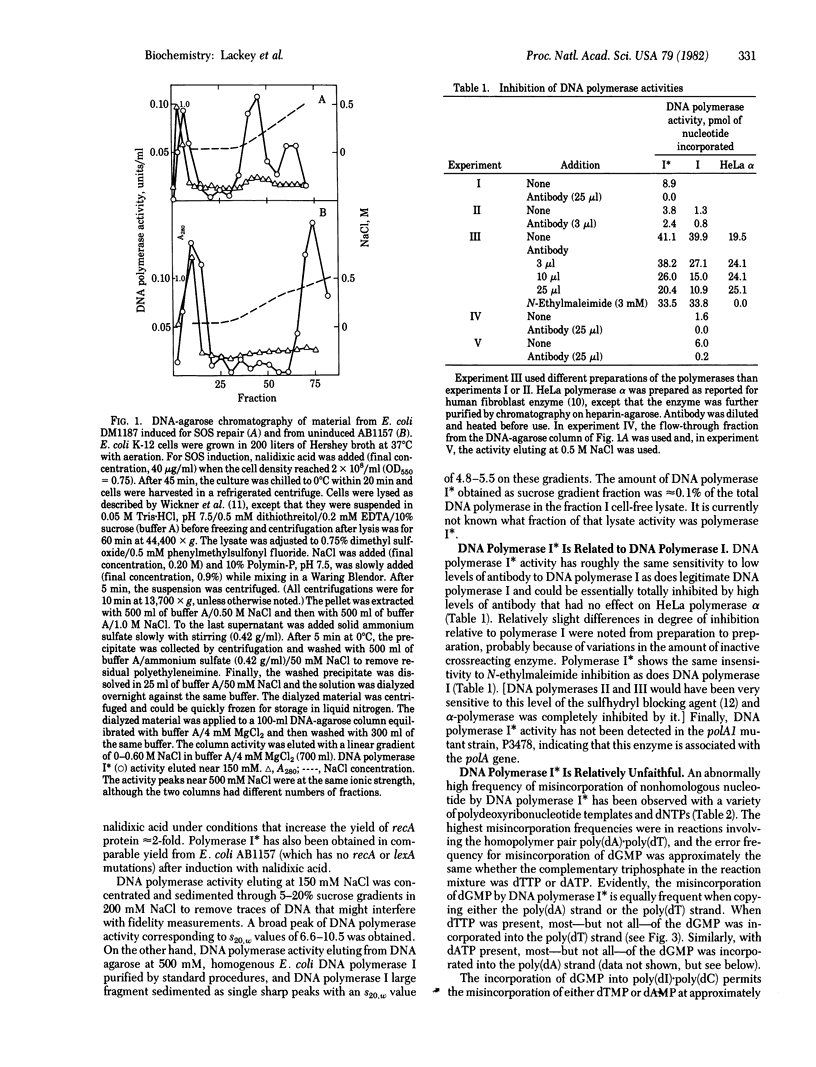
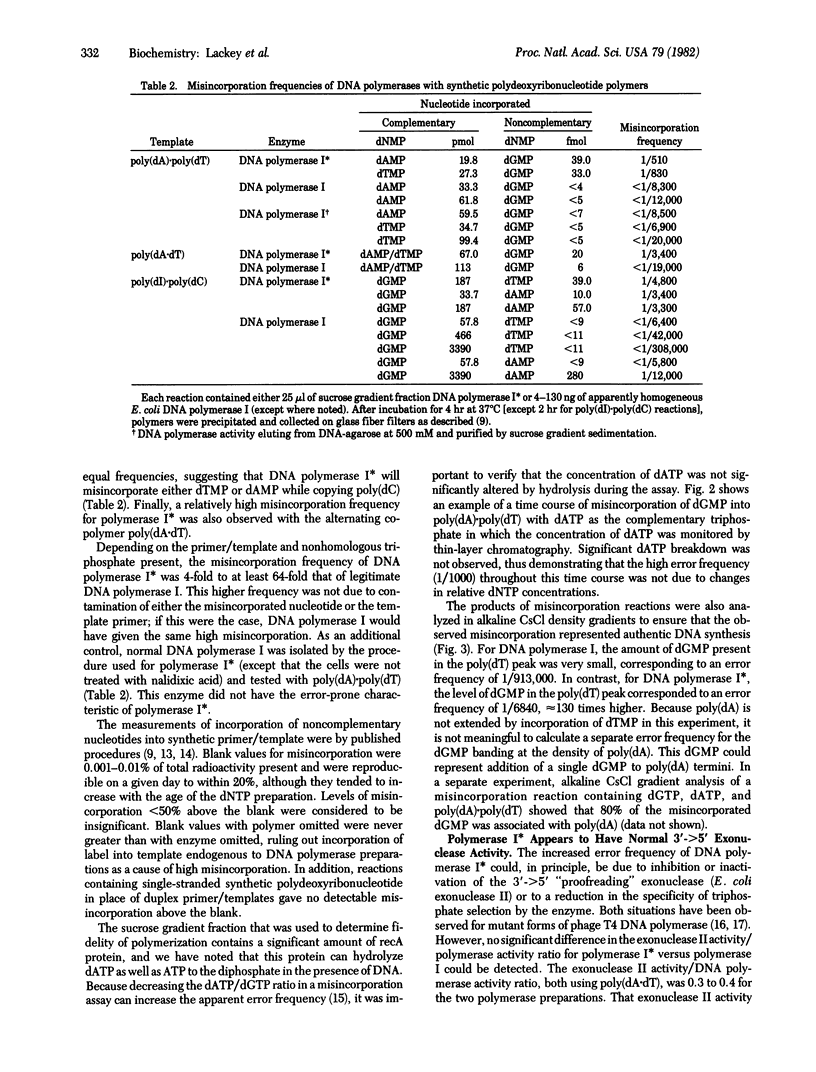
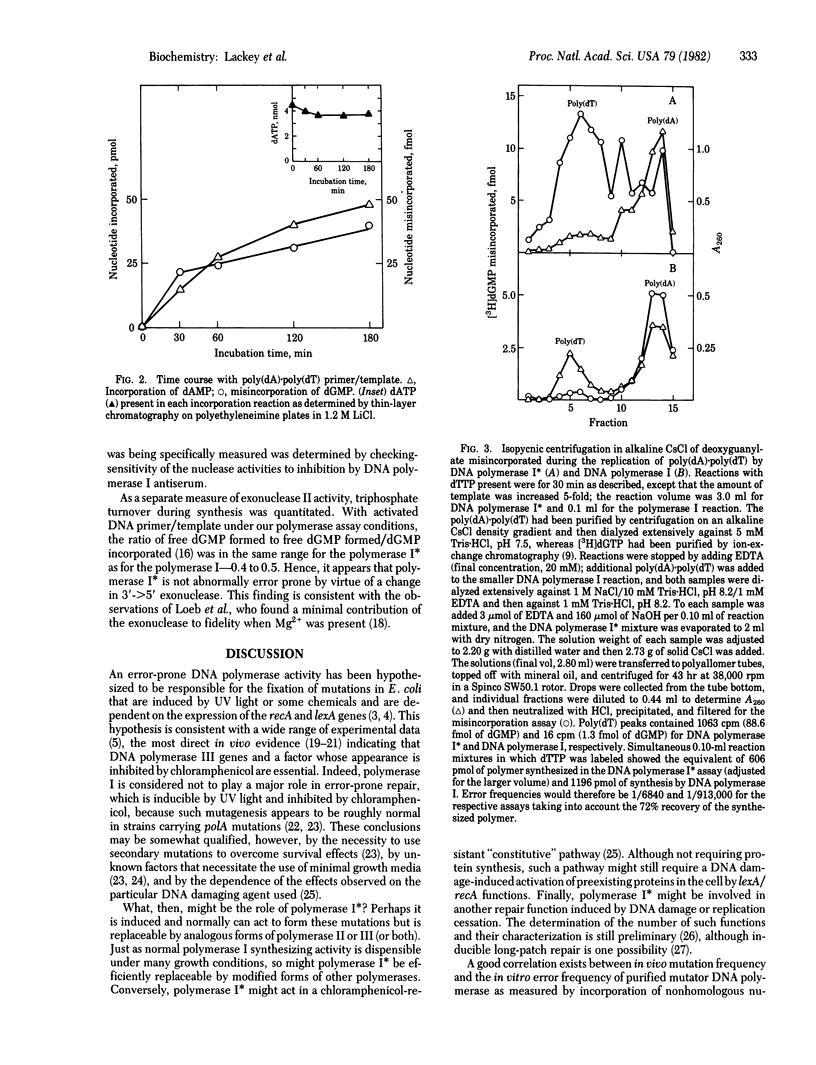
Abstract
A novel form of DNA polymerase I (deoxynucleosidetriphosphate:DNA deoxynucleotidyltransferase, DNA nucleotidyltransferase, EC 2.7.7.7) activity has been isolated from Escherichia coli cells that had been activated for expression of the DNA damage-inducible genes. Induction was by treatment of normal cells or cells carrying the spr-51 and tif-1 mutations with nalidixic acid. This activity, DNA polymerase I, seems to be a form of DNA polymerase I because it is insensitive to N-ethylmaleimide, is inhibited by antibody to DNA polymerase I, and does not appear in a polA1 strain. DNA polymerase I activity sediments through sucrose gradients as a broad peak with s20.w = 6.6--10.5, compared with an s20,w = 4.8--5.5 for DNA polymerase I. The fidelity during polymerization reactions of DNA polymerase I is relatively low with a variety of synthetic templates and deoxynucleoside triphosphates, although the enzyme appears to have a normal level of 3' greater than 5' exonuclease. Polymerase I has properties that might implicate it in some form of mutagenic DNA repair.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal S. S., Dube D. K., Loeb L. A. On the fidelity of DNA replication. Accuracy of Escherichia coli DNA polymerase I. J Biol Chem. 1979 Jan 10;254(1):101–106. [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barfknecht T. R., Smith K. C. The involvement of DNA polymerase I in the postreplication repair of ultraviolet radiation-induced damage in Escherichia coli K-12. Mol Gen Genet. 1978 Nov 16;167(1):37–41. doi: 10.1007/BF00270319. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. P. Mutagenic DNA repair in Escherichia coli. VIII. Involvement of DNA polymerase III in constitutive and inducible mutagenic repair after ultraviolet and gamma irradiation. Mol Gen Genet. 1978 Jun 1;162(1):35–41. doi: 10.1007/BF00333848. [DOI] [PubMed] [Google Scholar]
- Caillet-Fauquet P., Defais M., Radman M. Molecular mechanisms of induced mutagenesis. Replication in vivo of bacteriophage phiX174 single-stranded, ultraviolet light-irradiated DNA in intact and irradiated host cells. J Mol Biol. 1977 Nov 25;117(1):95–110. doi: 10.1016/0022-2836(77)90025-0. [DOI] [PubMed] [Google Scholar]
- Defais M., Caillet-Fauquet P., Fox M. S., Radman M. Induction kinetics of mutagenic DNA repair activity in E. coli following ultraviolet irradiation. Mol Gen Genet. 1976 Oct 18;148(2):125–130. doi: 10.1007/BF00268375. [DOI] [PubMed] [Google Scholar]
- Hall J. D., Mount D. W. Mechanisms of DNA replication and mutagenesis in ultraviolet-irradiated bacteria and mammalian cells. Prog Nucleic Acid Res Mol Biol. 1981;25:53–126. doi: 10.1016/s0079-6603(08)60483-3. [DOI] [PubMed] [Google Scholar]
- Hall Z. W., Lehman I. R. An in vitro transversion by a mutationally altered T4-induced DNA polymerase. J Mol Biol. 1968 Sep 28;36(3):321–333. doi: 10.1016/0022-2836(68)90158-7. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C., Cooper P. K., Smith C. A. Repair replication schemes in bacteria and human cells. Prog Nucleic Acid Res Mol Biol. 1981;26:181–196. doi: 10.1016/s0079-6603(08)60404-3. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S. On the role of deoxyribonucleic acid polymerase in determining mutation rates. Characterization of the defect in the T4 deoxyribonucleic acid polymerase caused by the ts L88 mutation. J Biol Chem. 1973 Feb 25;248(4):1417–1423. [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S., Ichikawa H., Iwo K., Kato T. Base-change mutagenesis and prophage induction in strains of Escherichia coli with different DNA repair capacities. Genetics. 1970 Oct;66(2):187–217. doi: 10.1093/genetics/66.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S. W., Linn S. Fidelity of fractionated deoxyribonucleic acid polymerases from human placenta. Biochemistry. 1980 Jan 8;19(1):220–228. doi: 10.1021/bi00542a033. [DOI] [PubMed] [Google Scholar]
- Linn S., Kairis M., Holliday R. Decreased fidelity of DNA polymerase activity isolated from aging human fibroblasts. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2818–2822. doi: 10.1073/pnas.73.8.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A., Dube D. K., Beckman R. A., Koplitz M., Gopinathan K. P. On the fidelity of DNA replication. Nucleoside monophosphate generation during polymerization. J Biol Chem. 1981 Apr 25;256(8):3978–3987. [PubMed] [Google Scholar]
- Moore P. D., Bose K. K., Rabkin S. D., Strauss B. S. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1981 Jan;78(1):110–114. doi: 10.1073/pnas.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P., Strauss B. S. Sites of inhibition of in vitro DNA synthesis in carcinogen- and UV-treated phi X174 DNA. Nature. 1979 Apr 12;278(5705):664–666. doi: 10.1038/278664a0. [DOI] [PubMed] [Google Scholar]
- Mount D. W. A mutant of Escherichia coli showing constitutive expression of the lysogenic induction and error-prone DNA repair pathways. Proc Natl Acad Sci U S A. 1977 Jan;74(1):300–304. doi: 10.1073/pnas.74.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray V., Holliday R. Increased error frequency of DNA polymerases from senescent human fibroblasts. J Mol Biol. 1981 Feb 15;146(1):55–76. doi: 10.1016/0022-2836(81)90366-1. [DOI] [PubMed] [Google Scholar]
- Muzyczka N., Poland R. L., Bessman M. J. Studies on the biochemical basis of spontaneous mutation. I. A comparison of the deoxyribonucleic acid polymerases of mutator, antimutator, and wild type strains of bacteriophage T4. J Biol Chem. 1972 Nov 25;247(22):7116–7122. [PubMed] [Google Scholar]
- Schaller H., Nüsslein C., Bonhoeffer F. J., Kurz C., Nietzschmann I. Affinity chromatography of DNA-binding enzymes on single-stranded DNA-agarose columns. Eur J Biochem. 1972 Apr 24;26(4):474–481. doi: 10.1111/j.1432-1033.1972.tb01789.x. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G. Inducible error-prone repair in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2753–2757. doi: 10.1073/pnas.72.7.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



