Abstract
Filamentous cyanobacteria of the order Nostocales display typical properties of multicellular organisms. In response to nitrogen starvation, some vegetative cells differentiate into heterocysts, where fixation of N2 takes place. Heterocysts provide a micro-oxic compartment to protect nitrogenase from the oxygen produced by the vegetative cells. Differentiation involves fundamental remodeling of the Gram-negative cell wall by deposition of a thick envelope and by formation of a neck-like structure at the contact site to the vegetative cells. Cell wall-hydrolyzing enzymes, like cell wall amidases, are involved in peptidoglycan maturation and turnover in unicellular bacteria. Recently, we showed that mutation of the amidase homologue amiC2 gene in Nostoc punctiforme ATCC 29133 distorts filament morphology and function. Here, we present the functional characterization of two amiC paralogues from Anabaena sp. strain PCC 7120. The amiC1 (alr0092) mutant was not able to differentiate heterocysts or to grow diazotrophically, whereas the amiC2 (alr0093) mutant did not show an altered phenotype under standard growth conditions. In agreement, fluorescence recovery after photobleaching (FRAP) studies showed a lack of cell-cell communication only in the AmiC1 mutant. Green fluorescent protein (GFP)-tagged AmiC1 was able to complement the mutant phenotype to wild-type properties. The protein localized in the septal regions of newly dividing cells and at the neck region of differentiating heterocysts. Upon nitrogen step-down, no mature heterocysts were developed in spite of ongoing heterocyst-specific gene expression. These results show the dependence of heterocyst development on amidase function and highlight a pivotal but so far underestimated cellular process, the remodeling of peptidoglycan, for the biology of filamentous cyanobacteria.
INTRODUCTION
Cyanobacteria constitute the largest, most diverse group of photosynthetic prokaryotes, comprising unicellular as well as filamentous and branched species with diverse morphology (42). Exhibiting a high potential for adaptation to changing environments, cyanobacteria are widely distributed in nearly all illuminated habitats. They perform oxygenic photosynthesis and fix CO2 via the Calvin cycle. Ammonia is assimilated via the glutamine synthetase-glutamate synthase (GS-GOGAT) cycle and is acquired from various combined nitrogen sources. In the absence of these sources, specialized strains can use atmospheric N2, which is reduced to ammonia by the highly oxygen-sensitive enzyme nitrogenase. Various strategies are employed by different strains to avoid oxygen poisoning of nitrogenase (12). Cyanobacteria of the order Nostocales restrict nitrogen fixation within specialized cells, the heterocysts. During the differentiation of vegetative cells to heterocysts, extensive morphological and biochemical alterations take place. In addition to the inactivation of oxygen-releasing photosystem II and enhancement of the oxygen-consuming respiration rate, modification of the cell wall is an essential step to establish a micro-oxic environment. As an early event during heterocyst differentiation, a multilayered envelope is formed on the preexisting cell wall to reduce oxygen diffusion from the aerobic environment. The outermost layer, which is formed first, is composed of heterocyst-specific polysaccharides (HEP). The inner laminated layer is built of heterocyst-specific glycolipids. At the junction sites to the neighboring oxygen-producing cells, the septa are narrowed and neck-like structures are formed. Special inner membranes, localized at the poles, reduce entering oxygen by high respiration activity (27, 48).
In Anabaena sp. strain PCC 7120 (here, Anabaena PCC 7120), a model organism for heterocyst formation, 5 to 10% of the vegetative cells in the filament differentiate into heterocysts in a semiregular pattern in the absence of combined nitrogen. Heterocysts feed the neighboring vegetative cells with fixed nitrogen and in return obtain fixed carbon, glutamate, and probably other metabolites (33, 41). Hence, Anabaena PCC 7120 represents a true multicellular organism, in which interdependent cells exchange metabolites. Coordination of the highly regulated developmental process requires transfer of regulatory molecules and compounds (14). The exact mechanism of cell-cell communication in Anabaena PCC 7120 is still unknown, but evidence for two different routes has been obtained. One is the transfer of solutes via a continuous periplasm; the other is exchange via cell-cell connections (14, 31, 32, 37). So far, several genes that could possibly encode cell-cell joining proteins have been characterized (1, 5, 11, 15, 34, 35, 39). Some of these proteins localize to the septum; however, it is not known how they could span the septum and whether the proteins are part of multiprotein complexes.
Murein hydrolases degrade specifically the bacterial murein sacculus by cleaving defined bonds in the peptidoglycan. They are involved in fundamental biological functions in bacteria: insertion of newly synthesized building blocks into nascent peptidoglycan, turnover of peptidoglycan, and the splitting of daughter cells during cell division. N-Acetylmuramyl-l-alanine amidases (Ami) are of particular importance for daughter cell separation in unicellular bacteria (22, 23, 45). They cleave the amide bond between the N-terminal l-Ala residue of the cell wall peptide and the N-acetylmuramic acid backbone (MurNAc) (22, 23, 45). An Escherichia coli AmiABC triple mutant forms chains of cells resembling filamentous bacteria (19). This raised the question of the function of these amidases in filamentous cyanobacteria. Nostoc and Anabaena strains harbor two adjacent copies of AmiC gene homologues in their genomes. Previously, we showed that in Nostoc punctiforme ATCC 29133 an AmiC2 mutant (NpF1846) displays pleiotropic phenotypes: the filaments are distorted, with irregular cell division planes and impaired intercellular transfer of the fluorophore calcein (30). To gain more insights into the general function of cell wall amidases in filamentous cyanobacteria, we inactivated orthologous amidase genes in the model organism Anabaena PCC 7120 and performed a functional characterization of the mutants, revealing a pivotal role for AmiC1 in Anabaena PCC 7120.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Anabaena PCC 7120 and the derived mutant strains were grown in medium BG11 (containing NaNO3) or BG110 (free of combined nitrogen) (42). Routinely, strains were grown in Erlenmeyer flasks with constant shaking (100 rpm) under photoautotrophic conditions (20 to 40 μEm−2 s−1) at 28°C. For nitrogen step-down experiments, cultures were grown in liquid BG110 medium supplied with 4 mM NH4Cl and 8 mM N-Tris(hydroxymethyl)methyl-2-aminoethane sulfonic acid (TES)-NaOH buffer, pH 7.5, bubbled with air enriched by 2% CO2. To induce heterocyst formation (N step-down), the cultures were washed 3 times with nitrogen-free medium and resuspended in nitrogen-free medium. Mutant strains were grown in media supplemented with antibiotics at the following concentrations: streptomycin (Sm), 2.5 μg/ml; spectinomycin (Sp), 2.5 μg/ml; and neomycin (Nm), 50 μg/ml. Strains of Escherichia coli were cultured as described previously (30). All bacterial strains used in this study are summarized in Table S1 in the supplemental material.
DNA isolation and construction of mutants.
Total DNA from Anabaena PCC 7120 was prepared as described previously (6). Plasmids were purified from E. coli with the peqGold Plasmid Miniprep kit I (Peqlab). DNA restriction and ligation were carried out with enzymes from Fermentas or New England BioLabs according to their instructions. Oligonucleotides used as primers in PCR are summarized in Table S3 in the supplemental material. All plasmids used in this study are listed in Table S2 in the supplemental material.
To analyze the subcellular localization of AmiC1 and AmiC2 in Anabaena PCC 7120, translational green fluorescent protein (GFP) fusions were constructed as follows. For the transport of the folded AmiC1-GFP into the periplasm, we replaced the Sec signal sequence of the amiC1 gene with a signal sequence for the Tat translocation pathway (Tat). To this end, the amiC1 gene without the predicted sec sequence, replaced by a tat overhang, was amplified by PCR with genomic DNA as the template and Oligo700 and Oligo727 as primers. Oligo698 and Oligo699 were used to amplify the Tat signal sequence from all3333, with an amiC1 overhang. In a third PCR, using both PCR products as the template and oligonucleotides Oligo698 and Oligo727 as primers, the fragments were fused, amplified, and cloned into vector pGEM-T, producing plasmid pIM451. A promoterless gfp gene was excised from pCSEL19 (38) as an EcoRV-PstI fragment and transferred into EcoRV- and PstI-digested pIM451, resulting in a translational fusion of tat-amiC1 with gfp (pIM452). To integrate tat-amiC1-gfp into pRL1049, which is a replicating plasmid for use in Anabaena PCC 7120 (4), a BamHI site was inserted via PCR using Oligo698 and Oligo706 as primers, resulting in plasmid pIM453.
For construction of AmiC2-GFP, Oligo698 and Oligo703 were used to amplify the Tat signal sequence from all3333, with an amiC2 overhang. The amiC2 gene without the predicted sec sequence, replaced by a tat overhang, was amplified by PCR with genomic DNA as the template and Oligo704 and Oligo731 as primers. A promoterless gfp gene was amplified from pCSEL19 using Oligo730 and Oligo706, resulting in a gfp fragment with an amiC2 overhang. In a subsequent PCR, using the PCR products as the template and oligonucleotides Oligo698 and Oligo706 as primers, the fragments were fused, amplified, and cloned into pJET (Fermentas), resulting in plasmid pIM454. tat-amiC2-gfp was isolated after EcoRI and BamHI digestion and ligated into the EcoRI and BamHI site of the self-replicating plasmid pRL1049, resulting in plasmid pIM455.
The putative promoter region of amiC was amplified by PCR using oligonucleotides Oligo807 and Oligo808, which are flanked by EcoRI, digested by EcoRI, and ligated into the EcoRI sites of pIM453 and pIM455, respectively. The resulting plasmids pIM471 and pIM472 were transferred to Anabaena PCC 7120 by triparental mating as described previously (9, 47), resulting in strains Anabaena(pIM471) and Anabaena(pIM472), respectively.
To inactivate alr0092 and alr0093, internal fragments of both genes were amplified by standard PCR using Anabaena DNA as the template; oligonucleotides F1 and R1 were primers for alr0092, and F2 and R2 were primers for alr0093. DNA fragments were cloned into pGEM-T (Promega), resulting in pIM475 and pIM476, respectively. After XhoI digestion, internal fragments of alr0092 and alr0093 were purified and ligated into the XhoI site of the mobilizable vector pRL277 (3), resulting in pIM477 and pIM478, respectively. Plasmids were transferred by triparental mating to Anabaena PCC 7120 and inserted into the genome via single recombination, resulting in strains SR477 (amiC1 mutant) and SR478 (amiC2 mutant). Segregation of the mutant alleles was confirmed by PCR.
For complementation of mutant strain SR477, a pIM471 derivative was used. Since plasmid pIM471, carrying PamiC1, amiC2-tat-amiC1-gfp on vector pRL1049, contains an Sm/Sp resistance cassette, the plasmid pRL442 (10) was digested with SmaI, and the isolated fragment containing the C.K3 cassette, which confers Km/Nm resistance, was ligated into the StuI site of pIM471. The resulting plasmid pIM496 was transferred by triparental mating to mutant strain SR477.
For overexpression experiments, the entire coding regions of amiC1 (alr0092) and amiC2 (alr0093) were amplified by standard PCR using DNA from Anabaena PCC 7120 as the template and primers F3 and R3 for the former and F4 and R4 for the latter. PCR products were cloned into pJET, resulting in pIM480 and pIM481, respectively. The petE promoter was amplified by PCR using Oligo767 and Oligo768 as primers and Anabaena DNA as the template. The resulting 358-bp fragment was restricted with EcoRV/BglII and cloned into the EcoRV/BglII sites of pIM480 and pIM481, respectively. The plasmids produced, pIM482 and pIM483, were digested with PstI and EcoRI, and the isolated PpetE-amiC1 or PpetE-amiC2 fragments were cloned into PstI- and EcoRI-digested pCSEL24, which integrates into the nucA region of the α-plasmid of Anabaena PCC 7120 (40). The resulting plasmids pIM484 and pIM485 were transferred to Anabaena PCC 7120 by conjugation, resulting in strains OE-IM484 and OE-IM485, respectively. The genes were induced with 0.2 μM CuSO4 for normal expression or 2 μM CuSO4 for overexpression (24, 40).
Gene expression analysis.
Total RNA of 50-ml cultures of Anabaena PCC 7120 and the derived mutant strains was extracted as described previously (30). Reverse transcriptase (RT)-PCR was performed by OneStep RT-PCR kit (Qiagen) according to the manufacturer's instructions using 60 ng RNA. Primers used for gene expression analysis are summarized in Table S3 in the supplemental material.
Lipid analysis.
Filaments of Anabaena PCC 7120 and the mutant strains were grown in 50-ml liquid cultures and starved for combined nitrogen for 3 days (N step-down). Total lipids were extracted with chloroform-methanol (2:1, vol/vol) and evaporated in a stream of air. Concentrated extracts were dissolved in 200 μl chloroform and chromatographed on thin-layer plates of silica gel (Merck) as described previously (46).
Microscopy and FRAP analysis.
Light, fluorescence, and electron microscopy analyses were performed as previously described (30). Calcein labeling was done according to Mullineaux et al. (37). All fluorescence recovery after photobleaching (FRAP) measurements were performed essentially as previously described (30).
RESULTS
Amidases of cyanobacteria.
In the filamentous heterocyst-forming cyanobacterium Nostoc punctiforme ATCC 29133, the amidase AmiC2, encoded by the second gene of an amidase gene cluster (encoding NpF1845-NpF1846), plays an important role in filament morphogenesis (30). The model strain Anabaena PCC 7120 harbors a similar cluster of two adjacent genes encoding AmiC homologues (alr0092 and alr0093), which exhibit similarities to amiC of E. coli, encoding an N-acetylmuramyl-l-alanine-amidase. The deduced amino acid sequences of both Anabaena PCC 7120 amidases have 60.8% amino acid sequence identity (similarity, 84.1%) and show about 24% identity to E. coli AmiC. The catalytic domain of AmiC is located at the C terminus of the deduced protein, whereas the N-terminal part of the Anabaena PCC 7120 and N. punctiforme homologues contains two AMIN (amidase N-terminal) domains (see Fig. S1A in the supplemental material). AMIN domains mediate the targeting of periplasmic or extracellular proteins to specific regions of the bacterial cell wall (8). In E. coli, AmiC contains only one AMIN domain, and it is essential for septal targeting of AmiC during cell division (2). The amidase architecture, with two septal targeting domains, is found in all heterocyst-forming cyanobacteria sequenced so far (see Fig. S1A in the supplemental material). In contrast to Nostocales, AmiC homologues in nonheterocystous filamentous cyanobacteria like Arthrospira platensis and Trichodesmium erythraeum as well as in unicellular species such as Synechococcus PCC 7002 or Cyanothece sp. PCC 8801 contain just one AMIN domain (see Fig. S1A). The genomic organization, with an upstream kefB gene, two amidase genes, and a downstream gene for glutamate racemase (murI), is conserved in the related strains Anabaena PCC 7120, Anabaena variabilis, N. punctiforme, and Nostoc azollae. In Nodularia spumigena, just one amidase gene exists in this region (see Fig. S1B).
Generation of amiC1 and amiC2 mutants and phenotypic characterization.
In order to gain deeper insights into the function of the AmiC paralogues in heterocystous cyanobacteria, we constructed Anabaena PCC 7120 strains SR477 and SR478, with mutations in amiC1 (alr0092) and amiC2 (alr0093), respectively (Fig. 1). PCR analysis revealed that no full-length copies of the wild-type genes alr0092 and alr0093 were present in the respective single recombinant mutants but that the integrated vector was present at the recombination site (see Fig. S2B and C in the supplemental material). RT-PCR was performed to test whether inactivation of one amidase gene affected the expression of the other amidase gene. As in the wild type, amiC1 was expressed in the SR478 mutant and amiC2 was expressed in the SR477 mutant (see Fig. S2D). Hence, the vector insertion in the neighboring gene by single recombination had no polar effect on the expression of the adjacent amidase gene.
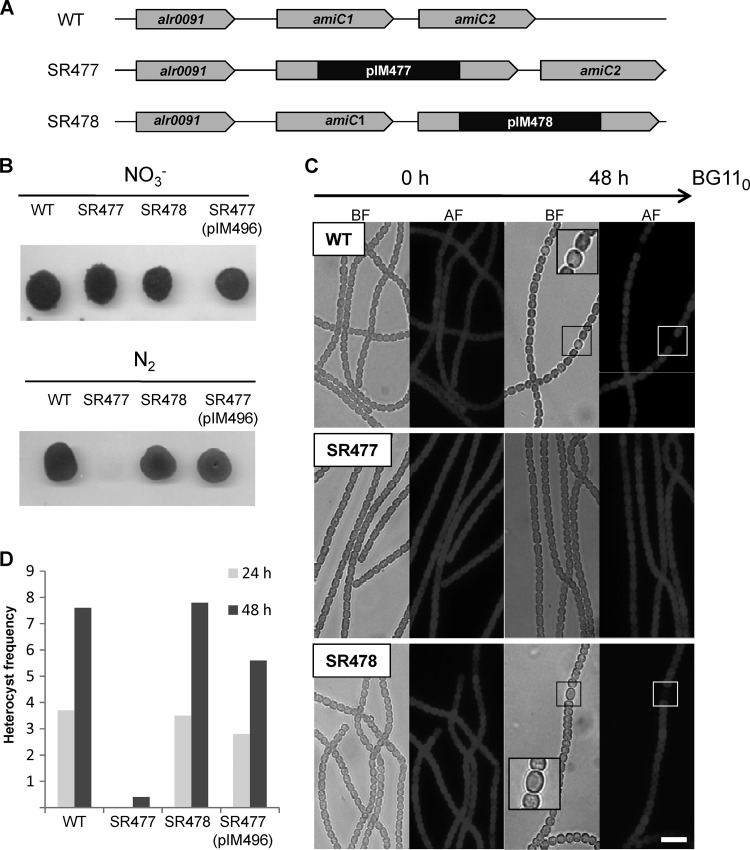
Fig 1.
Genotype and growth of Anabaena PCC 7120 wild type, amiC mutants SR477 andSR478, and complemented mutant SR477(pIM496) with different N sources. (A) Genomic organization of the amiC cluster in Anabaena PCC 7120 wild type (WT), amiC1 mutant strain SR477, and amiC2 mutant strain SR478. (B) Growth of pseudocolonies with and without NO3−: after nitrogen step-down, filaments were resuspended in BG11 (containing NO3−) or BG110 medium, and suspensions containing 100 ng chlorophyll were spotted on top of agar with (upper part) or without (lower part) NO3− and incubated under growth conditions for several days. (C) Light and autofluorescence microscopy images of Anabaena PCC 7120 wild type and amiC mutants SR477 and SR478 grown on BG11 at 0 h and 48 h after nitrogen step-down. BF, bright field; AF, autofluorescence. (D) Heterocyst frequency of Anabaena PCC 7120 wild type and mutant strains SR477, SR478, and SR477(pIM496). To calculate the frequency, 2,000 cells were counted. Bar = 10 μm.
In nitrate-supplemented BG11 medium, both amiC mutants grew photoautotrophically with growth rates comparable to those of wild-type Anabaena PCC 7120 and formed long filaments as did the wild type (Fig. 1). After nitrogen step-down, the AmiC1 mutant was not able to grow when N2 was the sole nitrogen source. Almost no heterocysts could be detected in the filaments. Nevertheless, morphologically the filaments did not show fragmentation. In contrast, the amiC2 mutant SR478 was able to develop heterocysts in a semiregular pattern along the filament and grew on N2 as did wild-type Anabaena PCC 7120 (Fig. 1). Thin-layer chromatography of lipid extracts from the wild-type and the mutant filaments showed that mutant strain SR477 failed to synthesize heterocyst-specific glycolipids upon nitrogen step-down (Fig. 2A). The glycolipids are synthesized during the first hours after nitrogen step-down in the maturing heterocysts and subsequently exported beyond the cytoplasmic and outer membrane by the type I secretion system DevBCA-TolC (36, 43). The formation of the heterocyst cell wall polysaccharide layer that takes place early in differentiation (27) could not be detected by alcian blue staining in this mutant (Fig. 2B). The HEP layer encompasses the glycolipid layer and hence is formed before the deposition of the glycolipids.
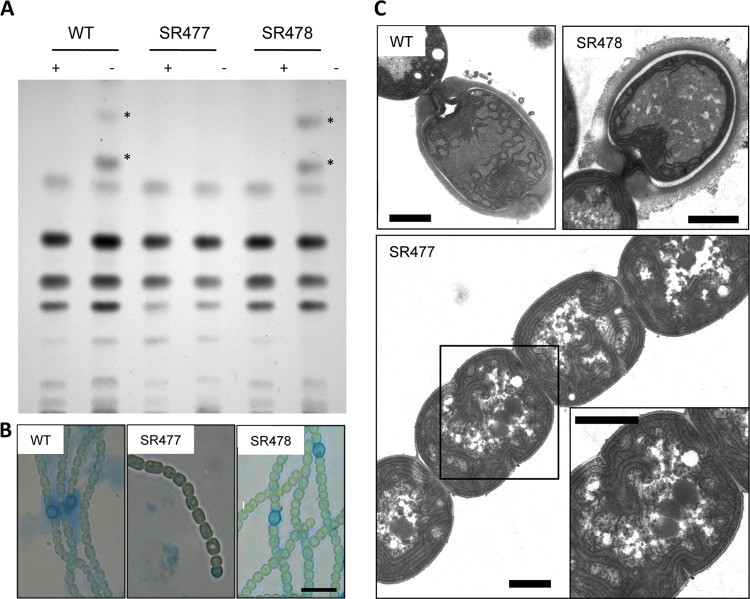
Fig 2.
Heterocyst formation in Anabaena PCC 7120 wild type and amiC mutant strains SR477 and SR478. (A) Thin-layer chromatography of lipid extracts of filaments of Anabaena PCC 7120 wild type (WT) and amiC mutant strains SR477 and SR478 grown in NH4Cl (+) and after 48 h of N step-down (−). Stars indicate the positions of the two heterocyst-specific glycolipids. (B) Light micrographs of alcian blue-stained filaments after incubation for 48 h on N2. (C) Transmission electron micrographs of ultrathin sections of Anabaena PCC 7120 wild type and amiC mutant strains SR477 and SR478 incubated for 48 h in nitrogen-free medium. Magnification of the boxed area is shown on the lower right. Bar = 10 μm (B) and 1 μm (C).
The inability of mutant SR477 to form heterocysts (Het− phenotype) was confirmed by ultrastructure analysis of ultrathin sections of the nitrogen-starved filaments. In electron micrographs, neither differentiating cells nor mature heterocysts could be detected. However, packed glycogen granules were present in all cells of the filaments, a typical phenotype for a Fox (fixation under oxic conditions [11]) mutant, which stores excess carbon during nitrogen starvation. In contrast, wild-type Anabaena PCC 7120 and amiC2 mutant SR478 showed mature heterocysts containing the typical envelope, the polar neck, and the polar body with cyanophycin (Fig. 2C). Hence, alcian blue stained the envelope, and the heterocyst glycolipids were detected in methanolic extracts after thin-layer chromatography (Fig. 2A and B).
To verify that the phenotype of the amiC1 mutant was caused by the integration of pIM477 in the open reading frame of alr0092, strain SR477 was complemented with plasmid pIM496 used in the localization studies described below. This plasmid bears a C-terminal translational fusion of alr0092 with gfp and was able to complement the Fox− phenotype of mutant strain SR477. The capability of heterocyst differentiation and diazotrophic growth was restored in strain SR477(pIM496) (Fig. 1B). The frequency of heterocysts was the same as in the wild type after 24 h of nitrogen step-down and about 20% lower after 48 h (Fig. 1D).
Transcription regulators HetR and NtcA are absolutely required for heterocyst differentiation. In hetR or ntcA mutants, repression of heterocyst-specific genes, like hepA or nifHDK, persists and development is arrested at an early time point (48). NtcA senses the low-nitrogen status and induces the expression of proteins involved in nitrogen metabolism and heterocyst differentiation (20). To analyze whether heterocyst differentiation in the amiC1 mutant is affected at the gene-regulatory level, RT-PCR was performed with total RNA isolated from filaments of wild-type Anabaena PCC 7120, strain SR477, and strain SR478 at 0 h, 3 h, 6 h, 9 h, and 24 h after nitrogen step-down. As shown in Fig. 3, early regulatory genes nctA and hetR are expressed during nitrogen starvation in all strains in almost identical time courses and at similar levels. The expression of the hepA gene, encoding a putative HEP exporter (21), was delayed in the mutant SR477 compared to the wild type or mutant SR478 (Fig. 3A). In the heterocyst-forming strains, the hepA transcript was detected in the 6-h sample and in the mutant SR477 in the 24-h sample. Transcripts of the nifD gene, which encodes a subunit of the nitrogenase, could be detected in all strains after 24 and 48 h of nitrogen step-down. The prerequisite for the transcription of the nifHDK operon is a nif gene rearrangement on the chromosome that takes place during heterocyst formation (17). As shown by PCR (Fig. 3B), it takes place in all three strains as expected from the transcription data (Fig. 3C).
Fig 3.
Expression of heterocyst-specific genes in wild-type Anabaena PCC 7120 and amiC mutants SR477 and SR478. (A) RT-PCR of ntcA, hetR, and hepA during nitrogen step-down. RNA was isolated from cultures incubated for different times (0, 3, 6, 9, and 24 h) from Anabaena PCC 7120 wild type, mutant SR477, and mutant SR478, and transcripts were examined after reverse transcription. Primers used for RT-PCR are hepA-for, hepA-rev, ntcA-for, ntcA-for, hetR-for, hetR-rev, rnpB-for, and rnpB-rev; the sequences of primers are listed in Table S3 in the supplemental material. The rnpB gene was used as a loading control with (rnpB+) and without (rnpB−) reverse transcriptase. (B) The nifHDK rearrangement during nitrogen step-down was shown by PCR using primers left and right from the 11-kb insertion element (18) as listed in Table S3 in the supplemental material, nifD-for and nifD-rev. Primers all4999-rev and all4999-rev for amplification of gene all4999 were used as the control for the chromosomal gene. Lanes: WT, Anabaena PCC 7120; 1, SR477; 2, SR478. (C) Expression of nifD at 24 h and 48 h after nitrogen step-down as shown by RT-PCR using isolated RNA as the template and primers nifD-for and oligo919 for reverse transcription from cultures incubated for 24 and 48 h on BG110. The rnpB gene was used as a loading control. Lanes: WT, Anabaena PCC 7120; 1, SR477; 2, SR478.
Subcellular localization of AmiC1-GFP and AmiC2-GFP in Anabaena PCC 7120.
In order to study the subcellular localization of the amidases in the filaments of Anabaena PCC 7120, a self-replicating plasmid that bears a translational fusion of the amidase to the GFP was transferred to Anabaena PCC 7120. Since the GFP folds efficiently only in the cytoplasm (13), the native Sec signal sequence was replaced by the signal sequence for the Tat translocation system of the periplasmic binding protein All3333.
To exclude the possibility that subcellular organization could be affected by replacing the signal sequence, we transferred the amiC1-gfp construct also to the amiC1 mutant SR477 as described above. Since the fusion protein was able to complement the amiC1 phenotype, the fusion to GFP presumably did not affect the activity or localization of AmiC1. Uehara et al. demonstrated that there was no difference between the localization of septal protein EnvC-GFP that was artificially targeted to the periplasm through the Tat export system and that of EnvC-mCherry when translocated to the periplasm via the Sec system (44). In both cases, EnvC was recruited to the division site.
To express the fusion proteins under native conditions, a 653-bp region upstream of amiC1 was cloned in frame in front of the tat-amiC1-gfp fusion and tat-amiC2-gfp fusion (Fig. 4A). The strains Anabaena(pIM471), carrying the tat-amiC1-gfp fusion, and Anabaena(pIM472), carrying the tat-amiC2-gfp fusion, were grown in liquid BG11 medium and analyzed by epifluorescence microscopy. Fluorescence from AmiC1-GFP and AmiC2-GFP was located in the cell periphery outside the red autofluorescence coming from the photosynthetic apparatus in the thylakoid membranes. Strong green fluorescence accumulated at the cross walls between neighboring cells and at sites midway to cells, where a new septum starts to form; less fluorescence could be detected in the side walls of the cells (Fig. 4B). Twelve hours after incubation in BG110 medium, AmiC1-GFP and AmiC2-GFP preferentially accumulated in the cell wall of proheterocysts, in particular in the septal area (Fig. 4C; see also Fig. S4 in the supplemental material). GFP was barely detectable in the septa between vegetative cells of these nitrogen-starved cultures, which do not grow until mature heterocysts are formed. Septa between vegetative cells of N2-grown cultures showed GFP accumulation similar to that of filaments growing on combined nitrogen. The amidases, however, disappeared in the septa and polar neck regions of mature and old heterocysts (Fig. 4C, 72 h).
Fig 4.
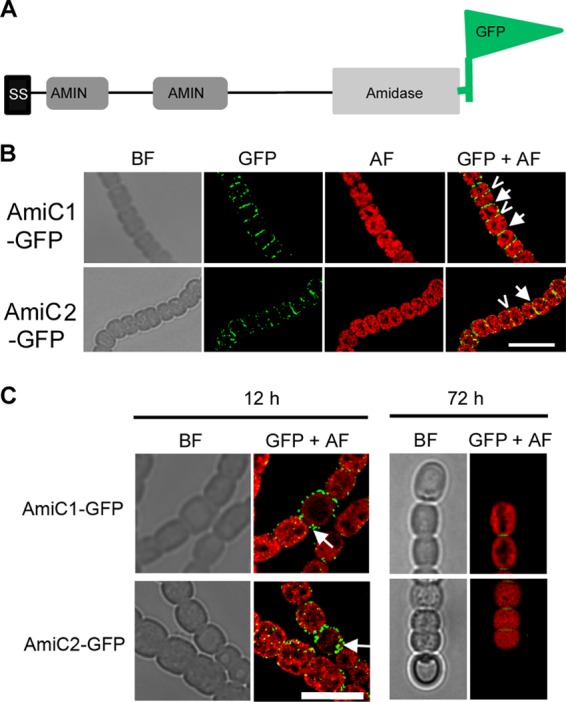
Subcellular localization of AmiC-GFP. (A) Scheme of the different domains of the AmiC protein and the C-terminal fusion to GFP. (B) Subcellular localization of AmiC1-GFP and AmiC2-GFP in BG11-grown filaments of Anabaena PCC 7120 bearing plasmids pIM471 and pIM472, respectively. Arrows show freshly divided cells, and arrowheads show early formation of cell division plane. (C) Subcellular localization of AmiC1 and AmiC2 12 h and 72 h after nitrogen step-down of Anabaena PCC 7120 bearing plasmids pIM471 and pIM472, respectively. BF, bright field; GFP, GFP fluorescence; AF, autofluorescence; GFP+AF, overlay of GFP fluorescence and autofluorescence. Bar = 10 μm.
Cell-cell communication.
FRAP measurements were used to examine the intercellular exchange inside the filament of Anabaena PCC 7120 (37). The fluorophore calcein can be loaded into the cytoplasm of the filaments as an indicator. Normally, after bleaching one cell in the filament, the calcein fluorescence recovers swiftly, accompanied by coincident reduction of fluorescence from the neighboring nonbleached cells, which is an indication of intercellular solute exchange. Using this method, we have previously shown that also in N. punctiforme a rapid fluorescence recovery occurs, showing the ability of cell-cell communication in this strain. No intercellular exchange occurred in an amiC2 mutant of N. punctiforme, leading to the conclusion that this amidase is necessary for the creation or for the functioning of cell-cell connections (30).
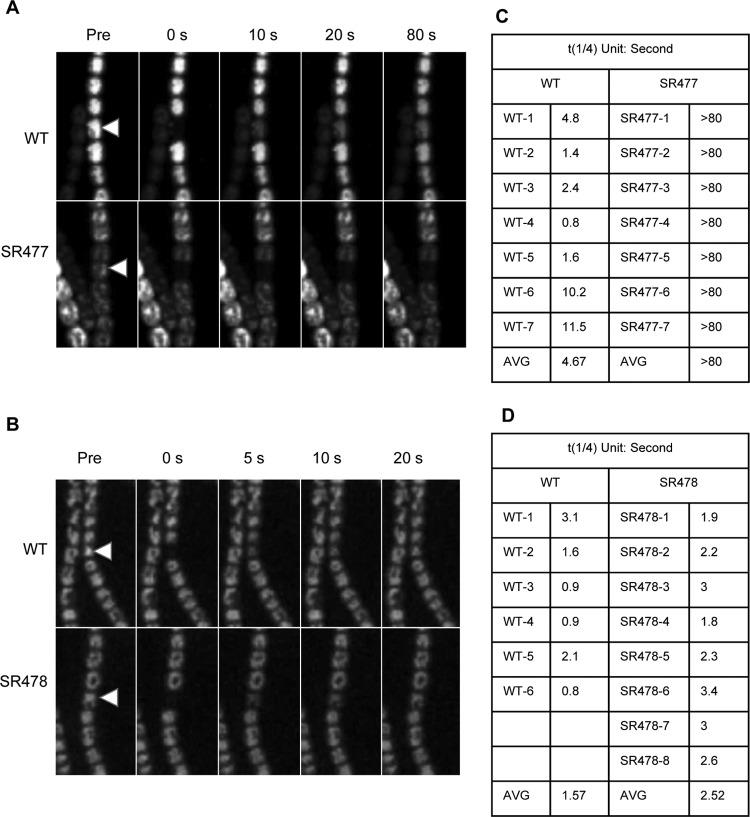
Here, we investigated the capacity of the amiC mutants of Anabaena PCC 7120 for intercellular transfer of calcein. In the calcein-loaded filaments of the wild type and the amiC2 mutant SR478, calcein transfer occurred rapidly. A few seconds after bleaching, an almost complete recovery was reached (Fig. 5). In contrast, in the amiC1 mutant no intercellular transfer was observed during the observation period of 80 s. This shows that a direct cell-to-cell exchange of calcein was abolished in the amiC1 mutant.
Fig 5.
Calcein FRAP in Anabaena PCC 7120 wild type and the amiC mutants. After loading of calcein-AM into the cells of the indicated strains and washing in medium, one cell (arrowhead) was bleached by a laser beam and the recovery of fluorescence monitored at the confocal microscope at different time points after bleaching. Shown are confocal images before (Pre) and directly after bleaching as well as at later time points in seconds. (A, B) Anabaena PCC 7120 wild type (WT) and amiC1 mutant SR477 (A) and amiC2 mutant SR478 (B). The arrowheads indicate the bleached cell. (C, D) To quantify FRAP, the quarter-time of equilibration (t1/4) was calculated for six wild-type filaments and eight SR478 filaments of independent measurements. t1/4 could not be accurately calculated for SR477 mutant cells, as these cells showed no substantial recovery within the recorded 80-s time interval. All quantifications were calculated as described previously (16).
Overexpression of amiC1 and amiC2.
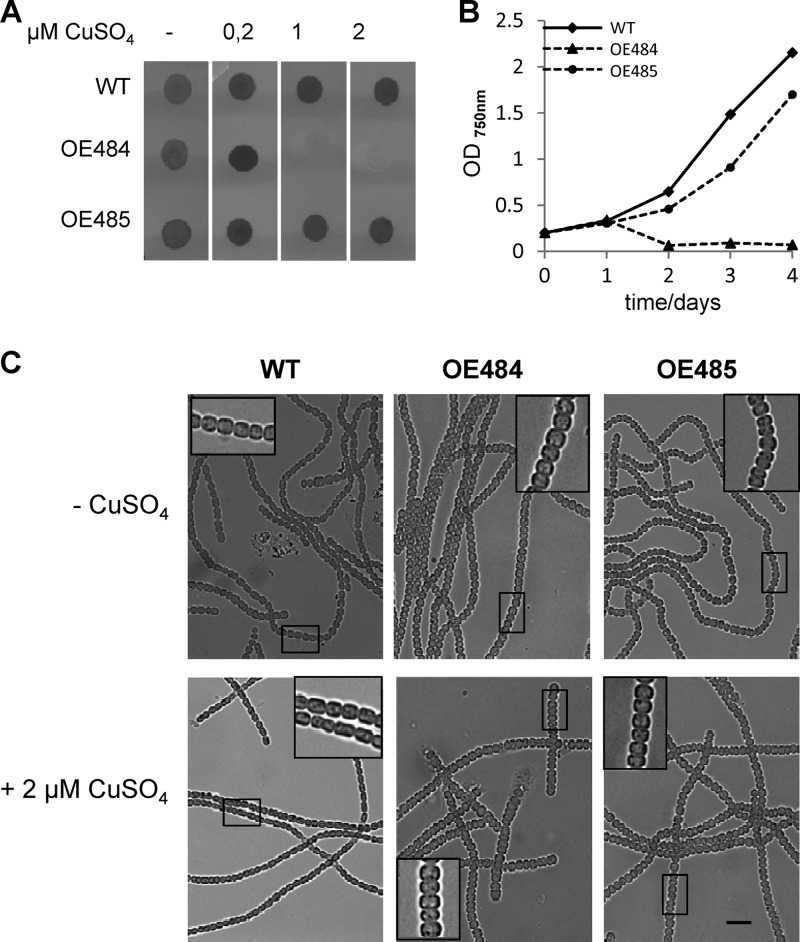
In addition to gene knockout analysis, some aspects of gene functions may be revealed by gene overexpression. It can be assumed that overexpression of cell wall-lytic enzymes leads to disintegration of the peptidoglycan and thereby to alterations in cell shape and size due to the counteracting cell turgor. In last consequence, the amidases would lyse the cells. For overexpression studies, both amiC genes were cloned under the control of a strong inducible promoter. The petE promoter from the plastocyanin-encoding petE gene was successfully used for such purposes, and its expression can be induced by copper addition (24). The amiC1 overexpression plasmid pIM484 was transferred into the wild-type background to yield strain OE484. Likewise, strain OE485 carried the amiC2 overexpression plasmid pIM485. RT-PCR confirmed that transcription of amiC1 and amiC2 was strongly increased in OE484 and OE485, respectively, in the presence of 2 μM CuSO4 in the medium (see Fig. S3A in the supplemental material). In addition, the RT-PCR assay showed that overexpression of one amidase gene had no effect on the expression of the other one (see Fig. S3A). Prolonged overexpression of amiC1 for several days led to cell death. In contrast, overexpression of amiC2 seemed to have just minor effects on the cells and did not lead to fragmentation or cell death (Fig. 6A and B). Microscopic examination showed an enlargement of the cells following overexpression of any AmiC protein, and in the case of AmiC1 several cells in the state of cell lysis could be observed (Fig. 6C). This indicates that excess activity of AmiC1 probably weakens and finally lyses the cell wall, a typical property of autolysins, which is in agreement with the proposed role of AmiC1 as a cell wall amidase. The phenotype of overexpressing AmiC2 is less severe, indicating that AmiC2 is less active than AmiC1.
Fig 6.
Overproduction of AmiC1 (strain OE484) and AmiC2 (strain OE485) in Anabaena PCC 7120 wild type (WT). For overexpression, the native promoters of amiC1 and amiC2 were replaced by the copper-inducible promoter of the petE gene. (A) Cell suspensions of each strain containing 100 ng chlorophyll were spotted on agar plates containing different concentrations of CuSO4 in copper-free BG11 medium and incubated under growth conditions for 10 days. (B) Growth curves of wild-type Anabaena PCC 7120 and the overproducing strains in liquid medium. Optical density was measured at 750 nm. (C) Light micrographs of Anabaena PCC 7120 wild type (WT) and overexpressing strains before and 2 days after adding 2 μM CuSO4. Magnifications of the boxed areas are shown. Bar = 10 μm.
DISCUSSION
In a previous study, we found a novel function for cell wall amidases in the filamentous cyanobacterium N. punctiforme. Instead of being involved in cleavage of the septal murein at the end of cell division, mutational analysis of AmiC2 (NpF1846) showed that this enzyme is pivotal for filament morphology, intercellular exchange of calcein, and cell differentiation. The amiC2 gene is the second of a gene cluster conserved in heterocystous cyanobacteria (see Fig. S1B in the supplemental material). Two genes of the cluster encode AmiC-like cell wall amidases, but so far we have not been able to obtain amiC1 mutant strains in N. punctiforme, probably because amiC1 is essential for growth (30).
Since similar clusters of amiC homologues are present in heterocystous cyanobacteria only, we were interested to investigate the effect of their mutations in the model organism Anabaena PCC 7120. The present functional study provides further support for the novel role of AmiC-type amidases in interfilament cell-cell communication in heterocystous cyanobacteria. Inactivation of open reading frames alr0092 (amiC1) and alr0093 (amiC2) did not affect the growth rate on media with combined nitrogen. Hence, neither amiC1 nor amiC2 alone is essential for cell division. It cannot be excluded that redundancy exists as in E. coli (19). In contrast to N. punctiforme, where a mutation of amiC2 leads to filament dystrophy and an aberrant cell division plane, in Anabaena PCC 7120 inactivation of the amidase genes had no effect on filament morphology. However, in medium without combined nitrogen, the amiC1 mutant failed to differentiate heterocysts and could not grow on N2 as the sole nitrogen source, similar to what was observed with the amiC2 mutant of N. punctiforme. Since the downstream gene alr0093 was still expressed in SR477, and because a wild-type copy of the gene could complement the mutant on a self-replicating plasmid, polar effects could be excluded (Fig. 1; see also Fig. S2 in the supplemental material). No sign of morphological differentiation of heterocysts was observed in strain SR477, as shown by microscopy and chemical analysis. However, the induction of the early regulators NtcA and HetR persisted, and their genes were upregulated after nitrogen step-down as in the wild type (Fig. 3). Even the expression of the late heterocyst-specific genes hepA and nifD took place in nitrogen-starved filaments of the mutant. Only in the case of hepA transcription could a delayed expression be observed in the AmiC1 mutant. These results showed that the inability to form heterocysts was caused neither by the loss of sensing nitrogen starvation nor by a defect of the genetic program of heterocyst development, governed by NtcA and HetR, as evidenced by the expression of heterocyst-specific genes.
Previously, Zhu and coworkers described a mutant with a transposon insertion in alr0093 (49). In this transposon mutant, the luxAB reporter gene controlled by the hepA promoter showed delayed expression during nitrogen depletion compared to the wild type. The mutant could not grow on N2 as the sole nitrogen source. Due to the sequence homology of alr0093 to amidases, Zhu et al. named the gene hcwA, for being involved in heterocyst cell wall formation (49). It is not clear why SR478 and the hcwA transposon mutant have different phenotypes. However, there are also reports of other genes in which mutations at different sites within the same gene lead to distinct phenotypes (7, 26). When Zhu et al. complemented the hcwA mutant with an intact copy of amiC2, mature heterocysts formed, but the level of hepA expression was still reduced. Reconstruction of the mutation of hcwA by double recombination with the recovered transposon led to filaments with apparently normal heterocysts, but the mutant was nonetheless Fox−. Therefore, it cannot be excluded that secondary or polar effects were responsible for the Fox phenotype in the hcwA mutant.
As in Anabaena PCC 7120, the homologous amidase genes amiC1 and amiC2 are in pairwise orientation in the same genomic cluster in other heterocystous cyanobacteria. The reason for the duplication of amiC is not clear. However, this study demonstrated that the two amidases seem to have different relevance for the cell. Both inactivation and overexpression of amiC1 showed stronger phenotypes than the respective amiC2 mutations. One possible explanation for the stronger phenotype is that AmiC1 has a higher activity or is more stable than AmiC2 and thus appears to be the dominant amidase in Anabaena PCC 7120. The knockout of amiC2 can probably be compensated by the activity of amiC1, whereas amiC2 cannot carry out all the functions of amiC1 if the latter is inactivated.
It has been shown earlier that rapid exchange of fluorophore from cell to cell within the filament of Anabaena PCC 7120 and N. punctiforme occurs and that this intrafilamentous cell-cell communication is an essential prerequisite for multicellular properties (14, 30, 34, 37). The complete loss of cell-to-cell communication is evidenced by the complete lack of calcein transfer in the FRAP experiments. How can a cell wall-lytic enzyme be in charge of such a basic event? We suggest that AmiC1 processes the newly synthesized septum to allow the formation of structures for cell-cell communication. This is a new property of cell wall amidases, which so far have been described to be involved in modulation and turnover of peptidoglycan and cleavage of the septal murein during cell division in unicellular bacteria. By overexpressing AmiC1 in the wild-type background, cell lysis could be observed as a result of the autolysin activity of AmiC1. Excess of this cell wall-splitting enzyme obviously prevented a stringent regulation of its site-specific and temporal activity. Using translational fusion to GFP, we showed that at normal expression levels, the amidase AmiC1 is present mainly in the septum of freshly dividing cells. This is also true for AmiC2 (Alr0093) and for AmiC2 (NpF1846) in N. punctiforme (30). In older septa, the amidases are not needed anymore and disappear completely in the septa between vegetative cells and mature heterocysts.
Two different, but not mutually exclusive roles of AmiC1 in heterocyst development can be assumed from the data collected in this study. As a first possibility, the amidase-dependent remodeling of peptidoglycan during heterocyst formation would per se play an essential role in this developmental process. According to this scenario, the septal cell wall remodeling is necessary for ongoing developmental processes such as heterocyst-specific polysaccharide or glycolipid synthesis. In agreement with this scenario, after transferring cells to combined nitrogen-free medium, AmiC1-GFP and AmiC2-GFP accumulated at first exclusively in proheterocysts, and particularly, in the polar channel of proheterocysts, where the largest morphological alterations take place to form the polar neck. The autolysin activity might also be required for the transfer of heterocyst envelope material, polysaccharides and glycolipids, across the cell wall. This could contribute to the Het− phenotype of the AmiC1 mutant. The relevance of peptidoglycan modification for heterocyst differentiation was also demonstrated by inactivation of genes encoding penicillin binding proteins pbp2 (alr5101), pbp3 (all2981), and pbp6 (alr4579) in Anabaena PCC 7120. Deletion of these cell wall-modifying enzymes also led to a Fox− phenotype (25, 28, 29). The inability to remodel peptidoglycan would thus stop differentiation processes at the level of cell structure remodeling, which would be dominant over ongoing NtcA/HetR-executed developmental gene expression program.
In a second scenario, the requirement for AmiC1 function for heterocyst differentiation would be due to amidase-dependent cell-cell communication. According to this scenario, molecular exchange processes between vegetative cells of the filament would be absolutely required for heterocyst induction and proheterocyst formation. This assumption is in good agreement with the observed phenotypes of several mutations that affect cell-cell communication without being involved in peptidoglycan metabolism. Mutants with mutations in septum-localized proteins SepJ, FraC, and FraD display severely impaired cell-cell communication and are also affected in the maturation of functional heterocysts (15, 34, 35, 37, 39). It seems that the lack of cell-cell communication does not affect the induction of the heterocyst-specific gene regulators, but it may affect the fine-tuned sequence of the developmental program, as suggested by the delayed expression of hepA. Finally, in the absence of communication between proheterocysts and neighboring cells, the NtcA/HetR-dependent gene products may not be able to execute their function properly, which would cause deficient heterocyst development.
In summary, the data from this study strengthen the assumption that the duplication of amiC genes in heterocystous cyanobacteria is directly related to the formation of multicellular filaments. Between N. punctiforme and Anabaena PCC 7120, the AmiC proteins may have different functions with respect to their role in cell division plane regulation, but in both cases, at least one of the amidases is indispensable for the formation of filaments with intercommunicating cells, which is a novel property of this enzyme class in bacteria.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by SFB766 (Deutsche Forschungsgemeinschaft).
We thank Peter Wolk, PRL, MSU, East Lansing, MI, for pRL plasmids; Alicia Muro-Pastor, CSIC, Sevilla, Spain, for pCSEL plasmids; and Claudia Menzel for technical assistance.
Footnotes
Published ahead of print 20 July 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Bauer CC, Buikema WJ, Black K, Haselkorn R. 1995. A short-filament mutant of Anabaena sp. strain PCC 7120 that fragments in nitrogen-deficient medium. J. Bacteriol. 177:1520–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bernhardt TG, de Boer PA. 2003. The Escherichia coli amidase AmiC is a periplasmic septal ring component exported via the twin-arginine transport pathway. Mol. Microbiol. 48:1171–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Black TA, Cai Y, Wolk CP. 1993. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol. Microbiol. 9:77–84 [DOI] [PubMed] [Google Scholar]
- 4. Black TA, Wolk CP. 1994. Analysis of a Het- mutation in Anabaena sp. strain PCC 7120 implicates a secondary metabolite in the regulation of heterocyst spacing. J. Bacteriol. 176:2282–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buikema WJ, Haselkorn R. 1991. Characterization of a gene controlling heterocyst differentiation in the cyanobacterium Anabaena 7120. Genes Dev. 5:321–330 [DOI] [PubMed] [Google Scholar]
- 6. Cai YP, Wolk CP. 1990. Use of a conditionally lethal gene in Anabaena sp. strain PCC 7120 to select for double recombinants and to entrap insertion sequences. J. Bacteriol. 172:3138–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chapman KE, Duggan PS, Billington NA, Adams DG. 2008. Mutation at different sites in the Nostoc punctiforme cyaC gene, encoding the multiple-domain enzyme adenylate cyclase, results in different levels of infection of the host plant Blasia pusilla. J. Bacteriol. 190:1843–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Souza RF, Anantharaman V, de Souza SJ, Aravind L, Gueiros-Filho FJ. 2008. AMIN domains have a predicted role in localization of diverse periplasmic protein complexes. Bioinformatics 24:2423–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elhai J, Wolk CP. 1988. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 167:747–754 [DOI] [PubMed] [Google Scholar]
- 10. Elhai J, Wolk CP. 1988. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene 68:119–138 [DOI] [PubMed] [Google Scholar]
- 11. Ernst A, et al. 1992. Synthesis of nitrogenase in mutants of the cyanobacterium Anabaena sp. strain PCC 7120 affected in heterocyst development or metabolism. J. Bacteriol. 174:6025–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fay P. 1992. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol. Rev. 56:340–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feilmeier BJ, Iseminger G, Schroeder D, Webber H, Phillips GJ. 2000. Green fluorescent protein functions as a reporter for protein localization in Escherichia coli. J. Bacteriol. 182:4068–4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flores E, Herrero A. 2010. Compartmentalized function through cell differentiation in filamentous cyanobacteria. Nat. Rev. Microbiol. 8:39–50 [DOI] [PubMed] [Google Scholar]
- 15. Flores E, et al. 2007. Septum-localized protein required for filament integrity and diazotrophy in the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 189:3884–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fuger P, Behrends LB, Mertel S, Sigrist SJ, Rasse TM. 2007. Live imaging of synapse development and measuring protein dynamics using two-color fluorescence recovery after photo-bleaching at Drosophila synapses. Nat. Protoc. 2:3285–3298 [DOI] [PubMed] [Google Scholar]
- 17. Golden JW, Robinson SJ, Haselkorn R. 1985. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature 314:419–423 [DOI] [PubMed] [Google Scholar]
- 18. Golden JW, Wiest DR. 1988. Genome rearrangement and nitrogen fixation in Anabaena blocked by inactivation of xisA gene. Science 242:1421–1423 [DOI] [PubMed] [Google Scholar]
- 19. Heidrich C, Ursinus A, Berger J, Schwarz H, Höltje JV. 2002. Effects of multiple deletions of murein hydrolases on viability, septum cleavage, and sensitivity to large toxic molecules in Escherichia coli. J. Bacteriol. 184:6093–6099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herrero A, Muro-Pastor AM, Valladares A, Flores E. 2004. Cellular differentiation and the NtcA transcription factor in filamentous cyanobacteria. FEMS Microbiol. Rev. 28:469–487 [DOI] [PubMed] [Google Scholar]
- 21. Holland D, Wolk CP. 1990. Identification and characterization of hetA, a gene that acts early in the process of morphological differentiation of heterocysts. J. Bacteriol. 172:3131–3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Höltje JV. 1995. From growth to autolysis: the murein hydrolases in Escherichia coli. Arch. Microbiol. 164:243–254 [DOI] [PubMed] [Google Scholar]
- 23. Höltje JV, Tuomanen EI. 1991. The murein hydrolases of Escherichia coli: properties, functions and impact on the course of infections in vivo. J. Gen. Microbiol. 137:441–454 [DOI] [PubMed] [Google Scholar]
- 24. Hu B, Yang G, Zhao W, Zhang Y, Zhao J. 2007. MreB is important for cell shape but not for chromosome segregation of the filamentous cyanobacterium Anabaena sp. PCC 7120. Mol. Microbiol. 63:1640–1652 [DOI] [PubMed] [Google Scholar]
- 25. Huang G, et al. 2005. Clustered genes required for the synthesis of heterocyst envelope polysaccharide in Anabaena sp. strain PCC 7120. J. Bacteriol. 187:1114–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim YH, et al. 2004. The role of Slr1443 in pilus biogenesis in Synechocystis sp. PCC 6803: involvement in post-translational modification of pilins. Biochem. Biophys. Res. Commun. 315:179–186 [DOI] [PubMed] [Google Scholar]
- 27. Kumar K, Mella-Herrera RA, Golden JW. 2010. Cyanobacterial heterocysts. Cold Spring Harb. Perspect. Biol. 2:a000315.doi:10.1101/cshperspect.a000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lázaro S, Fernández-Piñas F, Fernández-Valiente E, Blanco-Rivero A, Leganés F. 2001. pbpB, a gene coding for a putative penicillin-binding protein, is required for aerobic nitrogen fixation in the cyanobacterium Anabaena sp. strain PCC7120. J. Bacteriol. 183:628–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leganes F, et al. 2005. Wide variation in the cyanobacterial complement of presumptive penicillin-binding proteins. Arch. Microbiol. 184:234–248 [DOI] [PubMed] [Google Scholar]
- 30. Lehner J, et al. 2011. The morphogene AmiC2 is pivotal for multicellular development in the cyanobacterium Nostoc punctiforme. Mol. Microbiol. 79:1655–1669 [DOI] [PubMed] [Google Scholar]
- 31. Mariscal V, Flores E. 2010. Multicellularity in a heterocyst-forming cyanobacterium: pathways for intercellular communication. Adv. Exp. Med. Biol. 675:123–135 [DOI] [PubMed] [Google Scholar]
- 32. Mariscal V, Herrero A, Flores E. 2007. Continuous periplasm in a filamentous, heterocyst-forming cyanobacterium. Mol. Microbiol. 65:1139–1145 [DOI] [PubMed] [Google Scholar]
- 33. Meeks JC, Elhai J. 2002. Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiol. Mol. Biol. Rev. 66:94–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Merino-Puerto V, Mariscal V, Mullineaux CW, Herrero A, Flores E. 2010. Fra proteins influencing filament integrity, diazotrophy and localization of septal protein SepJ in the heterocyst-forming cyanobacterium Anabaena sp. Mol. Microbiol. 75:1159–1170 [DOI] [PubMed] [Google Scholar]
- 35. Merino-Puerto V, et al. 2011. FraC/FraD-dependent intercellular molecular exchange in the filaments of a heterocyst-forming cyanobacterium, Anabaena sp. Mol. Microbiol. 82:87–98 [DOI] [PubMed] [Google Scholar]
- 36. Moslavac S, et al. 2007. A TolC-like protein is required for heterocyst development in Anabaena sp. strain PCC 7120. J. Bacteriol. 189:7887–7895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mullineaux CW, et al. 2008. Mechanism of intercellular molecular exchange in heterocyst-forming cyanobacteria. EMBO J. 27:1299–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muro-Pastor AM, Olmedo-Verd E, Flores E. 2006. All4312, an NtcA-regulated two-component response regulator in Anabaena sp. strain PCC 7120. FEMS Microbiol. Lett. 256:171–177 [DOI] [PubMed] [Google Scholar]
- 39. Nayar AS, Yamaura H, Rajagopalan R, Risser DD, Callahan SM. 2007. FraG is necessary for filament integrity and heterocyst maturation in the cyanobacterium Anabaena sp. strain PCC 7120. Microbiology 153:601–607 [DOI] [PubMed] [Google Scholar]
- 40. Olmedo-Verd E, Muro-Pastor AM, Flores E, Herrero A. 2006. Localized induction of the ntcA regulatory gene in developing heterocysts of Anabaena sp. strain PCC 7120. J. Bacteriol. 188:6694–6699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Popa R, et al. 2007. Carbon and nitrogen fixation and metabolite exchange in and between individual cells of Anabaena oscillarioides. ISME J. 1:354–360 [DOI] [PubMed] [Google Scholar]
- 42. Rippka R, Dereules J, Waterbury JB, Herdman M, Stanier RY. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1–61 [Google Scholar]
- 43. Staron P, Forchhammer K, Maldener I. 2011. Novel ATP-driven pathway of glycolipid export involving TolC protein. J. Biol. Chem. 286:38202–38210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Uehara T, Parzych KR, Dinh T, Bernhardt TG. 2010. Daughter cell separation is controlled by cytokinetic ring-activated cell wall hydrolysis. EMBO J. 29:1412–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vollmer W, Joris B, Charlier P, Foster S. 2008. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol. Rev. 32:259–286 [DOI] [PubMed] [Google Scholar]
- 46. Winkenbach F, Wolk CP, Jost M. 1972. Lipids of membranes and of the cell envelope in heterocysts of a blue-green alga. Planta 107:69–80 [DOI] [PubMed] [Google Scholar]
- 47. Wolk CP, Vonshak A, Kehoe P, Elhai J. 1984. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc. Natl. Acad. Sci. U. S. A. 81:1561–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao J, Wolk CP. 2008. Developmental biology of heterocysts, 2006, p 397–418 In Whitworth DE. (ed), Myxobacteria: multicellularity and differentiation. ASM Press, Washington, DC [Google Scholar]
- 49. Zhu J, et al. 2001. HcwA, an autolysin, is required for heterocyst maturation in Anabaena sp. strain PCC 7120. J. Bacteriol. 183:6841–6851 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.