Abstract
Moloney leukemia virus type 10 protein (MOV10) is an RNA helicase that is induced by type I interferon. It inhibits HIV replication at several steps of its replicative cycle. Of interest, MOV10 is a component of mRNA processing (P) bodies, which inhibit retrotransposition (RTP) of intracisternal A particles (IAP). In this report, we studied the effects of MOV10 on IAP RTP and its dependence on P bodies. Indeed, MOV10 inhibited IAP RTP. It decreased significantly not only the products of reverse transcriptase but also its endogenous activity. MOV10 also associated with IAP RNA. Furthermore, although it was found in IAP virus-like particles, it did not affect their incorporation of IAP RNA, primer tRNAPhe (phenylalanine tRNA), or IAP Gag. Concerning P bodies, the exogenously expressed MOV10 had no effect on their size and number, and the inhibition of IAP RTP persisted despite the depletion of their RCK subunit. Thus, by interfering with reverse transcription, MOV10 inhibits IAP RTP, and this inhibition is independent of P bodies.
INTRODUCTION
Intracisternal A particles (IAPs) are long-terminal repeat (LTR)-retrotransposons (endogenous retroviruses [ERVs]) in the mammalian genome. About 100 of 1,000 to 2,000 IAP ERVs in the mouse genome are still active for autonomous intracellular retrotransposition (RTP). In humans, no recent RTP events of IAPs were reported. However, there is evidence that IAPs are transcriptionally active and can express proteins in human cell lines and tissues (10, 12). Indeed, IAP virus-like particles (VLPs) were found associated with monocytes and in salivary tissues in patients with CD4+ T-cell deficiencies and with Sjögren's syndrome, respectively (10, 12). These observations also suggested that active transcription and production of IAP proteins do not necessarily lead to integration, the final step of RTP, due to restrictions at different steps in its replicative cycle: (i) transcription, (ii) translation, (iii) reverse transcription (RT), and (iv) integration.
To prevent deleterious effects of RTP, host cells have developed multiple defense mechanisms. Innate immunity conferred by cellular restriction factors is an important antiviral defense against endogenous and exogenous retroviruses. One of these is the Moloney leukemia virus type 10 protein (MOV10), an RNA helicase that belongs to the DExD box superfamily (7). It consists of seven highly conserved helicase motifs. MOV10 is also a component of mRNA processing (P) bodies, which harbor Argonaute proteins (Ago1 and Ago2) and are involved in microRNA (miRNA)-guided mRNA silencing (17). MOV10 is also induced by type I interferon (20). Three groups have reported that MOV10 inhibits human immunodeficiency virus type 1 (HIV-1) replication at a postentry step (5, 9, 23). However, the molecular mechanism by which MOV10 functions to restrict HIV replication is not clear. Previously, we published that P bodies inhibit IAP RTP (14). Therefore, we now wanted to determine if MOV10 also inhibits IAP RTP and whether its effects depend on P bodies.
In this study, we found that MOV10 not only is incorporated into IAP VLPs but also inhibits IAP RTP by blocking its RT. MOV10 also associated with IAP RNA but did not affect IAP RNA, primer tRNAPhe, and IAP Gag incorporation into VLPs. Lastly, the exogenous expression of MOV10 had no effect on the size and number of P bodies, and it inhibited IAP RTP to a similar degree in the presence and absence of RCK.
MATERIALS AND METHODS
Cells, plasmids, and siRNAs.
Human embryonic kidney 293 (HEK 293) cells were maintained at 37°C with 5% CO2 in Dulbecco modified Eagle medium containing 10% fetal bovine serum (FBS) and 100 mM l-glutamine. The IAP reporter plasmid pFL was a kind gift from Kyoji Horie. pFlAG/HA-MOV10 was purchased from Addgene (Addgene Plasmid 10976). To clone MOV10.YFP, primers (F, 5′-CAG ATC TCG AGA TGC CCA GTA AGT TCA GC-3′, and R, 5′-CCG G TG GAT CCA GGA GCT CAT TCC TCC ACT C-3′) were used to amplify MOV10 coding sequences from pFlAG/HA-MOV10. The amplicon was ligated into the pEYFP-N1 (Clontech) after digesting with XhoI and BamHI. MOV10 small interfering RNA (siRNA) (ON-TARGETplus SMARTpool, L-014162-00)) and control siRNA (ON-TARGETplus nontargeting pool, D-001810-10-05) were purchased from Dharmacon. Human RCK siRNA and its control siRNA were described previously (14).
Transfections.
Lipofectamine 2000 (Invitrogen) was used to reverse transfect HEK 293 cells with 1 μg of pFL and 1 μg (unless otherwise indicated in the figure legend) of pFLAG/HA-MOV10 or the empty plasmid vector, according to the manufacturer's instructions. siRNAs were transfected at a final concentration of 40 nM.
Western blotting.
Western blotting was performed as described previously (13). Rabbit anti-MOV10 (10370-1-AP, protein-tech group), mouse anti-actin (ab8227; Abcam), rabbit anti-IAP Gag (a kind gift from Bryan Cullen), mouse anti-Ago2 (ab57113; Abcam), and goat anti-RCK (sc-51415; Santa Cruz Biotechnology) were used as primary antibodies.
RNA-IP.
RNA immunoprecipitation (RNA-IP) was performed as described previously (3). Briefly, HEK 293 cells were collected and washed in cold PBS. Cells were resuspended and lysed in 1.5 volumes of polysome lysis buffer (10 mM HEPES [pH 7.0], 100 mM KCl, 5 mM MgCl2, 25 mM EDTA, 0.5% octylphenoxypolyethoxyethanol [Igepal], 2 mM dithiothreitol [DTT], 0.2 mg/ml heparin, 50 U/ml RNase OUT [Invitrogen], 50 U/ml Superase IN [Ambion], 1 tablet of complete protease inhibitor [Roche]), followed by centrifugation at 14,000 × g at 4°C for 10 min. Protein A Sepharose beads (50 μl) (Amersham) were equilibrated in NT2 buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM MgCl2, 0.05% Igepal, 5% bovine serum albumin (BSA) (Equitech Bio), 0.02% sodium azide, and 0.02 mg/ml heparin. Rabbit anti-MOV10 or normal rabbit IgG (2.2 μg) was added to the protein A beads, and the mixtures were incubated for 12 h at 4°C. The beads were subsequently washed three times in NT2 buffer and resuspended in 10 ml NT2 buffer supplemented with 30 mM EDTA (pH 8.0), 1 mM DTT, 50 U/ml RNase OUT, and 50 U/ml Superase IN. HEK 293 cell extracts were added to the antibody-coated beads and incubated for 6 h at 4°C. The beads were then thoroughly washed four times in ice-cold NT2 buffer, and RNP complexes were eluted twice with 500 μl SDS-EDTA (50 mM Tris [pH 8.0], 100 mM NaCl, 10 mM EDTA, 1% SDS) containing 40 U/ml RNase inhibitor for 10 min at 65°C. To isolate RNA from the RNA-IP elution, the elution was incubated in 200 mM NaCl and 20 μg of proteinase K for 1 h at 42°C. A 980-μl volume of acid-phenol-chloroform was added, followed by centrifuging at 10,000 × g at room temperature for 3 min. The aqueous layer was transferred to a new microcentrifuge tube. RNA was precipitated by adding 98 μl of 3 M sodium acetate, 10 μg yeast tRNA, and 2,450 μl ice-cold ethanol to the aqueous layer and incubating the mixture for 2 h at 80°C, followed by centrifuging for 30 min at 4°C. The RNA pellet was washed with 70% ethanol and dissolved in diethyl pyrocarbonate (DEPC)-water.
IP.
Cells were lysed in lysis buffer containing 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2 mM EDTA, 1% NP-40, 10% glycerol, and protease inhibitor (P8340; Sigma). Lysate was precleared with A-Sepharose (GE Healthcare) and then incubated with rabbit anti-MOV10 antibody or normal rabbit IgG overnight, followed by 4 h of incubation with the protein A-Sepharose beads. Beads were washed three times in 0.5 ml lysis buffer, and proteins were eluted from the beads with SDS sample buffer by incubating for 5 min at 95°C. Immunoprecipitates were then resolved in SDS-PAGE gel followed by Western blotting.
Immunofluorescence staining.
Immunofluorescence was performed as described previously (14). Anti-eIF4E-T antibody was used as primary antibody, and donkey anti-goat IgGs conjugated to Alexa Fluor dye 594 (Molecular Probes, Eugene, OR) were used as secondary antibody. Samples were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and visualized with a Zeiss LSM 510 confocal microscope, with a 63X1.4-numerical-aperture lens (Carl Zeiss Inc.).
RTP assay.
The RTP assay was performed as described previously (14). Briefly, the RTP reporter plasmid pFL was transfected with pFlAG/HA-MOV10, or MOV10, or RCK siRNAs into HEK 293 cells. Three days later, cells were collected and subjected to fluorescence-activated cell sorting (FACS) analysis.
qPCR.
Total RNA or DNA was extracted, and quantitative PCR (qPCR) was performed using Stratagene Mx3500. The primers used in this study included primers for detecting IAP RNA levels, IAP Gag (F, 5′-ACC CAG GAA GCA GTC AGA GA-3′, and R, 5′-CCT TTA GGG CTT GAG CAC AG-3′); two sets of primers for detecting the IAP RT product, GFP (F1, 5′-TGA AGA AGT CGC TGA TGT CCT-3′, and R1, 5′-GAG CAA GCA GAT CCT GAA GAA-3′), and GFP (CF1, 5′-ACG CGG TAC ACG AAC ATC TC-3′, and CR1, 5′-TCG CCT TCG ACA TCC TGA GC-3′); human β-actin (F, 5′-AAA GAC CTG TACGCC AAC AC-3′, and R, 5′-GTC ATA CTC CTG CTT GCT GAT-3′); and tRNAPhe (F, 5′-GCC GAA ATA GCT CAG TTG GG-3′, and R, 5′-TGG TGC CGA AWY CCG GGA TC-3′).
Endogenous RT assay.
Endogenous reverse transcription (RT) activity was assayed using 30 μg of purified IAPs in 50 μl of reaction mixture: 50 mM Tris-HCl (pH 8.3), 10 mM MgCl2, 8 mM DTT, 400 μM (each) dinucleoside triphosphates (dNTPs), and 0.05% NP-40. The reaction mixture was incubated at 37°C for 3 h and was stopped by incubating in stop solution (10 mM Tris-HCl [pH 7.4], 10 mM EDTA, 20 μg/ml of salmon sperm DNA, and 50 μg/ml protease K) at 37°C for 15 min, followed by 10 min at 95°C. A 10-μl volume of the stopped reaction mixture was used as the template for qPCR to measure IAP cDNA from endogenous reverse transcription.
VLP isolation and extraction of RNA from VLPs.
Endogenous A particles were isolated from HEK 293 cells as described previously (15). Briefly, HEK 293 cells were collected from four 100-mm dishes and washed with cold PBS. Cells were lysed in 2 ml of buffer A (0.25 M sucrose, 0.6% Triton X-100, 0.05 M Tris-HCl [pH 7.6], 0.025 M KCl, and 0.005 M MgCl2). Nuclei were removed by centrifugation at 700 × g for 10 min. Cytoplasmic extract was collected in 0.01 M EDTA and was layered over a 17.5% (wt/wt) sucrose cushion in 10 mm Tris-HCl (pH 7.4) and 1 mm EDTA before centrifugation in an SW 41.T1 rotor for 60 min at 40,200 rpm. The A particles were collected as a Triton-EDTA-insoluble pellet and were resuspended in a small volume of sodium phosphate buffer (pH 7.1). All procedures were performed at 4°C. RNA was isolated from same amount of purified IAPs using TRIzoL reagent according to the manufacturer's instruction.
RESULTS
MOV10 inhibits IAP RTP in a dose-dependent manner.
To monitor IAP RTP, we used a previously described reporter IAP plasmid (pFL) (14). Briefly, pFL contains a green fluorescent protein (GFP) reporter gene, which is disrupted by the γ-globin intron and expresses GFP only if IAP has undergone RTP. Thus, RTP rate is determined by counting GFP-positive cells. To determine if MOV10 inhibits IAP RTP, we cotransfected HEK 293 cells with pFL (1 μg) and different amounts of pFLAG/HA-MOV10 plasmid ranging from 0 to 1 μg. Three days after the transfection, cells were collected. FACS was used to count GFP-positive cells and estimate the IAP RTP rate. As presented in Fig. 1A and B, IAP RTP was reduced in cells expressing MOV10 exogenously. Indeed, increasing amounts of pFLAG/HA-MOV10 correlated positively with this inhibition (Fig. 1A). These dose-dependent effects suggest that the inhibition of IAP RTP is MOV10 specific.
Fig 1.
MOV10 decreases IAP RTP in a dose-dependent manner. (A) Inhibition of IAP RTP increases with higher ratios of MOV10 over pFL plasmids (0.2:1, 0.5:1, 1:1) in cotransfections. Data are presented relative to control (% RTP set to 100) as averages ± standard deviations from two independent experiments. (B) MOV10 expression was detected by Western blotting. β-Actin was used as the loading control.
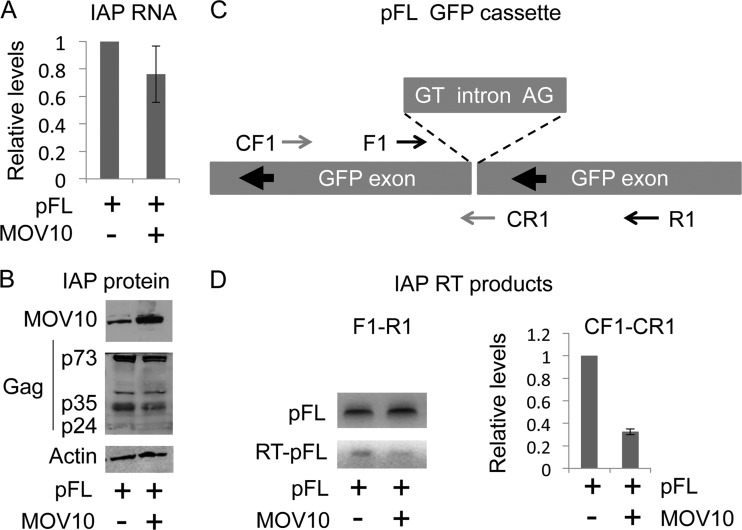
MOV10 decreases IAP RT products but not levels of IAP RNA and protein.
To limit deleterious effects of ERVs, mammalian cells use multiple strategies that target various stages of their replicative cycles. IAPs have an obligatory RNA-intermediate step similar to that of exogenous retroviruses like HIV, except that they lack an extracellular phase. Thus, the host applies similar defense mechanisms to that target: (i) transcription, (ii) translation, (iii) RT, and (iv) integration of new IAP cDNA. To pinpoint where MOV10 inhibits IAP, products from these different steps were examined. First, levels of IAP RNA and IAP Gag were determined by real-time qPCR and Western blotting, respectively. As presented in Fig. 2A and B, the exogenous expression of MOV10 resulted in little to no change in the levels of IAP RNA and IAP Gag. Next, we examined RT products (cDNA) of IAP by conventional qPCR using the primer set F1 and R1 (Fig. 2C), which detects amplicons from the parental pFL plasmid containing the γ-globin intron (Fig. 2D, top left panel) and the reverse-transcribed pFL cDNA, where this intron had been spliced out (Fig. 2D, bottom left panel). In contrast to levels of IAP RNA and proteins, markedly decreased amounts of IAP cDNA were detected in cells that expressed MOV10 exogenously (Fig. 2D, bottom left panel). To better quantify decreased IAP RT products, we also performed real-time qPCR using a primer set (Fig. 2C, CF1 and CR1), which only detects the amplicon from reverse-transcribed pFL. These IAP RT products were reduced 3-fold (Fig. 2D). Since the GFP cassette in pFL was inserted into the 3′ Env open reading frame of IAP (Fig. 2C) and we detected these RT products using our GFP primer sets, we conclude that early RT is inhibited by MOV10.
Fig 2.
Exogenous MOV10 expression decreases IAP RT products. (A) Exogenous MOV10 expression does not affect levels of IAP RNA. Relative levels of RNA were set to 1 in the control. β-Actin was used as the reference RNA. (B) Exogenous MOV10 expression does not affect levels of IAP Gag. MOV10 and IAP Gag proteins were detected by Western blotting. β-Actin was used as the control. (C) Schematic representation of the GFP cassette within pFL. GFP is in the antisense orientation, as indicated by large black arrows. The γ-globin intron is in the sense orientation with marked donor (GT) and acceptor (AG) splice sites. Small arrows indicate primer sets used in panel D. (D) Exogenous MOV10 expression inhibits IAP RT products in 293 cells. IAP RT products were detected by qPCR (left panel, black arrows, F1-R1) and real-time qPCR (right panel, gray arrows, CF1-CR1), using IAP genomic DNA as the template. F1-R1 detects two amplicons: one containing the intron (top left panel) and the other where the intron was spliced out (bottom left panel, RT-pFL). CF1-CR1 detects only amplicons from spliced and reverse-transcribed pFL. Relative levels of RT products by real-time qPCR were set to 1 in the control. Data in panels A and D represent averages ± standard deviations from three independent experiments.
MOV10 inhibits the endogenous IAP RT activity.
To confirm that MOV10 inhibited IAP RT, we examined the endogenous IAP RT activity in VLPs. HEK 293 cells were cotransfected with pFL (1 μg) and pFLAG/HA-MOV10 (1 μg) or the empty plasmid vector (1 μg). Three days later, IAP VLPs were isolated from cells as described previously (15). Endogenous RT (ERT) activity was assayed by incubating 30 μg of purified IAP VLPs in 50 μl of reaction mixture, which included dNTPs, for 3 h. RT was stopped, and 10 μl of this reaction mixture was analyzed by qPCR to measure levels of IAP cDNA. Consistent with the decreased steady-state cDNA levels shown in Fig. 2D, the endogenous IAP RT activity was markedly decreased in cells expressing MOV10 exogenously (Fig. 3).
Fig 3.
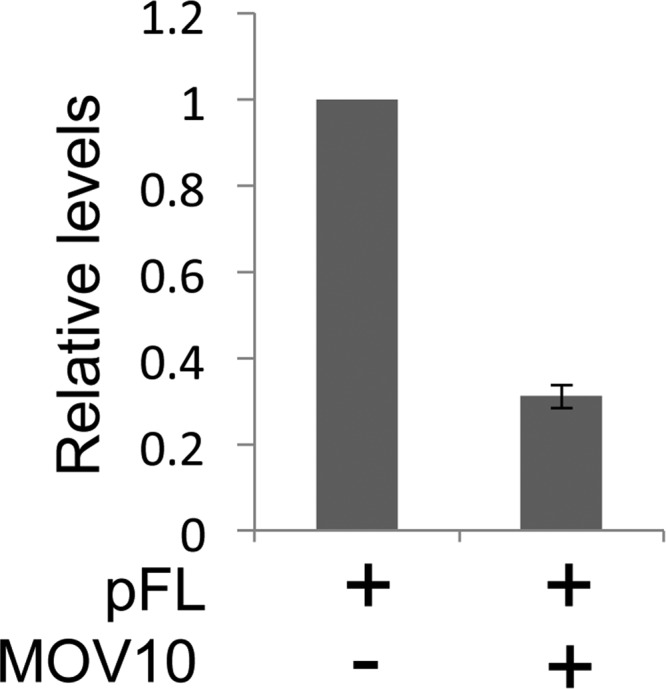
Exogenous MOV10 expression decreases endogenous IAP RT activity in VLPs. Levels of RT products synthesized by endogenous IAP RT were measured by real-time qPCR. Relative levels of RT products were set to 1 in the control. Data represent averages ± standard deviations from three independent experiments.
MOV10 is incorporated into IAP VLPs.
The exogenous expression of MOV10 inhibited IAP RT activity, which occurs in VLPs. To determine if MOV10 is also incorporated into IAP VLPs, they were isolated from cells. Next, we determined the levels of MOV 10 and IAP Gag in these VLPs and lysates by Western blotting. Indeed, MOV10 was enriched in VLPs, compared to its levels in total cell lysates (Fig. 4). Although the exogenous expression of MOV10 increased its incorporation, it did not change the levels of IAP Gag in VLPs (Fig. 4), suggesting that MOV10 does not interact with IAP Gag or that interactions between MOV10 and IAP Gag are not required for the incorporation of MOV10 into VLPs. Intriguingly, Ago2, another component of the RNA-induced silencing complex (RISC) that associates with MOV10 (17), was also enriched in IAP VLPs (Fig. 4), which is consistent with findings with HIV (4). However, the exogenous MOV10 expression did not increase Ago2 incorporation (Fig. 4), suggesting that its interaction with Ago2 is not required for its encapsidation in VLPs.
Fig 4.

MOV10 and Ago2 are incorporated into IAP VLPs, and MOV10 does not affect levels of IAP Gag. VLPs were isolated from 293 cells that expressed or not the exogenous MOV10 protein. MOV10, IAP Gag, Ago2, and β-actin proteins in VLPs (lanes 1 and 2) and total cell lysates (TCL, lanes 3 and 4) were detected with anti-MOV10, IAP Gag, Ago2, and β-actin antibodies by Western blotting. β-Actin was used as the loading control.
MOV10 associates with IAP RNA but not IAP Gag.
Next, we studied interactions between MOV10, IAP RNA, and IAP Gag. HEK 293 cells were cotransfected with pFL, pFLAG/HA-MOV10, or the empty plasmid vector. Two days posttransfection, cells were lysed and subjected to protein and RNA immunoprecipitations (IP and RNA-IP). Immunoprecipitations with anti-MOV10 antibodies were probed for the presence of Ago2 and IAP Gag. As presented in Fig. 5, Ago2, a known partner, but not IAP Gag coprecipitated with MOV10 (Fig. 5A). This observation suggests that MOV10 does not interact with IAP Gag. After expressing pFL and FLAG/HA-MOV10 in HEK 293 cells, we used anti-HA and anti-Flag antibodies to precipitate proteins bound to MOV10. These immunoprecipitations were subjected to liquid chromatography-tandem mass spectrometry (LC-MS-MS). No IAP Gag was identified in either immunoprecipitation (data not shown), confirming that MOV10 does not interact with IAP Gag. In contrast, RNA-IP revealed that IAP RNA was enriched 400-fold with anti-MOV10 antibodies compared to the IgG control (Fig. 5B and C), indicating that MOV10 interacts with IAP RNA.
Fig 5.

MOV10 associates with IAP RNA. (A) MOV10 does not interact with IAP Gag. IPs were performed with IgG or anti-MOV10 antibodies on lysates from cells expressing pFL and MOV10. Levels of MOV10, IAP Gag, and Ago2 were determined by Western blotting. (B) IAP RNA is enriched about 400-fold in immunoprecipitations with anti-MOV10 compared to IgG antibodies. RNA-IPs were performed with cell lysates overexpressing MOV10 and pFL. RNA was isolated from immunoprecipitations with IgG or anti-MOV10 antibodies and quantified by real-time qPCR. Data are presented as averages ± standard deviations from two independent experiments. (C) MOV10 was immunoprecipitated with anti-MOV10 antibodies in RNA-IPs. Western blotting was used to detect MOV10 in immunoprecipitations with IgG or anti-MOV10 antibodies.
MOV10 does not affect the incorporation of IAP RNA or primer tRNAPhe into VLPs.
Decreased RT by MOV10 could also result from decreased incorporation of IAP RNA, which serves as the template for RT, or tRNAPhe, the tRNA that primes IAP RT (18, 19). To determine if levels of IAP RNA or tRNAPhe in VLPs were decreased by MOV10, VLPs were isolated from cells expressing pFL with or without exogenous MOV10. Total RNA was isolated from purified IAP VLPs and subjected to real-time qPCR. PCR amplifications revealed that MOV10 did not affect levels of IAP RNA or tRNAPhe in VLPs (Fig. 6A and B). In addition, the ratio between tRNAPhe and IAP RNA remained the same (Fig. 6C). Thus, MOV10 does not inhibit IAP RT by affecting the incorporation of IAP RNA or primer tRNAPhe into new VLPs.
Fig 6.
MOV10 does not affect levels of tRNAPhe or IAP RNA in VLPs. RNA was extracted from VLPs isolated from 293 cells expressing the exogenous MOV10 protein. Real-time qPCR was performed to detect levels of tRNAPhe (A) and IAP RNA (B). (C) MOV10 does not affect the ratio of tRNAPhe and IAP RNA. Data are presented as averages ± standard deviations from three independent experiments.
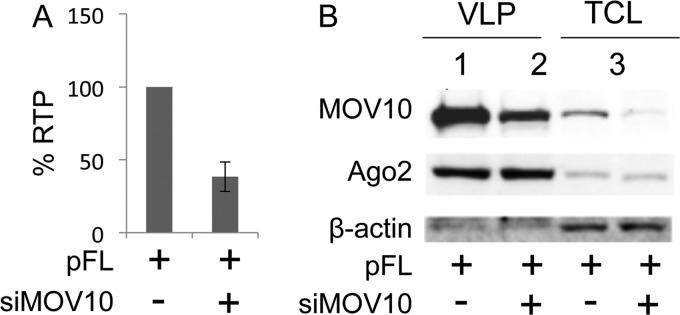
Knockdown of MOV10 inhibits IAP RTP.
To determine if the endogenous MOV10 protein also inhibits IAP RTP, we cotransfected HEK 293 cells with pFL and previously validated MOV10 siRNAs (9). Three days later, cells were collected for FACS and VLPs were isolated for Western blotting. Surprisingly, depleting MOV10 also inhibited IAP RTP (Fig. 7A), albeit to a lesser degree than by its exogenous expression, suggesting that MOV10 also plays a positive role in IAP RTP. This observation is consistent with findings with HIV (5, 9). Furthermore, this depletion decreased MOV10 but not Ago2 incorporation into VLPs (Fig. 7B). These observations again suggest that MOV10 and Ago2 are incorporated into VLPs independently, which is consistent with the data presented in Fig. 4.
Fig 7.
Depletion of MOV10 decreases IAP RTP and MOV10 but not Ago2 incorporation into VLPs. (A) Depleting MOV10 decreases IAP RTP to 38% of the control. Data are presented as averages ± standard deviations from three independent experiments. (B) Whereas depleting MOV10 decreases its incorporation into VLPs, Ago2 incorporation remains the same. VLPs (lanes 1 and 2) and total cell lysates (TCL) (lanes 3, left and right) were used to detect levels of MOV10 and Ago2 by Western blotting.
The exogenous MOV10 expression does not affect the size and number of P bodies.
Previously, we reported that the disruption of P bodies increases IAP RTP (14). MOV10 is a component of P bodies, and the exogenous expression of some of their subunits can affect their size and number (6, 8, 21). Thus, whereas the exogenous expression of P body component SMG7 increases their size (21), that of hDcp2, RCK/p54, or hEdc3 can reduce their number significantly (8). To determine if MOV10 could also affect P bodies, we examined these RNA granules in cells, which expressed the MOV10.YFP fusion protein exogenously, with anti-eIF4E-T antibodies by immunofluorescence. eIF4E-T is a resident of P bodies (1). As presented in Fig. 8, MOV10 did not affect the size or number of P bodies (Fig. 8A and B). Further comparisons between cells expressing or not MOV10.YFP exogenously from the same transfection confirmed this finding (Fig. 8B, compare cells 1 and 2 to cells 3 and 4). To further address whether P bodies are required for the MOV10 inhibition of IAP RTP, we also depleted RCK in these cells with siRNA (14). Three days later, MOV10 inhibited IAP RTP to a similar extent with or without RCK (Fig. 9). Taken together, these observations suggest that MOV10 inhibits IAP RTP by a mechanism that does not involve P bodies.
Fig 8.
MOV10 colocalizes with eIF4E-T to P bodies (panels B and C, MOV10.YFP), and its exogenous expression does not affect the number and size of these RNA granules. Mov10.YFP marks transfected cells (panel C, MOV10, green fluorescence), where it colocalizes with P bodies (panel B, red fluorescence, and panel D, merged, yellow fluorescence). The size and number of P bodies do not change with the exogenous expression of MOV10.YFP (panel A, control, and panel B, eIF4E-T, compare transfected cells 1 and 2 to nontransfected cells 3 and 4). Red fluorescent signals mark P bodies (panels A, B, and D). Green fluorescent signals indicate MOV10.YFP (panels C and D). In panel D, images B and C were merged digitally. White arrows indicate P bodies in cells expressing the exogenous MOV10.YFP protein. Data are representative of two independent experiments.
Fig 9.

MOV10 inhibits IAP RTP to a similar extent in the presence or absence of RCK. (A) MOV10 decreases IAP RTP to 23% and 30% of the control in the absence and presence, respectively, of RCK. Data are presented as averages ± standard deviations from three independent experiments. (B) Three days after the cotransfection of pFL with control or RCK siRNAs, levels of MOV10 and RCK proteins were detected by Western blotting. β-Actin was used as the loading control.
DISCUSSION
In this study, we demonstrated that the RNA helicase MOV10 inhibits IAP RTP. Indeed, the exogenous expression of MOV10 did not affect the levels of IAP mRNA and proteins but decreased those of IAP RT products. Consistent with decreased IAP RT products, the endogenous RT activity from IAP VLPs was markedly decreased. Whereas MOV10 associated with IAP RNA and was incorporated into IAP VLPs, it did not bind to IAP Gag. Importantly, MOV10 did not affect the size and number of P bodies. Depleting P body component RCK did not affect the inhibition of IAP RTP by MOV10. These findings suggest that MOV10 inhibits IAP RT by steric hindrance or structural changes in IAP RNA.
MOV10 was packaged efficiently into IAP VLPs. Moreover, it did not affect levels of IAP RNA or IAP Gag in cells or IAP VLPs, which is similar to findings with HIV (9, 23). However, unlike HIV, we detected interactions only between MOV10 and IAP RNA but not IAP Gag. Likewise, the binding between MOV10 and HIV RNA was important for its incorporation into HIV (5). Consistent with our observations with IAP, Ago2 is also incorporated into HIV (4). Of note, Ago2 and MOV10 are also components of RISC (17). They warrant an investigation of how other subunits of RISC and/or translational silencing are involved in the replicative cycle of endogenous and exogenous retroviruses.
MOV10 inhibits early and late HIV RT products (9, 23). Consistent with these findings, our data indicate that early RT is inhibited by MOV10, suggesting that MOV10 could have affected the incorporation of the primer tRNA. Phenylalanine tRNA (tRNAPhe) was identified as the primer tRNA for IAP in mouse and hamster cells (18, 19). However, we found that MOV10 did not interfere with tRNAPhe incorporation. Thus, MOV10 could decrease RT in VLPs due to steric hindrance or changes in IAP RNA secondary structure. The specific molecular mechanism by which MOV10 inhibits RT remains to be investigated.
Of interest, instead of increasing, depletion of MOV10 also inhibited IAP RTP, which is consistent with findings with HIV (5, 9). Thus, MOV10 could be involved in multiple steps of IAP and HIV RNA metabolism, where its exogenous expression and depletion affect distinct steps in viral replication. Alternatively, a complex containing MOV10 is required and is disrupted by both processes leading to these inhibitory effects. It will be of interest to determine the underlying mechanism by which depletion of MOV10 inhibits IAP RTP.
MOV10 is concentrated in cytoplasmic P bodies (17). However, the exogenous MOV10 expression did not change the size and number of P bodies, confirming that MOV10 is not an essential component of these RNA granules. In addition, the depletion of the essential P body component RCK did not affect the inhibition of IAP RTP by MOV10. These findings suggest that MOV10 inhibits IAP RTP independently of P bodies. In sum, MOV10 can restrict exogenous and endogenous retroviruses in mammals, where it acts primarily at the level of RT.
ACKNOWLEDGMENTS
We thank members of the Peterlin laboratory for helpful advice and discussions, Kyoji Horie for the pFL plasmid, and the HARC Center for Flag- and HA-IP followed by LC-MS/MS analysis.
This work was supported by grants from the NIH (P50 GMO82250, P01 AI090935, P50 GM081879, P50 GM082250) and the Campbell Foundation.
Footnotes
Published ahead of print 18 July 2012
REFERENCES
- 1. Andrei MA, et al. 2005. A role for eIF4E and eIF4E-transporter in targeting mRNPs to mammalian processing bodies. RNA 11:717–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reference deleted.
- 3. Baroni TE, Chittur SV, George AD, Tenenbaum SA. 2008. Advances in RIP-chip analysis: RNA-binding protein immunoprecipitation-microarray profiling. Methods Mol. Biol. 419:93–108 [DOI] [PubMed] [Google Scholar]
- 4. Bouttier M, et al. 2012. Retroviral GAG proteins recruit AGO2 on viral RNAs without affecting RNA accumulation and translation. Nucleic Acids Res. 40:775–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burdick R, et al. 2010. P body-associated protein Mov10 inhibits HIV-1 replication at multiple stages. J. Virol. 84:10241–10253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eulalio A, Behm-Ansmant I, Izaurralde E. 2007. P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 8:9–22 [DOI] [PubMed] [Google Scholar]
- 7. Fairman-Williams ME, Guenther UP, Jankowsky E. 2010. SF1 and SF2 helicases: family matters. Curr. Opin. Struct. Biol. 20:313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fenger-Gron M, Fillman C, Norrild B, Lykke-Andersen J. 2005. Multiple processing body factors and the ARE binding protein TTP activate mRNA decapping. Mol. Cell 20:905–915 [DOI] [PubMed] [Google Scholar]
- 9. Furtak V, et al. 2010. Perturbation of the P-body component Mov10 inhibits HIV-1 infectivity. PLoS One 5:e9081 doi:10.1371/journal.pone.0009081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garry RF, et al. 1990. Detection of a human intracisternal A-type retroviral particle antigenically related to HIV. Science 250:1127–1129 [DOI] [PubMed] [Google Scholar]
- 11. Reference deleted.
- 12. Gupta S, Ribak CE, Gollapudi S, Kim CH, Salahuddin SZ. 1992. Detection of a human intracisternal retroviral particle associated with CD4+ T-cell deficiency. Proc. Natl. Acad. Sci. U. S. A. 89:7831–7835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lenassi M, et al. 2010. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 11:110–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu C, Contreras X, Peterlin BM. 2011. P bodies inhibit retrotransposition of endogenous intracisternal A particles. J. Virol. 85:6244–6251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lueders KK, Kuff EL. 1975. Synthesis and turnover of intracisternal A-particle structural protein in cultured neuroblastoma cells. J. Biol. Chem. 250:5192–5199 [PubMed] [Google Scholar]
- 16. Reference deleted.
- 17. Meister G, et al. 2005. Identification of novel argonaute-associated proteins. Curr. Biol. 15:2149–2155 [DOI] [PubMed] [Google Scholar]
- 18. Mietz JA, Grossman Z, Lueders KK, Kuff EL. 1987. Nucleotide sequence of a complete mouse intracisternal A-particle genome: relationship to known aspects of particle assembly and function. J. Virol. 61:3020–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ono M, Ohishi H. 1983. Long terminal repeat sequences of intracisternal A particle genes in the Syrian hamster genome: identification of tRNAPhe as a putative primer tRNA. Nucleic Acids Res. 11:7169–7179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schoggins JW, et al. 2011. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472:481–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Unterholzner L, Izaurralde E. 2004. SMG7 acts as a molecular link between mRNA surveillance and mRNA decay. Mol. Cell 16:587–596 [DOI] [PubMed] [Google Scholar]
- 22. Reference deleted.
- 23. Wang X, et al. 2010. Moloney leukemia virus 10 (MOV10) protein inhibits retrovirus replication. J. Biol. Chem. 285:14346–14355 [DOI] [PMC free article] [PubMed] [Google Scholar]