Abstract
The pathogen Staphylococcus aureus undergoes phenotype switching in vivo from its normal colony phenotype (NCP) to a slow-growing, antibiotic-resistant small-colony-variant (SCV) phenotype that is associated with persistence in host cells and tissues. However, it is not clear whether phenotype switching is the result of a constitutive process that is selected for under certain conditions or is triggered by particular environmental stimuli. Examination of cultures of diverse S. aureus strains in the absence of selective pressure consistently revealed a small gentamicin-resistant SCV subpopulation that emerged during exponential-phase NCP growth and increased in number until NCP stationary phase. Treatment of replicating bacteria with the antibiotic gentamicin, which inhibited NCP but not SCV replication, resulted in an initial decrease in SCV numbers, demonstrating that SCVs arise as a consequence of NCP replication. However, SCV population expansion in the presence of gentamicin was reestablished by selection of phenotype-stable SCVs and subsequent SCV replication. In the absence of selective pressure, however, phenotype switching was bidirectional and occurred at a high frequency during NCP replication, resulting in SCV turnover. In summary, these data demonstrate that S. aureus phenotype switching occurs via a constitutive mechanism that generates a dynamic, antibiotic-resistant subpopulation of bacteria that can revert to the parental phenotype. The emergence of SCVs can therefore be considered a normal part of the S. aureus life cycle and provides an insurance policy against exposure to antibiotics that would otherwise eliminate the entire population.
INTRODUCTION
Phenotype switching from a normal to a slow-growing or dormant phenotype is a widely employed bet-hedging strategy to insure bacteria against environmental conditions that might otherwise eradicate the entire population (3, 17, 19, 37).
Staphylococcus aureus is responsible for a raft of superficial and invasive infections, including furuncles, tissue abscesses, bacteremia, and infective endocarditis (22). In spite of a robust immune response and apparently appropriate antimicrobial therapy, S. aureus frequently gives rise to chronic or recurrent infections (8, 22, 27, 30, 33, 34). The mechanisms by which S. aureus persists within host tissues are poorly understood but likely include both biofilm formation and intracellular residency, both of which confer a degree of protection against immune components and antimicrobial therapy (1, 9, 11, 23, 28).
Several clinical studies have linked S. aureus persistence with a phenotypic shift from the normal colony phenotype (NCP) to a slow-growing small-colony-variant (SCV) phenotype that exhibits reduced pigmentation and hemolysis (21, 30, 31, 33, 39). The clinical evidence for a role for SCVs in persistence is supported by a recent animal study that demonstrated the emergence of SCVs and concomitant loss of NCP in tissues (bone and kidney) over time using a persistent infection model (35).
The ability of SCVs to persist within host cells is hypothesized to be due to reduced expression of cytolytic toxins via downregulation of the quorum-sensing system Agr, which also results in enhanced expression of surface proteins associated with cell invasion (35, 36). As such, SCVs are better able to enter and persist within host cells than NCP S. aureus (11, 23, 36, 38, 39).
It appears that SCVs arise via mutations, often in genes associated with biosynthetic pathways (5, 7, 18, 31, 32). These mutations typically abrogate gene function and have been identified in genes involved in the biosynthesis of hemin, menadione, and thymidine (5, 7, 16, 18, 31, 32). As such, SCVs are typically auxotrophic, and the normal phenotype is restored upon supplementation of the culture medium with the appropriate catabolite. Furthermore, mutations of other genes have been shown to confer an SCV-like phenotype (12), and it is likely that several other pathways are involved in SCV formation, since SCVs exist with unknown auxotrophisms or for which no molecular basis has been elucidated (13, 15, 32). The nature of SCV-causing mutations are not well characterized but are often very unstable. As such, SCVs have high rates of reversion to the NCP, which is strongly selected for in vitro due to the increased growth rate of NCP S. aureus on laboratory media (24, 30, 35). It is also possible that some SCVs might be the product of phenotypic heterogeneity (persisters [2, 3]); however, there is currently no evidence for this, and persister bacteria do not usually replicate.
To examine the SCV phenotype in vitro and in animal model systems, stable mutants have been constructed to overcome the problem of SCV instability (4, 5, 38).
SCVs are often, but not always, resistant to aminoglycoside antibiotics such as gentamicin and frequently other classes of antimicrobial agents as well (24, 33). The ability to switch between phenotypes facilitates increased resistance to antibiotics without the fitness costs associated with maintenance of resistance genes (24).
Treatment of cultures with gentamicin has been reported to trigger the emergence and expansion of the SCV subpopulation (24). However, gentamicin also selects for SCVs via their increased MIC relative to NCP S. aureus. It was therefore not clear whether SCV emergence was due to selection for a preexisting SCV subpopulation or whether the antibiotic triggered switching from the NCP to SCV.
The aim of this work was to determine whether gentamicin-resistant SCVs comprise a naturally occurring subpopulation that expands under selective pressure or whether phenotype switching is triggered by environmental conditions such as exposure to gentamicin.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Wild-type (WT) S. aureus strains SH1000, N315, Wood 46, Newman, Col, and USA300 lac were cultured in 5 ml tryptic soy broth (TSB) in the presence or absence of gentamicin (0 to 2 μg ml−1 as described in Results) in 30-ml screw-cap tubes at 37°C with orbital shaking (180 rpm). S. aureus strains SH1001 (agr::tet) and SH1002 (sarA::kan) (14) were cultured as described for WT strains. Some strains were also cultured in 20-ml portions of TSB in 500-ml conical flasks with silicon foam covers and incubated at 180 rpm to ensure aeration. In those assays where a single strain was used, SH1000 was chosen, because it has been well characterized, with intact regulatory elements (14). It also has strong expression of pigment and hemolysins, aiding the identification of SCVs devoid of these features. Total staphylococcal CFU were enumerated by plating of serial dilutions of cultures onto tryptic soy agar (TSA). Total staphylococcal CFU refers to both NCP and SCV, since reversion from SCV to NCP frequently occurs on laboratory media. SCVs were isolated and enumerated by plating of serial dilutions onto TSA containing gentamicin (see below).
Isolation and enumeration of SCVs within S. aureus populations.
A selective medium that permitted SCV growth but not that of NCP (TSA containing 2 μg ml−1 gentamicin) was employed to isolate and enumerate SCVs within S. aureus populations. SCVs isolated via this method were phenotypically similar to those described previously, exhibiting significantly reduced colony size, pigmentation, and production of beta-hemolysin (see Fig. S1 in the supplemental material) (30, 31). To confirm that the organisms selected for were SCVs (rather than slow-growing resistant or tolerant mutants), stable variants were isolated and characterized further. All exhibited reduced growth rates, and several were auxotrophic for hemin, menadione, CO2, or fatty acids (data not shown). Such auxotrophic phenotypes have been reported previously in clinical samples (5, 13, 15, 30, 31). The molecular basis of CO2 and fatty acid auxotrophism is currently unknown but was identified by restoration of the NCP after growth in CO2 (5%) or on media supplemented with Tween 80 (0.01%), respectively (13, 15).
The majority of SCVs isolated using medium containing gentamicin reverted to the NCP when plated onto antibiotic-free TSA and cultured for 24 h (data not shown). Revertant bacteria did not display an enhanced MIC to gentamicin (data not shown).
Construction of Tetr S. aureus SH1000.
A tetracycline-resistant (Tetr) strain of S. aureus SH1000 was produced by transduction of a tetracycline resistance cassette from S. aureus RN4220 containing pTM304, which is integrated into the geh locus on the bacterial chromosome, using ϕ85 (40). This strain and plasmid are part of a phage-mediated transposon system but do not contain any transposable elements that might affect the mutation rate of the recipient (40). To ensure that acquisition of the tetracycline resistance cassette had no effect on growth or ability to produce SCVs, SH1000 Tetr was cultured in the presence or absence of gentamicin and the numbers of NCPs and SCVs determined (see Fig. S2 in the supplemental material). To ensure that tetracycline resistance did not provide a competitive disadvantage, equal numbers of SH1000 tetracycline-sensitive (Tets) and Tetr bacteria were used to inoculate TSB (5 ml), and the proportions of Tetr and Tets bacteria were determined after 24 h of incubation; serial dilutions of broth were plated onto TSA plates to obtain single colonies (50 to 100), which were subsequently patched onto TSA plates containing tetracycline, and the percentage of resistant colonies was determined (Fig. S2).
Phenotypic shift assay.
The shift between NCP and SCV during culture was assessed using WT S. aureus SH1000 Tets and SH1000 Tetr. TSB (5 ml) with or without gentamicin (2 μg ml−1) was inoculated with ∼5 × 106 WT Tets SH1000 (which included ∼10 SCVs). To this was added ∼50 SCV CFU derived from SH1000 Tetr. The Tetr SCVs were derived from a mixture of >500 Tetr SH1000 SCV colonies (isolated on gentamicin-containing solid medium from cultures grown in the absence of gentamicin) which were subsequently resuspended in 1 ml TSB containing 15% glycerol. The concentration of SCVs was determined by plating of serial dilutions, and the mixture was stored at −80°C in 100-μl aliquots to prevent SCV reversion to the NCP. When needed, an aliquot was thawed on ice, serial dilutions were made, and the appropriate dilution was used to add 50 SCV CFU to the culture medium.
This produced starter cultures whose SCV population was approximately 17% Tets and 83% Tetr, while the NCP population was >99.999% Tets. Cultures were incubated at 37°C for 24 h with shaking, and NCP and SCV bacteria were subsequently enumerated as described above. The proportion of each population that was Tetr was determined as detailed above.
Phenotype stability assay.
SCVs were isolated from cultures grown in the presence or absence of gentamicin by plating onto TSA containing gentamicin (2 μg ml−1). Colonies were picked and patched onto antibiotic-free TSA plates and incubated for 48 h at 37°C. Patches that maintained the SCV phenotype were classified as stable. Patches that were a mix of SCV and NCP colonies were defined as partially stable and those that exhibited a NCP were classified as revertant (see Fig. S3 in the supplemental material).
RESULTS
Gentamicin-resistant SCVs are present at low levels within S. aureus populations in the absence of selective pressure.
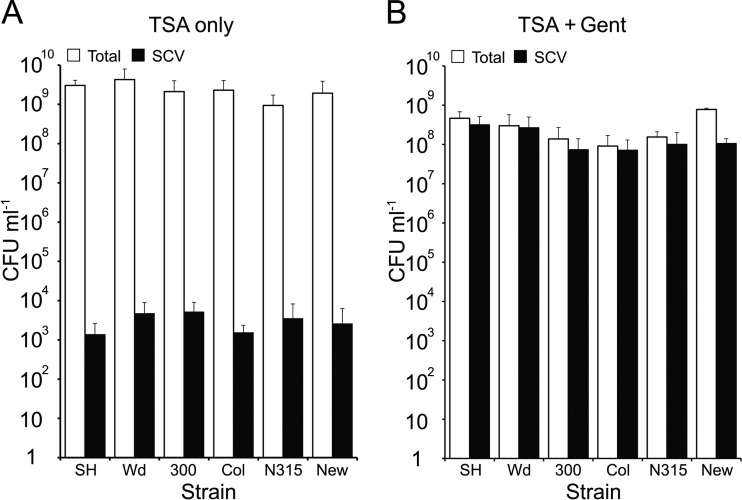
Examination of stationary-phase (24-h) broth cultures of diverse S. aureus strains using selective media revealed the presence of low levels (∼103 CFU ml−1) of gentamicin-resistant SCVs within the total population (∼3 × 109 CFU ml−1) (Fig. 1A). In contrast, culture of S. aureus in broth containing gentamicin (2 μg ml−1) resulted in populations with significantly larger SCV subpopulations (∼5 × 108 SCV CFU ml−1) (Fig. 1B). In keeping with previous reports (24), stationary-phase cultures treated with gentamicin contained large numbers of gentamicin-susceptible NCP S. aureus. This has previously been shown to be due to the acidification of the culture medium by SCVs, which occurs after several hours of SCV growth. The drop in pH renders the gentamicin ineffective and allows growth of gentamicin-susceptible NCPs in the presence of the antibiotic (25). To ensure that oxygenation levels of cultures grown in tubes did not affect the composition of the S. aureus populations, similar experiments were performed using conical flasks with silicon foam covers. As can be seen in Fig. S4 in the supplemental material, S. aureus cultured in this manner produced populations that grew to slightly higher densities but whose composition was essentially identical to those found in tubes in the presence or absence of gentamicin.
Fig 1.
Genetically diverse S. aureus cultures contain a small subpopulation of SCVs that expands under selective pressure. S. aureus strains SH1000 (SH), Wood (Wd), USA300 (300), Col, N315, and Newman (New) were grown in TSB in the absence or presence of gentamicin (Gent), and the total number of CFU and SCV CFU was determined. The bars represent mean averages of 8 independent cultures, and error bars represent the standard deviations of the means. In all cases, the number of SCVs in cultures after growth in the presence of gentamicin was significantly greater than those grown in the absence of antibiotic (P ≤ 0.0001).
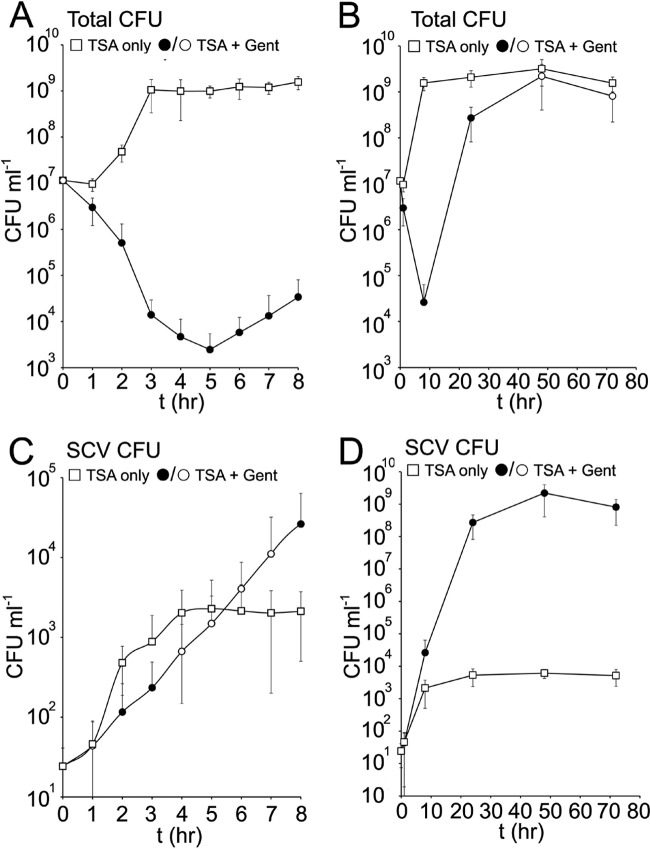
To elucidate the population dynamics during these 2 culture conditions, the size of the total and SCV populations was measured over time in the presence or absence of gentamicin. In the absence of the antibiotic, the total (NCP plus SCV) population expanded rapidly (between 1 and 3 h) with a mean generation time of ∼20 min (Fig. 2A). The SCV subpopulation also increased during this time, albeit at a lower rate (mean generation time of ∼38 min), up to approximately 2 × 103 CFU ml−1 (Fig. 2C). Once the total population entered stationary phase, the SCV subpopulation ceased expanding, and CFU counts remained stable for up to 72 h (Fig. 2B and D).
Fig 2.
The SCV subpopulation expands more slowly in the presence of gentamicin. S. aureus SH1000 was inoculated into TSB with or without gentamicin (Gent), and the total number of CFU and SCV CFU was determined over time (t) (shown in hours). Data were split into 2 graphs (0 to 8 h [A and C]; 0 to 72 h [B and D]) to improve resolution and highlight growth dynamics during the early and late stages of culture. Data points represent the averages of 8 independent cultures, and error bars represent the standard deviations of the means. Those data points from gentamicin-grown cultures that are significantly different (P ≤ 0.05) from the corresponding values in the gentamicin-negative cultures are represented by black symbols.
Inoculation of S. aureus into broth containing gentamicin resulted in an SCV population that expanded up to approximately 5 × 104 CFU ml−1 after 8 h and 5 × 108 CFU ml−1 after 24 h (Fig. 2C and D). However, the mean generation time of SCVs in gentamicin (∼48 min) was greater than that seen for SCVs in the absence of gentamicin. After 24 h, the composition of the populations remained stable for up to 72 h postinoculation (Fig. 2B and D).
The size of the SCV subpopulation is consistent and maintained.
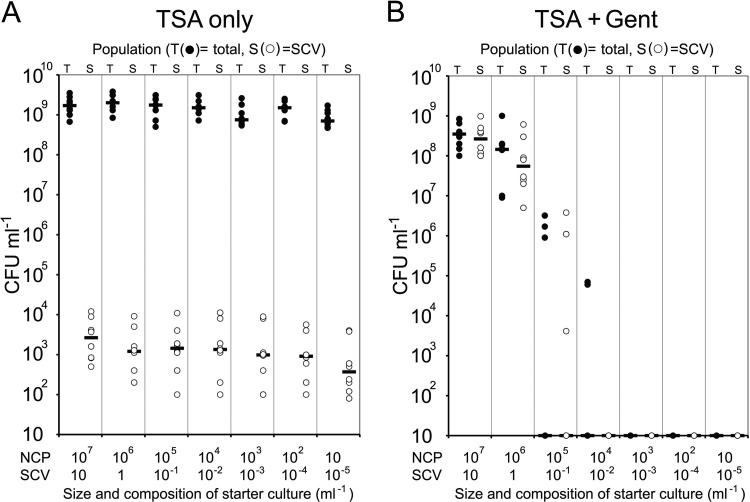
Because inocula used in the assays described above (Fig. 1 and 2) contained SCVs (approximately 10 SCV CFU ml−1 in the starter cultures), their emergence could be ascribed to simple expansion of a preexisting population. To determine whether the SCV subpopulation is actively generated and maintained in S. aureus populations, broths were inoculated with serial dilutions of a 24-h culture to the point at which no SCVs would be present and incubated for 24 h. In each case, S. aureus grew to approximately 3 × 109 CFU ml−1 (Fig. 3A). Despite the absence of SCVs in the most-dilute inocula, the stationary-phase broth cultures contained similar numbers of SCVs, although there was some variability between independent cultures (Fig. 3A). This suggests that not only are SCVs normally present in the absence of selective pressure, they are actively maintained at a certain level within the general S. aureus population. To examine whether SCV-deficient populations could expand in the presence of gentamicin, identical serial dilutions were used to inoculate broths containing the antibiotic (2 μg ml−1). Those diluted inocula that consistently contained SCVs (∼106 to 107 total CFU ml−1, 1 to 10 SCV ml−1) gave rise to populations that contained high levels of SCVs (Fig. 3B). In contrast, those inocula that did not contain SCVs (≤104 total CFU ml−1) were sterilized by the antibiotic, indicating that gentamicin selects for SCVs but does not trigger phenotype switching (Fig. 3B).
Fig 3.
SCVs appear in cultures grown from purely NCP inocula. Stationary-phase (16-h) S. aureus SH1000 cultures were serially diluted and used to inoculate TSB in the absence or presence of gentamicin. The approximate composition of each diluted inoculum is indicated. The cultures were incubated for 24 h, and the total CFU and SCV CFU was determined. Each data point represents the value for an independent culture. Bars represent the medians for the different groups of cultures. There was no significant difference in the total or SCV CFU counts between each of the inocula used to inoculate TSB without gentamicin (A). However, there were significant differences in CFU counts between TSB containing gentamicin inoculated with ≥106 CFU and those inoculated with ≤105 CFU (B). The limit of detection was 10 CFU ml−1.
SCVs emerge during NCP exponential-phase growth.
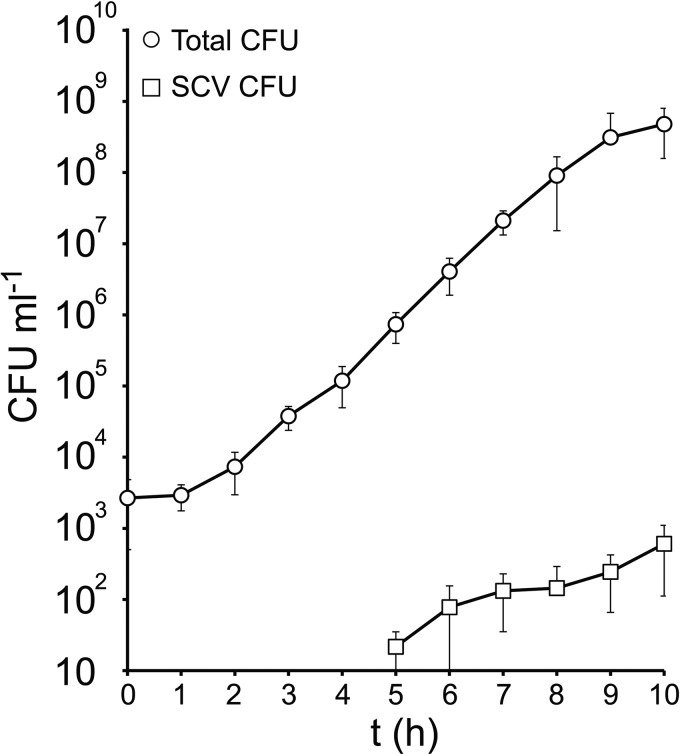
To determine when SCVs appeared in cultures that were inoculated with 100% NCP S. aureus, broths were inoculated with low concentrations of S. aureus such that SCVs would not be expected to be present (approximately 103 CFU ml−1, 1 in 1,000 chance of a single SCV being present). The broth cultures were incubated as described above, and the composition of the culture was then monitored over time. As expected, the total number of (predominantly NCP) S. aureus increased rapidly (Fig. 4). Although SCVs were not detected during the first few hours (1 to 4 h), they were consistently present at 5 h (in 7/8 independent cultures), albeit in different numbers. The size of the SCV subpopulation increased steadily during the remainder of the assay up to levels seen in previous experiments (Fig. 4).
Fig 4.
SCVs emerge during exponential-phase NCP growth. TSB was inoculated with a low number of S. aureus SH1000 bacteria (to reduce the probability of SCVs being present in the inoculum), and total and SCV CFU was measured over time. Data points represent the averages of 8 independent cultures. Error bars represent the standard deviations of the means. The limit of detection was 10 CFU ml−1.
SCV emergence requires NCP replication.
Because SCVs emerged and increased in number during exponential-phase growth, it was hypothesized that phenotype switching might be dependent upon replication of the NCP population, rather than a direct switch. To test this, cultures were grown to mid-exponential phase before the addition of gentamicin at a concentration that inhibited replication of NCP but not SCV S. aureus. Total and SCV populations were then enumerated (Fig. 5).
Fig 5.
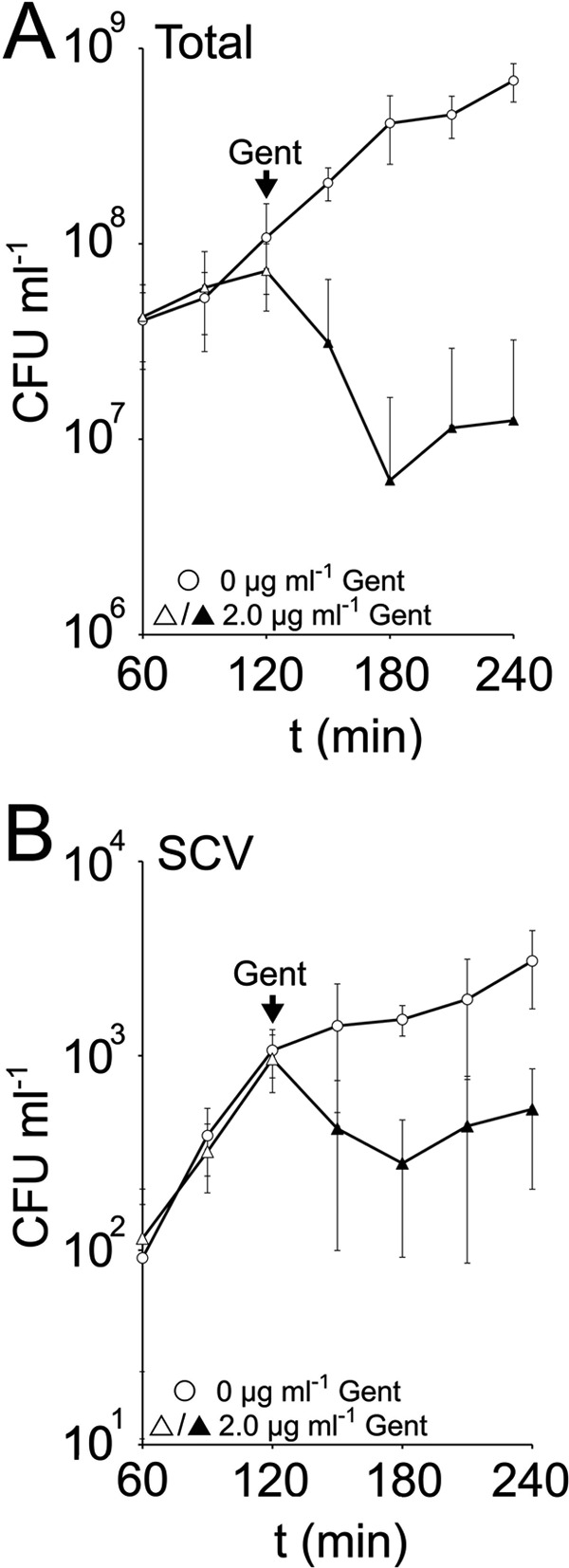
Expansion of the SCV subpopulation is primarily due to phenotype switching during NCP replication. TSB was inoculated with S. aureus SH1000, and the total and SCV CFU ml−1 was quantified over time. After 2 h, cultures were spiked with 0 or 2.0 μg ml−1 gentamicin (Gent), and measurements were continued. Data points represent the averages of 8 independent cultures. Error bars represent the standard deviations of the means. Those data points that are significantly (P ≤ 0.05) different from the culture without gentamicin are shown by black symbols.
Prior to the addition of gentamicin, the number of NCP and SCV S. aureus increased steadily over 2 h (Fig. 5). In the absence of gentamicin, the SCV subpopulation continued to increase over the following 30 min before the growth rate started to slow (Fig. 5B). In contrast, the addition of 2 μg ml−1 gentamicin resulted in a significant decrease in both total (NCP) CFU and a significant drop in the size of the gentamicin-resistant SCV subpopulation, presumably due to reversion to the gentamicin-susceptible NCP (Fig. 5). After 120 min of gentamicin treatment, the number of SCVs started to increase, and the NCP population stopped declining (Fig. 5).
This demonstrates that NCP replication drives expansion of the SCV subpopulation in the absence of selection and provides further evidence that gentamicin does not trigger phenotype switching.
Gentamicin selects for stable SCVs.
The data in Fig. 5 described above indicated that phenotype switching is a continuous process and occurs during NCP replication. However, this appeared to conflict with data in Fig. 2, which showed SCV population expansion despite NCP killing in the presence of gentamicin (Fig. 2).
Unstable gentamicin-resistant SCVs are likely to be killed during exposure to gentamicin when they revert to the gentamicin-susceptible NCP. Indeed, this explains the initial drop in SCV numbers when an antibiotic-free culture was exposed to gentamicin (Fig. 5). However, the SCV population eventually expands in the presence of gentamicin, presumably due to SCV replication (Fig. 2 and 5). As such, it was hypothesized that gentamicin selects for stable SCVs or at least those that revert less frequently than they replicate (partially stable). To test this, the stability of SCVs from cultures grown in the presence or absence of gentamicin was compared using a phenotype stability assay (Fig. 6).
Fig 6.
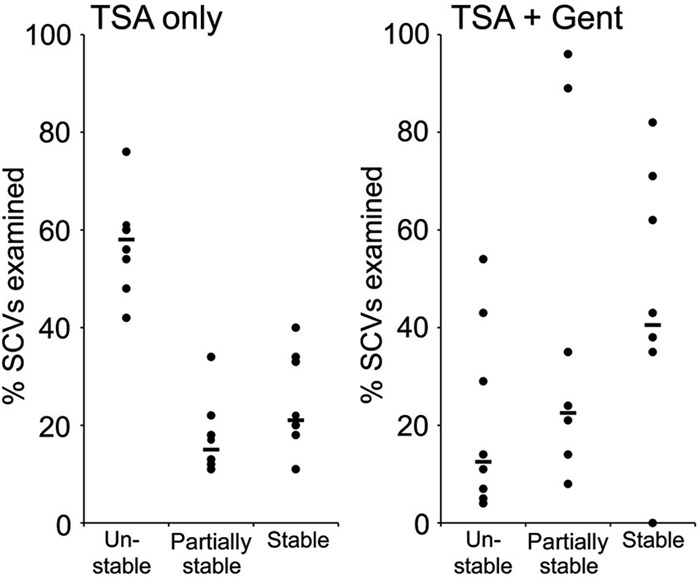
Gentamicin selects for stable SCVs. The cultures shown in Fig. 5 were grown for 20 h after they were spiked with gentamicin, and aliquots were plated onto TSA containing gentamicin to isolate SCVs. SCV colonies (50 to 100) were patched onto gentamicin-free TSA, and reversion to the NCP was assessed using a simple scoring system. Each data point represents the value for an independent culture. Bars represent the medians for the different groups. The number of unstable SCVs was significantly lower (P ≤ 0.01) in cultures grown with gentamicin. Data representing partial and fully revertant colonies were too variable to achieve statistical significance.
The stability of SCVs in the absence of gentamicin was largely consistent; approximately 60% were unstable (reverted to NCP), ∼20% were partially stable (some reversion), and ∼20% were stable (Fig. 6). In contrast, only ∼13% of SCVs from cultures treated with gentamicin were unstable. The proportion of stable and partially stable SCVs varied greatly in independent cultures, but median values were greater than those seen with SCVs from gentamicin-free cultures (Fig. 6). This indicates that gentamicin selects for SCVs with increased stability, which facilitates SCV population expansion via replication.
Quorum sensing is not required for SCV emergence.
Although SCV emergence was clearly linked with NCP replication, the consistent appearance of SCVs after 5 h (Fig. 4) raised the possibility that induction of the Agr quorum-sensing system (albeit at a low level) triggered phenotype switching. To test this, mutants defective in quorum sensing (agr) or the global regulator SarA (controls agr expression) were assessed to determine the relative sizes of their SCV subpopulations in the presence or absence of gentamicin (Fig. 7). In each case, the numbers of SCVs were similar to those seen with wild-type S. aureus, demonstrating that the Agr quorum-sensing system is not involved in triggering phenotype switching (Fig. 7).
Fig 7.
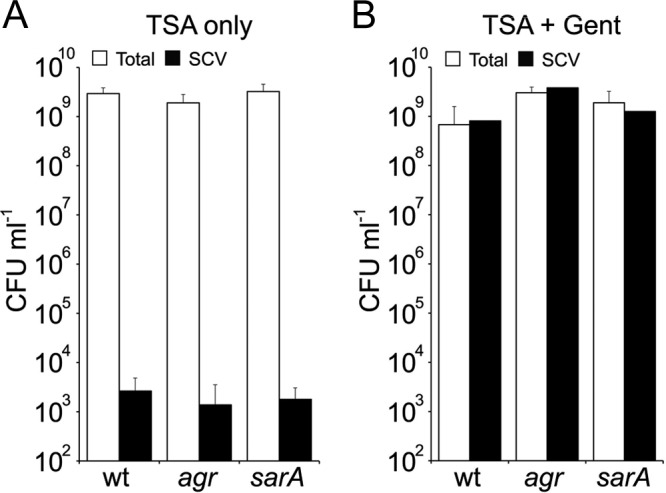
The quorum-sensing system Agr is not required for SCV emergence. S. aureus SH1000 (wild type [wt]), SH1001 (agr), and SH1002 (sarA) were cultured in TSB in the absence or presence of gentamicin, and the total and SCV CFU was quantified. Values for SH1001 and SH1002 were not significantly different from SH1000. The bars represent the averages of 8 independent cultures. Error bars represent the standard deviations of the means.
The SCV subpopulation is dynamic.
Previous data (Fig. 4 and 5) demonstrated that NCP replication was essential for the emergence and expansion of the SCV subpopulation. However, it was not clear to what extent the SCV subpopulation is the result of clonal expansion of a few SCVs that emerge during NCP replication or are the result of continuous switching between NCP and SCV.
To test this, broths were inoculated with a mixture of ∼50 Tetr (∼83% initial SCV population) and ∼10 Tets (∼17% initial SCV population) SCVs and approximately 106 Tets NCP CFU ml−1 (Fig. 8) and incubated for 24 h. The numbers of Tetr and Tets SCV were enumerated, along with the total population (Fig. 8). In the absence of gentamicin, both the total and SCV populations increased in size (Fig. 8A). However, the Tetr SCV population fell to approximately 11% of the total SCVs from the initial level of ∼83% (Fig. 8B). This correlated with a significant increase in Tetr bacteria in the total population (>200-fold increase), suggesting that the majority of the SCVs in the inoculum had reverted to the NCP and that the majority of the SCVs in the final subpopulation were derived from the Tets NCP population (Fig. 8).
Fig 8.
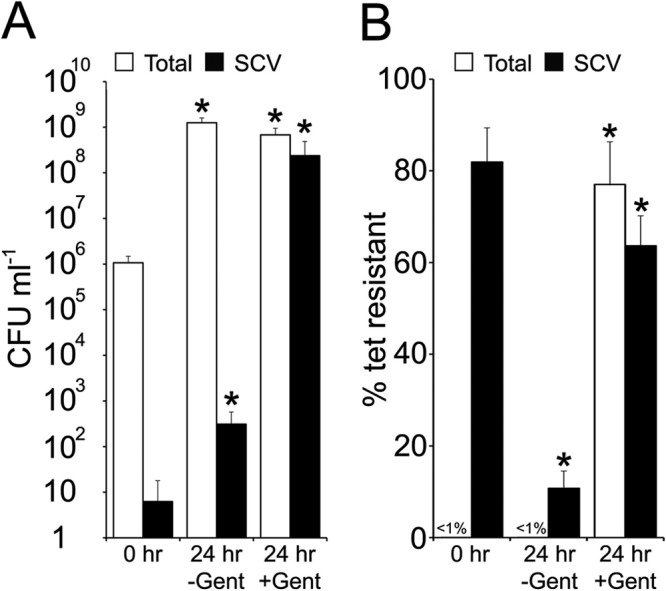
The SCV subpopulation is dynamic in the absence of selective pressure. Tets S. aureus SH1000 (106 CFU) was combined with Tetr SCVs and used to inoculate TSB in the absence (−) or presence (+) of gentamicin (Gent) and cultured for 24 h. After culture, total and SCV CFU were enumerated (A), and the percentage of tetracycline-resistant colonies was determined (B). The bars represent the averages of 8 independent cultures. Error bars represent the standard deviations of the means. Values that are significantly different from the value at 0 h are indicated by an asterisk.
In contrast, the presence of gentamicin selected for SCVs in the inoculum and severely restricted phenotype switching; approximately 62% of the final SCV population and 77% of the total population was Tetr (Fig. 8B).
As such, in the absence of selective pressure, SCV population expansion is driven primarily by phenotype switching, with replicating NCP S. aureus generating the majority of the SCV population. In contrast, expansion of the SCV subpopulation under selective pressure (gentamicin) occurs via SCV replication, facilitated by selection for SCVs with increased stability (Fig. 6).
Furthermore, these data explain the apparent differences in replication rate between SCVs in the absence or presence of gentamicin (Fig. 2); SCVs grown in the absence of gentamicin appear to replicate faster, because their population is increased by NCP switching to SCV, as well as SCV replication, while gentamicin-treated cultures expand by replication.
DISCUSSION
Bacterial phenotype switching is considered to be an insurance policy against harmful environmental change such as exhaustion of nutrients or antibiotic therapy (3, 19). The presence of a subpopulation of non- or slow-growing, antibiotic-tolerant or -resistant cells provides a mechanism for survival and repopulation of a niche in the event that the fast-growing, antibiotic-susceptible population is killed (2, 3, 17, 19).
Phenotype switching of S. aureus has been linked with persistence in host tissues, treatment failure, and the development of persistent and recurrent infections. However, it was not clear whether phenotype switching is a constitutive process that is selected for in vivo or occurs only in response to particular environmental triggers, including the antibiotic gentamicin (24).
The data presented here show that replicating S. aureus populations generate a small but consistently present subpopulation of slow-growing gentamicin-resistant cells during exponential-phase growth. The emergence of gentamicin-resistant SCVs is dependent upon NCP replication and appears not to be triggered by stimuli such as quorum sensing, antibiotics, or starvation.
This population expands under selective pressure and can revert back to the parental phenotype when antibiotics are discontinued. As such, the emergence of SCVs is a natural consequence of S. aureus replication that has important implications for the infection process.
While expansion of the SCV subpopulation in the absence of gentamicin occurs principally via phenotype switching, expansion of the SCV population in the presence of gentamicin appears to occur primarily via SCV replication. Although SCVs are typically unstable, the data presented here show that SCV stability increases under selective pressure, which facilitates population expansion via replication. This is in agreement with the data which shows that the majority of SCVs in cultures containing gentamicin were derived from the original SCV inoculum (Fig. 8). However, it is possible that some of the SCV population expansion in the presence of gentamicin occurs via switching from the NCP. It has been shown previously (25) that SCVs can, over time, alter the pH of the culture medium, ultimately reducing the effectiveness of gentamicin and enabling NCP survival and replication in the presence of the antibiotic. In fact, this may explain why the percentage of Tetr SCVs grown in the presence of gentamicin is slightly less than the starting percentage (Fig. 8), suggesting that some SCVs emerge from the NCP population in the presence of the antibiotic. However, the data indicate that the majority of SCV population expansion in the presence of gentamicin is driven by SCV replication.
The mechanism by which phenotype switching occurs is poorly understood, but SCVs typically arise via mutations that result in the disruption of biosynthetic pathways (5, 7, 16, 18, 31, 32). Despite this, previous work has shown that SCVs are able to revert with high frequency to the NCP (24), indicating that the SCV-causing genetic changes are unstable. The data presented in this report support and extend this observation by demonstrating that there is high turnover of the SCV subpopulation during NCP staphylococcal replication, showing that bidirectional switching occurs constitutively during bacterial replication in the absence of selective pressure.
While it may seem reasonable to expect mutations to occur during replication, there is convincing evidence that mutation rates increase in stationary phase compared with exponential phase (10, 20, 26, 29). As such, it is surprising that SCV numbers did not increase in stationary-phase cultures. Work with Escherichia coli indicates that mutations that occur during replication are caused by a different mechanism than mutations that occur in stationary phase (26). It is therefore possible that SCV-generating mutations are due to a specific mechanism that is active only during exponential-phase growth.
The creation of an SCV subpopulation is a dynamic process that results not only from NCP switching to SCV but also high levels of SCV reversion to the NCP. The continual turnover of SCVs during exponential-phase growth can be explained by their highly unstable nature and reduced growth rate, which provides a strong selective pressure to revert to the NCP. However, once the culture enters stationary phase and NCP replication stops, there are no further opportunities for SCV emergence, but also no selective pressure for SCV reversion to the NCP, resulting in a stable balance between NCP and SCV populations.
The link between NCP replication and SCV emergence means that the mechanism is hard-wired into the S. aureus life cycle and is an entirely normal event. Thus, the SCV insurance policy is created by default and does not require any particular stimulus (e.g., starvation) to trigger phenotype switching as has been described for type I persisters of E. coli (2). This means that the insurance policy is present before harsh environmental conditions occur. This is essential since the data presented here show that S. aureus is not able to respond to the presence of gentamicin by producing SCVs. The cost of maintaining the SCV subpopulation is that S. aureus sacrifices a proportion of its fast-growing, toxin-producing population to maintain the SCV subpopulation.
Recent evidence suggests that the transition from colonization to infection is underpinned by mutations that convert S. aureus from a commensal into a pathogen (41). As such, mutations that generate SCVs may be considered to perform a similar role in the transition from an aggressive pathogen to a less-pathogenic, antibiotic-resistant-phenotype form residing inside the cell. The data presented here demonstrate that S. aureus maintains an SCV population, but it is not clear whether S. aureus also maintains other subpopulations whose phenotype is associated with, e.g., virulence, colonization, or tolerance of/resistance to other antibiotics.
The generation of SCVs via mutation has the advantage of producing variants with a range of differing levels of stability and rates of reversion to the NCP, which very likely enhances the ability of S. aureus to persist and also revert at the earliest opportunity. Furthermore, the stability of SCVs can be modulated by environmental conditions; the selection of stable SCVs by prolonged exposure to gentamicin is vital for survival in the presence of the antibiotic, since it reduces reversion events that would diminish the size of the SCV population (since reversion results in death). However, limited reversion is essential as it allows S. aureus to determine whether permissive growth conditions have returned and restore the fast-growing, pathogenic NCP population at the earliest possible opportunity.
Phenotype switching provides a compelling explanation for the ability of S. aureus to cause recurrent infections days, weeks, or even years after the initial infection: S. aureus replicates during the early stages of a primary infection, leading to the production of an SCV subpopulation; this SCV subpopulation enters and persists within host cells, shielding them from host immune components and antimicrobial therapy which clears the NCP bacteria; over time these SCVs can persist within cells, replicating at low levels; SCV replication results in occasional reversion to NCP which, if conditions are permissive, can initiate a second round of symptomatic infection.
Although the data clearly show that phenotype switching is constitutive during replication, this does not rule out the possibility that the phenotype switching rate is modulated by environmental cues. Previous work has shown that loss of the mismatch repair (MMR) genes mutS and mutL results in enhanced levels of switching (32), and presumably, any environmental stimuli that downregulate expression of the MMR system would enhance the phenotype switching rate.
One question that this work does not address is whether all replicating NCP bacteria within a population are equally likely to generate an SCV or whether SCVs are produced by a small subpopulation of hypermutator strains. Work by Besier et al. (6) indicated that SCVs isolated from patients with cystic fibrosis are hypermutators, suggesting that they may have arisen from hypermutator parent strains. Such a phenomenon may also be important for the reversion from SCV to NCP. The likelihood of a given SCV reverting to the NCP may simply reflect differences in the genetic changes associated with the original SCV phenotype. For example, SCVs that arise via a single point mutation are presumably more likely to revert than those that have arisen via deletion of a region of DNA. However, it is also possible that some SCVs have a higher basal mutation rate than others, which governs the likelihood of reversion from SCV to NCP. Future work will address the role of hypermutators in SCV emergence and reversion to the NCP.
It is important to note that this work examined only SCVs that are able to resist gentamicin and therefore does not include all SCVs. However, many clinically isolated SCVs (including those that are auxotrophic for hemin, menadione, and thymidine) do have increased MICs for gentamicin (30, 31), and as such, the data presented here are relevant to the development of persistent and recurrent infections.
In summary, the data presented here demonstrate that S. aureus populations generate and maintain a small, dynamic subpopulation of gentamicin-resistant SCVs in the absence of selective pressure. This occurs via a constitutive process that is dependent upon bacterial replication. The constitutive production of SCVs is very likely to be important for the development of chronic and recurrent infections by establishing an antibiotic-resistant, intracellular dwelling reservoir that can persist within host tissues and subsequently revert to the parental phenotype.
Supplementary Material
ACKNOWLEDGMENTS
Financial support from the Department of Medicine of Imperial College London is gratefully acknowledged.
Ruth Massey (University of Bath), Tim Foster (Trinity College Dublin), Angelika Grundling (Imperial College), Simon Foster (University of Sheffield), and Timothy Meredith (Merck) are acknowledged for providing strains or phage. David Holden and Angelika Grundling (Imperial College) and Angela Nobbs (University of Bristol) are thanked for useful discussions and helpful comments on the manuscript.
Footnotes
Published ahead of print 3 August 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Archer NK, et al. 2011. Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence 2:445–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balaban NQ. 2011. Persistence: mechanisms for triggering and enhancing phenotypic variability. Curr. Opin. Genet. Dev. 21:768–775 [DOI] [PubMed] [Google Scholar]
- 3. Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. 2004. Bacterial persistence as a phenotypic switch. Science 305:1622–1625 [DOI] [PubMed] [Google Scholar]
- 4. Bates DM, et al. 2003. Staphylococcus aureus menD and hemB mutants are as infective as the parent strains, but the menadione biosynthetic mutant persists within the kidney. J. Infect. Dis. 187:1654–1661 [DOI] [PubMed] [Google Scholar]
- 5. Besier S, Ludwig A, Ohlsen K, Brade V, Wichelhaus TA. 2007. Molecular analysis of the thymidine-auxotrophic small colony variant phenotype of Staphylococcus aureus. Int. J. Med. Microbiol. 297:217–225 [DOI] [PubMed] [Google Scholar]
- 6. Besier S, et al. 2008. The thymidine-dependent small-colony-variant phenotype is associated with hypermutability and antibiotic resistance in clinical Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 52:2183–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chatterjee I, et al. 2008. In vivo mutations of thymidylate synthase (encoded by thyA) are responsible for thymidine dependency in clinical small-colony variants of Staphylococcus aureus. J. Bacteriol. 190:834–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen CJ, Su LH, Lin TY, Huang YC. 2010. Molecular analysis of repeated methicillin-resistant Staphylococcus aureus infections in children. PLoS One 5:e14431 doi:10.1371/journal.pone.0014431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Foreman A, et al. 2011. Adaptive immune responses in Staphylococcus aureus biofilm-associated chronic rhinosinusitis. Allergy 66:1449–1456 [DOI] [PubMed] [Google Scholar]
- 10. Galhardo RS, Hastings PJ, Rosenberg SM. 2007. Mutation as a stress response and the regulation of evolvability. Crit. Rev. Biochem. Mol. Biol. 42:399–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garzoni C, Kelley WL. 2009. Staphylococcus aureus: new evidence for intracellular persistence. Trends Microbiol. 17:59–65 [DOI] [PubMed] [Google Scholar]
- 12. Gaupp R, Schlag S, Liebeke M, Lalk M, Götz F. 2010. Advantage of upregulation of succinate dehydrogenase in Staphylococcus aureus biofilms. J. Bacteriol. 192:2385–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gómez-González C, et al. 2010. Clinical and molecular characteristics of infections with CO2-dependent small-colony variants of Staphylococcus aureus. J. Clin. Microbiol. 48:2878–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horsburgh MJ, et al. 2002. sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaplan ML, Dye W. 1976. Growth requirements of some small-colony-forming variants of Staphylococcus aureus. J. Clin. Microbiol. 4:343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kriegeskorte A, et al. 2011. Small colony variants of Staphylococcus aureus reveal distinct protein profiles. Proteomics 11:2476–2490 [DOI] [PubMed] [Google Scholar]
- 17. Kussell E, Kishony R, Balaban NQ, Leibler S. 2005. Bacterial persistence: a model of survival in changing environments. Genetics 169:1807–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lannergård J, et al. 2008. Identification of the genetic basis for clinical menadione-auxotrophic small-colony variant isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 52:4017–4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lewis K. 2007. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 5:48–56 [DOI] [PubMed] [Google Scholar]
- 20. Loewe L, Textor V, Scherer S. 2003. High deleterious genomic mutation rate in stationary phase of Escherichia coli. Science 302:1558–1560 [DOI] [PubMed] [Google Scholar]
- 21. Looney WJ. 2000. Small-colony variants of Staphylococcus aureus. Br. J. Biomed. Sci. 57:317–322 [PubMed] [Google Scholar]
- 22. Lowy FD. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532 [DOI] [PubMed] [Google Scholar]
- 23. Lowy FD. 2000. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 8:341–343 [DOI] [PubMed] [Google Scholar]
- 24. Massey RC, Buckling A, Peacock SJ. 2001. Phenotypic switching of antibiotic resistance circumvents permanent costs in Staphylococcus aureus. Curr. Biol. 11:1810–1814 [DOI] [PubMed] [Google Scholar]
- 25. Massey RC, Peacock SJ. 2002. Antibiotic-resistant subpopulations of the pathogenic bacterium Staphylococcus aureus confer population-wide resistance. Curr. Biol. 12:686–687 [DOI] [PubMed] [Google Scholar]
- 26. McKenzie GJ, Lee PL, Lombardo MJ, Hastings PJ, Rosenberg SM. 2001. SOS mutator DNA polymerase IV functions in adaptive mutation and not adaptive amplification. Mol. Cell 7:571–579 [DOI] [PubMed] [Google Scholar]
- 27. Peyrani P, et al. 2012. Clinical outcomes of osteomyelitis patients infected with methicillin-resistant Staphylococcus aureus USA-300 strains. Am. J. Orthop. 41:117–122 [PubMed] [Google Scholar]
- 28. Plouin-Gaudon I, et al. 2006. Intracellular residency is frequently associated with recurrent Staphylococcus aureus rhinosinusitis. Rhinology 44:249–254 [PubMed] [Google Scholar]
- 29. Ponder RG, Fonville NC, Rosenberg SM. 2005. A switch from high-fidelity to error-prone DNA double-strand break repair underlies stress-induced mutation. Mol. Cell 19:791–804 [DOI] [PubMed] [Google Scholar]
- 30. Proctor RA, van Langevelde P, Kristjansson M, Maslow JN, Arbeit RD. 1995. Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin. Infect. Dis. 20:95–102 [DOI] [PubMed] [Google Scholar]
- 31. Proctor RA, et al. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4:295–305 [DOI] [PubMed] [Google Scholar]
- 32. Schaaff F, Bierbaum G, Baumert N, Bartmann P, Sahl HG. 2003. Mutations are involved in emergence of aminoglycoside-induced small colony variants of Staphylococcus aureus. Int. J. Med. Microbiol. 293:427–435 [DOI] [PubMed] [Google Scholar]
- 33. Sendi P, et al. 2006. Staphylococcus aureus small colony variants in prosthetic joint infection. Clin. Infect. Dis. 43:961–967 [DOI] [PubMed] [Google Scholar]
- 34. Sreeramoju P, et al. 2011. Recurrent skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus requiring operative debridement. Am. J. Surg. 201:216–220 [DOI] [PubMed] [Google Scholar]
- 35. Tuchscherr L, et al. 2011. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol. Med. 3:129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tuchscherr L, et al. 2010. Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. J. Infect. Dis. 202:1031–1040 [DOI] [PubMed] [Google Scholar]
- 37. Veening JW, et al. 2008. Bet-hedging and epigenetic inheritance in bacterial cell development. Proc. Natl. Acad. Sci. U. S. A. 105:4393–4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. von Eiff C, et al. 1997. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J. Bacteriol. 179:4706–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. von Eiff C, et al. 2001. Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with Darier's disease. Clin. Infect. Dis. 32:1643–1647 [DOI] [PubMed] [Google Scholar]
- 40. Wang H, Claveau D, Vaillancourt JP, Roemer T, Meredith TC. 2011. High-frequency transposition for determining antibacterial mode of action. Nat. Chem. Biol. 7:720–729 [DOI] [PubMed] [Google Scholar]
- 41. Young BC, et al. 2012. Evolutionary dynamics of Staphylococcus aureus during progression from carriage to disease. Proc. Natl. Acad. Sci. U. S. A. 109:4550–4555 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.