Abstract
Adenovirus has been extensively exploited as a vector platform for delivering vaccines. However, preexisting antiadenovirus immunity is the major stumbling block for application of adenovirus-vectored vaccines. In this study, we found that freshly isolated peripheral blood mononuclear cells (PBMCs), mostly CD14+ cells, from adenovirus serotype 5 (Ad5)-seropositive primates (humans and rhesus macaques) can be efficiently infected with Ad5 in vitro. On the basis of this observation, a novel strategy based on adenoviral vector-infected PBMC (AVIP) immunization was explored to circumvent antivector immunity. Autologous infusion of Ad5-SIVgag-infected PBMCs elicited a strong Gag-specific cellular immune response but induced weaker Ad5-neutralizing antibody (NAb) in Ad5-seronegative macaques than in macaques intramuscularly injected with Ad5-SIVgag. Moreover, Ad5-seropositive macaques receiving multiple AVIP immunizations with Ad5-SIVenv, Ad5-SIVgag, and Ad5-SIVpol vaccines elicited escalated Env-, Gag-, and Pol-specific immune responses after each immunization that were significantly greater than those in macaques intramuscularly injected with these Ad5-SIV vaccines. After challenged intravenously with a highly pathogenic SIVmac239 virus, macaques receiving AVIP immunization demonstrated a significant reduction in viral load at both the peak time and set-point period compared with macaques without Ad5-SIV vaccines. Our study warranted further research and development of the AVIP immunization as a platform for repeated applications of adenovirus-vectored vaccines.
INTRODUCTION
Adenovirus-based vectors, especially human adenovirus serotype 5 (Ad5), have been extensively explored as vaccine and gene therapy vehicles for human immunodeficiency virus (HIV), hepatitis, influenza, and other infectious diseases and cancers and have been shown to elicit potent immune responses in animal models and humans (10, 18, 49, 50, 53, 55). However, a major obstacle for the practical application of Ad5 as a vaccine vector is that the preexisting antiadenoviral immunity, especially Ad5-neutralizing antibodies (NAb), can significantly reduce the immunogenicity of Ad5-vectored vaccines, as demonstrated in preclinical animal models and human clinical trials (37, 38, 42, 52).
It is known that Merck's Ad5-vectored HIV vaccine was terminated due to lack of protective efficacy in both Ad5-seronegative and Ad5-seropositive populations, and there was a modest susceptibility to HIV infection in the noncircumcised male vaccinees with high levels of preexisting Ad5 NAb. The reasons for this failure are still being extensively investigated, and one notable observation is that the Ad5-vectored vaccines elicited a relatively weak immune response in Ad5-seropositive recipients, while the preexisting anti-Ad5 immunity may play some adverse roles in noncircumcised males (4, 12, 24, 43, 44, 51, 60). In fact, all Ad5-seronegative recipients also became Ad5-seropostive after receiving one administration of Ad5-vectored vaccines. Ad5-vectored vaccines could therefore be attenuated for their abilities in stimulating vaccine-specific immune responses but also could augment immune responses specific to Ad5 vector, which may contribute to the poor outcome of Merck's Ad5-HIV vaccine clinical trials. Although it is still a puzzle regarding what constitutes an effective immune responses that can confer effective protection for an HIV vaccine (21, 29, 34, 39), stimulation of robust and balanced cellular and humoral responses targeted to HIV antigens has been considered to be the necessary pieces if an HIV vaccine will be effective in humans (5, 6, 20, 25, 46). However, it has been reported that approximately 40% of the adult population in America (42), 77% of the total population in Guangzhou, southern China (54), and more than 93% of the children in Sub-Saharan Africa (57) are Ad5 seropositive. Therefore, improving the immunogenicity of Ad-vectored vaccines in the Ad5-seropositive population and minimizing as well as circumventing host immune responses to Ad5 will be of great significance.
The development of novel Ad vectors based on rare serotypes from humans or other species, such as nonhuman primates, canines, and bovines, has been explored and received extensive interest to circumvent preexisting Ad5 NAb (16, 27, 30). However, unlike Ad5 vectors, which have accumulated an extensive safety record from many clinical trials, the use of any new adenoviruses may be associated with unknown safety risks, such as oncogenicity, in the future. Furthermore, all new Ad vectors can be used only once regardless of their serotypes, as the recipients would quickly generate specific NAb responses against these adenoviruses, thus attenuating their subsequent usages. Consequently, it is of great interest to explore a new strategy for the repeated application of Ad5-based vectors.
In the present study, we aimed to explore a new strategy that could potentially enable repeated usage of a Ad5-based vector for antigen delivery. We first investigated whether freshly isolated human and macaque peripheral blood mononuclear cells (PBMCs), especially CD14+ cells, from Ad5-seropositive people and rhesus macaques could be infected in vitro by Ad5 vectors. We then developed a novel immunization strategy, termed adenovirus vector-infected PBMCs (AVIP), to enable repeated application of Ad5-vectored vaccines for consistently eliciting immune responses toward target antigens. Rhesus macaques were used in a proof-of-concept study to demonstrate the feasibility of AVIP immunization for vaccine application. This study provided new insights to revitalize the development of Ad5-vectored vaccines.
MATERIALS AND METHODS
Human samples and animals.
The research conducted with human participants was approved by the Ethics Committee of the Guangzhou Institute of Biomedicine and Health (GIBH), Chinese Academy of Sciences, and human blood samples were taken from 34 healthy donors with written informed consents.
The use of the Chinese rhesus macaques in this study was carried out according to the principles of the NIH Guide for the Care and Use of Laboratory Animals and the policies and procedures of Guangzhou Institute of Biomedicine and Health (GIBH), Chinese Academy of Sciences. The protocol was approved by our Institutional Animal Care and Use Committee (Animal Welfare Assurance: A5748-01; IACUC permit number 2009039). A total of 27 Chinese rhesus macaques used in this study were 3 to 5 kg and 3 to 6 years old and were housed at the Animal Experimental Center of GIBH. All macaques were found to be free of simian immunodeficiency virus (SIV), simian T lymphotropic virus type 1 (STLV-1), and simian retrovirus (SRV) prior to assignment.
Recombinant adenovirus vectors.
Recombinant adenoviruses were generated using homologous recombination according to our previously published methods (8, 53), and an E1/E3-deleted Ad5 vector was used to generate Ad5-enhanced green fluorescent protein (EGFP), Ad5-secreted alkaline phosphatase (SEAP), and Ad5-vectored SIV vaccines, including Ad5-SIVenv, Ad5-SIVgag, and Ad5-SIVpol. All genes encoding SIVmac239 proteins were codon optimized for high-level expression in mammalian cells. Construction, amplification, purification, identification, and the 50% tissue culture infective dose (TCID50) assay for these recombinant adenoviruses were all performed in our lab.
PBMC isolation, adenovirus infection, and flow cytometric analysis.
Fresh blood samples from healthy human donors or the experimental macaques were collected into tubes containing anticoagulant (EDTA), and PBMCs were isolated with OptiPrep lymphocyte separation solution (Axis Shield Poc As, Oslo, Norway) by following the manufacturer's directions. A total of 1 × 106 cells were incubated with the appropriate dose of the Ad5 vector for 1 h at 1,000 × g centrifugation at room temperature. The infected cells were then cultured in complete 1640 medium for 24 h at 37°C in a 5% CO2 incubator. The cells were then incubated with fluorescent-labeled monoclonal antibodies for 20 min and detected with a BD FACSCalibur flow cytometer (BD Biosciences, United States). Data were acquired using Cell Quest software. All monoclonal antibodies (CD3-APC, CD14-PE, CD19-PE-cy5, CD56-PE) were purchased from BD Pharmingen, and 7-AAD stain was purchased from Jingmei Biological Engineering Limited, Shenzhen, China.
Quantitative PCR for the determination of Ad5 genome copies and SIVenv mRNA copies.
CD3+, CD19+, or CD14+ cells were magnetically separated from PBMCs (MACS; Miltenyi Biotec, Germany) by following the manufacturer's directions, and 1 × 106 PBMCs or CD3+, CD19+, or CD14+ cells were infected with an Ad5 vector, as described above, for 24 h. To determine the number of Ad5 genome copies, cells were washed and lysed to release the Ad5 genome. For the determination of SIVenv mRNA copies, total RNA was extracted from the cells and reverse transcribed into cDNA. Cell lysate and cDNA then served as templates for the quantitative PCR.
SYBR green-based quantitative PCR was carried out with OpticonTM2 (CFD-3220; MJ Research) with SYBR premix Ex Taq (TaKaRa, Japan). Cycle threshold (CT) values and melting curves were analyzed with Opticon Monitor 3 software (MJ Research). The pAd5 (E1−E3−) plasmid was serially diluted 10-fold and used to generate a standard curve to determine the number of adenovirus genome copies. The relative numbers of SIVenv mRNA copies were determined by comparison with the number of beta actin mRNA copies. The final data are reported as the mean values from triplicate experiments.
SEAP-based Ad5-neutralizing antibody assay.
Specific Ad5 NAb titers were measured as previously reported (2), with our minor modifications (54). Briefly, 293 cells were seeded at 3 × 104 cells per well in 96-well plates 1 to 2 days before the assay so that the cells were able to reach 95 to 100% confluence. A total of 4 × 106 viral particles (vp) of Ad5-SEAP virus was incubated with Ad5-SEAP virus alone or with serial dilutions of serum for 1 h at 37°C. Next, 200 μl of each sample was applied to 293 cells and incubated at 37°C for 24 h. A total of 50 μl of cell-free supernatant was then removed from each sample to detect SEAP activity using a Phospha-Light kit (Applied Biosystems, Foster City, CA). Relative light units (RLU) were monitored in a luminometer (MLX Microtiter, Dynex Technologies, Inc.).
Immunization and challenge.
A total of 27 Chinese rhesus macaques were used in two studies: studyI and studyII. In studyI, 12 macaques confirmed to be Ad5 seronegative were enrolled into three groups: (i) AVIP immunization with intravenous infusion of autologous PBMCs (107) incubated in vitro with 1011 vp of Ad5-SIVgag (n = 4 macaques); (ii) intramuscular injection of Ad5-SIVgag (1011 vp) (n = 4 macaques); (iii) negative control (n = 4 macaques). In studyII, 15 macaques previously immunized with Ad5-empty vector were enrolled into three groups: (i) AVIP immunization with intravenous infusion of autologous PBMCs (107) incubated in vitro with Ad5-SIV vaccines (1011vp) carrying env, gag, and pol (n = 6 macaques); (ii) intramuscular injection of Ad5-SIV vaccines (1011vp) carrying env, gag, and pol (n = 5 macaques); (iii) intramuscular injection of Ad5-empty as the control (n = 4 macaques).
For the challenge experiment, macaques in studyII were injected intravenously with 1,000 TCID50 of SIVmac239 virus at 6 weeks after the last immunization. After immunization and challenge, samples were collected at different time points to evaluate the SIV-specific immune responses and viral load.
IFN-γ ELISPOT assays.
Gamma interferon (IFN-γ) enzyme-linked immunosorbent spot (ELISPOT) assays for rhesus macaques were conducted by following our previously described protocol (8, 53). Briefly, 96-well plates (Millipore; Immobilon-P membrane) were coated with anti-IFN-γ monoclonal antibody (U-Cytech, Utrecht, Netherlands) overnight at 4°C and then washed with phosphate-buffered saline (PBS) and blocked with R10 medium (RPMI; 0.05 mM 2-mercaptoethanol, 1 mM sodium pyruvate, 2 mM l-glutamate, 10 mM HEPES, 10% fetal bovine serum) for 2 h at 37°C. Freshly isolated PBMCs were added at a density of 4 × 105 cells/well and stimulated in the absence or presence of the SIV antigen peptide pool (2 μg/ml/peptide) or 10 μg/ml concanavalin A (Sigma-Aldrich, St. Louis, MO). SIV peptide pools consisted of 15-amino-acid peptides, shifted by 10 amino acids, which were obtained through the NIH AIDS Research and Reference Reagent Program. Cells were then incubated for 24 h at 37°C in 5% CO2. Plates were washed with PBS-Tween 20 (PBST), and anti-IFN-γ polyclonal biotinylated detection antibody (U-Cytech) was added. Plates were incubated overnight at 4°C, and color was developed by incubating in NBT/BCIP (Pierce, Rockford, IL) for 10 min. Spots were counted under an ELISPOT reader (Bioreader 4000; BIOSYS, Germany), and data are reported as the number of spot-forming cells (SFC) per million PBMCs.
ICS.
Intracellular cytokine staining (ICS) was processed according to previously reported methods (8, 53). Briefly, anti-human CD28 (clone L293; BD Biosciences) and anti-human CD49d (clone L25; BD Biosciences) monoclonal antibodies were added to a final concentration of 1 μg/ml to a tube containing 2 × 106 PBMCs. The SIV peptide pool (2 μg/ml per peptide) or dimethyl sulfoxide (DMSO) was added for antigen-specific or mock stimulation, respectively. The suspensions were incubated at 37°C for 1 h, after which brefeldin A (BD Pharmingen) was added. The cells were then incubated for 16 h at 37°C and stained for 30 min at room temperature with CD3-PerCP, CD8-APC, and CD4-PE (BD Pharmingen). The cells were permeabilized in FACS Perm buffer for 10 min and stained for 30 min with IFN-γ–fluorescein isothiocyanate (FITC) (BD Pharmingen). Samples were analyzed with a FACS Calibur.
ELISA for SIV binding antibody.
Serum-binding antibodies to SIVmac239 were determined by enzyme-linked immunosorbent assay (19). The purified SIVmac239 virus was grown in CEM × 174 cells, which is a fusion cell line of human B cell line 721.174 and the human T cell line, and lysed with 1% (vol/vol) Triton X-100 followed by sonication. Protein concentrations were determined with a bicinchoninic acid (BCA) kit (Pierce) and diluted to 1 μg/ml in 0.1 M sodium carbonate buffer (pH 9.6) just prior to use. A total of 100 μl of the lysate was added to each well of a 96-well plate and incubated at 4°C overnight. Plates were then washed with PBST buffer and blocked with 200 μl of 5% (wt/vol) milk in PBST at 37°C. Next, 100 μl of the diluted samples was added to the plate and incubated for 2 h at 37°C. The plate was then washed with PBST buffer, and 100 μl of goat anti-human IgG labeled with horseradish peroxidase (HRP) diluted 1:5,000 was added for another hour. The color was developed by adding 100 μl of tetramethyl benzidine (TMB) (Chemicon, Temecula, CA) for 5 min and stopped with 100 μl of 0.5 M H2SO4. Absorbance was detected at 450 nm using a Synergy HT multimode plate reader (BioTek Instruments, Inc., Vermont).The binding titer was defined as the reciprocal of the serum dilution at which absorbance of the test serum was twice that of the negative-control serum diluted 1:50.
Viral load determination by quantitative RT-PCR.
Plasma SIV RNA was monitored by quantitative PCR as described previously (31, 53). Briefly, viral RNA was isolated from plasma using a QIAamp viral RNA minikit (Qiagen, Valencia, CA) and amplified using a QuantiTect SYBR green reverse transcription (RT)-PCR kit (Qiagen). The reactions were performed in an Opticon TM2 RT-PCR detection system (CFD-3220; MJ Research). Primers were designed to exactly match the SIVmac239 gag sequence. The viral RNA copy number was determined by extrapolation of threshold fluorescence values onto an internal standard curve prepared from serial dilutions of an in vitro-transcribed fragment of the SIVmac239 gag gene. The detection limits of assays were 100 copies per 1 ml of plasma.
Data analysis.
Flow cytometric data were analyzed using FlowJo version 7.6 software (Tree Star, Inc., Ashland, OR). Statistical analyses and graphical presentations were computed with GraphPrism 5.01 (GraphPad Software Inc., La Jolla, CA), and data of infection efficiency and immune responses between different groups were first analyzed by one-way analysis of variance (ANOVA) or two-way ANOVA, followed by Tukey's or Bonferroni post hoc test. For the small-sample data, it was analyzed by a Mann-Whitney U test. For these tests, the two-tailed P value was calculated, and P values of <0.05 were considered significant.
RESULTS
CD14+ cells in human PBMCs are the major cell type infected by adenovirus.
Previous studies have demonstrated that freshly isolated human PBMCs were poorly susceptible to Ad5 infection (11, 26, 33). However, a recent study showed that the infection efficiency could be enhanced under centrifugation (36). Using a recombinant replication-defective Ad5 carrying an EGFP reporter gene (Ad5-EGFP), we further optimized the condition for adenovirus infection of PBMCs in vitro. Infection efficiency was examined at various incubation conditions, including adenovirus dosage, temperature, and time. An optimal infection efficiency was achieved when isolated PBMCs were incubated with Ad5-EGFP at 250 to 1,250 vp/cell, 1,000 × g centrifugation, for 1 h at room temperature. Under this condition, the efficiency of Ad5-mediated gene transduction in PBMCs was remarkably enhanced without affecting cell viability.
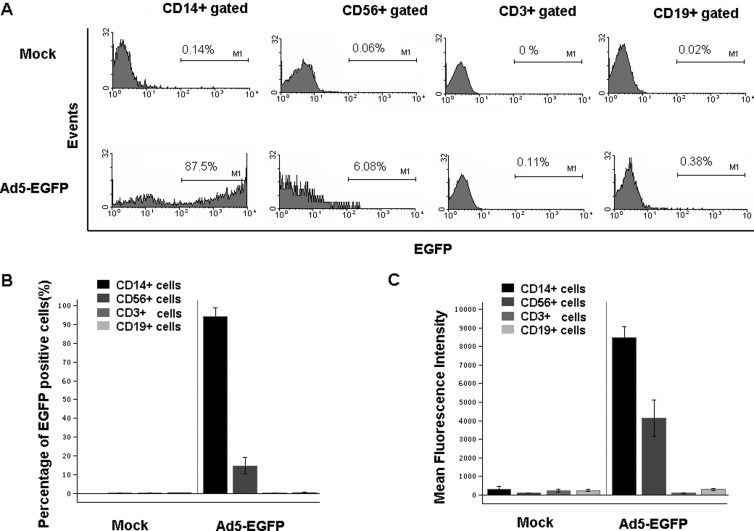
Further analysis was carried out to determine which cell populations could be infected by Ad5-EGFP based on cell surface markers (Fig. 1A). About 87% of CD14+ cells (mainly macrophages, monocytes, and dendritic cells) were EGFP positive, and about 14% of CD56+ cells (NK cells) were EGFP positive. However, neither CD3+ (T lymphocytes, 0.08%) nor CD19+ (B lymphocytes, 0.28%) had detectable EGFP expression (Fig. 1B). This finding was consistent with previous reports (11, 26, 33). Moreover, the EGFP expression level in CD14+ cells, as represented by mean fluorescence intensity (MFI) value, was significantly higher than that seen in other cells (Fig. 1C). The Ad5 genome copy number was also much higher in CD14+ cells than in other cell populations, as determined by quantitative PCR (Fig. 2E). These data demonstrated that CD14+ cells in PBMCs are the major cell type that can be effectively infected by Ad5 and subsequently express transgenes under our conditions.
Fig 1.
CD14+ cells in human PBMCs were the major cell type to be infected with Ad5. (A) Representative graphics of fluorescence-activated cell sorting (FACS) analysis. Human PBMCs were infected with Ad5-EGFP or Ad5-empty as a mock infection, and the proportions of EGFP-positive cells were determined in different cell populations by FACS analysis. Herein, EGFP-positive cells represent those cells that have been infected with Ad5. (B) Percentage of EGFP-positive cells in CD14+ (n = 24), CD56+ (n = 4), CD3+ (n = 24), and CD19+ (n = 24) cells. (C) Expression level of EGFP protein (represented with MFI value) in CD14+ (n = 24), CD56+ (n = 4), CD3+ (n = 24), and CD19+ (n = 24) cells. The bars represent the standard errors.
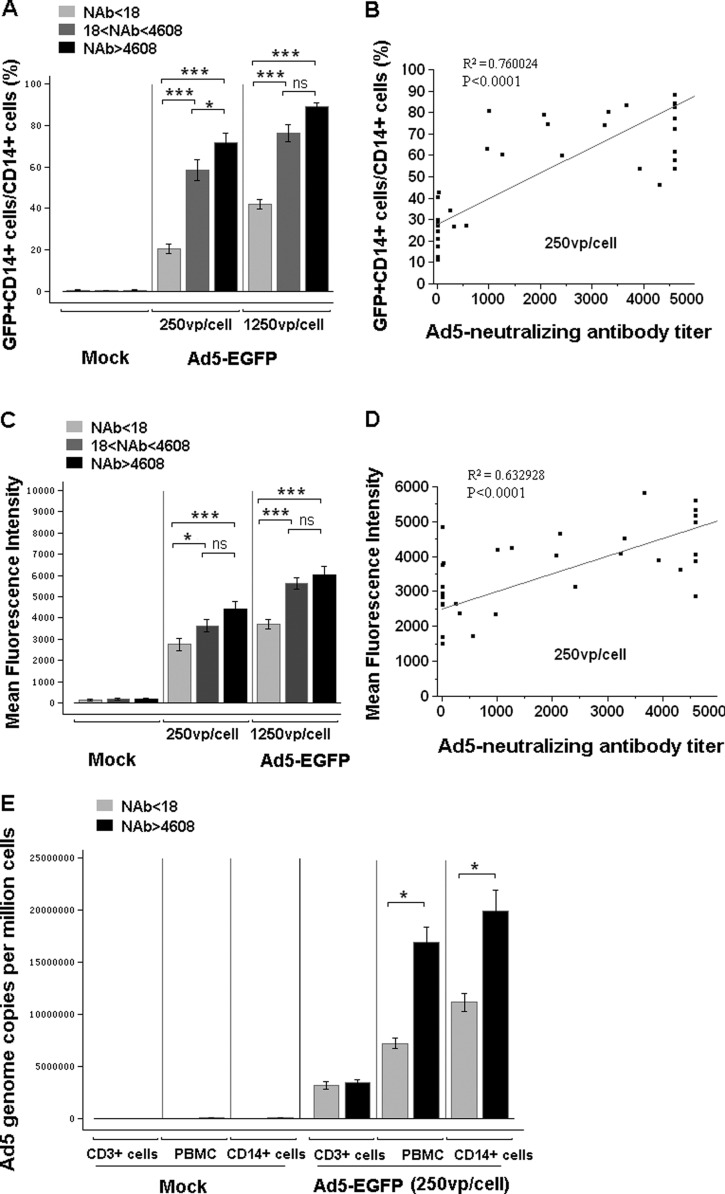
Fig 2.
CD14+ cells in human PBMCs from Ad5-seropositive people were more susceptible to Ad5 infection than those from Ad5-seronegative people. Human PBMCs were infected with Ad5-EGFP at 0 vp/cell, 250 vp/cell, and 1,250 vp/cell. The subjects were divided into three groups based on the level of preexisting NAb: Ad5 NAb naïve (NAb < 18; n = 11), Ad5 NAb median (18 < NAb titer < 4,608; n = 15), and Ad5 NAb high (NAb titer > 4,608; n = 8). Expression of EGFP in CD14+ cells was analyzed by FACS at 24 h postinfection. (A) Percentage of EGFP-positive cells in CD14+ cell population. (C) EGFP expression level of the CD14+ cell population. These data were analyzed statistically by one-way ANOVA followed by Tukey's multiple comparison test. Correlations of the percentage (B) or EGFP expression level (D) of CD14+ EGFP-positive cells in PBMCs with adenovirus-neutralizing antibody titer were determined using linear fit analysis. (E) Ad5 genome copy number in PBMCs from Ad5-seronegative (n = 4) or Ad5-seropositive (n = 4) people was determined by quantitative PCR after infection at 250 vp/cell. These data were analyzed statistically by a nonparametric Mann-Whitney U test. The bars represent the standard errors. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, no significance.
CD14+ cells in human PBMCs from Ad5-seropositive individuals were more susceptible to Ad5 infection than those from Ad5-seronegative individuals.
We next compared the efficiencies of Ad5 infections in CD14+ cells from Ad5-seropositive and Ad5-seronegative individuals. Interestingly, as indicated by the percentage and fluorescence intensity of EGFP-expressing cells (Fig. 2), the infection efficiencies of CD14+ cells from people with both median (18 < NAb titer < 4,608; n = 15) and high (NAb titer > 4,608; n = 8) levels of preexisting NAbs were significantly higher than those from Ad5-seronegative people (NAb < 18; n = 11). The significant difference in infection efficiency was found to be correlated to the titer of Ad5 NAb, with the highest EGFP expression in the highest NAb group (Fig. 2A to D). The infection efficiency between different populations could also be visualized under fluorescence microscopy (data not shown).
To dissect if the higher level of EGFP is due to increased adenoviral infection or an enhancement in gene expression, we employed quantitative PCR to measure the Ad5 genome copy number in cells from Ad5-seropositive and Ad5-seronegative individuals. There was significantly more Ad5 genome copies in both total PBMCs and CD14+ cells from people with high Ad5 NAb levels than in cells from Ad5-seronegative people (Fig. 2E). However, the number of Ad5 genome copies in CD3+ cells from all samples was low and comparable regardless of Ad5-seropositive or Ad5-seronegative status. This result further confirmed that CD14+ cells (including mainly macrophages, monocytes, and dendritic cells), but not lymphocytes, are the major cell populations that can be infected with Ad5 and confer a higher level of transgene expression in Ad5-seropositive individuals.
CD14+ cells from Ad5-seropositive rhesus macaques are more susceptible to Ad5 infection than cells from Ad5-seronegative macaques.
In line with the data observed in human PBMCs, CD14+ cells from Ad5-seropositive rhesus macaques were also more susceptible to Ad5-mediated EGFP expression than CD14+ cells from Ad5-seronegative macaques. As indicated by the percentage and fluorescence intensity of EGFP-expressing cells, Ad5-mediated EGFP expression levels in CD14+ cells from rhesus macaques with both median (18 < NAb titer < 4,608) and high (NAb titer > 4,608) levels of preexisting Ad5 NAb were significantly higher than those from Ad5 naïve rhesus macaques (NAb < 18). The significant difference in infection efficiency was found to be correlated to the Ad5 NAb titer, with the highest EGFP expression level in the group with the highest Ad5 NAb level (Fig. 3A and B).
Fig 3.
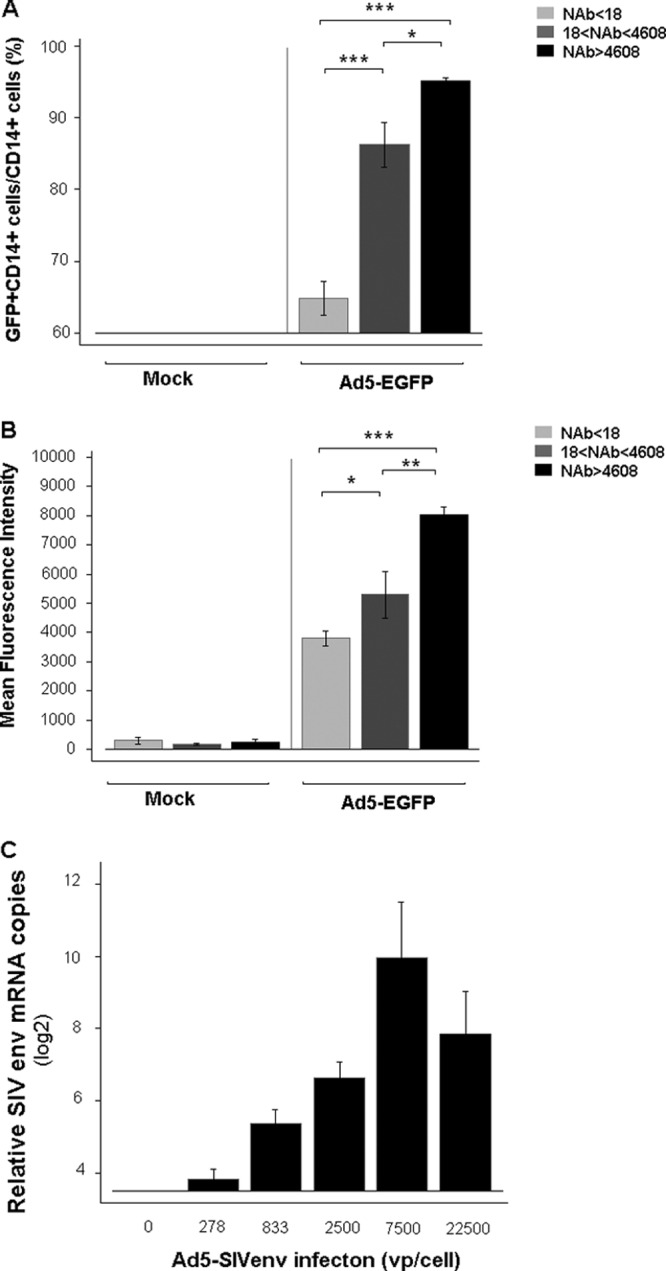
Ad5 could efficiently infect PBMCs from rhesus macaques with preexisting anti-Ad5 immunity. The macaques were divided into three groups based on the level of preexisting NAb: Ad5 NAb naïve (Nab < 18; n = 9), Ad5 NAb median (18 < NAb titer < 4,608; n = 7), and Ad5 NAb high (NAb titer > 4,608; n = 8). (A) The infected proportion of EGFP+ cells; (B) EGFP expression level of the CD14+ cell population; (C) the expression levels of SIVenv mRNA in macaque PBMCs were determined by quantitative PCR, as described in Materials and Methods. These data were analyzed statistically by one-way ANOVA followed by Tukey's multiple comparison test. The bars represent the standard errors. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We next assessed the ability of an Ad5-vectored vaccine carrying the gene encoding the SIVmac239 envelop to infect macaque PBMCs. Ad5-SIVenv could efficiently infect PBMCs from macaques with high-level preexisting antiadenoviral immunity. The expression level of the SIV envelop gene in macaque PBMCs showed a dose dependency on the Ad5-SIVenv virus (Fig. 3C). Infection with a very high dose caused cell death, as confirmed by 7-AAD staining, which decreased the expression level of transgene products. Therefore, for Ad5-mediated transgene expression in rhesus macaque PBMCs, infection conditions of 7,500 vp/cell and 1,000 × g centrifugation for 1 h at room temperature were used in subsequent experiments.
Comparable target antigen-specific but weaker Ad5 vector-specific immune responses were elicited with AVIP immunization in Ad5-seronegative macaques.
We subsequently assessed whether AVIP immunization, i.e., autologous infusion of Ad5-SIVgag-infected PBMCs in Ad5-seronegative rhesus macaques, would be as immunogenic as traditionally intramuscular injection of Ad5-SIVgag. After the first immunization, comparable SIVgag-specific IFN-γ ELISPOT responses were elicited in Ad5-seronegative macaques that received either intramuscular injection of Ad5-SIVgag or Ad5-SIVgag through delivery with AVIP (Fig. 4A). Interestingly, after the second immunization, the magnitude of SIVgag-specific IFN-γ ELISPOT responses in macaques that received intramuscular injection of Ad5-SIVgag failed to increase. However, the magnitude of SIVgag-specific IFN-γ ELISPOT responses in macaques that received Ad5-SIVgag through AVIP immunization continued to increase (Fig. 4A).
Fig 4.
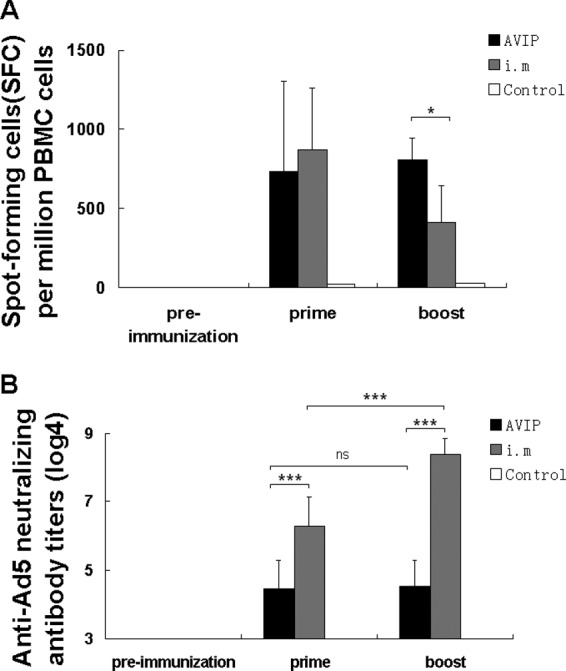
Delivery of Ad5-SIVgag through AVIP induced better Gag-specific immune response but less Ad5 NAb than intramuscular injection of Ad5-SIVag in Ad5-seronegative macaques. Twelve macaques, which had been confirmed to be Ad5 seronegative, were divided into three groups (n = 4 per group) as described in Materials and Methods (studyI), and specific immune responses against SIV antigen or Ad5 vector were assessed. (A) SIV-specific cellular immune responses were assessed by IFN-γ ELISPOT assays. (B) NAb against Ad5 vector was detected by Ad5-SEAP-based neutralizing assay, and results represent Ad5 NAb titers. These data were analyzed statistically by two-way ANOVA followed by a Bonferroni post hoc test. The bars represent the standard errors. ***, P < 0.001; ns, no significance.
It is of interest to note that there was a significantly lower titer of Ad5 NAb in macaques that received Ad5-SIVgag through AVIP immunization after the first immunization than in macaques that received intramuscular injection of Ad5-SIVgag (Fig. 4B). Intriguingly, after the second immunization, although the Ad5 NAb was boosted significantly in macaques that received intramuscular injection of Ad5-SIVgag vaccine, the Ad5 NAb was not significantly increased in macaques that received Ad5-SIVgag through the delivery of AVIP (Fig. 4B). It is also worthwhile to note that after the second immunization, SIVgag-specific IFN-γ ELISPOT responses in macaques that received Ad5-SIVgag vaccine through autologous infusion of AVIP was higher than in macaques that received intramuscular injection of Ad5-SIVgag (P = 0.0180), although there was a large variation among the macaques.
Delivery of Ad5-SIVenv through AVIP immunization induced robust antigen-specific cellular and humoral immune responses in Ad5-seropositive macaques.
To mimic the preexisting anti-Ad5 immunity in the human population, cohorts of 15 Mamu-A*01-negative Chinese-origin rhesus macaques which had previously been immunized with Ad5 vector were used in this study. The presence of both humoral and cellular responses against Ad5 was measured. There were moderate to high levels of adenovirus-neutralizing antibodies in all macaques (Fig. 5A), and robust anti-Ad5 cellular responses had also been confirmed by IFN-γ ELISPOT assays (Fig. 5B).
Fig 5.
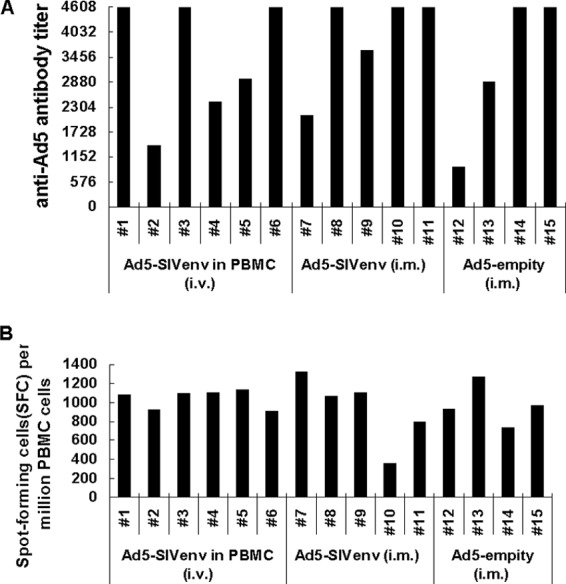
Ad5 NAb and Ad5-specific cellular immune responses in Ad5-seropostitive macaques. Fifteen macaques, which had previously been immunized with Ad5-empty vector, were enrolled in studyII. These macaques were divided into three groups in subsequent studies. Preexisting humoral and cellular responses against adenoviral vector were assessed in those macaques. (A) Humoral responses against Ad5. Ad5 NAb was detected by Ad5-SEAP-based neutralizing assay, and results represent Ad5 NAb titers. (B) Cellular responses against Ad5. Macaque PBMCs were stimulated with the lysate of Ad5-empty virus particles, and ELISPOT assays were then performed. The results are represented as spot-forming cells (SFC)/106 PBMCs.
These 15 macaques were first immunized with Ad5-SIVenv either through the intravenous infusion of AVIP or through intramuscular injection. SIV Env-specific IFN-γ ELISPOT responses were measured at 2 weeks and 4 weeks after immunization. Macaques that received the Ad5-SIVenv vaccine through the delivery of AVIP had more robust responses and greater increases than those that received Ad5-SIVenv intramuscularly (Fig. 6A). The SIV Env-specific cellular response was further assessed by intracellular cytokine staining at 4 weeks after immunization. Specific CD4+ T and CD8+ T cells against Env peptide pool stimulation were detected. Compared with the intramuscularly injected Ad5-SIVenv, Ad5-SIVenv delivered through AVIP produced significantly more robust CD8+ T cell responses (Fig. 6B).
Fig 6.
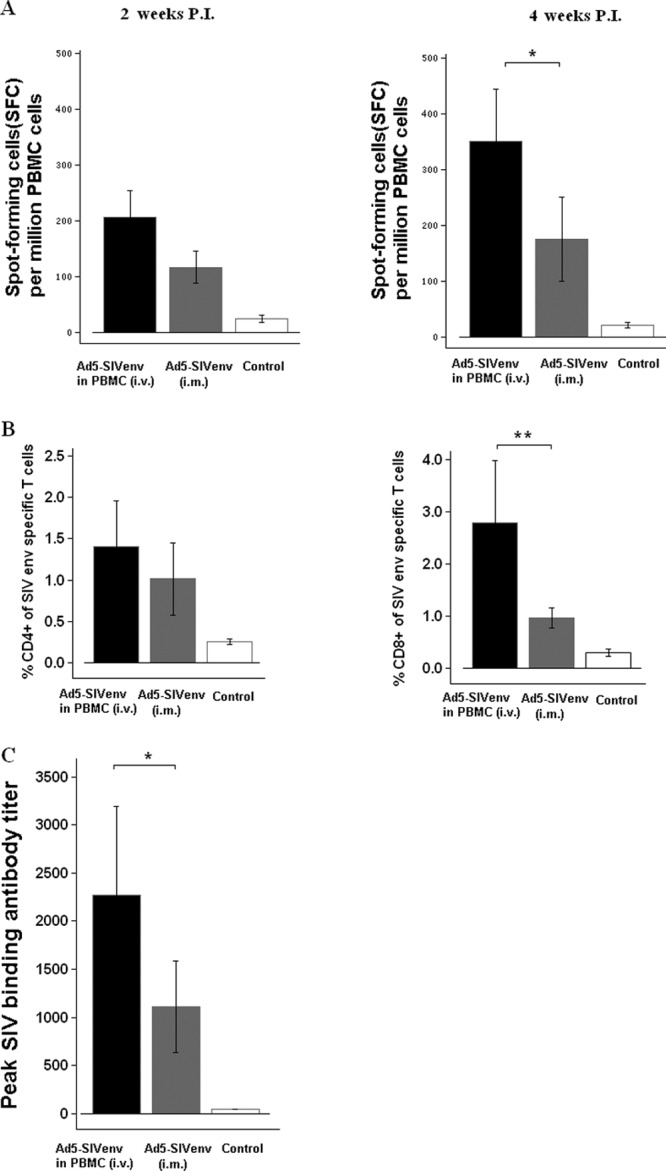
Delivery of Ad5-SIVenv through AVIP induced stronger Env-specific immune responses in Ad5-seropositive macaques. Fifteen Ad5-seropositive macaques from Fig. 5 were put into three groups: (i) Ad5-SIVenv delivered through AVIP; (ii) Ad5-SIVenv injected intramuscularly; and (iii) Ad5-empty injected intramuscularly as the control. SIV Env-specific immune responses were assessed at 2 weeks and 4 weeks after immunization. (A) Specific IFN-γ ELISPOT responses against SIV Env stimulation; (B) specific CD4+ and CD8+ T cell responses against Env peptide stimulation by intracellular cytokine staining; (C) SIV binding antibody responses by ELISA. Data were analyzed by a nonparametric Mann-Whitney U test. The bars represent the standard error. *, P < 0.05; **, P < 0.01.
We also measured SIV-specific antibody responses using ELISA. The SIV binding antibody titer in macaques that received Ad5-SIVenv-infected PBMCs were significantly higher than those in macaques that received Ad5-SIVenv intramuscularly (Fig. 6C). The control group with macaques that received intramuscular injection of Ad5-empty showed no Env-specific immune responses throughout the experiment.
Repeated delivery of Ad5-vectored SIV vaccines through AVIP immunization elicited robust antigen-specific immune responses in Ad5-seropositive macaques.
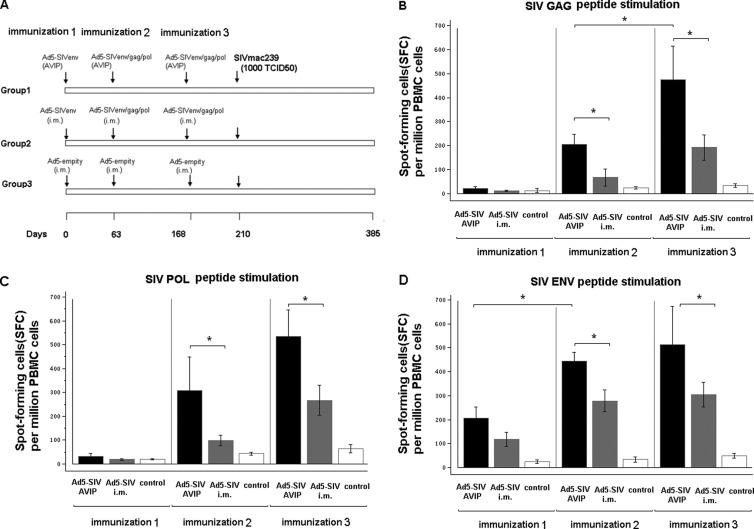
We next assessed if repeated delivery of Ad5-vectored vaccines through AVIP immunization could induce specific immune responses for subsequent priming and boosting of multiple antigens. At 9 weeks and 15 weeks after the first immunization with Ad5-SIVenv, the same three groups of Ad5-seropositive macaques used in the previous experiment (Fig. 6) received either an autologous infusion of PBMCs pulsed in vitro with a mixture of Ad5-SIVenv, Ad5-SIVgag, and Ad5-SIVpol; received an intramuscular injection of the same mixture of Ad5 SIV vaccines; or received Ad5-empty vector as a control (Fig. 7A).
Fig 7.
Repeated delivery of Ad5-vectored SIV vaccines through AVIP elicited robust antigen-specific immune responses in Ad5-seropositive macaques. (A) Immunization and challenge procedure. After the first immunization (Fig. 6), the same three groups of Ad5-seropositive macaques received the second and third immunizations: group 1 with Ad5-SIVenv, Ad5-SIVgag, and Ad5-SIVpol vaccines delivered through AVIP; group 2 with Ad5-SIVenv, Ad5-SIVgag, and Ad5-SIVpol vaccines injected intramuscularly; and group 3 with Ad5-empty injected intramuscularly as the control. Vaccine-elicited specific cellular immune responses were measured by IFN-γ ELISPOT assays following stimulation with SIV Gag, Pol, and Env peptide pools. (B) Gag-specific cellular immune responses; (C) Pol-specific cellular immune responses; (D) Env-specific cellular immune responses. Data were analyzed by a nonparametric Mann-Whitney U test. The bars represent the standard error. *, P < 0.05; **, P < 0.01.
Consistent with our earlier observations, robust cell-mediated immune responses to SIV Gag, Pol, and Env antigens were observed in macaques after receiving each immunization with Ad5-SIV vaccines through the delivery of AVIP. A consistent booster effect in cellular immune response to SIV antigen Env and Gag was observed in macaques after receiving the second delivery of Ad5-SIVenv through the AVIP approach (Fig. 7B and D). Importantly, Ad5-SIVenv delivered earlier via the AVIP approach showed no negative impact on the same antigen, Env, or different antigens, Gag and Pol, delivered through the AVIP approach (Fig. 7B, C, D). In contrast, macaques that received intramuscular injection of Ad5-SIV vaccines showed significantly weaker cellular immune responses to Gag, Pol, and Env than macaques that received AVIP immunization after each immunizations (Fig. 7B, C, D). These results demonstrated that the AVIP approach can circumvent preexisting anti-Ad5 immunity to enable repeated deliver of multiple Ad5-vectored vaccines for stimulating robust antigen-specific immune responses in rhesus macaques.
Delivery of Ad5-vectored SIV vaccines through AVIP immunization resulted in partial control in Ad5-seropositive macaques challenged by intravenous injection of SIVmac239.
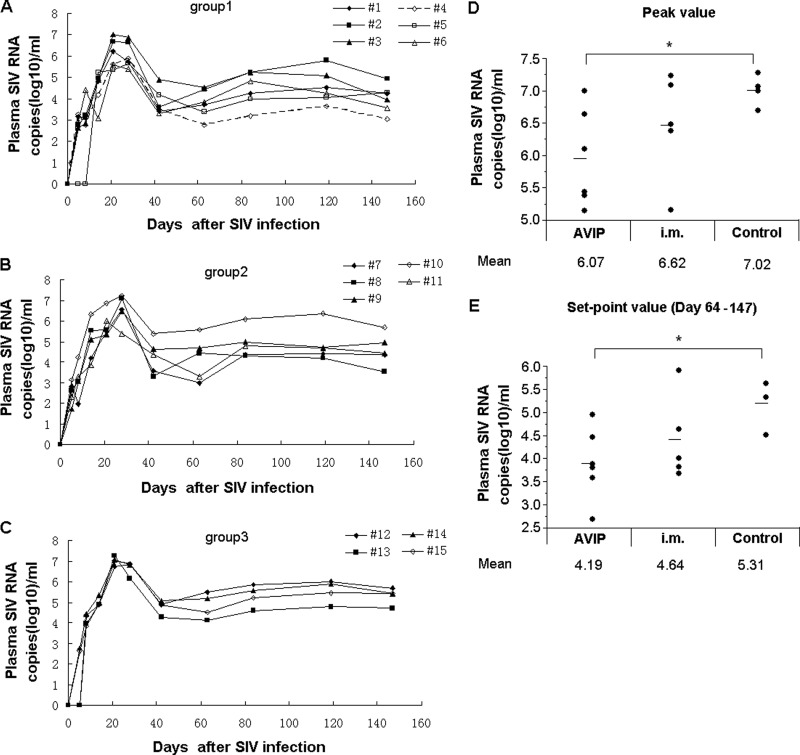
We finally assessed if Ad5-vectored SIV vaccines delivered through either AVIP immunization or intramuscular injection could result in any protective effects in Ad5-seropositive macaques. The same three groups of macaques were challenged intravenously with a high dose (1,000 TCID50) of highly pathogenic SIVmac239 virus at 6 weeks after the last immunization. The copy numbers of SIV RNA in the plasma of these animals were monitored over the course of 160 days (Fig. 8A to C). There were no significant difference in the peak and set-point SIV viral loads between the group that received immunization via intramuscular injection and the group that received Ad5-empty as the control, although some macaques that received intramuscular injection of Ad5-SIV vaccines showed a lower peak and set-point SIV viral load. There was a decreasing trend between the AVIP group and the intramuscular injection group, but the difference is not significant statistically for the peak SIV viral load (P = 0.1290). It is of great interest to observe that the AVIP immunization achieved a significant reduction in the peak and set-point SIV viral load compared to that of the control group. Compared with macaques that received Ad5-empty, AVIP immunization achieved a 0.95-log reduction of peak viral load (P = 0.0325; Fig. 8D) and a 1.12-log reduction of the set-point SIV viral load (P = 0.0190; Fig. 8E). These results suggest that the Ad5-SIV vaccines delivered through AVIP could confer better antigen-specific immune responses and protection in Ad5-seropositive macaques.
Fig 8.
AVIP immunization conferred better control of peak and set-point plasma SIV viral load in Ad5-seropositive macaques challenged with SIVmac239. The same three groups of macaques were challenged with intravenous injection of 1,000 TCID50 SIVmac239 at 6 weeks after last immunization. Plasma SIV RNA levels were measured by quantitative PCR at various time intervals. SIV RNA levels are depicted longitudinally for each group: (A) group 1 with Ad5-SIVenv, Ad5-SIVgag, and Ad5-SIVpol vaccines delivered through AVIP; (B) group 2 with Ad5-SIVenv, Ad5-SIVgag, and Ad5-SIVpol vaccines injected intramuscularly; (C) group 3 with Ad5-empty injected intramuscularly as the control. (D) Peak (day 14) SIV RNA levels for each group; (E) set-point (day 64 to 147) SIV RNA levels for each group. Data were analyzed by a nonparametric Mann-Whitney U test. The lines on the graph represent mean values. *, P < 0.05.
DISCUSSION
We reported herein a strategy, dubbed AVIP immunization, in which AVIPs were demonstrated for circumventing preexisting antiadenoviral immunity, especially Ad5 NAb, to enable repeated application of Ad5-vectored vaccines. The application of Ad5-vectored vaccines has been seriously hindered by preexisting anti-Ad5 immunity and also a potential side effect due to augmentation of preexisting anti-Ad5 immunity. Even in Ad5-seronegative individuals, the application of Ad5 is also limited, because strong antibody- and cell-mediated responses against Ad5 would develop right after one intramuscular injection, which will hinder the efficacy of any injection approaches that allow direct interaction of Ad5 NAb with Ad5. Many strategies have been reported with the aim to avoid the attenuated delivery efficacy, including developing vectors based on rare Ad serotypes (16, 27, 31), fiber-modified Ad5 vector (63), polyethylene glycol (PEG) (61), or alginate microsphere-coated Ad5 vectors (48) and so on (3). Although, in general, a significant booster effect was achieved, there were still some limitations for these methods. For example, there are concerns for using a rare Ad serotype due to the lack of a long-term safety record, especially oncogenicity. Furthermore, any new serotypes or fiber-modified Ad vectors are likely to encounter a new wave of NAb responses and therefore are limited to one-time administration. Therefore, we sought to develop an alternative approach to overcome the preexisting anti-Ad5 immunity, especially Ad5 NAb, to enable repeated usage of Ad5-vectored vaccines.
It has been well documented that in Ad5-seropositive individuals, neutralizing antibodies in the bloodstream can significantly decrease the Ad5-mediated gene transfer (45, 62). Furthermore, Lyons et al. (33) demonstrated that over 90% of the virus bound to human erythrocytes after direct delivery of the Ad5 vector into the bloodstream, and the binding interaction could inhibit Ad5 infection to target cells and significantly compromise delivery efficacy. Theoretically, our AVIP approach would avoid exposing the Ad5 vectors to Ad5 NAb and other unwanted interactions with components in blood because neutralizing antibodies, erythrocytes, and serum proteins have been eliminated during PBMC isolation. After in vitro incubation, the Ad5 vectors are internalized into mostly CD14+ cells, before being exposed to undesired interactions in vivo.
We first demonstrated the feasibility of delivering genes of interest in primates using Ad5 carrying an EGFP reporter gene through an ex vivo manipulation of PBMCs. One initial concern was that freshly isolated primate PBMCs do not express, or only express low levels of, receptors for Ad5 infection, including coxsackie-adenovirus receptor (CAR) and integrins αVβ3, so they are usually poorly susceptible to Ad5 infection (26, 33, 47). However, with an optimized condition, we found that PBMCs, especially CD14+ cells, from both humans and rhesus macaques could be infected by Ad5. To our surprise, CD14+ cells from Ad5-seropositive individuals could be infected by Ad5 more effectively than those from Ad5-seronegative individuals. The antibody-mediated enhancement of adenoviral infection has been documented (28, 56) but could not explain the enhancement of infection efficacy of CD14+ cells in this study, because all antibody was excluded after extensive washes during isolation of PBMCs. In an attempt to elucidate the underlying mechanism, we carried out an isolate-mix-isolate experiment to exclude the possibility of antibody-mediated enhancement of infection. The paired plasma and PBMCs from Ad5-seropositive and Ad5-seronegative individuals were isolated and exchanged with each other. After incubation for 1 h at ambient conditions, remixed PBMCs were reisolated and infected with Ad5-EGFP. PBMCs from Ad5-seronegative individuals did not increase EGFP expression after incubation with plasma from Ad5-seropositive individuals (data not shown), indicating that this enhancement effect was not simply caused by any residual Ad5 antibodies. We then attempted to determine the amount of receptors associated with adenovirus infection on CD14+ cells from Ad5-seropositive and Ad5-seronegative people but failed to detect any differences in both CAR and the αVβ3, αVβ5 integrins. Thus, the exact mechanism for this observation needs to be further investigated. Nevertheless, the observed enhanced Ad5 infection in PBMCs from Ad5-seropositive individuals would be advantageous for exploring the potential of using PBMCs as stealth vehicles for Ad5-mediated gene delivery.
One concern for this AVIP immunization strategy is that it might not be a suitable vaccine modality in developing countries, as it requires more sophisticated capability and medical facility in handling the procedure. On the other hand, there is a higher prevalence of preexisting anti-Ad5 immunity in populations of the developing countries (42, 54, 57), and so the implication of this modality is even more significant. We believe that if this modality can achieve positive results in the future human clinical trials in any countries willing to shed light on HIV vaccines or any infectious diseases with currently no efficacious vaccines, it will attract support for resources to develop a simple and user-friendly device to enable its application. At this proof-of-principle stage, this modality is aimed to complement what conventional vaccines could not achieve, so its application will be limited to diseases that have yet to have a better solution. We believe the AVIP immunization approach will have potential indications in “specialty” vaccines, including therapeutic vaccines for HIV, tuberculosis (TB), and cancer, that conventional modalities could not achieve. In both developed and developing country, it can be done as long as a clinic or hospital has a qualified facility and equipment. In fact, a variety of cell-based therapies using autologous dendritic cells (DCs), NK cells, and T cells have been widely explored worldwide for treating cancer patients in which the procedures are in general more complicated and time-consuming in the laboratory, whereas the AVIP requires only a brief pulse of PBMCs with Ad5-vectored vaccines in vitro followed by infusion back to the same individual. Therefore, using the AVIP strategy for delivering Ad5-vectored vaccines should be explored further in both nonhuman primates and human clinical trials.
An earlier study has suggested that DCs loaded with inactivated SIV virions could induce immune response and contribute to the control of SIV infection in macaques (7). However, such techniques require prolonged in vitro culture and maturation of DCs, which is actually more troublesome. In addition, infusion of autologous DCs pulsed with tumor antigens is being extensively explored as an experimental medicine for cancer immunotherapy (41, 59). Recently, peptide-pulsed PBMCs, either using overlapping peptides spanning the whole SIV mac239 proteome (13, 14) or inactivated SIVmac251 virions (35), was reported to be able to elicit antigen-specific immune responses in macaques. Similarly with our AVIP strategy, this technique does not require prolonged ex vivo culture of antigen-presenting cells but may either have safety concerns in using inactivated virions or limited epitope coverage in using peptides. We believe the AVIP strategy has the advantage of directly expressing, processing, and presenting antigens in antigen-presenting cells (APCs). Previous studies have demonstrated that dendritic cells (DCs), the most potent APCs, could be generated from CD14+ cells directly (22, 26, 32). Therefore, in our study, the Ad5-transduced CD14+ cells are likely the major source for producing and presenting antigens for eliciting effective antigen-specific CD4+ and CD8+ T cell responses. Indeed, our study demonstrated that using the AVIP approach to deliver Ad5-vectored vaccines can induce antigen-specific immune response in Ad5-seronegative macaques at a level comparable to direct intramuscular injection Ad5-vectored vaccines. More intriguingly, in Ad5-seropositive macaques, the AVIP immunization approach can enable repeated delivery of Ad5-vectored vaccines carrying different antigens to induce specific immune responses to each antigen consistently in macaques.
Surprisingly, another observation in our study is that there was a lower Ad5 NAb titer in Ad5-seronegative macaques after receiving AVIP immunization than in macaques receiving intramuscular injection of Ad5-vectored vaccines. Since penton, fiber, and hexon proteins on the Ad5 capsid are the major targets of Ad5 NAb (17, 23), one possible explanation to this lower titer of Ad5 NAb response might be attributed to having less Ad5 capsid proteins administered into animals. Since noninfectious viral particles would be washed away after the in vitro incubation with PBMCs, the antigen load that could induce Ad5 NAb in this AVIP strategy would be much less than that in the traditional strategy of intramuscular injection of Ad5 vectors, which contain substantial amounts of noninfectious viral particles. Further investigation will be needed to define the detailed mechanism of AVIP immunization, which should facilitate the repeat application of Ad5-vectored vaccines.
As a proof-of-concept, this study evaluated not only immunogenicity but also the protective efficacy of multiple Ad5-vectored SIV vaccines in Ad5-seropositive macaques by repeated AVIP immunizations. Recently, the rhesus macaques challenged with highly pathogenic SIVmac239 has been used as the most stringent nonhuman primate model for evaluating HIV/SIV vaccine candidates (1, 40, 59). Protection against SIVmac239, especially through intravenous challenge, has been extremely difficult to achieve (9, 15, 58). Therefore, a more practical challenge route would be to use low-dose intrarectal challenge or intravaginal challenge in the protection study. Nevertheless, in our present study, macaques challenged intravenously with SIVmac239 virus showed that AVIP immunization strategy helped to control pathogenic SIVmac239 infection compared with control animals (Fig. 8). At present, one unanswered question is which immune response is most likely to be responsible for controlling HIV/SIV virus. A popular view in the field of HIV vaccine research is that an effective HIV vaccine should be able to induce a great magnitude, breadth, and polyfunctionality of balanced cellular immune responses, as well as humoral responses that include neutralizing antibodies and perhaps ADCC. In this study, we demonstrated that the AVIP strategy can effectively elicit cellular immune responses which at least partially contribute to the observed viral control in Ad5-seropositive macaques. Since the aim of this study is to develop a strategy to circumvent antiadenovirus immunity, the detailed immune responses responsible for the observed viral control might be studied in the future.
Taken together, we observed that CD14+ cells from Ad5-seropositive individuals were more susceptible to Ad5 infection than those from Ad5-seronegative individuals and developed a novel strategy based on AVIP immunization to enable repeated application of Ad5-vectored vaccines to elicit immune responses toward multiple antigens. We achieved the proof-of-concept in a recognized challenge model of SIVmac239 infection using Ad5-seropositive rhesus macaques. Our study warranted further development of this strategy into a simple and practical method for repeated applications of Ad5-vectored vaccines for HIV and other infectious diseases that have yet to have an effective vaccine.
ACKNOWLEDGMENTS
We gratefully acknowledge colleagues Zheng Xuehua, Liu Yichu, Zhang Maochao, and Li Pingchao for their technical assistance throughout the project, and the staff at the Animal Center of GIBH for animal experiments. We also gratefully acknowledge Merck Co. for technical guidance in ELISPOT, ICS, and quantitative adenovirus neutralization assays. The SIV Env, Gag, and Pol peptide pools were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
This study was supported by the National Natural Science Foundation of China (81000737), the National Key Science & Technology Specific Projects of China (2012ZX10001-009-001-002), the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX1-YW-10), the National Science Fund for Distinguished Young Scholars of China (30688004), and the Bureau of Science and Technology of Guangzhou Municipality S&T Support Program (2010J-E381).
Footnotes
Published ahead of print 1 August 2012
REFERENCES
- 1. Ambrose Z, KewalRamani VN, Bieniasz PD, Hatziioannou T. 2007. HIV/AIDS: in search of an animal model. Trends Biotechnol. 25:333–337 [DOI] [PubMed] [Google Scholar]
- 2. Aste-Amézaga M, et al. 2004. Quantitative adenovirus neutralization assays based on the secreted alkaline phosphatase reporter gene: application in epidemiologic studies and in the design of adenovector vaccines. Hum. Gene Ther. 15:293–304 [DOI] [PubMed] [Google Scholar]
- 3. Bangari DS, Mittal SK. 2006. Current strategies and future directions for eluding adenoviral vector immunity. Curr. Gene Ther. 6:215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benlahrech A, et al. 2009. Adenovirus vector vaccination induces expansion of memory CD4 T cells with a mucosal homing phenotype that are readily susceptible to HIV-1. Proc. Natl. Acad. Sci. U. S. A. 106:19940–19945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Betts MR, et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bogers WM, et al. 2008. Systemic neutralizing antibodies induced by long interval mucosally primed systemically boosted immunization correlate with protection from mucosal SHIV challenge. Virology 382:217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown K, et al. 2003. Adenovirus-transduced dendritic cells injected into skin or lymph node prime potent simian immunodeficiency virus-specific T cell immunity in monkeys. J. Immunol. 171:6875–6882 [DOI] [PubMed] [Google Scholar]
- 8. Casimiro DR, et al. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305–6313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Casimiro DR, et al. 2005. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J. Virol. 79:15547–15555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen L, et al. 1995. Breast cancer selective gene expression and therapy mediated by recombinant adenoviruses containing the DF3/MUC1 promoter. J. Clin. Invest. 96:2775–2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen L, et al. 1996. Selective transgene expression for detection and elimination of contaminating carcinoma cells in hematopoietic stem cell sources. J. Clin. Invest. 98:2539–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen J. 2007. Did Merck's failed HIV vaccine cause harm? Science 318:1048–1049 [DOI] [PubMed] [Google Scholar]
- 13. De Rose R, et al. 2008. Delivery of immunotherapy with peptide-pulsed blood in macaques. Virology 378:201–204 [DOI] [PubMed] [Google Scholar]
- 14. De Rose R, et al. 2008. Control of viremia and prevention of AIDS following immunotherapy of SIV-infected macaques with peptide-pulsed blood. PLoS Pathog. 4:e1000055 doi:10.1371/journal.ppat.1000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Engram JC, et al. 2009. Vaccine-induced, simian immunodeficiency virus-specific CD8+ T cells reduce virus replication but do not protect from simian immunodeficiency virus disease progression. J. Immunol. 183:706–717 [DOI] [PubMed] [Google Scholar]
- 16. Fitzgerald JC, et al. 2003. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J. Immunol. 170:1416–1422 [DOI] [PubMed] [Google Scholar]
- 17. Gahéry-Ségard H, et al. 1998. Immune response to recombinant capsid proteins of adenovirus in humans: anti-fiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J. Virol. 72:2388–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghosh SS, Gopinath P, Ramesh A. 2006. Adenoviral vectors: a promising tool for gene therapy. Appl. Biochem. Biotechnol. 133:9–29 [DOI] [PubMed] [Google Scholar]
- 19. Gorelick RJ, et al. 2000. Protection of Macaca nemestrina from disease following pathogenic simian immunodeficiency virus (SIV) challenge: utilization of SIV nucleocapsid mutant DNA vaccines with and without an SIV protein boost. J. Virol. 74:11935–11949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hansen SG, et al. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 15:293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heeney JL, Plotkin SA. 2006. Immunological correlates of protection from HIV infection and disease. Nat. Immunol. 7:1281–1284 [DOI] [PubMed] [Google Scholar]
- 22. Herold J, Tillmanns H, Xing Z, Strasser RH, Braun-Dullaeus RC. 2006. Isolation and transduction of monocytes: promising vehicles for therapeutic arteriogenesis. Langenbecks Arch. Surg. 391:72–82 [DOI] [PubMed] [Google Scholar]
- 23. Hong SS, Habib NA, Franqueville L, Jensen S, Boulanger PA. 2003. Identification of adenovirus (Ad) penton base neutralizing epitopes by use of sera from patients who had received conditionally replicative Ad (Addl1520) for treatment of liver tumors. J. Virol. 77:10366–10375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hutnick NA, et al. 2009. Baseline Ad5 serostatus does not predict Ad5 HIV vaccine-induced expansion of adenovirus-specific CD4+ T cells. Nat. Med. 15:876–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalams SA. 2003. Cellular immunity for prevention and clearance of HIV infection. Curr. Mol. Med. 3:195–208 [DOI] [PubMed] [Google Scholar]
- 26. Leen A, et al. 2007. Contact-activated monocytes: efficient antigen presenting cells for the stimulation of antigen-specific T cells. J. Immunother. 30:96–107 [DOI] [PubMed] [Google Scholar]
- 27. Lemiale F, et al. 2007. Novel adenovirus vaccine vectors based on the enteric-tropic serotype 41. Vaccine 25:2074–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leopold PL, Wendland RL, Vincent T, Crystal RG. 2006. Neutralized adenovirus-immune complexes can mediate effective gene transfer via an Fc receptor-dependent infection pathway. J. Virol. 80:10237–10247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Letvin NL, et al. 2007. No evidence for consistent virus-specific immunity in simian immunodeficiency virus-exposed, uninfected rhesus monkeys. J. Virol. 81:12368–12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu J, et al. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lun WH, et al. 2004. Loss of virus-specific CD4(+) T cells with increases in viral loads in the chronic phase after vaccine-based partial control of primary simian immunodeficiency virus replication in macaques. J. Gen. Virol. 85:1955–1963 [DOI] [PubMed] [Google Scholar]
- 32. Lyakh LA, et al. 2002. Adenovirus type 5 vectors induce dendritic cell differentiation in human CD14+ monocytes cultured under serum-free conditions. Blood 99:600–608 [DOI] [PubMed] [Google Scholar]
- 33. Lyons M, et al. 2006. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol. Ther. 14:118–128 [DOI] [PubMed] [Google Scholar]
- 34. Maness NJ, et al. 2008. Comprehensive immunological evaluation reveals surprisingly few differences between elite controller and progressor Mamu-B*17-positive simian immunodeficiency virus-infected rhesus macaques. J. Virol. 82:5245–5254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mason RD, et al. 2009. Inactivated simian immunodeficiency virus-pulsed autologous fresh blood cells as an immunotherapy strategy. J. Virol. 83:1501–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mayne GC, et al. 2003. Centrifugation facilitates transduction of green fluorescent protein in human monocytes and macrophages by adenovirus at low multiplicity of infection. J. Immunol. Methods 278:45–56 [DOI] [PubMed] [Google Scholar]
- 37. McCoy K, et al. 2007. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J. Virol. 81:6594–6604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Molnar-Kimber KL, et al. 1998. Impact of preexisting and induced humoral and cellular immune responses in an adenovirus-based gene therapy phase I clinical trial for localized mesothelioma. Hum. Gene Ther. 9:2121–2133 [DOI] [PubMed] [Google Scholar]
- 39. Moog C. 2008. Immune responses that correlate with HIV-1 protection? AIDS 22:1461–1462 [DOI] [PubMed] [Google Scholar]
- 40. Morgan C, et al. 2008. The use of nonhuman primate models in HIV vaccine development. PLoS Med. 5:e173 doi:10.1371/journal.pmed.0050173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nguyen-Pham TN, Lee YK, Kim HJ, Lee JJ. 2012. Immunotherapy using dendritic cells against multiple myeloma: how to improve? Clin. Dev. Immunol. 2012:397648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nwanegbo E, et al. 2004. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab. Immunol. 11:351–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O'Brien KL, et al. 2009. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat. Med. 15:873–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perreau M, Pantaleo G, Kremer EJ. 2008. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. J. Exp. Med. 205:2717–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Piedra PA, Poveda GA, Ramsey B, McCoy K, Hiatt PW. 1998. Incidence and prevalence of neutralizing antibodies to the common adenoviruses in children with cystic fibrosis: implication for gene therapy with adenovirus vectors. Pediatrics 101:1013–1019 [DOI] [PubMed] [Google Scholar]
- 46. Qin L, Gilbert PB, Corey L, McElrath MJ, Self SG. 2007. A framework for assessing immunological correlates of protection in vaccine trials. J. Infect. Dis. 196:1304–1312 [DOI] [PubMed] [Google Scholar]
- 47. Richardson C, et al. 2005. Susceptibility of B lymphocytes to adenovirus type 5 infection is dependent upon both coxsackie-adenovirus receptor and alphavbeta5 integrin expression. J. Gen. Virol. 86:1669–1679 [DOI] [PubMed] [Google Scholar]
- 48. Sailaja G, HogenEsch H, North A, Hays J, Mittal SK. 2002. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther. 9:1722–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shiver JW, et al. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency virus immunity. Nature 415:331–335 [DOI] [PubMed] [Google Scholar]
- 50. Shiver JW, Emini EA. 2004. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 55:355–372 [DOI] [PubMed] [Google Scholar]
- 51. Steinbrook R. 2007. One step forward, two steps back—will there ever be an AIDS vaccine? N. Engl. J. Med. 357:2653–2655 [DOI] [PubMed] [Google Scholar]
- 52. Sumida SM, et al. 2004. Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J. Virol. 78:2666–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sun C, et al. 2010. Induction of balance and breadth in the immune responses is beneficial to control SIVmac239 replication in rhesus monkey. J. Infect. 60:371–381 [DOI] [PubMed] [Google Scholar]
- 54. Sun C, et al. 2011. Epidemiology of adenovirus type 5 neutralizing antibodies in healthy people and AIDS patients in Guangzhou, southern China. Vaccine 29:3837–3841 [DOI] [PubMed] [Google Scholar]
- 55. Tatsis N, Ertl HC. 2004. Adenoviruses as vaccine vectors. Mol. Ther. 10:616–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tirado SM, Yoon KJ. 2003. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 16:69–86 [DOI] [PubMed] [Google Scholar]
- 57. Thorner AR, et al. 2006. Age dependence of adenovirus-specific neutralizing antibody titers in individuals from Sub-Saharan Africa. J. Clin. Microbiol. 44:3781–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vogel TU, et al. 2003. Multispecific vaccine-induced mucosal cytotoxic T lymphocytes reduce acute-phase viral replication but fail in long-term control of simian immunodeficiency virus SIVmac239. J. Virol. 77:13348–13360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vulink A, Radford KJ, Melief C, Hart DN. 2008. Dendritic cells in cancer immunotherapy. Adv. Cancer Res. 99:363–407 [DOI] [PubMed] [Google Scholar]
- 60. Watkins DI, Burton DR, Kallas EG, Moore JP, Koff WC. 2008. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat. Med. 14:617–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Weaver EA, Barry MA. 2008. Effects of shielding adenoviral vectors with polyethylene glycol (PEG) on vector-specific and vaccine-mediated immune responses. Hum. Gene Ther. 19:1369–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wohlfart C. 1988. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J. Virol. 62:2321–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xin KQ, et al. 2005. Prime-boost vaccination with plasmid DNA and a chimeric adenovirus type 5 vector with type 35 fiber induces protective immunity against HIV. Gene Ther. 12:1769–1777 [DOI] [PubMed] [Google Scholar]