Abstract
The cellular receptor utilized by adenovirus serotype 26 (Ad26) has remained unclear. Here we show that Ad26 transduction is CD46-dependent and is efficiently blocked by anti-CD46 but not anti-CAR antibodies, demonstrating that Ad26 utilizes CD46 as a primary cellular receptor. Moreover, following Ad26 vaccination of rhesus monkeys, we did not observe sustained activation of peripheral or mucosal vector-specific CD4+ T lymphocytes. These data contribute to our understanding of Ad26 as a candidate vaccine vector.
TEXT
Human adenovirus serotypes 26 and 35 (Ad26 and Ad35) are currently being evaluated as vaccine vectors for both HIV-1 and other pathogens in phase 1 clinical trials. Ad26 and Ad35 vectors are substantially different than Ad5 vectors in terms of seroepidemiology, in vivo tropism, innate immune profiles, and adaptive immune phenotypes (2, 3, 11, 16). It is well accepted that Ad5 utilizes the coxsackievirus and adenovirus receptor (CAR) (4, 13) and that Ad35 utilizes CD46 as a primary cellular receptor (8). However, the cellular receptor utilized by Ad26 has remained unclear, although a recent publication has suggested that Ad26 utilizes CAR and not CD46 for cell entry (6).
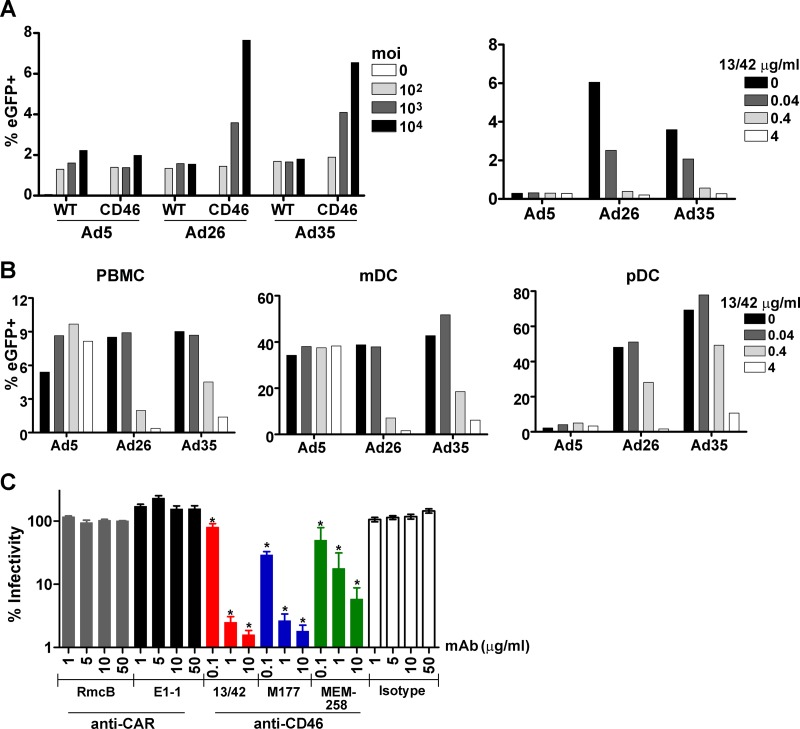
To address this question, we first evaluated the capacity of Ad5, Ad26, and Ad35 vectors encoding enhanced green fluorescent protein (eGFP) to infect peripheral blood mononuclear cells (PBMC) from wild-type and CD46 transgenic mice. PBMC from these mice were incubated with Ad vectors at multiplicities of infection (MOIs) from 102 to 104 for 24 h, and expression of eGFP was evaluated by flow cytometry. Transduction by Ad26 and Ad35, but not Ad5, was markedly enhanced in CD46 transgenic PBMC compared with wild-type PBMC, suggesting that Ad26 and Ad35 utilize CD46 for cell entry (Fig. 1A, left panel). Moreover, Ad26 and Ad35 transduction, but not Ad5 transduction, was efficiently blocked by the anti-CD46 monoclonal antibody (MAb) 13/42 (Fig. 1A, right panel) (14).
Fig 1.
Ad26 uses CD46 and not CAR as a primary cellular receptor. Ad5, Ad26, and Ad35 vectors expressing enhanced green fluorescent protein (eGFP) were incubated with mouse or human cells for 24 h, and the expression of eGFP was evaluated by flow cytometry. (A) PBMC from wild-type mice (WT) and CD46 transgenic mice (CD46) were infected with Ad vectors at multiplicities of infection (MOIs) of 0, 102, 103, and 104 (left). PBMC from CD46 transgenic mice were preincubated with the anti-CD46 MAb 13/42 at concentrations of 0, 0.04, 0.4, or 4 μg/ml for 1 h and then infected with Ad vectors at an MOI of 103 (right). (B) Human PBMC were preincubated with the anti-CD46 MAb 13/42 and then infected with Ad vectors at an MOI of 103. CD11c and CD123 were utilized to identify myeloid dendritic cell (mDC) and plasmacytoid dendritic cell (pDC) subpopulations. Panels A and B show representative data of two experiments. (C) Human PBMC were preincubated with the anti-CAR MAbs (RmcB, E1-1), the anti-CD46 MAbs (13/42, M177, MEM-258), or an isotype control MAb and were then infected with Ad26 at an MOI of 103. Mean and standard error of the mean are shown from 4 independent experiments. *, P < 0.01, one-way ANOVA with Dunnett's posttest.
We next assessed the capacity of Ad5, Ad26, and Ad35 vectors expressing eGFP to transduce human PBMC. Consistent with the mouse studies, we observed that the anti-CD46 MAb 13/42 efficiently blocked Ad26 and Ad35 transduction, but not Ad5 transduction, of human PBMC, myeloid dendritic cells (mDC), and plasmacytoid dendritic cells (pDC) (Fig. 1B). To confirm these findings, we tested the capacity of a panel of anti-CAR and anti-CD46 MAbs to block Ad26 transduction of human PBMC in 4 independent experiments. Dramatic inhibition of Ad26 transduction was observed with the anti-CD46 MAbs 13/42 (14), M177 (15), and MEM-258 (10) (P < 0.01, one-way analysis of variance [ANOVA] with Dunnett's posttest). In contrast, no detectable inhibition of Ad26 transduction was observed with the anti-CAR MAbs RmcB (9) or E1-1 (7), even when utilized at a high concentration of 50 μg/ml (Fig. 1C). These results demonstrate that Ad26 utilizes CD46 and not CAR as a primary cellular receptor for transduction of human PBMC. Moreover, the anti-CD46 MAbs 13/42 and M177 blocked > 99% of Ad26 transduction (Fig. 1C), suggesting that the contribution of additional secondary cellular receptors is likely minimal.
The results of the Step study of the Merck Ad5-Gag/Pol/Nef vaccine raised a hypothesis that Ad5 immunization may have activated Ad5-specific CD4+ T lymphocytes in individuals with baseline Ad5-specific neutralizing antibodies (NAbs) and that these activated vector-specific CD4+ T lymphocytes may have trafficked to mucosal tissues and served as increased target cells for HIV-1 infection (5). We therefore assessed the degree of activation of peripheral and mucosal vector-specific CD4+ T lymphocytes following Ad26 immunization of rhesus monkeys both with and without baseline Ad26 immunity (12). Twelve adult rhesus monkeys received intranasal inoculations of 1011 viral particles (vp) of replication-competent Ad5 and Ad26 (n = 6) or saline (n = 6) at weeks −8 and −4. The preimmunized animals developed high Ad5 NAb titers (327 to 1,182, median 1,004) and low Ad26 NAbs titers (19 to 141, median 63), as well as vector-specific T lymphocyte responses (data not shown). The difference between the Ad5 and Ad26 NAb titers reflects the fact that the replication-competent Ad26 vector replicates poorly in monkeys. Nevertheless, this profile of high Ad5 NAb titers and low Ad26 NAb titers models what is typically observed in individuals in sub-Saharan Africa (3). Monkeys both with and without baseline Ad5/Ad26 immunity were then injected intramuscularly with 3 × 1010 vp replication-incompetent Ad26 vectors encoding SIVmac239 Gag/Pol/Env.
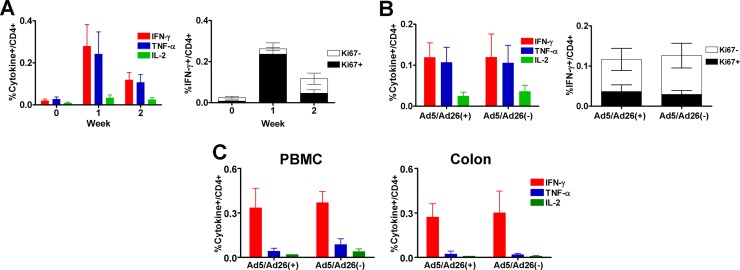
At week 2, monkeys were sacrificed to facilitate comprehensive immunologic and pathological studies of mucosal tissues. Ad26-specific CD4+ T lymphocyte responses and cellular activation as measured by Ki67 expression peaked at 1 week postimmunization and then declined rapidly (Fig. 2A). Moreover, monkeys both with and without baseline Ad5/Ad26 immunity exhibited similar magnitude and activation of vector-specific CD4+ T lymphocyte responses following stimulation with pooled Ad26 hexon peptides in PBMC (Fig. 2B) or Ad26 virus in both PBMC and colorectal biopsy specimens (Fig. 2C). These findings suggest that cellular activation induced by Ad26 vaccination in rhesus monkeys was transient and was not detectably enhanced by baseline Ad5/Ad26 immunity.
Fig 2.
Ad26 immunization induces only transient cellular immune activation in rhesus monkeys. Twelve adult rhesus monkeys received intranasal inoculation of 1011 vp replication-competent Ad5 and Ad26 (n = 6) or saline (n = 6) at weeks −8 and −4 and then were injected intramuscularly with 3 × 1010 replication-incompetent Ad26 encoding SIVmac239 Gag/Pol/Env at week 0. (A) Vector-specific CD4+ T lymphocyte responses following Ad26 vaccination were determined by multiparameter intracellular cytokine staining assays (12) using Ad26 hexon peptides for stimulation (left). Ki67 expression in vector-specific gamma interferon (IFN-γ) secreting CD4+ T lymphocytes was also determined (right). (B). Vector-specific CD4+ T lymphocyte responses are shown from baseline Ad5/Ad26-seropositive and -seronegative monkeys at 2 weeks postimmunization following stimulation with hexon peptides (left). Ki67 expression in IFN-γ secreting CD4+ T lymphocytes is also shown (right). (C) Cytokine secretion of peripheral (left) and colorectal (right) CD4+ T lymphocytes stimulated with whole Ad26 virus at an MOI of 104 at 2 weeks postimmunization.
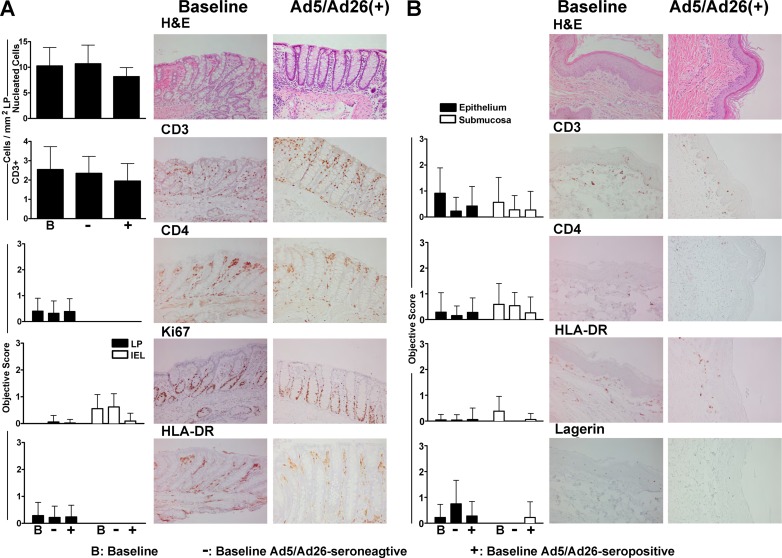
We next characterized inflammatory cell populations in colorectal and foreskin mucosal biopsy specimens before and after vaccination in these monkeys. Multiple serial cross sections of colorectal mucosa were collected, and cell populations were assessed by both quantitative image analysis and objective scoring criteria as we have described previously (12). Total cellular infiltrates and subpopulations were stained with hematoxylin and eosin (H&E) as well as with Abs against T cell (CD3, CD4), proliferation (Ki67), and activation (HLA-DR) markers. No differences in the quantity or nature of colorectal cellular inflammatory infiltrates were detected following vaccination of Ad5/Ad26-seronegative or -seropositive monkeys compared with baseline samples (Fig. 3A). We further explored cellular inflammatory infiltrates in pinch biopsy specimens of the inner penile foreskin region of the foreskin (prepuce) of these monkeys (12). Cellular infiltrates were evaluated in serial sections of foreskin by histopathology and immunohistochemistry utilizing Abs against CD3, CD4, HLA-DR, and Langerin, a marker for Langerhans cells. The extent and distribution of inflammatory subpopulations in foreskin proved comparable in baseline and vaccinated animals regardless of baseline Ad5/Ad26 immunity (Fig. 3B). These data indicate that Ad26 vaccination of rhesus monkeys resulted in only transient cellular activation in the periphery with no detectable mucosal inflammation in colorectal or foreskin tissues by week 2 following vaccination.
Fig 3.
Cell populations in colorectal and foreskin mucosa from rhesus monkeys before and after Ad26 vaccination. (A) Serial sections from colorectal mucosa at baseline and following vaccination were stained with H&E and Abs against CD3, CD4, Ki67, and HLA-DR. Stained cells were evaluated in lamina propria (LP) or epithelium (intraepithelial lymphocytes [IEL]) by quantitative image analysis and objective scoring criteria as described previously (12). Each measurement represents the mean score or cell count per area and standard deviation (left). Photomicrographs of representative tissues from baseline and 2 weeks postimmunization of baseline Ad5/Ad26-seropositive monkeys are also shown. (B). Foreskin sections from the inner penile foreskin were stained with H&E and Abs against CD3, CD4, HLA-DR, and Langerin and analyzed by objective scoring criteria (12). Photomicrographs of representative tissues are also shown.
In summary, we have demonstrated that Ad26 utilizes CD46 and not CAR as a primary cellular receptor. Ad26 transduction of human PBMC was CD46-dependent and was efficiently blocked by anti-CD46 but not anti-CAR MAbs. This is consistent with our previous observation that Ad26 transduced B16F10-CD46 transfectants more efficiently than native B16F10 cells (1). In contrast, a previous study reported that Ad26 transduced CHO-CAR transfectants more efficiently than CHO-HVEM transfectants and suggested that Ad26 in fact utilized CAR rather than CD46 for cell entry (6). However, levels of secondary receptor expression and confirmatory MAb blocking studies were not reported in that study (6). We also observed no increase in mucosal cellular infiltrates and no durable activation of vector-specific CD4+ T lymphocytes in baseline Ad5/Ad26-seropositive monkeys compared with seronegative monkeys following Ad26 vaccination. However, since substantial differences exist between nonhuman primates and humans, these questions also need to be addressed in clinical trials. Overall, these data provide key insights into Ad26 biology and inform further clinical development of Ad26 vaccine vectors.
ACKNOWLEDGMENTS
We thank J. Liu, M. Iampietro, D. Lynch, S. Clark, and L. Maxfield for their generous advice and assistance.
We acknowledge support from the National Institutes of Health (grants AI066305, AI066924, AI078526, and AI095985), the Bill and Melinda Gates Foundation, and the Ragon Institute of MGH, MIT, and Harvard.
We declare no financial conflicts of interest.
Footnotes
Published ahead of print 18 July 2012
REFERENCES
- 1. Abbink P, et al. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnberg N. 2009. Adenovirus receptors: implications for tropism, treatment and targeting. Rev. Med. Virol. 19:165–178 [DOI] [PubMed] [Google Scholar]
- 3. Barouch DH, et al. 2011. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 29:5203–5209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergelson JM, et al. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320–1323 [DOI] [PubMed] [Google Scholar]
- 5. Buchbinder SP, et al. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen H, et al. 2010. Adenovirus-based vaccines: comparison of vectors from three species of Adenoviridae. J. Virol. 84:10522–10532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ebbinghaus C, et al. 2001. Functional and selective targeting of adenovirus to high-affinity Fcgamma receptor I-positive cells by using a bispecific hybrid adapter. J. Virol. 75:480–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gaggar A, Shayakhmetov DM, Lieber A. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408–1412 [DOI] [PubMed] [Google Scholar]
- 9. Hsu KH, Lonberg-Holm K, Alstein B, Crowell RL. 1988. A monoclonal antibody specific for the cellular receptor for the group B coxsackieviruses. J. Virol. 62:1647–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kolev MV, et al. 2010. Upregulating CD59: a new strategy for protection of neurons from complement-mediated degeneration. Pharmacogenomics J. 10:12–19 [DOI] [PubMed] [Google Scholar]
- 11. Liu J, et al. 2008. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J. Virol. 82:4844–4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Masek-Hammerman K, et al. 2010. Mucosal trafficking of vector-specific CD4+ T lymphocytes following vaccination of rhesus monkeys with adenovirus serotype 5. J. Virol. 84:9810–9816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roelvink PW, et al. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909–7915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schneider-Schaulies J, Dunster LM, Schwartz-Albiez R, Krohne G, ter Meulen V. 1995. Physical association of moesin and CD46 as a receptor complex for measles virus. J. Virol. 69:2248–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seya T, Hara T, Matsumoto M, Akedo H. 1990. Quantitative analysis of membrane cofactor protein (MCP) of complement. High expression of MCP on human leukemia cell lines, which is down-regulated during cell differentiation. J. Immunol. 145:238–245 [PubMed] [Google Scholar]
- 16. Waddington SN, et al. 2008. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132:397–409 [DOI] [PubMed] [Google Scholar]