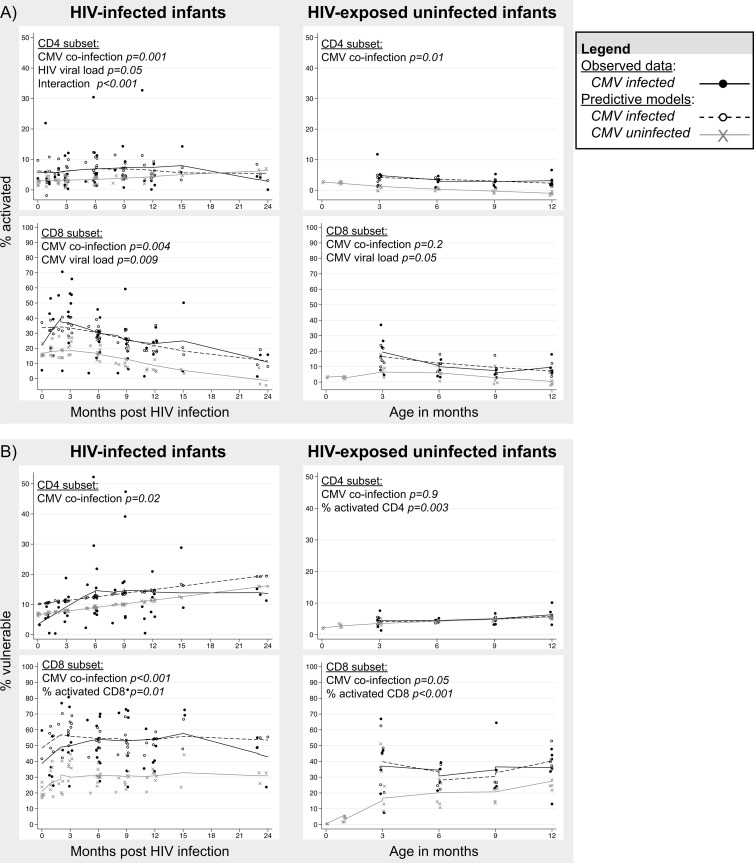
Generalized estimating equations (GEE) were used to determine predictors of activated and apoptosis-vulnerable cell frequencies; outcomes were continuous and used the identity link and Gaussian errors (see Table S2A in the supplemental material). All models used an exchangeable correlation matrix and robust standard errors. GEE beta coefficients were used to predict longitudinal frequencies of activated and apoptosis-vulnerable T cells in the presence and absence of CMV infection (Fig. 4).
Fig 4.
Longitudinal models of T cell activation and apoptotic vulnerability. Beta coefficients from GEE models were used to create predictive models using the general linear model (see Table S2 in the supplemental material). Scatter plots and fitted curves show observed data and predictive models for the outcomes of the percentage of activated cells (A) and the percentage of vulnerable cells (B) in HIV-infected and HIV-exposed uninfected infants. HIV-EU infants exited the study at 12 months, and HIV-infected infants were followed for an additional year. No HIV-EU infants acquired CMV before 3 months, so observed data and CMV-infected predictive models are not plotted for the month 0 and 1 time points. P values are shown for the effect of CMV coinfection in all GEE models; otherwise, P values are shown only for significant effects. Interaction refers to HIV viral load × CMV infection (yes/no).
Figure 4A shows that in the presence of CMV coinfection, HIV-infected infants are predicted to have substantially higher frequencies of activated T cells. In HIV-infected infants, the frequency of activated CD4+ T cells was predicted by CMV coinfection at the concurrent visit (see Table S2 in the supplemental material) (P = 0.001) and also HIV-1 viral load (P = 0.05). The effect of CMV coinfection was further enhanced by the level of the HIV-1 viral load (interaction term, P < 0.001), suggesting a synergistic effect of CMV coinfection and HIV-1 viral load on CD4+ T cell activation. The frequency of activated CD8+ T cells was dependent upon both the presence of CMV coinfection (P = 0.004) and CMV viral load (P = 0.009) but was not affected by HIV-1 viral load.
In HIV-EU infants, CMV coinfection was also associated with the frequency of activated CD4+ T cells (P = 0.01), and the CMV viral load was associated with the frequency of activated CD8+ T cells (P = 0.05).
CMV coinfection is associated with frequencies of apoptosis-vulnerable T cells.
Figure 4B shows that in the presence of CMV coinfection, HIV-infected infants are predicted to have substantially higher frequencies of apoptosis-vulnerable T cells. In HIV-infected infants, the frequencies of apoptosis-vulnerable CD4+ and CD8+ T cells were associated with the presence of CMV coinfection (see Table S2B in the supplemental material) (P = 0.02 and P < 0.001, respectively). Interestingly, HIV-1 viral load was not a significant predictor of apoptosis-vulnerable CD4+ or CD8+ T cells. We may have failed to find this association due to a correlation between HIV-1 and CMV viral loads and because T cell activation is in the causal pathway between HIV and/or CMV viral load and apoptosis. The frequency of apoptosis-vulnerable CD8+ T cells was also associated with the frequency of activated CD8+ T cells (P = 0.01).
In the HIV-EU controls, we observed a strong association between the frequencies of activated T cells and apoptosis-vulnerable T cells in both the CD4 and CD8 subsets, suggestive of AICD. CMV coinfection was significantly associated with apoptosis vulnerability only in the CD8 subset (P = 0.05).
In conclusion, we found that acute CMV infection was accompanied by substantial increases in the frequencies of activated and apoptosis-vulnerable T cells and that levels of activated and apoptosis-vulnerable T cells during acute HIV-1 infection were largely determined by the presence of CMV coinfection. Furthermore, HIV-1 viral load and CMV coinfection synergistically increased the frequency of activated CD4+ T cells, suggesting that CMV coinfection may play an important role in CD4+ T cell depletion during acute infant HIV-1 infection. These data support the hypothesis that CMV-induced T cell activation and Fas-mediated apoptosis potentially contribute to the increased HIV-1 disease progression observed in CMV-coinfected infants. As ART becomes more widely accessible to this population for both PMTCT and treatment, it will be important to determine the impact of maternal and infant ART on CMV epidemiology and pathogenesis.
Supplementary Material
ACKNOWLEDGMENTS
This publication was made possible by National Institutes of Child Health and Disease (NICHD) grants HD-23412 and HD-054314. J.A.S. is supported by National Institute of Allergy and Infectious Diseases (NIAID) grant AI087369. B.L.L.-P. was supported by the Fogarty International Center (FIC) grant TW06080. Additional funding was provided by the MRC grant to the Human Immunology Unit of the Weatherall Institute of Molecular Medicine. J.A.S. and B.L.L.-P. were scholars in the AIDS International Training and Research Program, NIH D43 TW000007, funded by the FIC and the Office of Research on Women's Health. G.C.J.-S. and S.L.R.-J. have each received the Pediatric AIDS Foundation Elizabeth Glaser Scientist Award. V.C.E. is funded by a grant from the MRC Centre for Clinical Virology. This publication resulted, in part, from research supported by the University of Washington Center for AIDS Research (CFAR), an NIH-funded program (P30 AI027757) which is supported by the following NIH institutes and centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, and NIA.
We acknowledge the contributions of the research personnel, laboratory staff, and data management teams in Nairobi and Seattle. We are grateful to the Nairobi City Council Clinics for their participation and cooperation and to the Departments of Pediatrics and Child Health and Medical Microbiology at the University of Nairobi for providing facilities for laboratory and data analysis. We thank Stephen Taylor of the Oxford University Computational Biology Research Group for the design and construction of data systems for the management of flow cytometry data. We thank Julie Overbaugh for providing HIV-1 viral loads and laboratory facilities for CMV viral loads. We are grateful to the Kizazi Working Group for reading and providing comments on the manuscript. Most of all, we thank the women and children who participated in this study.
None of the authors have a conflict of interest to declare.
The funding sources were not involved in the analyses or interpretation of data.
Jennifer Slyker performed the experimental work at the University of Oxford but analyzed the data and composed the manuscript at her current department at the University of Washington.
Footnotes
Published ahead of print 8 August 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Abrams E, et al. 1998. Association of HIV load early in life with disease progression among HIV-infected infants. J. Infect. Dis. 178:101–108 [DOI] [PubMed] [Google Scholar]
- 2. Baumler CB, et al. 1996. Activation of the CD95 (APO-1/Fas) system in T cells from human immunodeficiency virus type-1-infected children. Blood 88:1741–1746 [PubMed] [Google Scholar]
- 3. Bohler T, et al. 1997. Activation of the CD95 system increases with disease progression in human immunodeficiency virus type 1-infected children and adolescents. Pediatr. Infect. Dis. J. 16:754–759 [DOI] [PubMed] [Google Scholar]
- 4. Bohler T, Wintergerst U, Linde R, Belohradsky BH, Debatin KM. 2001. CD95 (APO-1/Fas) expression on naive CD4(+) T cells increases with disease progression in HIV-infected children and adolescents: effect of highly active antiretroviral therapy (HAART). Pediatr. Res. 49:101–110 [DOI] [PubMed] [Google Scholar]
- 5. Boudet F, Lecoeur H, Gougeon ML. 1996. Apoptosis associated with ex vivo down-regulation of Bcl-2 and up-regulation of Fas in potential cytotoxic CD8+ T lymphocytes during HIV infection. J. Immunol. 156:2282–2293 [PubMed] [Google Scholar]
- 6. Deayton JR, et al. 2004. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. Lancet 363:2116–2121 [DOI] [PubMed] [Google Scholar]
- 7. Deeks SG, et al. 2004. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 104:942–947 [DOI] [PubMed] [Google Scholar]
- 8. DeVange Panteleeff D, et al. 1999. Rapid method for screening dried blood samples on filter paper for HIV type 1 DNA. J. Clin. Microbiol. 37:350–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Douek DC, Roederer M, Koup RA. 2009. Emerging concepts in the immunopathogenesis of AIDS. Annu. Rev. Med. 60:471–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doyle M, Atkins JT, Rivera-Matos IR. 1996. Congenital cytomegalovirus infection in infants infected with human immunodeficiency virus type 1. Pediatr. Infect. Dis. J. 15:1102–1106 [DOI] [PubMed] [Google Scholar]
- 11. Emery S, et al. 2000. Evaluation of performance of the Gen-Probe HIV type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J. Clin. Microbiol. 38:2688–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Emery VC, et al. 1999. Quantitative effects of valacyclovir on the replication of cytomegalovirus (CMV) in persons with advanced human immunodeficiency virus disease: baseline CMV load dictates time to disease and survival. J. Infect. Dis. 180:695–701 [DOI] [PubMed] [Google Scholar]
- 13. Giorgi JV, et al. 1999. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 179:859–870 [DOI] [PubMed] [Google Scholar]
- 14. Giorgi JV, et al. 1993. Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. J. Acquir. Immune Defic. Syndr. 6:904–912 [PubMed] [Google Scholar]
- 15. John-Stewart GC, et al. 2009. HIV-1-specific cytotoxic T lymphocytes and breast milk HIV-1 transmission. J. Infect. Dis. 199:889–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khaitan A, Unutmaz D. 2011. Revisiting immune exhaustion during HIV infection. Curr. HIV/AIDS Rep. 8:4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kovacs A, et al. 1999. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. N. Engl. J. Med. 341:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Labalette M, Salez F, Pruvot FR, Noel C, Dessaint JP. 1994. CD8 lymphocytosis in primary cytomegalovirus (CMV) infection of allograft recipients: expansion of an uncommon CD8+ CD57− subset and its progressive replacement by CD8+ CD57+ T cells. Clin. Exp. Immunol. 95:465–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lohman BL, et al. 2005. Longitudinal assessment of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon responses during the first year of life in HIV-1-infected infants. J. Virol. 79:8121–8130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mattes FM, et al. 2005. Kinetics of cytomegalovirus load decrease in solid-organ transplant recipients after preemptive therapy with valganciclovir. J. Infect. Dis. 191:89–92 [DOI] [PubMed] [Google Scholar]
- 21. Miles DJ, et al. 2007. Cytomegalovirus infection in Gambian infants leads to profound CD8 T-cell differentiation. J. Virol. 81:5766–5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Newell ML, et al. 2004. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 364:1236–1243 [DOI] [PubMed] [Google Scholar]
- 23. Nigro G, et al. 1996. Rapid progression of HIV disease in children with cytomegalovirus DNAemia. AIDS 10:1127–1133 [PubMed] [Google Scholar]
- 24. Obimbo EM, et al. 2004. Predictors of early mortality in a cohort of human immunodeficiency virus type 1-infected African children. Pediatr. Infect. Dis. J. 23:536–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Obimbo EM, et al. 2009. Pediatric HIV-1 in Kenya: pattern and correlates of viral load and association with mortality. J. Acquir. Immune Defic. Syndr. 51:209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rees JC, Lifton MA, Light JA. 1989. Changes in lymphocyte subset distribution aid in the differential diagnosis of renal allograft dysfunction. J. Clin. Lab. Anal. 3:222–231 [DOI] [PubMed] [Google Scholar]
- 27. Resino S, Bellon JM, Gurbindo MD, Munoz-Fernandez MA. 2004. CD38 expression in CD8+ T cells predicts virological failure in HIV type 1-infected children receiving antiretroviral therapy. Clin. Infect. Dis. 38:412–417 [DOI] [PubMed] [Google Scholar]
- 28. Shearer WT, et al. 1997. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. N. Engl. J. Med. 336:1337–1342 [DOI] [PubMed] [Google Scholar]
- 29. Siegel DL, et al. 1989. Discriminating rejection from CMV infection in renal allograft recipients using flow cytometry. Clin. Immunol. Immunopathol. 51:157–171 [DOI] [PubMed] [Google Scholar]
- 30. Sloand EM, et al. 1997. Role of Fas ligand and receptor in the mechanism of T-cell depletion in acquired immunodeficiency syndrome: effect on CD4+ lymphocyte depletion and human immunodeficiency virus replication. Blood 89:1357–1363 [PubMed] [Google Scholar]
- 31. Slyker JA, et al. 2011. Phenotypic characterization of HIV-specific CD8+ T cells during early and chronic infant HIV-1 infection. PLoS One 6:e20375 doi:10.1371/journal.pone.0020375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Slyker JA, et al. 2009. Acute cytomegalovirus infection in Kenyan HIV-infected infants. AIDS 23:2173–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Slyker JA, et al. 2009. The detection of cytomegalovirus DNA in maternal plasma is associated with mortality in HIV-1-infected women and their infants. AIDS 23:117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spector SA, Wong R, Hsia K, Pilcher M, Stempien MJ. 1998. Plasma cytomegalovirus (CMV) DNA load predicts CMV disease and survival in AIDS patients. J. Clin. Invest. 101:497–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van de Berg PJ, et al. 2010. Human cytomegalovirus induces systemic immune activation characterized by a type 1 cytokine signature. J. Infect. Dis. 202:690–699 [DOI] [PubMed] [Google Scholar]
- 36. Yoshino T, et al. 1994. Inverse expression of bcl-2 protein and Fas antigen in lymphoblasts in peripheral lymph nodes and activated peripheral blood T and B lymphocytes. Blood 83:1856–1861 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.