Abstract
Autophagy is a programmed homeostatic response to diverse types of cellular stress that disposes of long-lived proteins, organelles, and invading microbes within double-membraned structures called autophagosomes. The 2′,5′-oligoadenylate/RNase L system is a virus-activated host RNase pathway that disposes of or processes viral and cellular single-stranded RNAs. Here we report that activation of RNase L during viral infections induces autophagy. Accordingly, infections with encephalomyocarditis virus or vesicular stomatitis virus led to higher levels of autophagy in wild-type mouse embryonic fibroblasts (MEF) than in RNase L-null MEF. Similarly, direct activation of RNase L with a 2′,5′-oligoadenylate resulted in p62(SQSTM1) degradation, LC3BI/LC3BII conversion, and appearance of autophagosomes. To determine the effect of RNase L-mediated autophagy on viral replication, we compared viral yields in wild-type and RNase L-null MEF in the absence or presence of either chemical inhibitors of autophagy (bafilomycin A1 or 3-methyladenine) or small interfering RNA (siRNA) against ATG5 or beclin-1. At a low multiplicity of infection, induction of autophagy by RNase L during the initial cycle of virus growth contributed to the suppression of virus replication. However, in subsequent rounds of infection, autophagy promoted viral replication, reducing the antiviral effect of RNase L. Our results indicate a novel function of RNase L as an inducer of autophagy that affects viral yields.
INTRODUCTION
Autophagy (or “self-eating”) is an essential and ancient cellular process for degrading and recycling components of long-lived proteins, organelles, and microbial invaders (7, 22). These materials are sequestered in the cytoplasm within double-membrane vesicles termed autophagosomes that fuse with lysosomes, causing the contents to be degraded. While autophagy is often induced in response to viral infections, the impact of autophagy on pathogenesis and virus replication can be unpredictable. For instance, intracerebral injection of Sindbis virus in mice induced autophagy, causing degradation of viral proteins while promoting viral clearance and protecting against virus-mediated neuronal cell death (32). However, while animal survival was enhanced by autophagy there was no effect on virus replication. There is also a complex, reciprocal relationship between autophagy and the mammalian immune system (22). The fact that many viruses have evolved strategies for countering and/or subverting autophagy supports a role for autophagy in the host antiviral response (31, 43). As an example of cross talk between autophagy and the innate immune response, autophagy mediates cellular recognition of some single-strand RNA (ssRNA) viruses, leading to alpha interferon (IFN-α) induction in plasmacytoid dendritic cells (20). In addition, downstream events in IFN-regulated pathways regulate the autophagocytic process itself. For instance, PKR is an IFN-inducible serine/threonine protein kinase that inhibits translation by phosphorylating eIF2α and that promotes autophagocytic degradation of HSV-1 (37, 38). Effects of PKR on both translation and autophagy are blocked by the HSV-1 neurovirulence gene product ICP34.5, which redirects protein phosphatase 1α to dephosphorylate eIF2α. Here we implicate a second IFN-regulated pathway, the 2′,5′-oligoadenylate (OAS)/RNase L system (3, 34), in the control of autophagy.
OASs are IFN-inducible pathogen recognition receptors (PRR) that produce 2′,5′-linked oligonucleotides of the formula px5′A(2′p5′A)n (x = 1 to 3; n ≥ 2) (2-5A) from ATP in response to viral double-stranded RNA (dsRNA) (15). The viral activators of OAS include replicative intermediates, dsRNA genomes, annealed ssRNAs of opposite polarity, and double-stranded regions in otherwise ssRNA. 2-5A is the ligand and activator of RNase L, a nearly ubiquitous enzyme in mammalian cells, that lies dormant until viral infections occur (45). Upon binding 2-5A, RNase L is converted from an inactive monomer to an active dimer that cleaves single-stranded regions of RNA (5). Once activated, RNase L cleaves viral and host ssRNAs, principally at UpAp and UpUp dinucleotides, leaving 3′-phosphoryl (3′-p) and 5′-hydroxyl (5′-OH) groups at the termini of small, often partially double-stranded RNA cleavage products (8, 40). Some of these RNA cleavage products signal through RIG-I like receptors (RLR), RIG-I and MDA5, to amplify and perpetuate production of type I IFNs (25). The most readily observable effect of RNase L in intact cells is the cleavage of rRNA in intact ribosomes producing characteristic and discrete RNA fragments (35, 39). Damaged ribosomes are typically disposed of in a type of autophagy known as ribophagy (17).
The relationship between autophagy and innate immunity, in particular the findings of prior studies on PKR, prompted us to investigate a possible role for RNase L in autophagy. We show that autophagy in response to viral infections is deficient in cells lacking RNase L. In addition, direct activation of RNase L by 2-5A treatment of cells caused specific rRNA cleavage events correlating with induction of autophagy. Moreover, by using either chemical inhibitors of autophagy or small interfering RNAs (siRNAs) against autophagy-related mRNAs we show that the induction of autophagy by RNase L modulates viral yields.
MATERIALS AND METHODS
Reagents and antibodies.
Bafilomycin A1 (BafA1), 3-methyladenine (3-MA), and rapamycin were purchased from Calbiochem (San Diego, CA). Chloroquine (CQ) was purchased from Sigma-Aldrich (St. Louis, MO). Antibodies against LC3B (no. 3868), beclin-1 (no. 3495), and normal goat serum (no. 5425) were purchased from Cell Signaling Technology, Inc. (Danvers, MA), and antibody to ATG5 (no. NB110-53818) was from Novus Biologicals (Littleton, CO). P62/SQSTM1 (GP62-C) antibody was purchased from Progen Biotechnik (Heidelberg, Germany). β-Actin antibody was purchased from Sigma-Aldrich (St. Louis, MO). Alexa Fluor 488-conjugated goat anti-rabbit IgG (heavy plus light chain) (no. A11034) secondary antibody was purchased from Invitrogen/Life Technologies (Grand Island, NY). Paraformaldehyde solution (16%), electron microscopy grade (no. 15710) was purchased from Electron Microscopy Sciences (Hatfield, PA). Vectashield mounting medium with 4′,6′-diamidino-2-phenylindole (DAPI) (no. H-1200) was purchased from Vector Laboratories Inc. (Burlingame, CA). SMARTpool siRNAs against mouse ATG5 and nonspecific control siRNA were purchased from Dharmacon/Thermo Fisher Scientific (Chicago, IL), and siRNAs against human RNase L (sc-45965) and mouse beclin-1 (sc-29798) and corresponding control siRNA (sc-37007) were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Lipofectamine 2000 and Dharmafect 2 transfection reagents were purchased from Invitrogen/Life Technologies and Dharmacon, respectively. Complete, EDTA-free protease inhibitor cocktail tablets (catalog no. 11697498001) were purchased from Roche Applied Science (Indianapolis, IN). Synthesis and purification of 5′-triphosphorylated and dephosphorylated 2′,5′-oligoadenylates (2-5A) was performed as we described (11).
Cell culture.
Wild-type (WT) and mouse embryonic fibroblasts (MEF) with a disrupted RNase L gene (Rnasel−/− MEF) (46) were derived from mouse embryos of the same genetic background (C57/bl6) after backcrossing mice 10 generations (heterozygous mice were used to breed both the WT and Rnasel−/− mice to minimize genetic differences). Primary MEF were transformed with cDNA encoding simian virus 40 (SV40) T antigen and were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). Hey1b human ovarian carcinoma cells (a gift from Alexander Marks, Toronto, Canada) were grown in the same medium. MEF expressing LC3 conjugated to green fluorescent protein (GFP) (a kind gift of N. Mizushima, Tokyo, Japan) (27) were grown in Dulbecco's modified Eagle's medium in the presence of 10% FBS, as were L929 mouse fibroblasts, CV1 African green monkey kidney cells, and HeLa M cells expressing wild-type (WT) human RNase L or mutant RNase L (R667A) (4). Transfection efficiencies were determined by transfecting pLenti-C-GFP plasmid (Origene) with Lipofectamine 2000, and then cells were counted by using fluorescence microscopy.
Viral infection of cells.
Encephalomyocarditis virus (EMCV) (9) (a gift from Ian Kerr, London, United Kingdom) and vesicular stomatitis virus (VSV, Indiana strain) (a gift from Amiya Banerjee, Cleveland, OH) were grown in L929 cells and CV1 cells, respectively. WT and Rnasel−/− MEF and HeLa M cells were infected with EMCV or VSV in RPMI 1640 medium containing 1% serum. After 1 h, supernatants were removed, and cells were washed at room temperature with phosphate-buffered saline (PBS), replenished with complete RPMI 1640 medium containing 10% FBS, and incubated for different times as described in the text. Viral titers were determined by plaque assays on L929 indicator cells.
UV inactivation of virus.
For UV inactivation of EMCV, a 1-ml sample of virus (108 PFU/ml) was irradiated for 1 h in 60-mm petri dishes at a distance of 30 cm in a UV cross-linker (Spectro Linker XL-1000 from Spectronics Corporation, Westbury, NY) at 300 μJ/cm2. Loss of virus infectivity was established by performing plaque assays on L929 indicator cells. WT and Rnasel−/− MEF were infected with UV-inactivated virus at a multiplicity of infection (MOI) of 1 for 12 and 16 h.
Measuring effects of autophagy inhibitors on viral yields.
Cells were pretreated with either 200 nM BafA1, 50 μM CQ, or 5 mM 3-MA for 2 h followed by virus infection, and proteins in cell extracts were subsequently analyzed by Western blotting. Alternatively, WT and Rnasel−/− MEF were pretreated with either 200 nM BafA1 or 5 mM 3-MA for 2 h. Cells were infected with EMCV (MOI = 0.05) for 1 h in RPMI medium containing 1% FBS. The cells were subsequently washed with PBS and replenished with complete medium containing 10% FBS. After an additional incubation, conditioned medium containing released virus particles was collected from each well and centrifuged for 5 min at 1,000 × g at room temperature to remove cellular debris. Viral titers were determined by plaque assays on indicator cells. WT and Rnasel−/− MEF were transfected with siRNAs (30 nM) using Dharmafect 2 according to the manufacturer's protocol (Dharmacon). After 48 h, the cells were infected with EMCV for 1 h in medium with 1% FBS and washed, and after an additional incubation in complete medium with 10% FBS, the cell supernatants were collected to determine viral titers by plaque assays.
Treatment of cells with 2-5A.
Hey1b cells and GFP-LC3-expressing MEF were transfected with various concentrations (see the text) of 2′-5′p3A3 or 2′-5′A3 using Lipofectamine 2000 (Invitrogen/Life Technologies) as described previously (26). Briefly, cells were grown in antibiotic-free RPMI medium, 2′-5′p3A3 or 2′-5′A3 was complexed with Lipofectamine 2000 in serum-free medium, and cells were transfected and incubated for 6 h before harvesting.
rRNA cleavage assays.
2-5A-mediated rRNA cleavage was monitored as described previously (26). Briefly, Hey1B cells were transfected with 10 μM either 2′-5′p3A3 or 2′-5′A3 using Lipofectamine 2000. Cells were incubated for different times, after which total RNA was isolated using TRIzol (Invitrogen) and the RNA was separated and analyzed on RNA chips using an Agilent 2100 Bioanalyzer. Also, HeLa M cells expressing WT or mutant (R667A) RNase L were infected with EMCV (MOI = 0.01) for 16 h after which total RNA was isolated TRIzol (Invitrogen) and resolved on RNA chips.
Cell lysis and immunoblotting.
Prior to lysis, cells were washed twice in cold phosphate-buffered saline (PBS). Cell extracts were prepared with lysis buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 0.1 mM EDTA; and 1% NP-40) supplemented with protease/phosphatase inhibitors (1 mM sodium orthovanadate in complete EDTA-free protease inhibitor from Roche) followed by incubation on ice for 20 min. Lysates were subjected to centrifugation at 12,000 × g for 10 min, and the supernatants collected and protein quantified by Bradford assays. Cell lysates (30 μg) were fractionated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, and proteins were transferred to polyvinylidene difluoride membranes (0.45 μm) and probed with their respective antibodies according to the different manufacturer's recommendations. Images from immunoblots were analyzed with Image J software (rsbweb.nih.gov/ij/). Relative levels of P62 and LC3BII were quantified and normalized to levels of β-actin.
Immunostaining and microscopy.
Hey1B cells were grown on glass coverslips and following transfection with 2-5A were fixed with 4% paraformaldehyde for 15 min. Cells were washed three times with PBS and blocked in blocking buffer (5% normal goat serum; 0.3% Triton X-100 in PBS) for 60 min. After blocking, cells were incubated with LC3B antibody (1:100) diluted in antibody dilution buffer (1% bovine serum albumin [BSA]; 0.3% Triton X-100 in PBS) at 4οC overnight. Cells were subsequently washed with PBS and incubated with Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody (1:500) for 1 h in the dark at room temperature. After washing with PBS, coverslips containing cells were mounted on glass slides with Vectashield mounting medium containing DAPI and observed with a confocal microscope. MEF expressing GFP-LC3 were grown on glass coverslips, and after 2-5A transfection, the cells were fixed with 4% paraformaldehyde for 15 min. Following fixation, the cells were washed three times with PBS and mounted on glass slides with Vectashield mounting medium containing DAPI and observed with a confocal microscope. LC3 vesicles or GFP-LC3-positive vesicles were scored by analysis of z-stack overlays from multiple fields. Overlay images were imported into Image-Pro Plus (v6.1; Media Cybernetics) and autophagosome analysis for each image was performed in an automated manner using customized visual basic Image-Pro Plus macros.
Transmission electron microscopy was performed in the Cleveland Clinic Imaging Core facility. Briefly, Hey1B cells were transfected with 2-5A and immediately fixed in 2.5% glutaraldehyde and 4% formaldehyde, pH 7.3, for 24 h followed by post fixation with 1% osmium tetroxide for 1 h. After en bloc staining and dehydration with ethanol, the samples were embedded with Eponate 12 medium (Ted Pella Inc., Redding, CA). Ultrathin sections of 85 nm were cut with a diamond knife, stained with uranyl acetate and lead citrate, and then observed with a Philips CM12 electron microscope operated at 60 kV.
RESULTS
Virus-mediated autophagy is deficient in cells lacking RNase L.
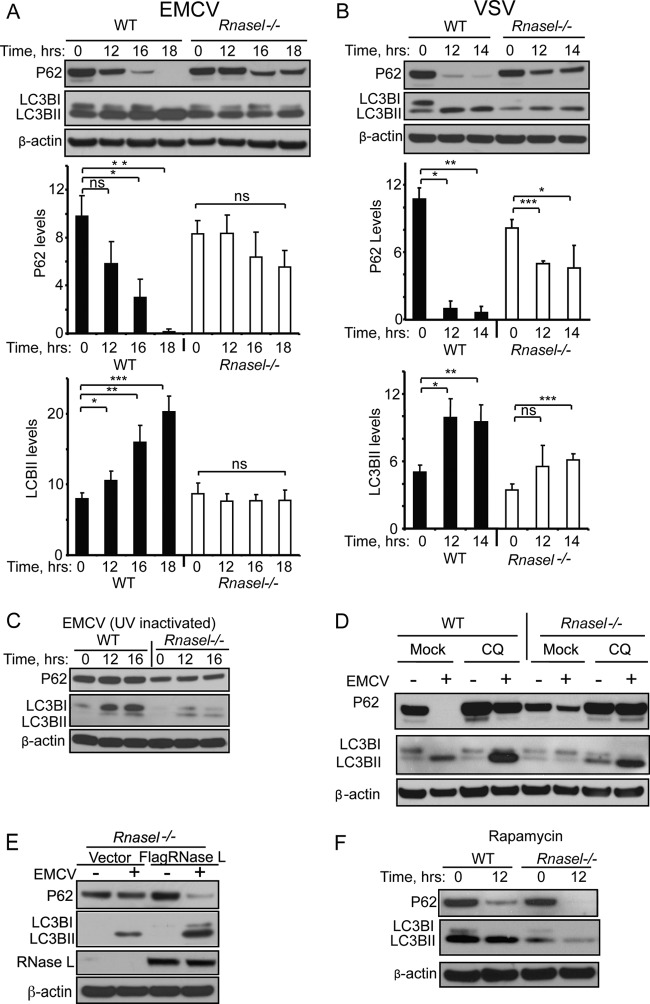
To determine the effect of RNase L on autophagy during viral infections, degradation of p62 (SQSTM1) and conversion of LC3BI to LC3BII were monitored in WT and Rnasel−/− MEF infected with either EMCV or VSV. P62 is an adaptor that binds to ubiquitin and LC3, and its measurement correlates well with other parameters of autophagic flux (28, 33). Conversion of the autophagy factor LC3BI (ATG8) to its phosphatidylethanolamine-conjugated form, LC3BII, results in its association with the autophagosome membrane (13). The rabbit monoclonal antibody against LC3B used here preferentially binds to the LC3BII form. EMCV infection led to dramatic induction of autophagy in WT MEF, whereas there was only a minimal level of virus-induced autophagy in the Rnasel−/− MEF as determined by measuring p62 degradation and LC3BI/LC3BII conversion (Fig. 1A). VSV was also highly effective in inducing autophagy in WT MEF as determined by measuring p62 degradation and LC3BI/LC3BII conversion (Fig. 1B). In comparison, there was a reduced level of autophagy in VSV-infected Rnasel−/− MEF. UV-inactivated EMCV (21) failed to induce autophagy both in WT and Rnasel−/− MEF, and therefore induction of autophagy required replication-competent virus (Fig. 1C). Autophagic flux was monitored by infecting WT and Rnasel−/− MEF with EMCV in the continuous presence of chloroquine (CQ), an agent that impairs lysosomal acidification (10). Accordingly, CQ treatment impaired p62 degradation in response to virus infection (Fig. 1D). In addition, CQ treatment caused LC3BII to accumulate to higher levels after EMCV infections in WT MEF compared to the Rnasel−/− MEF, demonstrating that RNase L contributes to induction of autophagic flux (Fig. 1D). The role of RNase L in virus-induced autophagy was further examined by infecting Rnasel−/− MEF with EMCV following ectopic expression of RNase L. Transfection efficiency was estimated to be 86% in Rnasel−/− MEF using a GFP-expressing plasmid (data not shown). Induction of autophagy was higher in the presence of RNase L as determined by measuring both p62 degradation and LC3BI/LC3BII conversion (Fig. 1E). To investigate whether RNase L contributed to autophagy in response to a nonviral inducer, cells were treated with rapamycin (29). However, autophagy initiated by rapamycin was only minimally affected by the presence or absence of RNase L as determined by measuring p62 degradation and LC3BI/LC3BII conversion (Fig. 1F). These findings suggest that RNase L contributes to autophagy mediated by EMCV or VSV infections but not by rapamycin treatment.
Fig 1.
Viral induction of autophagy is impaired in the absence of RNase L. (A and B) Immunoblots for P62, LC3B, and β-actin from WT and Rnasel−/− MEF were infected with EMCV (MOI = 0.01) or VSV (MOI = 0.01) for the indicated times. P62 and LC3BII levels normalized to the β-actin levels were determined from three independent biological replicates. Error bars show standard deviation (SD) and P values were determined by two-tailed Student's t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant). (C) Immunoblots for P62, LC3B, and β-actin from WT and Rnasel−/− MEF infected with UV-inactivated EMCV (MOI = 1). (D) Immunoblots for P62, LC3B and β-actin from WT and Rnasel−/− MEF treated with chloroquine (CQ) beginning 2 h prior to infection and continuing for an additional 16 h with or without EMCV infection. (E) Immunoblots for P62, LC3B, Flag-tagged RNase L, and β-actin from Rnasel−/− MEF transiently transfected with human Flag-tagged RNase L or empty vector followed by EMCV (MOI = 0.01) infection for 16 h. (F) Immunoblots for P62, LC3B, and β-actin from WT and Rnasel−/− MEF treated with rapamycin (5 μM).
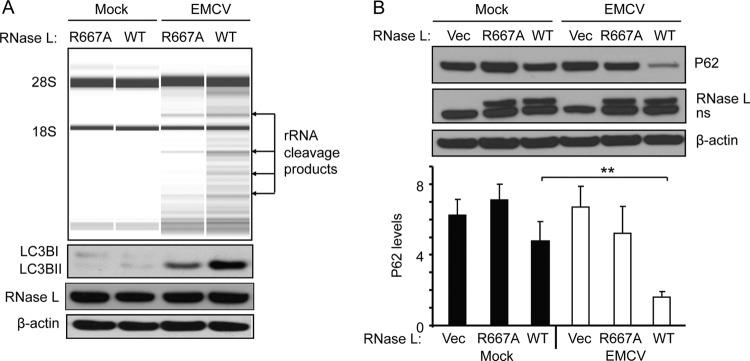
To determine if the RNase function of RNase L was necessary for autophagy, virus-mediated autophagy was compared in human HeLa M cells stably transfected with empty vector, WT RNase L cDNA, or mutant (R667A) RNase L cDNA (Fig. 2) (4). The parental HeLa M cells are deficient for RNase L (Fig. 2B). EMCV infections of HeLa M cells expressing WT RNase L produced robust cleavage of rRNA (35) accompanied by the appearance of LC3BII in the immunoblots of cell extracts (Fig. 2A). In contrast, in HeLa M cells expressing the R667A mutant RNase L, EMCV infection resulted in less rRNA cleavage and lower levels of LC3BII. In addition, p62 degradation in response to EMCV infection was significantly enhanced in HeLa M cells expressing WT RNase L compared with that in the parental HeLa M cells lacking RNase L or expressing the nuclease-dead R667A mutant RNase L (Fig. 2B). These findings indicate that the RNase activity of RNase L contributes to autophagy induction by viral infections.
Fig 2.
Cleavage of rRNA by RNase L during EMCV infections and effect of WT and mutant RNase L on autophagy. (A) HeLa M cells stably expressing WT or nuclease-dead mutant (R667A) RNase L were infected with EMCV at MOI of 0.01 for 16 h. Total RNA was isolated and rRNA cleavage was monitored in RNA chips (top panel). LC3B, RNase L, and β-actin were monitored in immunoblots (bottom panels). (B) In a separate experiment, HeLa M cells stably transfected with empty vector (vec) or vector expressing WT RNase L or R667A RNase L were infected with EMCV at MOI of 0.01 for 16 h. P62, RNase L, and β-actin in immunoblots (top) and P62 levels normalized to the β-actin levels (bottom) were determined from three independent biological replicates. Error bars (SD) and P values determined by two-tailed Student's t test (**, P < 0.01) are shown.
Direct activation of RNase L by 2-5A causes autophagy.
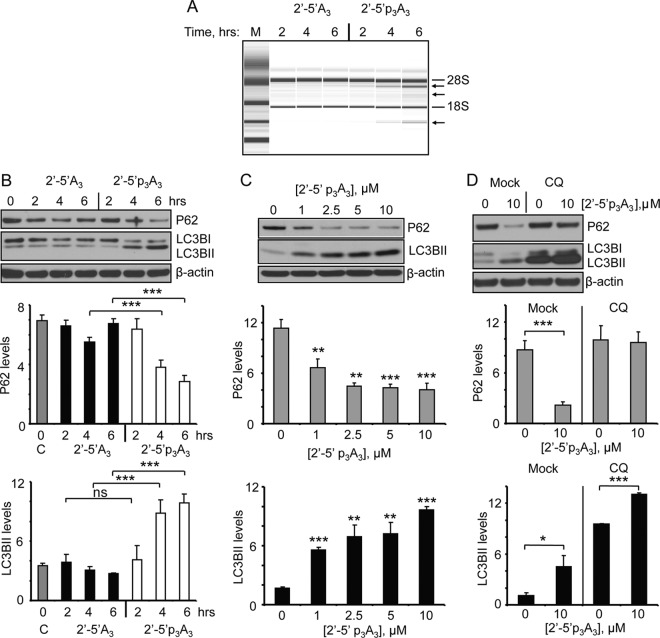
The preceding results demonstrated involvement of RNase L in autophagy in response to EMCV or VSV infection. To determine if RNase L activation by itself would lead to autophagy, cells were transfected with a 2′-5′ oligoadenylate activator of RNase L (2′-5′p3A3) or with a dephosphorylated form (2′-5′A3) which does not activate RNase L (6). The human ovarian carcinoma cell line, Hey1b, was selected for these studies because it expresses relatively high levels of RNase L. Activation of RNase L in intact Hey1b cells was monitored by rRNA cleavage products generated from intact ribosomes (35). Whereas no rRNA cleavage products were observed in cells treated with inactive dephosphorylated 2′-5′A3, specific rRNA cleavage products were apparent following 4 or 6 h treatment with 10 μM 2′-5′p3A3 (Fig. 3A). Interestingly, autophagy as determined by p62 degradation and LC3BI/LC3BII conversion was efficiently induced by the active form of 2-5A, 2′-5′p3A3, but not by the inactive form, 2′-5′A3, nor by the transfection reagent alone (Fig. 3B). P62 degradation and LC3BI/LC3BII conversion were increased after 4 to 6 h of 2-5A treatment. Increasing levels of autophagy were observed at concentrations of 2′-5′p3A3 ranging from 1 to 10 μM, as determined by monitoring p62 degradation and LC3BI/LC3BII conversion at 6 h (Fig. 3C). Autophagic flux was examined by transfecting Hey1b cells with 2-5A following continuous CQ treatment. 2-5A treatment in the presence of CQ prevented p62 decay while significantly increasing levels of accumulated LC3BII compared to the CQ or 2-5A treatment alone (Fig. 3D). These results show that activation of RNase L by itself causes autophagy.
Fig 3.
Direct activation of RNase L by 2-5A induces autophagy in Hey1b cells. (A) Cells were treated with 10 μM inactive 2-5A (2′-5′A3) or active 2-5A trimer (2′-5′ p3A3) for the indicated times, and rRNA cleavage was observed in RNA chips. M, RNA ladder markers (Agilent). (B) Cells were treated with 10 μM 2′-5′A3 or 2′-5′p3A3 for the indicated times, and induction of autophagy was monitored by visualizing P62 and LC3BII levels in immunoblots (top). Average levels of P62 and LC3BII are shown normalized to β-actin levels (middle and bottom). (C) Cells were transfected with the indicated concentrations of 2′-5′p3A3 for 6 h and P62, LC3BII and β-actin levels were measured from immunoblots as in panel B. (D) Immunoblots for P62, LC3B, and β-actin from Hey1b cells treated with chloroquine (CQ) beginning 2 h prior to mock transfection or transfection with 2′-5′p3A3 and continuing for an additional 6 h, analyzed as for panel B. Graphs were constructed by using three independent biological replicates. Error bars (SD) and P values determined by two-tailed Student's t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001) are shown. C, control with transfection reagent only.
Morphological evidence that RNase L activation induces autophagy.
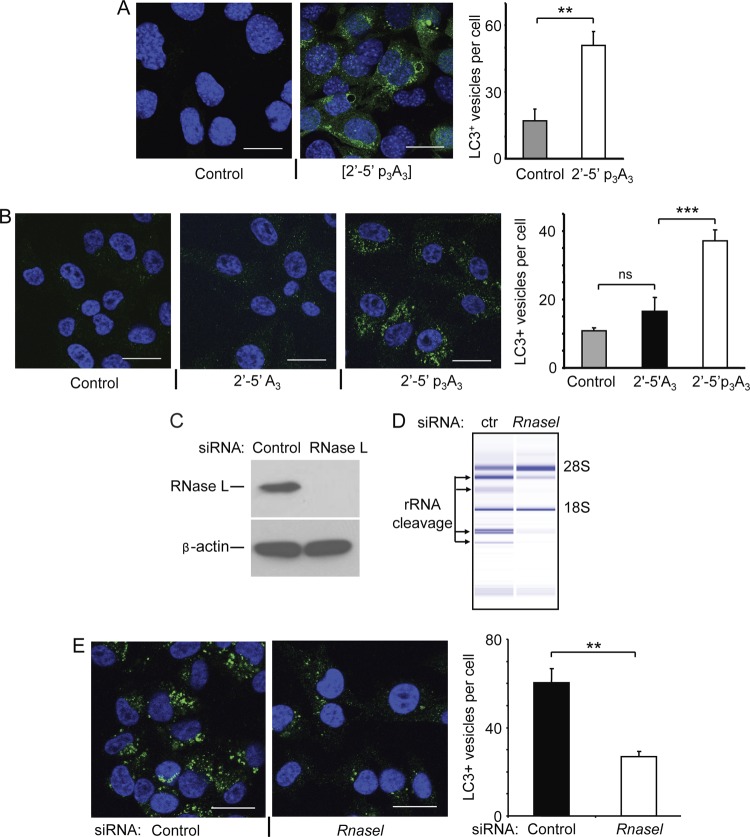
Induction of autophagy in intact cells is often monitored by visualizing punctate structures in the cytoplasm. Punctate bodies are a well established marker of autophagy resulting from conversion of the autophagy factor LC3BI (ATG8) to its phosphatidylethanolamine-conjugated form LC3BII, leading to its association with the autophagosome membrane (13). Direct activation of RNase L in WT MEF expressing GFP-LC3B (27) by transfection with 2′-5′p3A3 induced autophagy, as observed by the formation green puncta compared to the vehicle control (nuclei were stained in blue with DAPI) (Fig. 4A). Moreover, quantitation of LC3-positive puncta reveals that transfection of 2′-5′p3A3 results in threefold-greater numbers of punctate structures compared to the vehicle control.
Fig 4.
2-5A treatment results in a punctate structure characteristic of autophagy. (A) MEF expressing GFP-LC3 were either mock transfected (control) or treated with 2′-5′p3A3 (10 μM) for 6 h as indicated. Cells were fixed, nuclei were stained with DAPI (blue), and the GFP-LC3 punctate structures were visualized under confocal microscope at a magnification of ×63. Average numbers of LC3-positive vesicles per cell were quantified by analyzing control and 2-5A-treated cells from three biological replicates. (B) Hey1b cells were either mock transfected (control) or transfected with 10 μM inactive 2-5A [2′-5′A3] or active 2-5A trimer [2′-5′ p3A3] for 6 h as indicated. Cells were fixed and immunostained with antibody specific to LC3B (green), and the nuclei were stained with DAPI (blue) and visualized under a confocal microscope at a ×63 magnification. The numbers of LC3-positive vesicles per cell are averages from three biological replicates. (C) RNase L levels determined by immunoblotting in Hey1b cells treated with siRNA against Rnasel or control siRNA. (D) RNase L activity by rRNA cleavage assays was determined in control or siRNA-treated Hey1b cells transfected with 10 μM 2′-5′p3A3 for 6 h. (E) Hey1b cells treated as for panel D were fixed and immunostained with LC3B antibody (green), and nuclei were stained with DAPI (blue) and visualized with a confocal microscope at a ×63 magnification. Average numbers of LC3-positive vesicles per cell were quantified from three biological replicates. Error bars (SD) and P values determined by two-tailed Student's t test (**, P < 0.01; ***, P < 0.001; ns, not significant) are shown. Bars, 25 μm.
Similarly, Hey1b cells were treated with inactive and active forms of 2-5A and the endogenous level of LC3B-positive punctate structures were visualized by immunostaining the cells with rabbit monoclonal antibody against LC3B that preferentially binds to the LC3BII form. The green punctate structures corresponding to the LC3B-positive autophagic vesicles show induction of autophagy following 2′-5′p3A3 treatment but not when cells were treated with either the vehicle control or inactive dephosphorylated 2-5A (2′-5′A3) (Fig. 4B). Further, quantification revealed that there was more than a threefold increase in numbers of LC3B-positive vesicles in 2′-5′p3A3-treated cells compared to the control cells. No significant difference in numbers of LC3B-positive vesicles was observed between control cells and cells transfected with inactive 2-5A (Fig. 4B). To further establish the role of RNase L in autophagy, we depleted RNase L in Hey1B cells by siRNA treatment. RNase L was depleted to undetectable levels in the siRNA treated cells, as determined by immunoblotting with antibody against RNase L (Fig. 4C). Activation of RNase L by 2-5A transfection was monitored by rRNA cleavage products in RNA chips. There was effective activation of RNase L by 2-5A in the control cells but not in the cells treated with siRNA against RNase L (Fig. 4D). Furthermore, decreased numbers of green punctate structures corresponding to the LC3B-positive autophagic vesicles were observed in cells treated with siRNA against Rnasel and later transfected with 2-5A (Fig. 4E). Quantitation of LC3-positive puncta reveals that Rnasel knockdown decreased numbers of LC3B-positive vesicles more than twofold.
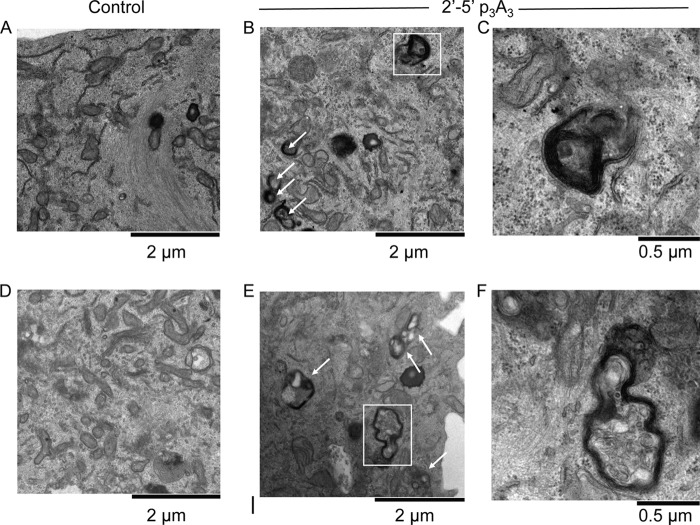
To extend the morphological analyses, we sought to visualize the ultrastructure of autophagosomes. Accordingly, Hey1b cells were examined by transmission electron microscopy following 2′-5′p3A3 treatment (Fig. 5). It was readily apparent that double-membraned autophagosomes were induced by 2′-5′p3A3 treatment compared to the vehicle control as shown from two different fields at a magnification of ×22000 (Fig. 5A, B, D, and E). It was also observed that many of the autophagosomes had already fused with lysosomes to form autolysosomes after 6 h of 2′-5′p3A3 treatment as visualized by dark cellular contents (representative of cellular bodies of low pH) either fused or partially encapsulated by double membrane vesicles (Fig. 5C and F). In addition, autophagocytosed intracellular membranes were sometimes visible following 2′-5′p3A3 treatment (Fig. 5C). Cumulatively, our microscopic studies correlates with our biochemical results showing that activation of RNase L by 2′-5′p3A3 leads to autophagy with formation of characteristic morphological structures.
Fig 5.
Electron micrographs showing autophagosomes and autolysosomes in 2-5A-treated Hey1b cells. (A and D) Mock-transfected controls; (B and E) 2′-5′p3A3-treated cells after 6 h (magnification, ×22,000). (C and F) Representative autolysosome (C) and autophagosome (F) from 2′-5′p3A3-treated cells from panels B and E, respectively (magnification, ×66,000). Arrows show autophagosomes.
Autophagy induction by RNase L modulates viral growth.
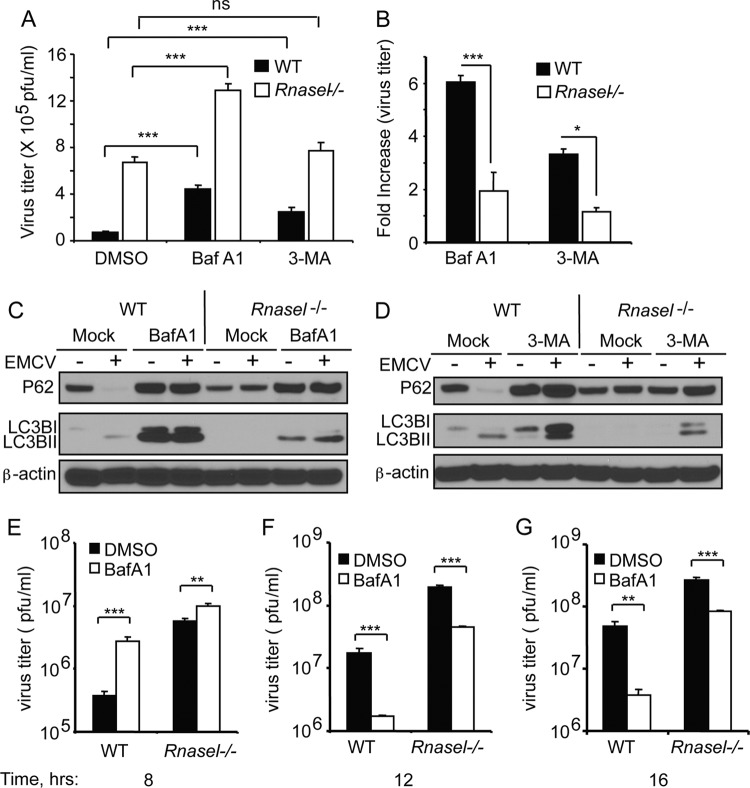
Because RNase L is an important mediator of cellular antiviral activity, we sought to determine if the antiviral mechanism of RNase L involves autophagy. Therefore, EMCV infections of MEF were performed in the absence or presence of chemical inhibitors of autophagy, BafA1 (which blocks vacuolar proton ATPases and prevents fusion of autophagosomes and lysosomes) (42) and 3-MA (an inhibitor of class III PI3 kinase-dependent formation of macroautophagy) (41). Initial experiments were performed at a low MOI (0.05) for a single cycle of virus replication (8 h) (Fig. 6A to D). EMCV replication was enhanced in Rnasel−/− MEF compared to WT MEF, as we reported previously (46). Interestingly, viral yields were increased by BafA1 or 3-MA treatments in both WT and Rnasel−/− MEF (Fig. 6A and B). However, the increase in viral titers was significantly higher in WT MEF (6-fold with BafA1 and 3.5-fold with 3-MA) than in Rnasel−/− MEF (2-fold with BafA1 and no significant difference with 3-MA) (Fig. 6B). The efficacy of the inhibitors was verified by immunoblotting for P62 and LC3B (Fig. 6C and D). Also, as expected BafA1 treatment inhibited P62 breakdown and caused accumulation of LC3B, whereas 3-MA treatment also inhibited P62 degradation while impairing conversion of LC3BI to LC3BII (Fig. 6C and D). To determine effects of RNase L on autophagy during the course of virus replication, viral yields were measured after 8, 12, and 16 h in WT and Rnasel−/− MEF in presence or absence of BafA1 (Fig. 6E and G and Table 1). By 8 h postinfection, about 2 h after completion of the initial round of virus replication, RNase L suppressed viral yields 14.7- and 3.6-fold in the absence or presence of BafA1, respectively (Table 1). At that time, BafA1 treatment enhanced viral yields 7.1-fold and 1.75-fold in WT and Rnasel−/− MEF, respectively. Therefore, during the first round of virus replication autophagy induced by RNase L suppressed virus growth. However, by 12 h postinfection RNase L suppressed virus growth by 11.2- and 26.5-fold in the absence or presence of BafA1, respectively. In addition, by 12 h postinfection, BafA1 treatment suppressed viral yields 10-fold and 4.2-fold in WT and Rnasel−/− MEF, respectively. By 16 h, upon completion of the second round of infection after all cells had lysed from infection, RNase L had suppressed virus growth by 5.4-fold and 22.4-fold in the absence or presence of BafA1, respectively. Finally, by 16 h, BafA1 treatment inhibited viral yields by 12.5-fold and 3-fold in the WT and Rnasel−/− MEF, respectively. Therefore, during the second round of virus replication autophagy promoted virus replication, thereby reducing the antiviral effect of RNase L.
Fig 6.
Suppression of autophagy by chemical inhibitors modulates the antiviral activity of RNase L. (A and B) WT and Rnasel−/− MEF were treated with BafA1 (200 nM) or 3-MA (5 mM) for 2 h prior to infection with EMCV (MOI = 0.05). Viral yields and increases in viral titers were determined from four biological replicates. Error bars, SD; DMSO, dimethyl sulfoxide (solvent control). (C and D) Inhibition of autophagy as described for panel A by BafA1 and 3-MA, respectively, was determined by monitoring P62 and LC3B in immunoblots. (E to G) WT and Rnasel−/− MEF were treated with BafA1 (200 nM) for 2 h prior to infection with EMCV (MOI = 0.05) for different times. Viral titers (error bars, SD) were determined from three biological replicates. **, P < 0.01; ***, P < 0.001; ns, not significant.
Table 1.
Effects of RNase L and autophagy on viral growtha
| Time (h) | Viral yield (PFU/ml) in: |
Change in viral yield (WT MEF/Rnasel−/− MEF) |
||||||
|---|---|---|---|---|---|---|---|---|
| WT MEF |
Rnasel−/− MEF |
RNase L effect |
Autophagy effect |
|||||
| DMSO (a) | BafA1 (b) | DMSO (c) | BafA1 (d) | −BafA1 (c/a) | +BafA1 (d/b) | WT MEF (b/a) | Rnasel−/− MEF (d/c) | |
| 8 | 3.8 × 105 | 2.7 × 106 | 5.6 × 106 | 9.8 × 106 | 14.7 | 3.6 | 7.1 | 1.75 |
| 12 | 1.7 × 107 | 1.7 × 106 | 1.9 × 108 | 4.5 × 107 | 11.2 | 26.5 | 0.1 | 0.24 |
| 16 | 4.8 × 107 | 3.8 × 106 | 2.6 × 108 | 8.5 × 107 | 5.4 | 22.4 | 0.08 | 0.33 |
Results are averages from three biological replicates. Virus titers and statistical parameters are from the experiments whose results are shown in Fig. 6E to G.
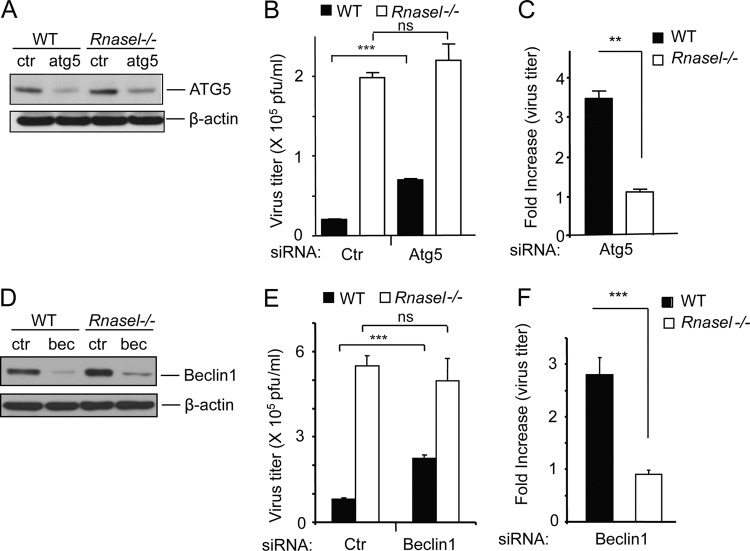
To confirm the role of autophagy in the antiviral activity of RNase L in the initial round of infection, the autophagy-related proteins ATG5 (1, 19) and beclin-1 (24, 30) were depleted with siRNAs in WT and Rnasel−/− MEF (Fig. 7A and D). As expected, EMCV replication after a single cycle of viral replication was enhanced up to 10-fold in Rnasel−/− MEF compared to WT MEF in the presence of nontargeted control siRNA. In accordance with the inhibitor data, depleting cells of ATG5 and beclin-1 levels with siRNAs enhanced EMCV replication 3.5- and 2.7-fold, respectively, in WT MEF (Fig. 7B and E). In contrast, there was little or no effect of downregulating levels of ATG5 and beclin-1 in the Rnasel−/− MEF. These results suggest that autophagy contributes to the antiviral mechanism of RNase L at early times after infection.
Fig 7.
Depletion of ATG5 and beclin-1 by siRNA treatments impairs the antiviral activity of RNase L. (A and D) Effects of siRNA against ATG5 mRNA and beclin-1 (bec) mRNA, respectively, in WT and Rnasel−/− MEF as determined in immunoblots in comparison to β-actin levels. Ctr, nontargeted siRNA control. (B and E) After siRNA or control (ctr) siRNA treatments, cells were infected with EMCV (MOI = 0.05). Viral yields were measured at 8 h postinfection. (C and F) Increases in the viral titers presented in panels B and E were determined by comparing viral yields between siRNA- and control siRNA-treated cells. Values and SD (error bars) were determined from three biological replicates. **, P < 0.01; ***, P < 0.001; ns, not significant.
DISCUSSION
RNase L activation during viral infections enhances autophagy and modulates virus growth.
Induction of autophagy in response to viral infections involves different PRRs, including PKR (37, 38), Toll-like receptors (TLRs) (20), and RIG-I-like receptors (12, 14, 36). OAS-1, -2, and -3 are PRRs activated by dsRNA from the infecting virus (reviewed in reference 18). dsRNA stimulates OAS to produce 2-5A that activates RNase L. Here we show for the first time that RNase L activation enhances autophagy during viral infections. Two different types of RNA viruses were used in this study, a positive-strand virus in the family Picornaviridae (EMCV) and a negative-strand virus in the family Rhabdoviridae (VSV). While other types of viruses were not investigated here, we predict that RNase L contributes to autophagy in response to infections by different viruses that stimulate the OAS/RNase L system. The fact that viral induction of autophagy was reduced in cells expressing the nuclease-dead mutant of RNase L, R667A, strongly implicates RNA cleavage as the triggering event. We also show that direct activation of RNase L by 2-5A causes autophagy. While the specific RNA cleavage events that lead to autophagy are unknown, cleavage of rRNA in intact ribosomes is a possible initiating event. Damaged ribosomes are sometimes packaged into autophagosomes by a process known as ribophagy (17). In this regard, we did observe a correlation between rRNA cleavage caused by RNase L and autophagy. It is possible that autophagy induced by rRNA cleavage following viral infection would promote transportation of viral particles into autophagosomes for lysosomal degradation. Alternatively, small RNAs produced by RNase L might induce autophagy by interacting with a PRR that interacts with autophagy-related proteins (12). Interestingly, both autophagy and the OAS/RNase L system may be viewed as cellular salvaging or recycling pathways. Autophagy results in the degradation of cellular components, while the OAS/RNase L pathway contributes to the breakdown of RNA and ribonucleoprotein complexes, in particular the ribosome. It is therefore perhaps not too surprising that autophagy and OAS/RNase L are interconnected cellular pathways that function during the host antiviral response.
Because autophagy promotes the replication of a wide range of different picornaviruses (reviewed in reference 16), including EMCV (44), we were surprised to observe that chemical inhibitors of autophagy or siRNA against ATG5 and beclin-1 increased rather than reduced EMCV yields during the initial virus replication cycle. It has been suggested that EMCV subverts autophagy during its infectious cycle by replicating its RNA on the surface of autophagosome-like vesicles (44). We have considered what could account for these differences from our study. Our data suggest that an antiviral function of autophagy may prevail depending on the specific EMCV strain, the cell type, and details of how the experiments were performed, including MOI and time postinfection when viral yields are measured. We used a different strain of EMCV (9) and different cell lines from the study that showed that autophagy promoted EMCV replication (44). In addition, we observed using BafA1 treatment of WT MEF that autophagy could either promote or inhibit virus growth depending on when virus was harvested. While autophagy reduced, up to 7-fold, EMCV yields after a single round of virus replication (completed by 8 h postinfection), autophagy increased viral yields at later times postinfection (12 and 16 h) (Fig. 6 and 7 and Table 1). Therefore, autophagy induced by RNase L was antiviral at early times of infection; however, at later times subversion of autophagy by EMCV prevailed to promote viral growth, thereby reducing the antiviral effect of RNase L.
It is possible that at early times of infection autophagy degrades viral proteins by the autolysosomal pathway, resulting in restriction of viral growth. However, perhaps cytokine induction during the first round of viral infection induces genes which, in the subsequent virus growth cycle, affect the dynamics between virus growth and autophagy, in turn allowing viral subversion of autophagy.
Involvement of autophagy in the antiviral activity of RNase L.
Several mechanisms have been proposed for the antiviral activity of the OAS/RNase L pathway (34). For viruses with single-stranded RNA genomes, a single RNA cleavage event will prevent the viral genome from being replicated. However, degradation of viral mRNAs could potentially suppress other types of viruses. Similarly, damage by RNase L to host machinery required for virus replication, in particular ribosomes, will suppress viral replication. Somewhat surprisingly, cellular (self) RNA cleavage products produced through the activity of RNase L can serve to amplify and perpetuate IFN production through the RIG-I or MDA5 signaling pathways (25). In addition, extensive cleavage of cellular RNAs by RNase L during acute infections cause apoptosis, which limits viral spread (2, 46). Our findings show a novel biological activity of RNase L as an inducer of autophagy. The implications are that RNase L not only causes degradation of single-stranded RNA but also contributes to the disposal of organelles, long-lived proteins, and microbes, perhaps including bacteria (23), during the host response to invading pathogens.
ACKNOWLEDGMENTS
These investigations were supported by U.S. Public Health Service grant CA044059 from the National Cancer Institute (to R.H.S.) and the Mal and Lea Bank Chair fund (to R.H.S.).
We thank Babal Kant Jha for the 2-5A and Christine McDonald for data analysis (Cleveland Clinic).
Footnotes
Published ahead of print 8 August 2012
REFERENCES
- 1. Boya P, et al. 2005. Inhibition of macroautophagy triggers apoptosis. Mol. Cell. Biol. 25:1025–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Castelli JC, et al. 1997. A study of the interferon antiviral mechanism: apoptosis activation by the 2-5A system. J. Exp. Med. 186:967–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chakrabarti A, Jha BK, Silverman RH. 2011. New insights into the role of RNase L in innate immunity. J. Interferon Cytokine Res. 31:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dong B, Niwa M, Walter P, Silverman RH. 2001. Basis for regulated RNA cleavage by functional analysis of RNase L and Ire1p. RNA 7:361–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dong B, Silverman RH. 1995. 2-5A-dependent RNase molecules dimerize during activation by 2-5A. J. Biol. Chem. 270:4133–4137 [DOI] [PubMed] [Google Scholar]
- 6. Dong B, et al. 1994. Intrinsic molecular activities of the interferon-induced 2-5A-dependent RNase. J. Biol. Chem. 269:14153–14158 [PubMed] [Google Scholar]
- 7. Dreux M, Chisari FV. 2010. Viruses and the autophagy machinery. Cell Cycle 9:1295–1307 [DOI] [PubMed] [Google Scholar]
- 8. Floyd-Smith G, Slattery E, Lengyel P. 1981. Interferon action: RNA cleavage pattern of a (2′-5′)oligoadenylate-dependent endonuclease. Science 212:1030–1032 [DOI] [PubMed] [Google Scholar]
- 9. Hoskins JM, Sanders FK. 1957. Propagation of mouse encephalomyocarditis virus in ascites tumour cells maintained in vitro. Br. J. Exp. Pathol. 38:268–272 [PMC free article] [PubMed] [Google Scholar]
- 10. Iwai-Kanai E, et al. 2008. A method to measure cardiac autophagic flux in vivo. Autophagy 4:322–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jha BK, et al. 2011. Inhibition of RNase L and RNA-dependent protein kinase (PKR) by sunitinib impairs antiviral innate immunity. J. Biol. Chem. 286:26319–26326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jounai N, et al. 2007. The Atg5 Atg12 conjugate associates with innate antiviral immune responses. Proc. Natl. Acad. Sci. U. S. A. 104:14050–14055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kabeya Y, et al. 2000. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19:5720–5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawai T, Akira S. 2009. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 21:317–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kerr IM, Brown RE. 1978. pppA2′p5′A2′p5′A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc. Natl. Acad. Sci. U. S. A. 75:256–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klein KA, Jackson WT. 2011. Picornavirus subversion of the autophagy pathway. Viruses 3:1549–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kraft C, Deplazes A, Sohrmann M, Peter M. 2008. Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nat. Cell Biol. 10:602–610 [DOI] [PubMed] [Google Scholar]
- 18. Kristiansen H, Gad HH, Eskildsen-Larsen S, Despres P, Hartmann R. 2011. The oligoadenylate synthetase family: an ancient protein family with multiple antiviral activities. J. Interferon Cytokine Res. 31:41–47 [DOI] [PubMed] [Google Scholar]
- 19. Kuma A, et al. 2004. The role of autophagy during the early neonatal starvation period. Nature 432:1032–1036 [DOI] [PubMed] [Google Scholar]
- 20. Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. 2007. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science 315:1398–1401 [DOI] [PubMed] [Google Scholar]
- 21. Levanon A, Kohn A, Inbar M. 1977. Increase in lipid fluidity of cellular membranes induced by adsorption of RNA and DNA virions. J. Virol. 22:353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levine B, Mizushima N, Virgin HW. 2011. Autophagy in immunity and inflammation. Nature 469:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li XL, et al. 2008. An essential role for the antiviral endoribonuclease, RNase-L, in antibacterial immunity. Proc. Natl. Acad. Sci. U. S. A. 105:20816–20821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liang XH, et al. 1999. Induction of autophagy and inhibition of tumorigenesis by beclin-1. Nature 402:672–676 [DOI] [PubMed] [Google Scholar]
- 25. Malathi K, Dong B, Gale M, Jr, Silverman RH. 2007. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 448:816–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malathi K, Paranjape JM, Ganapathi R, Silverman RH. 2004. HPC1/RNASEL mediates apoptosis of prostate cancer cells treated with 2′,5′-oligoadenylates, topoisomerase I inhibitors, and tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 64:9144–9151 [DOI] [PubMed] [Google Scholar]
- 27. Mizushima N, Kuma A. 2008. Autophagosomes in GFP-LC3 transgenic mice. Methods Mol. Biol. 445:119–124 [DOI] [PubMed] [Google Scholar]
- 28. Mizushima N, Yoshimori T, Levine B. 2010. Methods in mammalian autophagy research. Cell 140:313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Noda T, Ohsumi Y. 1998. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J. Biol. Chem. 273:3963–3966 [DOI] [PubMed] [Google Scholar]
- 30. Orvedahl A, et al. 2007. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 1:23–35 [DOI] [PubMed] [Google Scholar]
- 31. Orvedahl A, Levine B. 2009. Autophagy in mammalian antiviral immunity. Curr. Top. Microbiol. Immunol. 335:267–285 [DOI] [PubMed] [Google Scholar]
- 32. Orvedahl A, et al. 2010. Autophagy protects against Sindbis virus infection of the central nervous system. Cell Host Microbe 7:115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pankiv S, et al. 2007. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 282:24131–24145 [DOI] [PubMed] [Google Scholar]
- 34. Silverman RH. 2007. Viral encounters with 2′,5′-oligoadenylate synthetase and RNase L during the interferon antiviral response. J. Virol. 81:12720–12729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Silverman RH, Skehel JJ, James TC, Wreschner DH, Kerr IM. 1983. rRNA cleavage as an index of ppp(A2′p)nA activity in interferon-treated encephalomyocarditis virus-infected cells. J. Virol. 46:1051–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tal MC, Iwasaki A. 2009. Autophagic control of RLR signaling. Autophagy 5:749–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Talloczy Z, et al. 2002. Regulation of starvation- and virus-induced autophagy by the eIF2alpha kinase signaling pathway. Proc. Natl. Acad. Sci. U. S. A. 99:190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Talloczy Z, Virgin HW, IV, Levine B. 2006. PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy 2:24–29 [DOI] [PubMed] [Google Scholar]
- 39. Wreschner DH, James TC, Silverman RH, Kerr IM. 1981. Ribosomal RNA cleavage, nuclease activation and 2-5A(ppp(A2′p)nA) in interferon-treated cells. Nucleic Acids Res. 9:1571–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wreschner DH, McCauley JW, Skehel JJ, Kerr IM. 1981. Interferon action–sequence specificity of the ppp(A2′p)nA-dependent ribonuclease. Nature 289:414–417 [DOI] [PubMed] [Google Scholar]
- 41. Xavier S, et al. 2010. BAMBI is expressed in endothelial cells and is regulated by lysosomal/autolysosomal degradation. PLoS One 5:e12995 doi:10.1371/journal.pone.0012995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang PM, et al. 2010. Inhibition of autophagy enhances anticancer effects of atorvastatin in digestive malignancies. Cancer Res. 70:7699–7709 [DOI] [PubMed] [Google Scholar]
- 43. Yordy B, Iwasaki A. 2011. Autophagy in the control and pathogenesis of viral infection. Curr. Opin. Virol. 1:196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang Y, Li Z, Xinna G, Xin G, Yang H. 2011. Autophagy promotes the replication of encephalomyocarditis virus in host cells. Autophagy 7:613–628 [DOI] [PubMed] [Google Scholar]
- 45. Zhou A, Hassel BA, Silverman RH. 1993. Expression cloning of 2-5A-dependent RNAase: a uniquely regulated mediator of interferon action. Cell 72:753–765 [DOI] [PubMed] [Google Scholar]
- 46. Zhou A, et al. 1997. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. EMBO J. 16:6355–6363 [DOI] [PMC free article] [PubMed] [Google Scholar]