Abstract
Feline calicivirus (FCV) is an important pathogen of domestic cats and a frequently used model of human caliciviruses. Here we use an epidemiologically rigorous sampling framework to describe for the first time the phylodynamics of a calicivirus at regional and national scales. A large number of FCV strains cocirculated in the United Kingdom at the national and community levels, with no strain comprising more than 5% and 14% of these populations, respectively. The majority of strains exhibited a relatively restricted geographical range, with only two strains (one field virus and one vaccine virus) spreading further than 100 km. None of the field strains were identified outside the United Kingdom. Temporally, while some strains persisted locally for the majority of the study, others may have become locally extinct. Evolutionary analysis revealed a radial phylogeny with little bootstrap support for nodes above the strain level. In most cases, spatially and temporally diverse strains intermingled in the phylogeny. Together, these data suggest that current FCV evolution is not associated with selective competition among strains. Rather, the genetic and antigenic landscape in each geographical location is highly complex, with many strains cocirculating. These variants likely exist at the community level by a combination of de novo evolution and occasional gene flow from the wider national population. This complexity provides a benchmark, for the first time, against which vaccine cross-protection at both local and national levels can be judged.
INTRODUCTION
One of the major challenges of molecular epidemiology is to progress from qualitative descriptions of pathogen diversity to an understanding of the processes that generate the temporal and spatial distributions of pathogen strains and to explain how these are affected by both epidemiology and interactions with the host immune response (15). The interpretation of such data will, however, inevitably be affected by the samples chosen for analysis. Although structured sampling techniques are well established in epidemiology, they appear to be used less commonly in molecular epidemiology, and sampling is often carried out on samples obtained by convenience. For this study, we obtained a large, stratified random sample of feline caliciviruses (FCVs), which are important pathogens of cats that exist at a high prevalence in the general cat population. Evolutionary analysis was used to explore the complex temporal and spatial dynamics of this virus at the national and community levels in the United Kingdom and to relate the dynamics to the international spread of the virus.
FCV is a member of the Caliciviridae (14, 33). It has a 7.7-kb positive-sense RNA genome with three open reading frames (ORFs), encoding the nonstructural proteins, the major capsid protein, and a minor structural protein. Genetically, FCV strains belong to one diverse genogroup, with little evidence for subspecies clustering. This genetic diversity is accompanied by antigenic diversity, although there is sufficient cross-reactivity that all isolates are deemed to belong to a single serotype (28). Several vaccines, both live and inactivated, are licensed for disease control, although they do not prevent infection (30). Infection is generally associated with relatively mild oral and respiratory tract disease. However, the virus's genetic plasticity has led to the repeated emergence of variants (or pathotypes), some of which can be lethal (8, 18, 25, 33, 42, 43). In addition to its significance for feline health, FCV is frequently used as a model for human norovirus, an important cause of vomiting and diarrhea in people (36).
Several studies have explored the evolution of FCV in different populations. In individual cats, positive selection for FCV antigenic change is believed to contribute to the establishment of persistent infections (7, 19, 21, 39). Evolutionary rates of the variable regions of the capsid protein have been estimated to be up to 1.3 × 10−2 to 2.6 × 10−2 substitution per nucleotide per year (7), one of the highest evolutionary rates reported for any virus. In large groups of cats, such as those in breeding households, immunological selection may operate at the level of the colony, leading to extremely high levels of observed strain diversity and long-term endemic infection (5, 7). As a result of persistent infections, the prevalence of FCV is high, ranging from approximately 10% in the general cat population to as much as 90% in some colonies, making it relatively easy to obtain FCV isolates from randomly sampled cats (27, 33, 54).
Studies on FCV diversity and evolution have led to a generally accepted level of genetic distance by which strains can be differentiated. Nucleotide sequences of the variable and immunodominant regions C to E of the capsid gene (44) differ at more than 20% of sites when epidemiologically unrelated viruses are compared, and these are considered distinct strains (33). Epidemiologically related viruses such as those found in acute outbreaks of disease are usually less than 1 to 2% divergent (and rarely more than 5% divergent) and are considered variants of the same strain (31, 35). However, during endemic infections, such as those that exist in some household colonies, variants of a single strain have been shown to vary by up to 18% (7). Using such definitions, we have shown that individual cats are generally infected with a single strain but may be infected with two, leading to the possibility of interstrain recombination at the ORF1-ORF2 junction (9). Within household colonies and rescue shelters, many strains often cocirculate, with the number of strains probably reflecting the size of the colony and its degree of isolation and turnover (6, 34, 38).
The evolution of FCV is well studied at the individual and small colony scales. The aim of this study was to investigate the evolution and diversity of FCV at the community and national levels and to test whether the observations from small populations apply over larger temporal and spatial scales. In order to address these questions, we have undertaken a comprehensive repeat cross-sectional national study of FCV genetic diversity, using cats attending randomly selected veterinary practices from across the United Kingdom, in tandem with an in-depth longitudinal community study centered on two local veterinary practices. Taken together with questionnaire data, the data in this work represent an unprecedented molecular epidemiological study of the evolution of an RNA virus of veterinary importance over previously unexplored spatial and temporal scales.
MATERIALS AND METHODS
Cross-sectional national survey.
Two individual cross-sectional surveys were carried out to collect oropharyngeal (OP) samples from cats attending veterinary practices during a 12-month period in 2005 to 2006. In order to reduce the risk of bias in the sample collection, three veterinary practices were chosen by random selection from each of the 23 geographical regions of the United Kingdom (identified in the 2004 Royal College of Veterinary Surgeons practice register) and were asked to participate in this study (Fig. 1A). If a practice declined to take part in the study, then another practice from the same region was randomly selected. Each practice was asked to take oropharyngeal swabs between October and December 2005 from cats belonging to 30 owners who gave their informed consent (cross-sectional study 1 [CS1]). Each practice was asked to repeat the sampling procedure in April to June 2006 (CS2). The dates chosen for sampling were based on convenience and did not relate to any perceived seasonal pattern of FCV dynamics. The mean geographical distance between practices was 317 km (range, 7 to 1,096 km) (Fig. 1B).
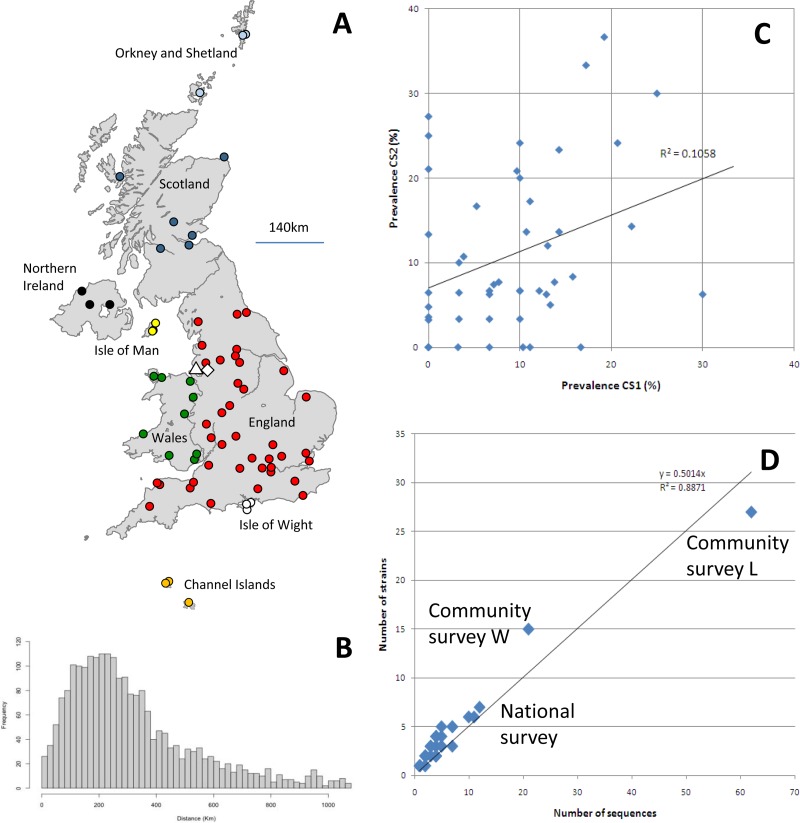
Fig 1.
(A) Locations of the veterinary practices sampled in the two national cross-sectional surveys (filled circles) and the longitudinal community surveys (open triangle [L] and open diamond [W]). The colors of the circles correspond to the node tips in Fig. 2. (B) Pairwise geographic distances between all veterinary practices in the national survey. (C) Comparison of the prevalences of FCV per practice in CS1 and CS2. Only practices submitting 10 or more swabs were included in the analysis. (D) Number of sequences isolated per practice (national and community) compared to the number of strains isolated per practice.
Longitudinal community survey.
To obtain a more detailed understanding of the local diversity and dynamics of FCV, oropharyngeal samples were collected over a 14-month period by sampling cats attending two veterinary practices in northwestern England (L and W) (Fig. 1A). These practices were chosen based on compliance and convenience. Each practice was asked to take oropharyngeal swabs from up to 30 cats a week for cats belonging to compliant owners who gave their informed consent and who visited their surgery for the 60 weeks between 22 October 2006 and 14 December 2007.
For both the national and local community virus collections, the geographical location of each sample was determined by the owner's postcode, and a short questionnaire which included the cat's age, sex, breed, vaccination status, and brief medical status relating to mouth ulcers and respiratory disease was administered. This questionnaire was completed by each cat's owner or veterinary surgeon. Oropharyngeal swabs were collected into 2 ml of virus transport medium, batched, and sent to our laboratories for virus isolation.
Virus isolation.
Feline calicivirus and feline herpesvirus 1 (FeHV-1) were isolated as described previously on feline embryo A (FEA) cells or Crandall Reese feline kidney (CRFK) cells (20, 50) and were stored at −80°C for further analysis.
RNA extraction, reverse transcription-PCR (RT-PCR), and consensus sequencing.
RNAs were extracted from positive cell cultures (second passage or less) (QIAmp viral RNA minikit; Qiagen) and transcribed into cDNAs (Superscript III; Invitrogen) by use of 500 ng of random hexamers (Abgene) as the first-strand primer, according to the manufacturers' instructions. A 529-nucleotide region of the capsid gene, equivalent to residues 6406 to 6934 of FCV strain F9 (4) and incorporating the immunodominant region E (23, 40, 44), was amplified as previously described (7). We have previously shown that sequences generated from cell culture are >99% accurate compared to sequences obtained directly from OP swabs (7).
In order to allow comparisons between polymerase and capsid sequences, a PCR targeting part of the relatively conserved 3′ polymerase-encoding region of the FCV genome was carried out on representative FCV isolates from CS1 for which the corresponding capsid sequence was available. A single-stage PCR was performed to amplify a 486-nucleotide region of the 3′ polymerase-encoding region of the FCV genome, equivalent to residues 4766 to 5251 of FCV strain F9 (4), essentially according to previously described protocols (9).
All capsid and polymerase amplicons were purified (QIAquick PCR purification kit; Qiagen Ltd.) and sequenced bidirectionally using corresponding PCR primers according to standard protocols (ABI Prism BigDye Terminator, version 3.0, cycle sequencing kit; Applied Biosystems). For each amplicon, a consensus sequence was produced using ChromasPro v1.32 (Technelysium Pty. Ltd.). Ambiguities identified consistently on both strands of sequence were included in the consensus sequence. All primer sites were removed prior to sequence analysis. For the capsid amplicons, a final useable sequence of 420 nucleotides, corresponding to codons 383 to 522 of the FCV capsid (44) and including variable regions C and E, was used for analysis.
Nucleotide sequence and phylogenetic analysis.
Geographical distances between isolates were calculated using free mapping tools (http://www.freemaptools.com/) and R (The R Project for Statistical Computing, Vienna, Austria [http://www.r-project.org/]).
To assign sequences to strains, we used the previously defined nucleotide distance threshold of 20% for hypervariable region E of the capsid (31, 35). Pairwise genetic distances between sequences were calculated using the Jukes-Cantor approach as implemented in MEGAv4.0 (47).
For phylogenetic analyses, sequence alignments were initially estimated using MUSCLE v3.6 (11) and manually corrected using GENEDOC v2.7. Maximum likelihood trees with 1,000 bootstrap replicates were calculated using PHYML 3.0 (16), using the most appropriate model of evolution, which was identified using the model selection approach implemented in Topali v2.5 (22). Amino acid phylogenies were estimated using MEGAv4.0. Sequences from the community study were analyzed alongside published FCV sequences from historical isolates dating between 1958 and 1995 and retrieved from GenBank (13). Quantification of phylogenetic structure was undertaken using likelihood mapping as implemented in TREEPUZZLE (45).
Associations between phylogeny and the sampling locations of sequences were explored (for the community data sets) using the parsimony methods implemented in BaTS v2 (24), using published global and ancestral sequences as controls. Tests for nucleotide sequence saturation were carried out using plots of the transition/transversion ratio versus divergence and using the Xia test for saturation as implemented in DAMBE (51).
Finally, to explore the level of phylogenetic signal across the genome, JC neighbor-joining phylogenies estimated from polymerase and capsid sequences were compared. Selection pressures on codons were estimated using the SLAC method as implemented in DATAMONKEY (10).
Epidemiological analysis.
Univariable and multivariable multilevel logistic regression analyses were carried out using MLWIN (v2.1; Centre for Multilevel Modeling, University of Bristol). The samples were clustered within region and within veterinary practice, and hence these factors were included in the models as random effects. Separate analyses were undertaken to identify risk factors for three outcomes of interest: FCV carriage, presence (at time of sampling) of mouth ulcers, and presence (at time of sampling) of signs of upper respiratory tract disease (URTD). For each analysis, initial univariable screening was undertaken for all potential risk factors for which data were available. Potential risk factors considered included the age of the cat (categorized into <48 months and >48 months, based on generalized additive models [not shown]), sex, breed, vaccination status, time of study (i.e., CS1 or CS2), and FCV carriage (the latter for the models for ulcers and URTD only). Those variables with P values of <0.25 in univariable screening were considered in the multivariable model by backward elimination. In all cases, models were fitted using Markov chain Monte Carlo (MCMC) simulation, using a Metropolis-Hastings sampler with diffuse priors (41). The number of iterations used was determined by examination of the Raftery-Lewis and Brooks-Draper Nhat statistics. In each case, a chain length of 50,000 integrations was sufficient, so this length was used throughout. The burn-in was set at 1% of the chain length (12). Model fit was assessed by examining the posterior distributions of the fixed and random variables included in the mixed-effects models. In each case, the fits were smooth and regular and approximated a normal distribution.
A quadratic assignment procedure correlation test (UCInet v 6.288; Analytic Technologies, Boston, MA) was used to assess the relationship between genetic distance (%) and geographical distance. This method computes standard measures of correlation between the genetic and geographic distance matrices and then computes an estimate of the significance of the correlation by permuting the elements of one of the matrices 5,000 times and counting the number of correlations between the observed and permuted matrices that are larger or smaller than the empirical estimate.
Nucleotide sequence accession numbers.
Nucleotide sequences obtained in this study have been submitted to GenBank under accession numbers JX123959 to JX124213.
RESULTS
Characteristics of study population.
For the national survey, of the 69 practices recruited for CS1, 57 (83%) returned samples (range, 3 to 34 samples; mean, 26 samples). Of these, 52 practices also submitted samples for CS2. For those regions where practices did not return samples for CS1, a replacement practice was recruited for CS2 (n = 12). Of the 69 practices recruited for CS2, 57 (83%) returned samples (range, 4 to 33 samples; mean, 25 samples).
In total, 2,818 swabs were received in the CS1 and CS2 national surveys, including swabs from 23 cats that were sampled twice, with 21 testing negative on both occasions, 1 testing negative in CS1 and positive in CS2, and 1 testing positive in both. The overall FCV prevalence in this population by virus isolation was 9.3% for CS1 (0 to 33% for individual practices) and 11.0% for CS2 (0 to 37% for individual practices) (Table 1). There appeared to be little correlation between the prevalence obtained in CS1 and CS2 for each practice (r2 = 0.11) (Fig. 1C), nor was there an effect of season/date of sampling.
Table 1.
Summary of samples, sequences, and strains identified in each population
| Study | No. of cats sampled | No. (%) of FCV-positive cats | No. (%) of isolates sequenced | No. of strains |
|---|---|---|---|---|
| National surveys | ||||
| CS1 | 1,468 | 137 (9.3) | 85 (62) | 71 |
| CS2 | 1,350 | 149 (11.0) | 86 (58) | 58 |
| Community surveys | ||||
| L | 633 | 88 (13.9) | 62 (70) | 27 |
| W | 414 | 38 (9.2) | 21 (55) | 15 |
For the community survey, a total of 1,047 samples were submitted over a 60-week period (n = 633 for practice L and n = 414 for practice W). The overall FCV prevalence by virus isolation within these communities was 13.9% for practice L and 9.2% for practice W.
Risk factors for FCV carriage and for signs of FCV disease.
There was a significant effect of age on FCV carriage, with a reduction in risk each month for the first 4 years, followed by a plateau. This resulted in an odds ratio of 0.9 per year (0.99 per month) for each of the first 4 years of life (Table 2). There was also a significant effect of FCV vaccination (Wald χ2 = 22.042; P < 0.001), with vaccinated cats being approximately half as likely to test positive for FCV as those that were not vaccinated. The group whose vaccination status was unknown was not significantly different from the unvaccinated cats.
Table 2.
Final multivariable models of risk factors associated with FCV carriage, presence of oral ulcers, and presence of signs of URTD
| Model and factor | Beta value | SE | OR | Lower CI | Upper CI | Wald chi-square value | P value |
|---|---|---|---|---|---|---|---|
| Multivariable model for FCV carriage | |||||||
| Age (per month for up to 48 months) | −0.009 | 0.003 | 0.991 | 0.985 | 0.997 | ||
| Vaccination status | |||||||
| No | Reference | 22.042 | <0.001 | ||||
| Yes | −0.568 | 0.121 | 0.567 | 0.447 | 0.718 | ||
| Unknown | −0.223 | 0.185 | 0.800 | 0.557 | 1.150 | ||
| Multivariable model for oral ulcers | |||||||
| Age (per month for up to 48 months) | 0.028 | 0.005 | 1.028 | 1.018 | 1.039 | 26.5 | <0.001 |
| Vaccination status | |||||||
| No | Reference | 10.243 | 0.001 | ||||
| Yes | −0.532 | 0.207 | 0.587 | 0.392 | 0.881 | ||
| Unknown | 0.265 | 0.301 | 1.303 | 0.723 | 2.351 | ||
| FCV carriage | |||||||
| No | Reference | 155.301 | <0.001 | ||||
| Yes | 2.508 | 0.201 | 12.280 | 8.282 | 18.210 | ||
| Multivariable model for signs of URTD | |||||||
| Age (per month for up to 48 months) | 0.007 | 0.004 | 1.007 | 0.999 | 1.015 | 3.185 | 0.07 |
| FCV carriage- vaccination interaction | |||||||
| FCV negative, not vaccinated | Reference | 68.403 | <0.001 | ||||
| FCV negative, vaccinated | −0.471 | 0.174 | 0.624 | 0.444 | 0.878 | ||
| FCV negative, unknown vaccination status | −0.258 | 0.321 | 0.773 | 0.412 | 1.449 | ||
| FCV positive, not vaccinated | 1.473 | 0.249 | 4.362 | 2.678 | 7.107 | ||
| FCV positive, vaccinated | −0.862 | 0.498 | 0.422 | 0.159 | 1.121 | ||
| FCV positive, unknown vaccination status | −0.332 | 0.853 | 0.717 | 0.135 | 3.819 |
There was a significant association between FCV carriage and vaccination, age, and the presence of oral ulceration and signs of URTD (Table 2). For oral ulceration, age significantly increased the odds (after allowing for FCV carriage and vaccination status). Those cats positive for FCV were around 12 times as likely to have oral ulceration, whereas vaccinated cats were approximately half as likely to have oral ulceration. The relationship between URTD and vaccination and FCV status was more complex due to an interaction between these risk factors. In short, compared to unvaccinated, FCV-negative cats (the reference group), there was a significantly higher odds of signs of URTD in unvaccinated, FCV-positive cats and a significantly lower odds in vaccinated, FCV-negative cats. Other categories were not significantly different from the reference group.
Sequence analysis for strain identification.
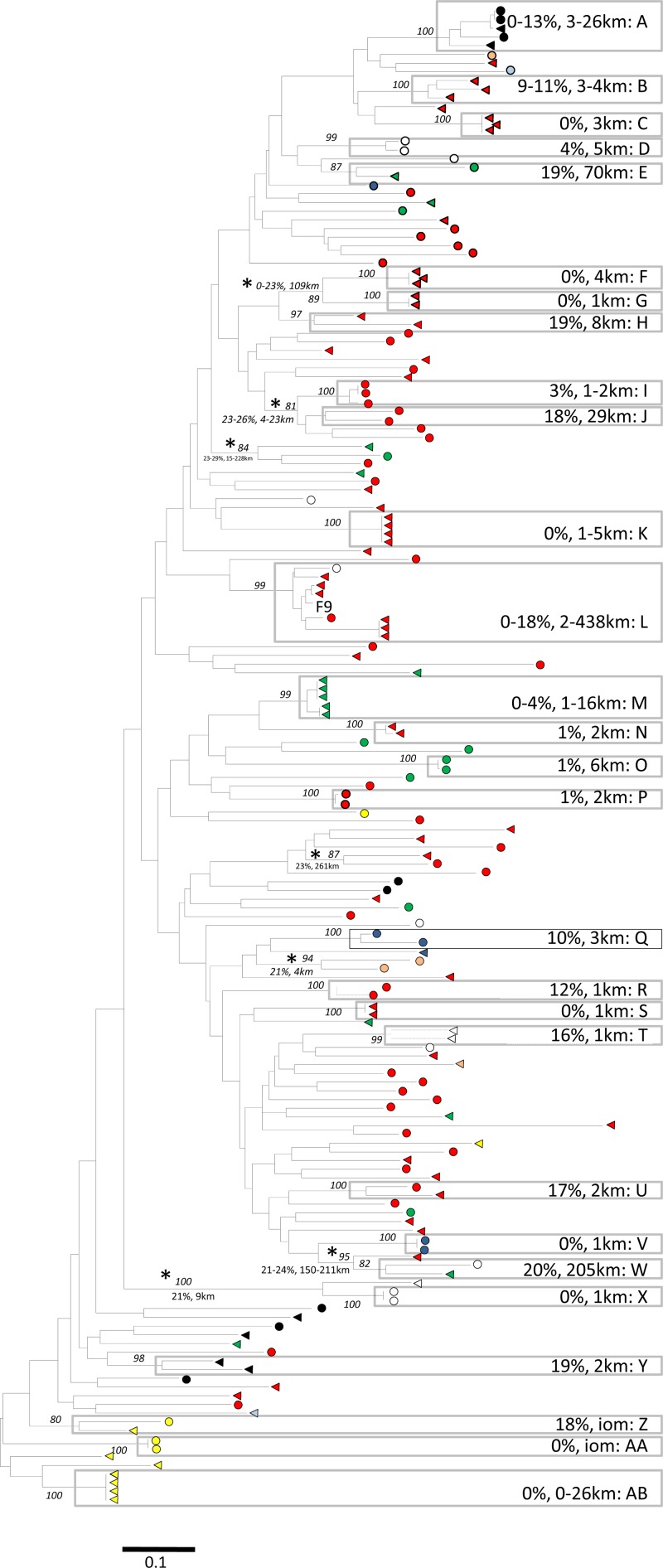
Partial sequences of the immunodominant region of the FCV capsid were obtained from 55 to 70% of the isolates obtained for each population (Table 1). Amplification success rates reached 88% when we included either capsid or polymerase PCR results (data not presented). Using a JC pairwise genetic divergence of 20% as the threshold between variants of the same strain, a total of 171 strains were identified within the national and community surveys (Table 1). In total, 45 strains contained more than one variant, including 28 from the national study and 17 from the community study. Forty-four of these 45 strains were supported by bootstrap values of >80%; the exception was clade J in the cross-sectional study (Fig. 2). No strains were common to both the national and community studies (data not presented), except for a vaccine strain (F9) that appeared in both populations. Apart from FCV F9, no strains identified here were also seen outside the United Kingdom (based on BLAST searches of GenBank [data not presented]). All but five of these strains (E, J, L, and W in the national survey [Fig. 2 and Table 3] and LW in the community survey [see Fig. 4]) were restricted to a single practice. Only in rare instances (7 cases in the national study and 1 case [L12] in the community study) were sequences with pairwise nucleotide divergences of >20% found clustered together with bootstrap scores of >80% (Fig. 2; also see Fig. 4).
Fig 2.
Unrooted maximum likelihood tree of 172 FCV capsid consensus sequences obtained from the national study (including the sequence of FCV vaccine strain F9 [GenBank accession no. M86379]). Circles indicate sequences from CS1, and triangles indicate sequences from CS2. The colors of the node tips correspond to the regions of sampling shown in Fig. 1. Gray boxed areas represent strains A and AB (as defined using a JC genetic distance of <20%). All other nodes with bootstrap support of >80% are indicated by asterisks. Minimum-maximum genetic diversity (%) and minimum-maximum geographical distances (km) are indicated next to the corresponding strains. Evolutionary distances were calculated using a GTR model and the maximum likelihood method, with the scale bar indicating the number of estimated substitutions per site. Numbers at major nodes are % bootstrap values (for 1,000 replicates; only values of >80% are shown). iom, Isle of Man.
Table 3.
Summary of strains from the national survey that were either present in both cross-sectional surveys or present at more than one practicea
| Strain | Presence in cross-sectional survey |
No. of practices where strain was present | No. of sequences | Maximum distance |
||
|---|---|---|---|---|---|---|
| CS1 | CS2 | km | DNA (%) | |||
| A | ✓ | ✓ | 1 | 5 | 3–26 | 0–13 |
| E | ✓ | ✓ | 2 | 2 | 70 | 19 |
| J | ✓ | 2 | 2 | 29 | 18 | |
| Lc | ✓ | ✓ | 5 | 8 | 2–438 | 0–18 |
| U | ✓ | ✓ | 1 | 2 | 2 | 17 |
| W | ✓ | ✓ | 2 | 2 | 205 | 20 |
| Z | ✓ | ✓ | 1 | 2 | IOMb | 18 |
Strain identity is the same as that in Fig. 2.
Isle of Man. No postcode data were available; the size of the island is 52 km by 22 km (http://www.gov.im/isleofman/geography.xml).
Strain L is phylogenetically related to FCV F9.
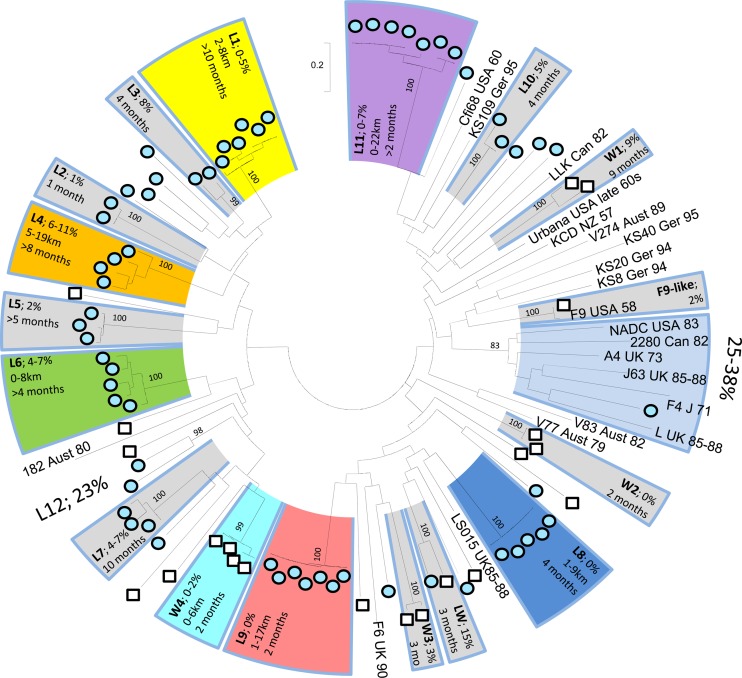
Fig 4.
Unrooted maximum likelihood phylogeny of 83 FCV consensus sequences obtained from the community study and 21 published FCV sequences obtained from GenBank (accession numbers are shown in reference 13). Strains (L1 to L11, W1 to W4, and L plus W) are represented by shaded arcs. For each strain, the JC genetic diversity (%) and, where available, the geographic dispersal (km) and number of calendar months in which it was present are indicated within the box. Published sequences are presented by name, country, and year of origin. Numbers at major nodes are bootstrap values of >800 of 1,000 replicates (expressed as percentages).
Using this definition of a strain, there was a clear linear relationship (r2 = 0.89) between the number of sequences obtained from a practice and the number of strains within it, with the number of strains being equal to approximately half the number of sequences (r2 = 0.7398 for the national survey alone [data not presented]). This suggests that despite the extensive nature of our survey, the extent of the genetic diversity in any given practice remains far from fully characterized (Fig. 1D).
Evolutionary analysis. (i) National survey.
The results of the phylogenetic analysis of the CS1 and CS2 samples are shown in Fig. 2. This diverse phylogeny contained 71 and 58 strains, identified in CS1 and CS2, respectively (Table 1). The phylogeny was strongly radial or star-like in nature (short external branches and long internal branches); correspondingly, there is a consistent lack of bootstrap support for most nodes near the root of the phylogeny. To quantify the lack of phylogenetic structure, we performed a likelihood mapping analysis (not shown) which concluded that approximately 25% of quartets are unresolvable or poorly resolvable.
Since the sequences are highly diverse, this lack of phylogenetic structure is unlikely due to a lack of polymorphism. Indeed, visual inspection of the alignments suggests that individual nucleotide sites are either invariant or very highly variable. We therefore performed further tests to determine if the lack of phylogenetic structure was caused by sequence saturation. For the national study, plots of the transition/transversion ratio against divergence indicated substantial saturation in the capsid sequences for genetic distances of >0.5 but provided little evidence of saturation in the pol data set (data not presented). However, with the Xia test for saturation (51), both alignments showed significant levels of nucleotide saturation. As a result of this saturation, we also estimated a phylogeny by using amino acid sequences, which showed that 35 of the 45 strains were supported by high bootstrap values (over 90) (data not shown). Of the remaining 10 strains, 7 were still monophyletic, but with lower bootstrap support, and 3 were no longer monophyletic. In addition to clarifying the role of sequence saturation, these results support the decision to classify FCV strains by use of a pairwise genetic divergence threshold of 20%.
Returning to Fig. 2, the most prevalent strains made up only 3% (strain A; 5 of 171 strains) and 5% (strain L [F9 vaccine strain]; 8 of 171 strains) of the total sequenced population. Even within individual practices, high levels of strain diversity sometimes existed, with up to five strains identified in some practices in a single sampling period (with averages of 2.0 and 1.7 for CS1 and CS2, respectively) (data not shown).
The sequences from CS1 and CS2 were not phylogenetically distinct, with sequences from both surveys intermingled in the tree and low bootstrap support for nodes joining most pairs of strains. There were no differences between nucleotide distances in comparing viral sequences obtained within and between the two surveys (within-CS1 distances, 0 to 53% [average, 38%]; within-CS2 distances, 0 to 55% [average, 38%]; and distances between CS1 and CS2, 4 to 55% [average, 38%]) (see Fig. S1 in the supplemental material). The program BaTS revealed some correlation between the date of sampling (CS1 versus CS2) and the phylogeny (P < 0.005 using the AI and PS statistics), although significance was lost when multiple variants of individual strains were removed from the alignment.
In addition, BaTS uncovered evidence for clustering of strains according to geographic region (Fig. 1A and 2) (P < 0.005 using the AI and PS statistics). With the exception of the Orkney Islands and Wales, this was evident for all geographic regions (P < 0.05). With that said, all regions contained multiple strains, even smaller islands such as the Isle of Man, the Channel Islands, and the Isle of Wight.
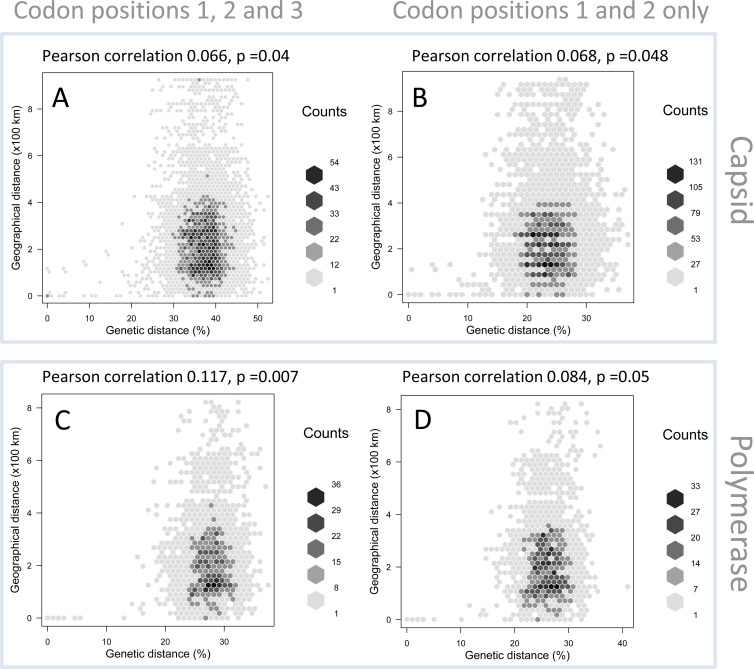
Given the weak structure and bootstrap support for the estimated phylogenies, we further explored the spatial clustering of strains by plotting pairwise genetic distances versus geographic distances. Regardless of the genomic region (pol or capsid gene) used or whether 3rd codon positions were excluded, we observed a weak association between genetic and spatial distances (Pearson correlation, 0.066 to 0.117) (Fig. 3). The weak signal that was present was driven mainly by variants of individual strains (divergence of <20%) confined to a single practice. In contrast, sequence pairs above the strain threshold (pairwise divergence of >20%) showed no association with distance.
Fig 3.
Comparison of genetic distances (%) and geographical distances (km) for FCV sequences obtained from the national cross-sectional studies, based on capsid (A and B) and polymerase (C and D) sequences. Panels A and C are based on codon positions 1, 2, and 3, and panels B and D are based on codon positions 1 and 2.
Of the 28 strains for which more than one variant was identified, 21 were restricted to a single practice and obtained during a single cross-sectional sampling period. The maximum geographical range among these 21 strains was 16 km (strain M) (Fig. 2). For the other seven strains, six were found during both CS1 and CS2, suggesting that they had persisted in the population, and four were present in more than one practice (Table 3). Only two strains were found at locations >100 km distant (strains L and W), suggesting that they had spread more widely. The most numerous (n = 8) and widely dispersed (up to 438 km) strain was FCV F9, a strain commonly used in live vaccines.
Previous studies have suggested that FCV and other caliciviruses have a predilection for recombination at the ORF1-ORF2 junction (3, 9). To assess the significance of recombination in this population, the polymerase sequence was obtained for 48 of the samples whose capsid sequence was also available in CS1. Phylogenetic analysis of these two ORFs failed to identify any recent recombination events (see Fig. S2 in the supplemental material), although the radial phylogeny with associated low bootstrap support for internal nodes observed in both trees likely precludes the identification of older recombination events in this data set. Furthermore, the selection analysis revealed no evidence of positive selection; rather, 127 of 140 codons were predicted to be under purifying selection (P < 0.05) (data not shown).
(ii) Community survey.
The results of phylogenetic analysis for the community samples are shown in Fig. 4. As with the national survey, the genetic diversity within both communities was high, with 27 and 15 strains in total identified in veterinary practices L and W, respectively (Table 1). All but one of these strains (strain LW) were confined to a single community/practice. No strain made up more than 14% of the sequences obtained from its practice (strains L1, L9, and L11 each comprised 7 of 62 [11%] sequences, and strains W5 and W7 each comprised 3 of 21 [14%] sequences). One cat from practice L tested positive three times (strain L5). A single F9-like virus was found in the samples from community W.
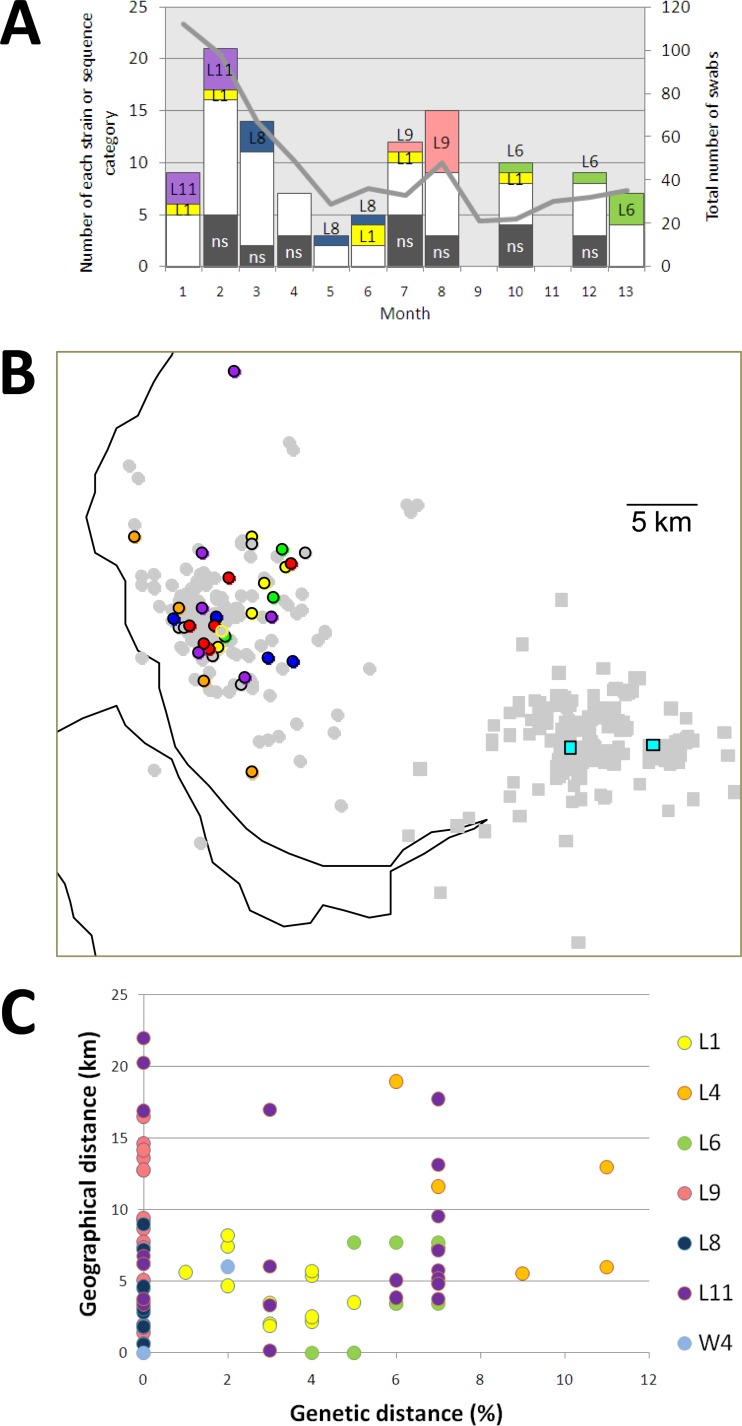
At the community level, 15 of the 16 strains for which more than one variant was identified were seen in more than 1 month (range, 2 to >10 months). Longitudinal sampling allowed us to assess the persistence of the most numerous strains (five or more variants) within community L (Fig. 5A). Strain L1 was found at a low prevalence (6 of 74 sequences [8%]) over a 10-month period, suggesting its persistence in this community. In contrast, strain L11 was seen only in the first 2 months, at a relatively high prevalence (7 of 25 sequences [28%]), suggesting that it may have become extinct in this population, whereas strain L6 was absent for the first 9 months (0 of 68 sequences) of the study and was observed only at a high prevalence in the last 3 months (5 of 19 strains; 26%), suggesting that this virus may have been newly introduced into this community. Strains L8 and L9 appeared only transiently, during the middle of the sampling period.
Fig 5.
Temporal and spatial distributions of strains from the community survey. (A) Bar chart showing the number of selected strain types (>3 sequences) in community L (same colors as in Fig. 4). White areas represent all strains seen only once; shaded areas (ns) are isolates that were refractory to capsid sequencing. The gray line represents the number of swabs received per month. (B) Spatial distribution of selected sequences obtained in the community study, based on postcode data. Gray circles and squares represent all samples from communities L and W, respectively. Colored circles represent locations of strains from community L, and colored squares represent locations of strains from community W. (C) Genetic distance (JC %) compared to geographical distance (km) for strains in the community study. Colored circles represent individual strains.
The geographical footprint of strains in the community survey is shown in Fig. 5B and C. For the 7 strains for which complete postcode information was available, the maximum geographic range was 22 km (strain L11). There appeared to be no correlation between the genetic diversity and the spatial distribution of variants within strains (Fig. 5C).
Of the three populations of viruses included in the phylogeny (i.e., L, W, and published world sequences), none were monophyletic (Fig. 4). However, there was a tendency for the sequences obtained in this study to be phylogenetically distinct from those obtained from GenBank. Most notably, there is a cluster containing six published global reference sequences and one sequence from our survey (from community L).
Strong bootstrap support for nodes containing sequences obtained in this study was generally restricted to variants of the same strain. Bayesian phylogenetic analysis suggested some geographical structure above the “strain” level (P < 0.01 using BaTS when only one sequence per strain is retained). This spatial structure is apparently driven by sequences from community L (mean observed clade size, 7.4; P = 0.01) and the global reference sequences mentioned above (mean observed clade size, 3.5; P = 0.05). Sequences from community W lacked any geographical correlation with the phylogeny.
DISCUSSION
Our aim is to understand the genetic diversity of FCV across a range of temporal and spatial phylodynamic scales. In previous studies, we have shown that FCV is subject to positive selection within individual cats (7, 39). Within cat colonies, high FCV prevalence is often associated with high levels of strain diversity, with colony persistence achieved by evolution within a small number of persistently infected individuals, and by reinfection of other members of the population (7, 34). We have suggested that immune-mediated positive selection thus operates at the colony level to generate viral diversity. In this study, we determined the extent to which these strains persist and mix in the wider population. Specifically, we measured the genetic diversity and persistence of FCV in rigorously sampled communities, as well as nationally. The prevalence and risk factors (effects of age and vaccination status) obtained in this study are in close agreement with those in previous work for both owned and unowned cats, suggesting that these results may be generalizable to other cat populations (6, 7, 27).
It is clear from the current study that the number of strains circulating in these populations is high, with 123 strains observed over 9 months and 41 strains observed over 14 months in the national and community studies, respectively. This level of strain complexity was surprising and previously unseen. It is associated with a radial, star-like phylogeny which exhibits low bootstrap support for most nodes above the strain level. Even individual practices in the cross-sectional survey had up to five strains at any one time, and the Isle of Man, which is only 21 km by 37 km, contained 12 strains.
This complex pattern of strain cocirculation is unusual and quite distinct from that seen for the related noroviruses, which are divided into five genogroups and a total of 29 genotypes (52). The diversity within a norovirus genogroup is ∼15 to 45%, based on uncorrected amino acid distances over the entire capsid. Amino acid diversity of <15% represents variants of the same genotype, and a diversity of >45% represents viruses in distinct genogroups. The capsid amino acid diversity between strains of FCV ranges from 9 to 19% (13), which is somewhat comparable to that for a single genogroup of norovirus, with FCV strains (as we define them) somewhat equivalent to norovirus genotypes (e.g., II.1 and II.12) (52). However, in this study, we identified over 100 strains in the United Kingdom alone, and our sampling suggests that the true number could be very much higher. This indicates a greater degree of lineage cocirculation for FCV than for noroviruses. However, norovirus studies are generally performed on clinical samples obtained by convenience; it would be informative to gather similar structured data for the human viruses to see if these data would better define the temporal and spatial circulation of these viruses.
Within this overall complexity, the maximum prevalence of any given field strain was 5% and 14% in the national and community studies, respectively. This diversity is reminiscent of that found within feline rescue shelters with good biosecurity, which in the absence of large-scale within-shelter transmission have been suggested to act as samplers of community pathogen diversity (6, 38). This complex pattern of strain coexistence again contrasts with the case for the noroviruses, for which individual genotypes predominate in any given season. Most notably, genotype II.4 has comprised 44% of 773 norovirus outbreaks in the United States over a 12-year period (1994 to 2006), with 51 to 85% of recent outbreaks being associated with GII.4 (46, 48, 53). The national and international epidemiological dominance of genotype II.4 has been attributed to a higher error rate of its viral RNA polymerase and an associated higher rate of evolution within the virus capsid (2). In contrast, extensive lineage cocirculation of FCV indicates that no strain has been able to outcompete the others. This could be explained by geographic subdivision of thehost population preventing strains from competing directly. However, this seems unlikely, since we have shown that even small islands and individual veterinary practices can have many coexisting strains with apparently overlapping geographical footprints. It is more likely that insufficient immunological interaction between FCV strains would allow cats to undergo cycles of reinfection or coinfection, both of which have been observed in the field (7). These observations are consistent with the low or absent cross-protection between some strains in vitro and with a failure of the host immune response to prevent heterologous infection (28, 29). Such weak antigenic cross-protection is predicted to cause chaotic or cyclical patterns of strain persistence in populations (17), may explain the lack of positive selection observed in the present community and national studies, and contrasts with previous studies of individual and colony cats infected with single strains (7, 39).
In this study, for the first time, we have mapped FCV isolates at a high resolution by using the owner's postcode, allowing us to define the geographical distribution of individual strains. The most widely dispersed lineage (strain L) contained variants of FCV F9, a common strain included in several live attenuated vaccines (4, 33). This vaccine virus is occasionally shed by vaccinated individuals (26), and variants of this strain are regularly, albeit occasionally, found in the general cat population (1, 6, 7, 37). As such, the geographical range of this strain is unsurprising and likely represents the widespread use of live F9 vaccines rather than natural virus transmission. To date, this level of apparently vaccine-derived viruses is tolerated, although pressure for change may come from those companies that market inactivated vaccines (30).
For those strains confined to an individual practice, the maximum geographical spread was 26 km in the national survey and 22 km in the community survey. This is likely to partly reflect the catchment area of a practice. A more informative estimate of the footprint of a given strain may be obtained from the four strains that were present in more than one practice. The geographical ranges for these strains were 29 km (strain J), 70 km (strain E), and 205 km (strain W) in the national survey and 29 km (strain LW) in the community survey, proving that FCV strains can be transmitted more widely, albeit rarely. The mechanism for these occasional widespread dissemination events remains unknown, but the most likely possibility is the movement of animals, especially those that are persistently infected (7, 33, 49), though indirect transmission may also occur. These data suggest that FCV has some limited ability for widespread geographical spread, and this is further supported by Bayesian analysis, which shows some limited correlation between geography and phylogeny for both the national and community studies. However, we cannot reject the hypothesis that geographically dispersed variants are more widespread, for two reasons. First, high overall strain diversity means that if the prevalence of a strain is sufficiently low in the population, then it is unlikely to be detected in multiple locations by our study design. Second, the majority of practices in the national study were over 300 km apart, reflecting the shape of the United Kingdom. This suggests that intermediate geographic distances may have been represented relatively poorly in this study design, restricting our ability to detect spread of viruses beyond individual veterinary practice catchment areas.
Knowing the date that each cat was sampled meant we could precisely map the temporal persistence of FCV strains. In the national survey, there was no evidence of phylogenetic clustering by date of sampling, with only 6 of 28 strains being identified in both the cross-sectional surveys. In the community survey, shedding patterns suggested that some strains persisted at a low prevalence over many months. In contrast, within the limits of our study design, others appeared to exist at a high prevalence for shorter periods but were undetectable at other times, suggesting their arrival in the community or extinction from it. Our data indicate highly dynamic strain behavior at the local community level, where high prevalence is maintained by the cocirculation of multiple strains, associated with ongoing strain extinction and introduction, with some other strains persisting for 10 months or more. The mechanisms that drive these dynamics are as yet unclear, but it is likely that population immunity plays an important role.
Bayesian analyses suggested that the overall temporal structure in our data was comparatively weak. Although there was evidence that older published sequences clustered distinctly from our community survey isolates, this was not consistent across the analyses. In addition to a lack of temporal signal, our phylogenies were star-like (radial) in shape, lacking bootstrap support for internal nodes. We concluded that a combination of high evolutionary rates coupled with strong evolutionary constraint at other nucleotide positions has led to sequence saturation at many of those sites that are able to vary and resulted in a loss of statistical support for temporal evolution, phylogenetic structure, and genetic isolation by distance. This may be an issue for other studies focusing on hypervariable regions of viral surface proteins.
Throughout this and previous studies, we have used the term “strain” to define closely related viral sequences that share a clear epidemiological link. This remains a useful classification for discussing the dynamics of the virus over short, clinically relevant time scales. However, at what point does the genetic distance between two sequences become too large to reliably indicate epidemiological linkage? Previously, we suggested that new strains take ∼5 to 10 years to evolve (7). Our sequence saturation results suggest that beyond that time, FCV phylogenies based on subgenomic capsid (and pol) gene sequences become uninformative about transmission history. Thus, the radial FCV phylogenies that we and others have observed represent distinct strains emerging from an opaque cloud of diversity, within which ancestral relationships cannot be resolved due to sequence saturation. The cloud represents all transmission events older than ∼10 years; hence, only variants of strains sampled within a decade of each other form statistically supported clades. Thus, a key conclusion of our study is that it will be necessary to sequence longer genome regions, possibly full genomes, to overcome saturation and provide greater phylogenetic resolution. Sequence-nonspecific methods may also allow us to address the fraction of FCV isolates that remain refractory to amplification (32).
The structured and detailed sampling methods used in this study improve our understanding of FCV phylodynamics and enable us to propose a new model for the global evolution and diversification of FCV strains. Strain diversification occurs in cats and colonies, probably aided by immune-mediated positive selection (7). Over many years, this has generated a large pool of distinct strains which act as a reservoir of genetic and antigenic diversity. However, sequence saturation among these means that the phylogenetic relationships among strains cannot reliably be discerned until larger genome regions are sequenced. At the community/practice level, there are two possible explanations for the high strain diversity observed. Strains could have evolved locally, with little widespread dissemination. If this is so, sequence saturation would have to be strong enough to erase nearly all evidence of a correlation between genetic and geographic distances. Alternatively, strains could be spread rapidly on a larger (perhaps national) level, with communities simply sampling from this diversity based on gaps in their local population immunity. Our data provide partial support for both of these models, with rare strains transmitted over relatively large distances and with evidence for some spatial clustering. Whichever is correct, it seems unlikely that endemic FCV evolution is driven by strong strain selection at the national level, which would favor strain turnover and limited strain diversity at any one time. Cats are exposed to a wide variety of antigenically variable FCV strains, including vaccine strains, and the dynamics of herd immunity that result are likely very complex. Clearly, a further understanding of the cross-reactivity of responses to different FCV strains is necessary and will also facilitate vaccine design. We hope that our study design, in which epidemiological principles are used to inform sample collection, may provide a framework for others seeking to understand the temporal and spatial evolution of viruses.
Supplementary Material
ACKNOWLEDGMENTS
Karen Coyne was funded by the PetPlan Charitable Trust.
We are very grateful to all the veterinary practitioners who willingly helped in this ambitious project, especially to the two practices partaking in the longitudinal community study. We are grateful to Marco Salemi for assistance with the saturation analyses and to Fiona Physick for assistance with the polymerase gene sequences.
Footnotes
Published ahead of print 1 August 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Abd-Eldaim M, Potgieter L, Kennedy M. 2005. Genetic analysis of feline caliciviruses associated with a hemorrhagic-like disease. J. Vet. Diagn. Invest. 17:420–429 [DOI] [PubMed] [Google Scholar]
- 2. Bull RA, Eden JS, Rawlinson WD, White PA. 2010. Rapid evolution of pandemic noroviruses of the GII.4 lineage. PLoS Pathog. 6:e1000831 doi:10.1371/journal.ppat.1000831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bull RA, Tanaka MM, White PA. 2007. Norovirus recombination. J. Gen. Virol. 88:3347–3359 [DOI] [PubMed] [Google Scholar]
- 4. Carter MJ, et al. 1992. The complete nucleotide sequence of a feline calicivirus. Virology 190:443–448 [DOI] [PubMed] [Google Scholar]
- 5. Coyne KP, et al. 2006. Long-term analysis of feline calicivirus prevalence and viral shedding patterns in naturally infected colonies of domestic cats. Vet. Microbiol. 118:12–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coyne KP, et al. 2007. Longitudinal molecular epidemiological analysis of feline calicivirus infection in an animal shelter: a model for investigating calicivirus transmission within high-density, high-turnover populations. J. Clin. Microbiol. 45:3239–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coyne KP, Gaskell RM, Dawson S, Porter CJ, Radford AD. 2007. Evolutionary mechanisms of persistence and diversification of a calicivirus within endemically infected natural host populations. J. Virol. 81:1961–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coyne KP, et al. 2006. Lethal outbreak of disease associated with feline calicivirus infection in cats. Vet. Rec. 158:544–550 [DOI] [PubMed] [Google Scholar]
- 9. Coyne KP, et al. 2006. Recombination of feline calicivirus within an endemically infected cat colony. J. Gen. Virol. 87:921–926 [DOI] [PubMed] [Google Scholar]
- 10. Delport W, Poon AF, Frost SD, Kosakovsky Pond SL. 2010. Datamonkey 2010: a suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics 26:2455–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geyer CJ. 1992. Practical Markov chain Monte Carlo. Stat. Sci. 7:473–511 [Google Scholar]
- 13. Glenn M, et al. 1999. Nucleotide sequence of UK and Australian isolates of feline calicivirus (FCV) and phylogenetic analysis of FCVs. Vet. Microbiol. 67:175–193 [DOI] [PubMed] [Google Scholar]
- 14. Green KY, et al. 2000. Taxonomy of the caliciviruses. J. Infect. Dis. 181(Suppl 2):S322–S330 [DOI] [PubMed] [Google Scholar]
- 15. Grenfell BT, et al. 2004. Unifying the epidemiological and evolutionary dynamics of pathogens. Science 303:327–332 [DOI] [PubMed] [Google Scholar]
- 16. Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696–704 [DOI] [PubMed] [Google Scholar]
- 17. Gupta S, Ferguson N, Anderson R. 1998. Chaos, persistence, and evolution of strain structure in antigenically diverse infectious agents. Science 280:912–915 [DOI] [PubMed] [Google Scholar]
- 18. Hurley KE, et al. 2004. An outbreak of virulent systemic feline calicivirus disease. J. Am. Vet. Med. Assoc. 224:241–249 [DOI] [PubMed] [Google Scholar]
- 19. Johnson RP. 1992. Antigenic change in feline calicivirus during persistent infection. Can. J. Vet. Res. 56:326–330 [PMC free article] [PubMed] [Google Scholar]
- 20. Knowles JO, Dawson S, Gaskell RM, Gaskell CJ, Harvey CE. 1990. Neutralisation patterns among recent British and North American feline calicivirus isolates from different clinical origins. Vet. Rec. 127:125–127 [PubMed] [Google Scholar]
- 21. Kreutz LC, Johnson RP, Seal BS. 1998. Phenotypic and genotypic variation of feline calicivirus during persistent infection of cats. Vet. Microbiol. 59:229–236 [DOI] [PubMed] [Google Scholar]
- 22. Milne I, et al. 2004. TOPALi: software for automatic identification of recombinant sequences within DNA multiple alignments. Bioinformatics 20:1806–1807 [DOI] [PubMed] [Google Scholar]
- 23. Neill JD. 1992. Nucleotide sequence of the capsid protein gene of two serotypes of San Miguel sea lion virus: identification of conserved and non-conserved amino acid sequences among calicivirus capsid proteins. Virus Res. 24:211–222 [DOI] [PubMed] [Google Scholar]
- 24. Parker J, Rambaut A, Pybus OG. 2008. Correlating viral phenotypes with phylogeny: accounting for phylogenetic uncertainty. Infect. Genet. Evol. 8:239–246 [DOI] [PubMed] [Google Scholar]
- 25. Pedersen NC, Elliott JB, Glasgow A, Poland A, Keel K. 2000. An isolated epizootic of hemorrhagic-like fever in cats caused by a novel and highly virulent strain of feline calicivirus. Vet. Microbiol. 73:281–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pedersen NC, Hawkins KF. 1995. Mechanisms for persistence of acute and chronic feline calicivirus infections in the face of vaccination. Vet. Microbiol. 47:141–156 [DOI] [PubMed] [Google Scholar]
- 27. Porter CJ, et al. 2008. Comparison of the ability of feline calicivirus (FCV) vaccines to neutralise a panel of current UK FCV isolates. J. Feline Med. Surg. 10:32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Povey C, Ingersoll J. 1975. Cross-protection among feline caliciviruses. Infect. Immun. 11:877–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Povey RC. 1974. Serological relationships among feline caliciviruses. Infect. Immun. 10:1307–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Radford AD, et al. 2009. Feline calicivirus infection. ABCD guidelines on prevention and management. J. Feline Med. Surg. 11:556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Radford AD, et al. 1997. The use of sequence analysis of a feline calicivirus (FCV) hypervariable region in the epidemiological investigation of FCV related disease and vaccine failures. Vaccine 15:1451–1458 [DOI] [PubMed] [Google Scholar]
- 32. Radford AD, et al. Application of next-generation sequencing technologies in virology. J. Gen. Virol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Radford AD, Coyne KP, Dawson S, Porter CJ, Gaskell RM. 2007. Feline calicivirus. Vet. Res. 38:319–335 [DOI] [PubMed] [Google Scholar]
- 34. Radford AD, et al. 2003. High genetic diversity of the immunodominant region of the feline calicivirus capsid gene in endemically infected cat colonies. Virus Genes 27:145–155 [DOI] [PubMed] [Google Scholar]
- 35. Radford AD, Dawson S, Wharmby C, Ryvar R, Gaskell RM. 2000. Comparison of serological and sequence-based methods for typing feline calicivirus isolates from vaccine failures. Vet. Rec. 146:117–123 [DOI] [PubMed] [Google Scholar]
- 36. Radford AD, Gaskell RM, Hart CA. 2004. Human norovirus infection and the lessons from animal caliciviruses. Curr. Opin. Infect. Dis. 17:471–478 [DOI] [PubMed] [Google Scholar]
- 37. Radford AD, et al. 2001. Endemic infection of a cat colony with a feline calicivirus closely related to an isolate used in live attenuated vaccines. Vaccine 19:4358–4362 [DOI] [PubMed] [Google Scholar]
- 38. Radford AD, et al. 2001. Molecular analysis of isolates of feline calicivirus from a population of cats in a rescue shelter. Vet. Rec. 149:477–481 [DOI] [PubMed] [Google Scholar]
- 39. Radford AD, et al. 1998. Quasispecies evolution of a hypervariable region of the feline calicivirus capsid gene in cell culture and in persistently infected cats. J. Gen. Virol. 79:1–10 [DOI] [PubMed] [Google Scholar]
- 40. Radford AD, Willoughby K, Dawson S, McCracken C, Gaskell RM. 1999. The capsid gene of feline calicivirus contains linear B-cell epitopes in both variable and conserved regions. J. Virol. 73:8496–8502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rasbach J, et al. 2000. A user's guide to MLwiN. Institute of Education, London, United Kingdom [Google Scholar]
- 42. Reynolds BS, et al. 2009. A nosocomial outbreak of feline calicivirus associated virulent systemic disease in France. J. Feline Med. Surg. 11:633–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schulz BS, et al. 2011. Two outbreaks of virulent systemic feline calicivirus infection in cats in Germany. Berl. Munch. Tierarztl. Wochenschr. 124:186–193 [PubMed] [Google Scholar]
- 44. Seal BS, Ridpath JF, Mengeling WL. 1993. Analysis of feline calicivirus capsid protein genes: identification of variable antigenic determinant regions of the protein. J. Gen. Virol. 74:2519–2524 [DOI] [PubMed] [Google Scholar]
- 45. Strimmer K, von Haeseler A. 1997. Likelihood-mapping: a simple method to visualize phylogenetic content of a sequence alignment. Proc. Natl. Acad. Sci. U. S. A. 94:6815–6819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sukhrie FH, et al. 2011. Using molecular epidemiology to trace transmission of nosocomial norovirus infection. J. Clin. Microbiol. 49:602–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 48. Verhoef L, et al. 2011. An integrated approach to identifying international foodborne norovirus outbreaks. Emerg. Infect. Dis. 17:412–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wardley RC, Povey RC. 1977. The clinical disease and patterns of excretion associated with three different strains of feline caliciviruses. Res. Vet. Sci. 23:7–14 [PubMed] [Google Scholar]
- 50. Wardley RC, Povey RC. 1977. The pathology and sites of persistence associated with three different strains of feline calicivirus. Res. Vet. Sci. 23:15–19 [PubMed] [Google Scholar]
- 51. Xia X, Xie Z, Salemi M, Chen L, Wang Y. 2003. An index of substitution saturation and its application. Mol. Phylogenet. Evol. 26:1–7 [DOI] [PubMed] [Google Scholar]
- 52. Zheng DP, et al. 2006. Norovirus classification and proposed strain nomenclature. Virology 346:312–323 [DOI] [PubMed] [Google Scholar]
- 53. Zheng DP, Widdowson MA, Glass RI, Vinje J. 2010. Molecular epidemiology of genogroup II-genotype 4 noroviruses in the United States between 1994 and 2006. J. Clin. Microbiol. 48:168–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zicola A, Saegerman C, Quatpers D, Viandier J, Thiry E. 2009. Feline herpesvirus 1 and feline calicivirus infections in a heterogeneous cat population of a rescue shelter. J. Feline Med. Surg. 11:1023–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.