Abstract
Epstein-Barr virus (EBV) is a highly prevalent herpesvirus associated with epithelial cancers, including nasopharyngeal carcinoma (NPC). The EBV protein latent membrane protein 2 (LMP2) is expressed in NPC tumor tissue and has been shown to induce transformation, inhibit differentiation, and promote migration of epithelial cells. In this study, the effect of LMP2A on migration of human epithelial cells was further analyzed. LMP2A expression induced migration in human foreskin keratinocytes (HFK) and HaCaT keratinocytes measured by wound healing scratch assay and chemoattractant-induced Transwell migration assay. The induction of migration by LMP2A required the ITAM signaling domain of LMP2A and activation of the Syk tyrosine kinase. LMP2A-induced Transwell migration required the Akt signaling pathway, and activation of Akt by LMP2A required the ITAM signaling domain of LMP2A. LMP2A also induced phosphorylation of the Akt target GSK3β, a Wnt signaling mediator that has been shown to regulate the activity of focal adhesion kinase (FAK), a tyrosine kinase activated by clustering and ligand interaction of integrins. Inhibition of either FAK or its signaling mediator Src kinase inhibited LMP2A-induced migration. Interestingly, αV-integrin was greatly increased in membrane-enriched fractions by LMP2A, and a neutralizing antibody to αV-integrin blocked migration, suggesting that the effects of LMP2A on membrane-localized αV-integrin promoted migration. The results of this study indicate that LMP2A expression in human epithelial cells induces αV-integrin-dependent migration through a mechanism requiring ITAM-mediated Syk and Akt activation and inducing membrane translocation or stabilization of αV-integrin and FAK activation. The specific effects of LMP2A on an integrin with a diverse repertoire of ligand specificities could promote migration of different cell types and be initiated by multiple chemoattractants.
INTRODUCTION
Epstein-Barr virus (EBV) is a widespread gammaherpesvirus characterized by primary infection of the oral epithelium and establishment of life-long latency. EBV is associated with the development of several cancers, including Burkitt lymphoma, Hodgkin lymphoma, and the epithelial cancer nasopharyngeal carcinoma (NPC) (5, 6, 22–24, 36). During latent infection, multiple viral proteins are expressed, including the nuclear proteins EBNA1, -2, -3A, -3B, and -3C and LP and the latent membrane proteins LMP1, -2A, and -2B (25). LMP2 is consistently detected in NPC tumor tissue at the RNA and protein levels and is thought to contribute to the development of NPC through its effects on epithelial cell growth (5, 6, 22, 24).
LMP2A likely contributes to a malignant phenotype in epithelial cells through its effects on transformation, migration, and inhibition of differentiation (8, 11, 27). Malignant cells are highly migratory and invasive, and LMP2A has also been shown to affect migration (1, 16, 21). In one study using tonsillar epithelial cells, LMP2 induced migration in a scratch assay and invasion through Matrigel by upregulating α6-integrin (21). LMP2A has also been shown to promote Transwell migration to collagen in HEK293 and HaCaT cells in a manner involving Syk activity and the LMP2A ITAM domain (16). LMP1 also promotes migration and features of epithelial-mesenchymal transition in epithelial cells, and LMP1 can be coexpressed in NPC with LMP2 (29, 30). In the context of an NPC tumor, LMP1 and LMP2 may have distinct effects that cooperate to promote an invasive phenotype. Migration and invasion during tumor progression involves several factors, including integrin recycling, integrin activation, epithelial-mesenchymal transition, and extracellular matrix (ECM) remodeling (2). Integrins expressed on the surfaces of cells interact with extracellular matrix (ECM) proteins and activate intracellular signaling pathways that control cytoskeletal rearrangement and motility (7).
LMP2A contains 12 hydrophobic transmembrane domains with a long cytoplasmic N-terminal domain that contains several signaling motifs. These include two PY domains that interact with WW-containing ubiquitin ligases such as Itch (13, 28), an ITAM domain that interacts with the tyrosine kinase Syk (9), and a YEEA domain that in B cells interacts with the tyrosine kinase Lyn (10, 18). In B cells, LMP2A provides cell survival signals by mimicking B cell receptor signaling and activating Syk through the ITAM domain (9). The Akt pathway is also activated by LMP2A and contributes to B cell survival (31). Akt is a serine/threonine protein kinase involved in controlling many cellular functions, including cell survival and proliferation (32). LMP2A also activates Akt signaling in epithelial cells and is required for LMP2A-induced transformation (11, 27). Akt has been linked to migration through effects on Ras-induced Fyn expression and focal adhesion kinase (FAK) activation (35). FAK is activated by integrin-ECM interaction and contributes to the regulation of adhesion and migration.
In this study, the effects of LMP2A on epithelial cell migration of immortalized, nontumorigenic HaCaT cells and HFK were analyzed using both scratch assays and Transwell migration to FBS, fibronectin, and collagen. The induction of migration required the ITAM motif and activation of Syk, Akt, and FAK. Additionally, LMP2A induced ITAM/Syk-dependent membrane translocation and stabilization of αV-integrin and neutralizing antibody to this integrin blocked migration. It is likely that depending upon the cell context, LMP2A may modulate other integrins. However, αV-integrin may be particularly significant, as it has a large repertoire of ligands which would enable LMP2A to promote widespread migration to different chemoattractants with effects that are potentially distinct from those of different ligands that regulate migration. This study further defines the molecular events through which LMP2A induces migration and reveals that LMP2A, through the ITAM motif, activates Syk and Akt, which modulate the membrane localization of integrins to activate FAK and induce migration.
MATERIALS AND METHODS
Cell lines.
Dedifferentiated HaCaT keratinocytes were maintained in calcium-free Dulbecco's modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 10% chelated fetal bovine serum (FBS), 1% glutamine, and 1% penicillin-streptomycin in a humidified growth chamber with 95% CO2. FBS was chelated with Chelex 100 resin (Bio-Rad Laboratories, Hercules, CA) for 1 h on a rocker at 4°C and sterile filtered to remove calcium. Human foreskin keratinocytes (HFK) were maintained in keratinocyte-SFM (Invitrogen, Carlsbad, CA) supplemented with 1% penicillin-streptomycin. Cell lines were stably transduced with retroviral vectors expressing pBabe vector control, LMP2A, or LMP2A containing mutations for the signaling regions PY and ITAM as described previously (8).
Wound healing scratch assays.
HaCaT cells and HFK were plated on 6-well culture dishes and grown to confluence. Wells were scratched with a sterile 1,000-μl pipet tip, wound healing was visualized after 24 h, and bright-field images were captured using a 5× objective from fixed and crystal violet-stained cells using a Zeiss fluorescence microscope from the Lineberger Comprehensive Cancer Center Tissue Culture Facility.
Transwell migration assays.
Transwell migration chambers (Becton, Dickinson Labware, Franklin Lakes, NJ) were coated overnight at 4°C with the same chemoattractant that was used the following day to induce migration. For cells induced to migrate to fibronectin, Transwell chambers were coated overnight with fibronectin (10 μg/ml) prepared in sterile phosphate-buffered saline (PBS), and the following day, cells were allowed to migrate to medium containing 1% FBS and fibronectin (20 μg/ml). For cells induced to migrate to collagen, Transwell chambers were coated overnight with collagen (10 μg/ml) prepared in sterile PBS, and the following day, cells were allowed to migrate to medium containing 1% FBS and collagen (20 μg/ml). For cells induced to migrate to FBS, Transwell chambers were not precoated, and cells were allowed to migrate to medium containing 10% FBS. For all Transwell experiments, cells were suspended in medium containing 1% FBS, plated at 1.5 × 105 cells/chamber, and allowed to migrate overnight at 37°C. After 24 h, the membranes of the Transwell migration chambers were washed and then fixed with ice-cold methanol, and cells were stained with the fluorescent DNA stain 4′,6-diamidino-2-phenylindole (DAPI) and visualized using a Zeiss upright fluorescence microscope from the Lineberger Comprehensive Cancer Center Tissue Culture Facility. For inhibitor experiments, cells were treated with concentrations that were previously found to be inhibitory and that were determined to be nontoxic in the treatments presented here. The Syk inhibitor piceatannol (25 μM), the Akt inhibitor triciribine (5 μM), the GSK3 inhibitor LiCl (10 mM), the FAK inhibitors FAK14 (1 μM) and PF573226 (500 nM), and the Src inhibitor PP2 (1 μM) were tested for effects on cell viability at the concentrations used in the migration studies by trypan blue exclusion 24 h after treatment at 37°C (see Fig. 4C). For neutralizing-antibody experiments, cells were treated with 25 μg/ml antibody (α5-integrin [Millipore, Billerica, MA] and αV-integrin [BioLegend, San Diego, CA]) at 4°C for 1 h prior to initiation of migration and for the duration of the assay. All Transwell migration data are presented as average numbers of migrated cells/field, and each experiment was performed in quadruplicate. Statistical data are presented from Student's t test analysis of data pairings.
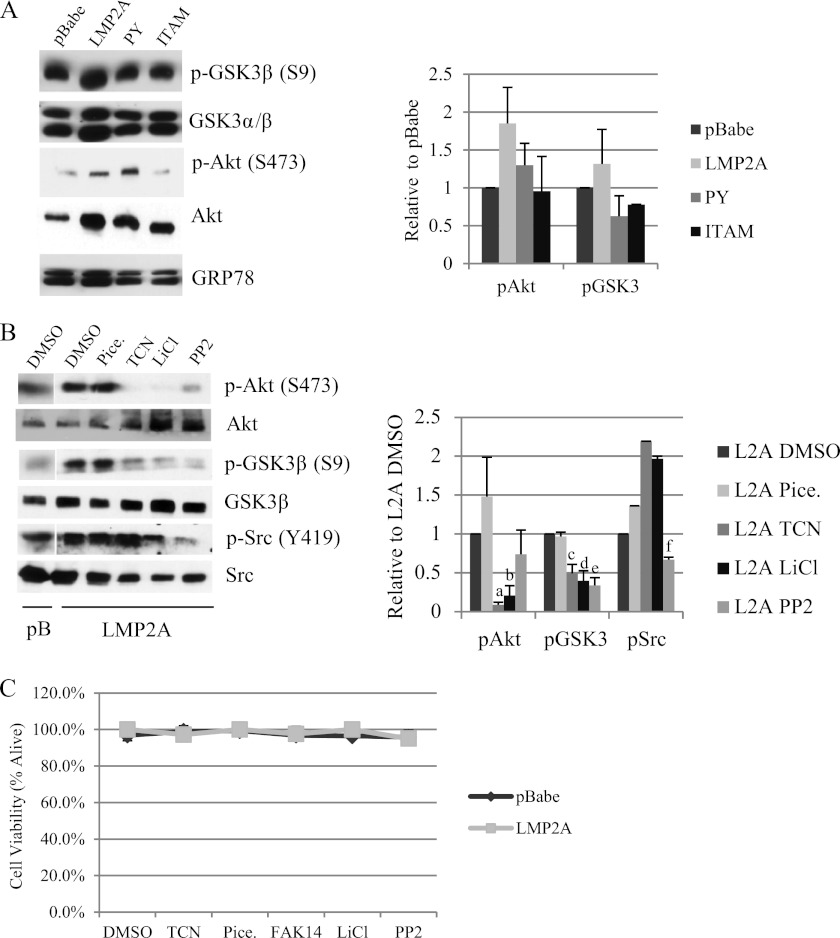
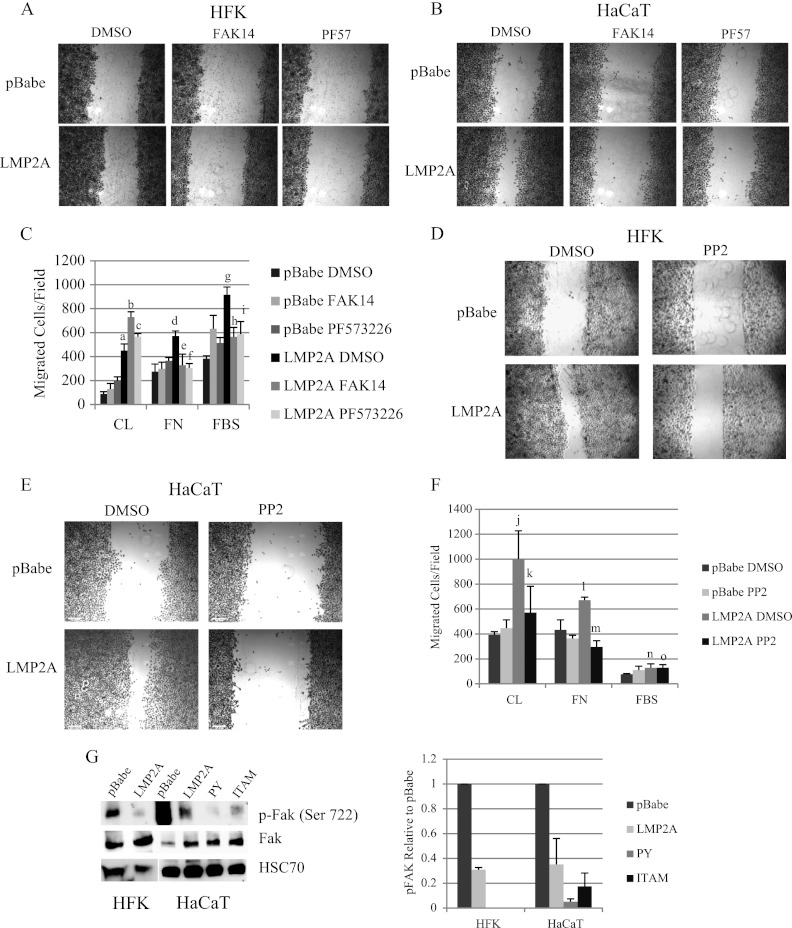
Fig 4.
LMP2A induces Akt signaling and activation of focal adhesion kinase. (A) Protein expression of Wnt signaling mediators was measured by Western blotting in cytoplasmic lysates from HaCaT cells expressing pBabe, wild-type LMP2A, and the LMP2A PY and ITAM mutants. Equal loading was determined by expression of the endoplasmic reticulum protein GRP78. Expression of phosphoproteins was normalized to total levels and is expressed relative to LMP2A DMSO vehicle controls; averages from three experiments are presented. (B) Protein levels of Akt, GSK3β, and Src were determined by Western blotting using antibodies to total protein or phosphorylated residues. Whole-cell lysates from pBabe and wild-type LMP2A expressing HaCaT cells were prepared from cells following treatment with DMSO vehicle control, the Syk inhibitor piceatannol (25 μM), the Akt inhibitor triciribine (TCN; 5 μM), the GSK3 inhibitor LiCl (10 mM), and the Src inhibitor PP2 (500 nM). Expression of phosphoprotein was normalized to total levels and expressed relative to values for LMP2A DMSO vehicle controls, and quantitation of two experiments is presented. a, P = 4.42 × 10−9 versus LMP2A DMSO; b, P = 0.00027 versus LMP2A DMSO; c, P = 0.0272 versus LMP2A DMSO; d, P = 0.0267 versus LMP2A DMSO; e, P = 0.011 versus LMP2A DMSO; f, P = 0.00587 versus LMP2A DMSO. (C) HaCaT cells expressing pBabe and wild-type LMP2A were treated overnight with DMSO vehicle control, triciribine (5 μM), piceatannol (25 μM), FAK14 (1 μM), LiCl (10 mM), and PP2 (500 nM). Cells were collected 24 h after treatment and cell viability was determined by trypan blue exclusion.
Lysate preparation and Western blots.
Whole-cell lysates were prepared with radioimmunoprecipitation assay (RIPA) buffer for 30 min on ice, followed by a 16,000 × g spin for 10 min. Membrane-enriched lysates were prepared by Dounce homogenization of cell pellets in ice-cold 1 mM EDTA containing protease inhibitors. Lysates were centrifuged for 10 min at 4°C and 300 × g. Supernatants were collected and centrifuged at 4°C and 20,000 × g for 20 min. Membrane pellets were resuspended in 1 mM EDTA. Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred to nitrocellulose. Following a 1-h block of the membrane in 5% milk–Tris-buffered saline (TBS)–Tween, membranes were incubated in primary antibody overnight (1:1,000 anti-LMP2A [Abcam, Cambridge, MA], 1:2,000 HSC70 [Santa Cruz Biotechnology, Santa Cruz, CA], 1:250 α2-integrin [BD Biosciences], 1:1,000 phospho-GSK3β [Ser9; Cell Signaling Technology, Danvers, MA], 1:1,000 GSK3 [Upstate, now Millipore, Billerica, MA], 1:1,000 phospho-Akt [Ser473;] 1:1,000 Akt [Cell Signaling Technology, Danvers, MA], 1:200 GRP78 [Santa Cruz Biotechnology, Santa Cruz, CA], 1:5,000 α5-integrin [BD Biosciences], 1:250 αV-integrin [BD Biosciences], 1:2,500 β1-integrin [BD Biosciences], 1:250 β6-integrin [GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom], 1:200 phospho-FAK [Santa Cruz Biotechnology, Santa Cruz, CA], and 1:1,000 FAK [Cell Signaling Technology, Danvers, MA]). Secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were added for 1 h and incubated at room temperature. Bands were visualized using Pierce SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL), and densitometry was acquired using Image J (National Institutes of Health, Bethesda, MD).
Immunofluorescence assays.
HaCaT cells were seeded on 8-well glass chamber slides and were allowed to attach overnight. Adherent cells were then washed with PBS and were fixed for 15 min at 4°C with ice cold acetone-methanol (1:1). Fixed cells were incubated for 20 min in blocking buffer at room temperature (3% BSA in PBS). Primary antibodies were diluted 1:50 in PBS and were incubated on blocked slides for 1 h at room temperature in a humidified chamber. For secondary antibody-only controls, slides were incubated in PBS. Following primary antibody incubation, slides were washed three times in PBS for 5 min and were incubated with 1:100 fluorescein isothiocyanate (FITC)-conjugated secondary antibody for 1 h at room temperature in a humidified chamber. Following secondary antibody incubation, slides were washed three times for 5 min in PBS, incubated with 1:10,000 of DAPI to stain nuclei, and mounted with antifade mounting medium. Images were acquired using a Zeiss 710 confocal laser scanning microscope located at the University of North Carolina Microscopy Services Laboratory. All images shown were acquired with a 63× oil objective.
RESULTS
The effects of LMP2A on epithelial cell migration were determined in two types of assays. The wound healing migration assay measures cell movement that is similar to epithelial cell migration in vivo in that when a confluent layer of cells is “scratched” or “wounded,” cells move in sheets to close the wound while maintaining cell-cell and cell-matrix contacts. In contrast, the Transwell migration assays quantitates migration of single cells to a chemoattractant independent of cell-cell or cell-matrix attachments.
LMP2A expression induces epithelial cell migration.
LMP2A has been previously shown to induce migration as assessed using a scratch assay (21) and by Transwell assay using fibronectin (1) and collagen (16) as chemoattractants. The induction of both types of migration and migration to two different chemoattractants suggests that LMP2A may induce distinct types of migration through a common mechanism. Previous studies using collagen as a chemoattractant indicated a requirement for the Syk tyrosine kinase signaling pathway (16) and induction of the laminin-binding α6β4 integrin (21). To further dissect the effects of LMP2A on migration, the effects of LMP2A and activation of specific signaling pathways were assessed in two nontumorigenic, immortalized cell lines using both scratch assay migration and Transwell migration to different chemoattractants.
To evaluate the effects of LMP2A in a wound healing assay, full-length LMP2A was stably expressed in the telomerase-immortalized human foreskin keratinocyte (HFK) line, and full-length LMP2A and mutants of the PY or ITAM motifs were stably expressed in the spontaneously immortalized HaCaT cell line. Immunoblot analysis indicated approximately equivalent expression of LMP2A and the mutants (Fig. 1A), and levels of LMP2A were comparable to that expressed by the Burkitt lymphoma cell line Jijoye. A comparison of wound healing after 24 h indicated that LMP2A induced migration and closure of the wound in both cell lines, in contrast to pBabe vector control cells (Fig. 1B and C). In HaCaT cells, the wound closure and migration induced by LMP2A expression was impaired in cells expressing the PY and ITAM mutants, suggesting that both signaling domains were important for increased migration (Fig. 1C).
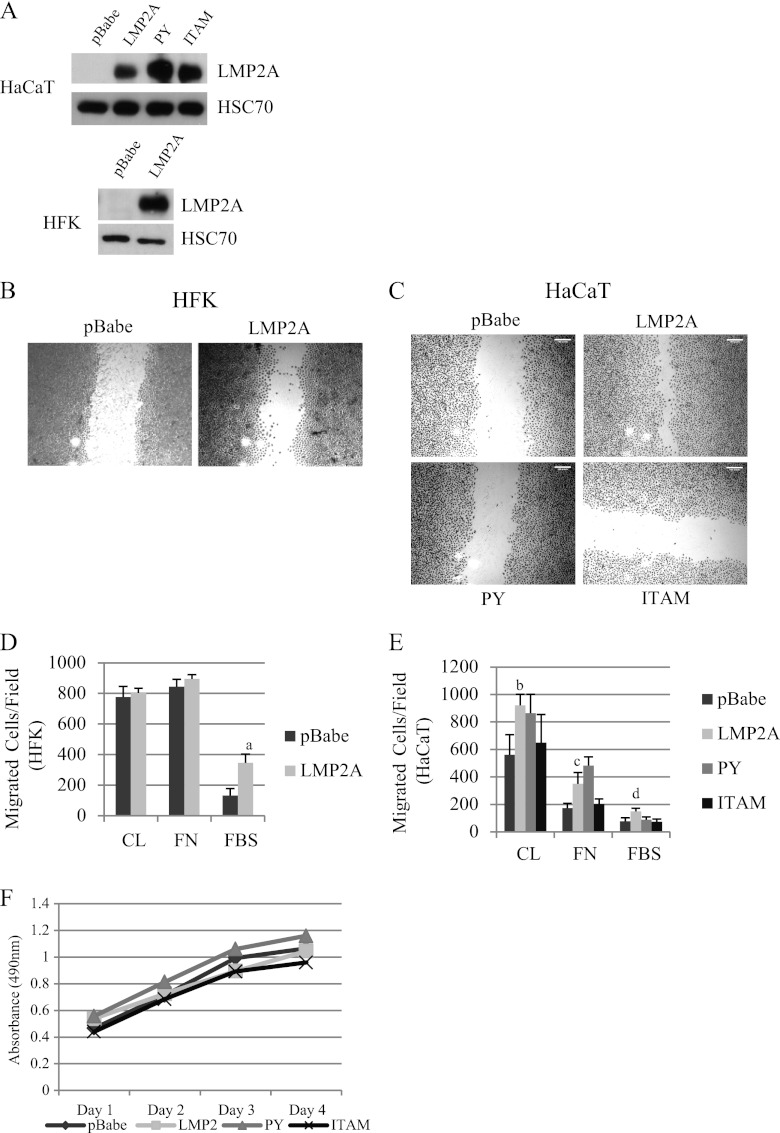
Fig 1.
LMP2A induces ITAM-dependent migration. (A) HaCaT cells were transduced to stably express the pBabe vector, wild-type LMP2A, and PY and ITAM mutants of LMP2A. HFK were transduced to stably express the pBabe vector and wild-type LMP2A. LMP2A levels were compared to the level in the Burkitt lymphoma cell line Jijoye. LMP2A expression was determined by Western blotting using HSC70 expression to demonstrate equal loading. (B) Wound healing of HFK expressing pBabe and LMP2A was determined by scratch assay. Images were acquired at a ×5 magnification from crystal violet-stained cells 24 h after scratching (n = 5). (C) Wound healing of HaCaT cells expressing pBabe, LMP2A, LMP2A PY, and LMP2A ITAM was determined by scratch assay (n = 6). (D) Transwell migration of HFK expressing pBabe and LMP2A was determined using collagen (CL), fibronectin (FN), and FBS as chemoattractants as described in Materials and Methods. Data are averages (n = 2). a, P = 0.001 versus pBabe. (E) Transwell migration of HaCaT cells expressing pBabe, LMP2A, LMP2A PY, and LMP2A ITAM was determined using collagen (CL), fibronectin (FN), and FBS as chemoattractants as described in Materials and Methods. Data are averages (n = 2). b, P = 0.0051 versus pBabe; c, P = 0.0071 versus pBabe; d, P = 0.0054 versus pBabe. (F) Proliferation of HaCaT cells expressing pBabe, LMP2A, LMP2A PY, and LMP2A ITAM was measured by MTS assay.
To compare LMP2A-induced wound healing with chemoattractant-directed migration, epithelial cells were induced to migrate for 24 h to FBS, collagen, and fibronectin. HFK pBabe control cells migrated efficiently to collagen and fibronectin, and LMP2A expression did not enhance this migration (Fig. 1D). In contrast, HFK migration induced by FBS was considerably lower in pBabe control cells than that induced by fibronectin and collagen but was significantly induced by LMP2A (Fig. 1D). In HaCaT cells, LMP2A expression significantly induced migration to collagen and fibronectin (Fig. 1E). Migration to FBS was also induced but not as significantly as in HFK. Similar assessment of PY and ITAM expressing cells revealed that the PY mutant did not impair LMP2A-induced migration, while migration of cells containing the ITAM mutant to all chemoattractants tested was reduced (Fig. 1E). To evaluate whether potential effects on cellular proliferation were a factor contributing to LMP2A-induced migration, HaCaT cells expressing the pBabe vector control, LMP2A, or the PY and ITAM mutants were analyzed using an 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS; inner salt) assay for 4 days. Cellular proliferation was not affected by LMP2A or the LMP2A mutants (Fig. 1F), suggesting that induction of migration by LMP2A in scratch and Transwell assays was a result of cell movement rather than proliferation.
LMP2A-induced migration requires the tyrosine kinase Syk.
The decreased wound healing and Transwell migration to FBS, collagen, and fibronectin of HaCaT cells expressing the ITAM mutant of LMP2A suggested that signaling mediated through the ITAM domain was required to induce migration. The ITAM domain has been shown to interact with the tyrosine kinase Syk (9). To evaluate the requirement for Syk activation in LMP2A-induced migration, LMP2A expressing cells were induced to migrate in the presence of the Syk inhibitor piceatannol. The Syk inhibitor blocked LMP2A-induced wound healing in HFK (Fig. 2A) and HaCaT cells (Fig. 2B), which were not affected by the dimethyl sulfoxide (DMSO) vehicle control. Piceatannol also significantly inhibited Transwell migration to FBS, fibronectin, and collagen in pBabe control vector- and LMP2A-expressing HaCaT cells (Fig. 2C) and to FBS in pBabe- and LMP2A-expressing HFK (Fig. 2D), indicating that all single-cell chemoattractant-directed migration and wound healing migration induced by LMP2A in the scratch assay required Syk signaling.
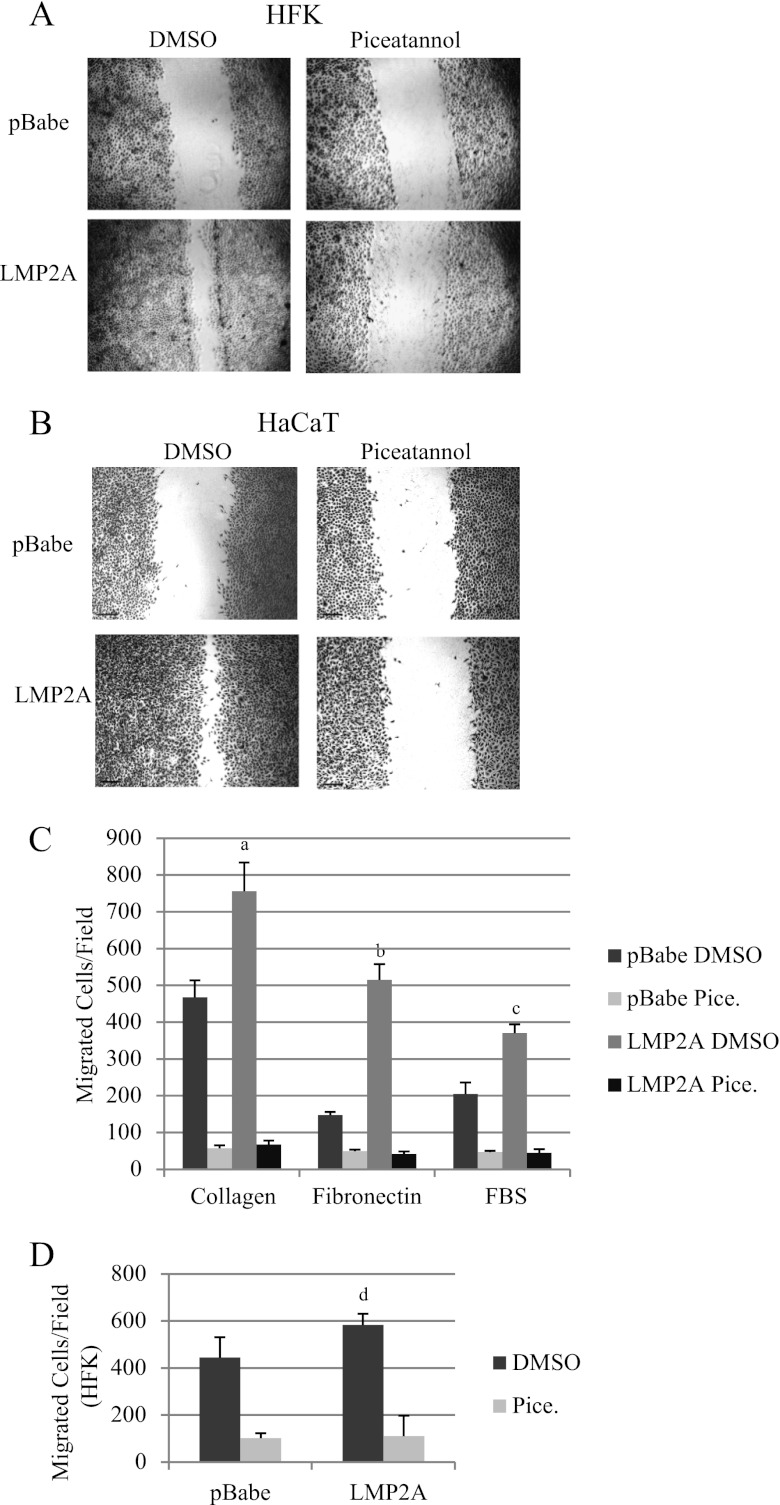
Fig 2.
LMP2A induces Syk-dependent migration. Wound healing of HFK (A) and HaCaT cells (B) was determined by scratch assay in the presence of DMSO vehicle control or the Syk inhibitor piceatannol (25 μM; n = 3 [HFK]; n = 4 [HaCaT]). (C) Transwell migration of HaCaT cells to collagen, fibronectin, and FBS was determined in the presence of piceatannol and DMSO vehicle control as described in Materials and Methods. Data are averages (n = 2). a, P = 0.01034 versus pBabe DMSO; b, P = 6.7 × 10−5 versus pBabe DMSO; c, P = 0.00265 versus pBabe DMSO. (D) HFK Transwell migration to FBS was determined in the presence of piceatannol and DMSO vehicle control as described in Materials and Methods. Data are averages (n = 2). d, P = 0.03026 versus pBabe DMSO.
LMP2A-induced migration requires Akt signaling.
The LMP2A ITAM signaling domain has been shown to be required for activation of Akt (31). Therefore, the contribution of Akt was evaluated using the Akt inhibitor triciribine. In wound healing assays in HFK, triciribine impaired LMP2A-induced migration, indicating that Akt activation is important for LMP2A-mediated migration (Fig. 3A). In HaCaT cells, triciribine partially blocked LMP2A-induced migration measured by scratch assay (Fig. 3B). Phosphorylation of the Akt target GSK3β by Akt inhibits GSK3β kinase activity, and it is thought that GSK3β induces an inhibitory phosphorylation of FAK that would impair migration (4). To assess the contribution of GSK3β in LMP2A-induced migration, wound healing scratch assays were performed in the presence of the GSK3β inhibitor lithium chloride (LiCl). Similarly to triciribine inhibition of Akt, LiCl unexpectedly inhibited LMP2A-induced migration in HaCaT cells and HFK (Fig. 3A and B). It is likely that GSK3β may impact migration in positive ways that are required for migration.
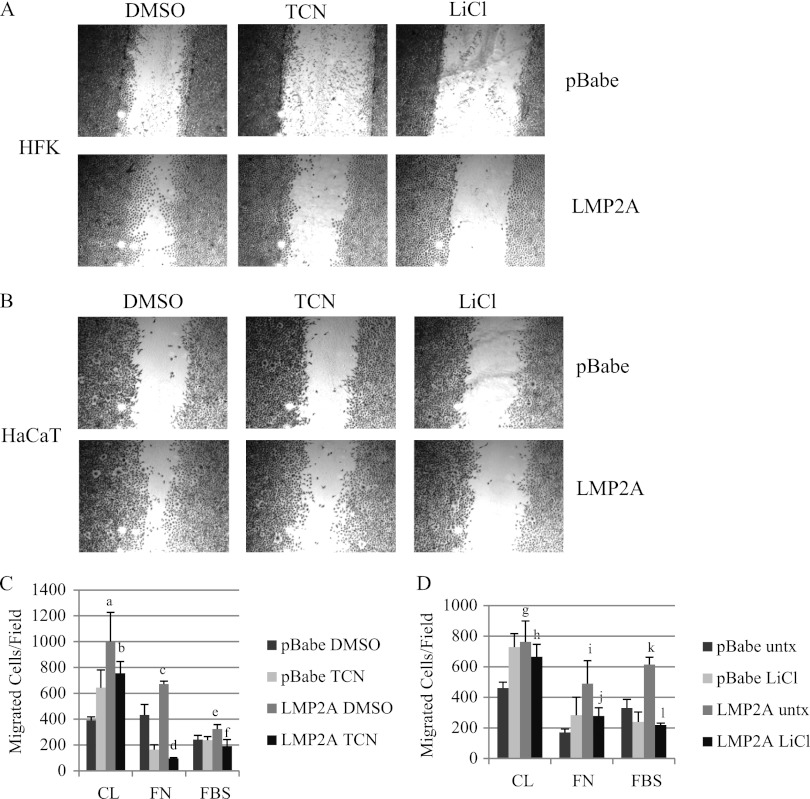
Fig 3.
LMP2A-induced migration involves Akt and GSK3β signaling. Wound healing of HFK (A) and HaCaT cells (B) expressing pBabe vector and wild-type LMP2A in the presence of the Akt inhibitor triciribine (TCN; 5 μM), the GSK3 inhibitor LiCl (10 mM), or DMSO vehicle control was determined by scratch assay. Images represent three individual experiments for LiCl and four individual experiments for triciribine. (C) Transwell migration of HaCaT cells to collagen (CL) and fibronectin (FN) and migration of HFK to FBS was determined in the presence of triciribine (TCN) and DMSO vehicle control as described in Materials and Methods. a, P = 0.00174 versus pBabe DMSO; b, P = 0.09066 versus LMP2A DMSO; c, P = 0.01624 versus pBabe DMSO; d, P = 1.6 × 10−7 versus LMP2A DMSO; e, P = 0.01248 versus pBabe DMSO; f, P = 0.00487 versus LMP2A DMSO. (D) Transwell migration of HaCaT cells to collagen (CL) and fibronectin (FN) and migration of HFK to FBS was determined in the presence of LiCl and DMSO vehicle control as described in Materials and Methods. g, P = 0.00528 versus pBabe DMSO; h, P = 0.26713 versus LMP2A DMSO; i, P = 0.00571 versus pBabe DMSO; j, P = 0.03775 versus LMP2A DMSO; k, P = 0.00025 versus pBabe DMSO; l, P = 3.4 × 10−6 versus LMP2A DMSO. All Transwell migration data are averages (n = 2; experiments were performed in quadruplicate).
To evaluate the contribution of Akt and GSK3β signaling in chemoattractant-directed migration, epithelial cells were induced to migrate to FBS, collagen, and fibronectin in the presence of triciribine. Inhibition of Akt signaling decreased LMP2A-induced migration to collagen in HaCaT cells; however, the decrease was not statistically significant (Fig. 3C). However, triciribine treatment completely blocked migration to fibronectin to levels below pBabe (Fig. 3C), suggesting that Akt was required for fibronectin-induced migration but contributed only to collagen-induced migration. Triciribine also impaired LMP2A-induced migration to FBS in HFK (Fig. 3C). Similar to the effects on wound healing, LiCl treatment mimicked Akt inhibition and blocked LMP2A induced migration of HaCaT cells to fibronectin but not collagen and decreased FBS stimulated migration of HFK (Fig. 3D).
LMP2A induces ITAM-dependent Akt signaling and activity of focal adhesion kinase and Src.
To evaluate the effects of LMP2A and the PY and ITAM mutants on activation of Akt and GSK3β, levels of phosphorylated and total protein were determined by immunoblotting. LMP2A-expressing cells had elevated levels of Akt phosphorylated at serine 473 and GSK3β phosphorylated at serine 9, while total GSK3α/β levels were not affected and Akt levels were elevated by LMP2A (Fig. 4A). The levels of phosphorylated Akt were decreased in the ITAM-expressing cells and less so in the PY cells. Phosphorylated GSK3β levels were decreased in both the PY and ITAM-expressing cells (Fig. 4A). These data confirm previous studies indicating that the ITAM motif is required for LMP2A activation of Akt.
To assess the effects of the inhibitors used in the migration studies on their respective signaling targets, HaCaT cells expressing LMP2A were treated with piceatannol, triciribine, lithium chloride, and the Src family kinase inhibitor, PP2, at the same concentration that was tested for migration effects, and the effects on the protein levels and phosphorylation of Akt, GSK3β, and Src were determined by immunoblotting. To determine the amount of increase in phosphorylation and activation of the specific protein, the amount of phosphoprotein detected was determined relative to the total protein level and expressed relative to that in the LMP2A DMSO vehicle controls. The data from three experiments are presented graphically. Piceatannol, which clearly blocked migration, did not decrease the levels of phosphorylated Akt, GSK3β, or Src (Fig. 4B) induced by LMP2A. This lack of effect on the LMP2-induced phosphorylation of other targets supports the specificity of piceatannol treatment. These findings also reveal that although the ITAM is required for Akt activation, Syk activity is not required for activation of Akt. Triciribine treatment significantly decreased p-Akt and also reduced phosphorylation levels of the Akt target p-GSK3β (Fig. 4B) but did not decrease levels of p-Src. Lithium chloride treatment completely blocked phosphorylation of Akt and significantly decreased phosphorylated GSK3β but did not decrease phosphorylated Src (Fig. 4B). The block of activation of Akt in the presence of LiCl supports the biologic findings that showed inhibition of migration resulting from inhibition of Akt by triciribine or treatment with LiCl. The Src inhibitor PP2 significantly decreased phosphorylation of Src but also decreased the relative phosphorylation levels of Akt and GSK3β (Fig. 4B), suggesting that Src may contribute to effects on Akt and GSK3β. It is thought that in B cells, the Src family kinase Lyn activates the ITAM domain to induce recruitment and activation of Syk to LMP2A. The decrease in Akt activation by the Src inhibitor suggests that Src family kinase activity and ITAM activation may both be required for Akt activation, although activation of Src and Akt is independent of Syk. These data indicate that the treatment with the kinase inhibitors blocked phosphorylation of expected targets but did not inhibit LMP2-mediated effects on other targets.
To determine effects of the inhibitors on cell viability, cells were treated for 24 h at the concentrations that were evaluated in biologic and molecular assays. At the effective concentration, none of the drugs used in this study had any effect on viability. These findings confirm that the effects on migration and phosphorylated protein levels were due to effects of the drugs on their targets and not cellular toxicity (Fig. 4C).
LMP2A-induced migration involves FAK and Src signaling.
GSK3β signaling has been shown to control activation of focal adhesion kinase (FAK) (4). FAK is activated by integrin clustering upon interaction with extracellular matrix proteins, and active FAK recruits signaling mediators, including Src, to control cellular migration. To determine if FAK and Src signaling contributed to LMP2A-induced migration, wound healing and Transwell migration assays were conducted in the presence of two FAK inhibitors (FAK14 and PF573226) and a Src inhibitor (PP2). Inhibition of FAK blocked wound healing migration in HFK (Fig. 5A) and HaCaT cells (Fig. 5B). Both FAK inhibitors also significantly impaired LMP2A-induced migration to fibronectin in HaCaT cells and to FBS in HFK (Fig. 5C) but did not affect LMP2A-induced migration to collagen (Fig. 5C), suggesting that only FBS and fibronectin-directed migration required FAK signaling. Inhibition of Src signaling with PP2 inhibited LMP2A-induced wound healing in both HFK and HaCaT cells (Fig. 5D and E). Src inhibition blocked LMP2A-induced migration to both fibronectin and collagen in HaCaT cells but did not impair migration to FBS in HFK (Fig. 5F). This difference suggests that distinct signaling mediators are activated by FAK during FBS-induced migration in HFK. These findings indicate a general requirement for FAK and distinct requirements for Src activation in wound healing and Transwell migration in epithelial cells.
Fig 5.
LMP2A induces FAK and Src-dependent migration. Wound healing in HFK (A) and HaCaT cells (B) expressing pBabe vector and wild-type LMP2A in the presence of the FAK inhibitors FAK14 (1 μM) and PF573228 (500 nM) and DMSO vehicle controls was determined by scratch assay. Images are representative of three individual experiments for both cell lines with each drug. (C) Transwell migration of HaCaT cells to collagen (CL) and fibronectin (FN) and migration of HFK to FBS were determined in the presence of FAK14, PF573228, and DMSO vehicle control as described in Materials and Methods and are expressed as averages (n = 2). a, P = 1.54 × 10−5 versus pBabe DMSO; b, P = 0.00021 versus LMP2A DMSO; c, P = 0.00808 versus LMP2A DMSO; d, P = 0.00412 versus pBabe DMSO; e, P = 0.03214 versus LMP2A DMSO; f, P = 0.0015 versus LMP2A DMSO; g, P = 0.00011 versus pBabe DMSO; h, P = 0.00704 versus LMP2A DMSO; i, P = 0.0221 versus LMP2A DMSO. Wound healing in HFK (D) and HaCaT cells (E) expressing pBabe vector and wild-type LMP2A in the presence of the Src inhibitor PP2 (500 nM) and DMSO vehicle control was determined by scratch assay. (F) Transwell migration of HaCaT cells to collagen (CL) and fibronectin (FN) and migration of HFK to FBS was determined in the presence of PP2 and DMSO vehicle control as described in Materials and Methods and is expressed as averages (n = 2). j, P = 0.00174 versus pBabe DMSO; k, P = 0.03209 versus LMP2A DMSO; l, P = 0.01624 versus pBabe DMSO; m, P = 0.00021 versus LMP2A DMSO; n, P = 0.01583 versus pBabe DMSO; o, P = 0.95127 versus LMP2A DMSO. (G) Protein levels of phospho-FAK (serine 722), total FAK, and the loading control HSC70 were determined by Western blotting of whole-cell lysates prepared from HFK expressing pBabe vector and wild-type LMP2A and from HaCaT cells expressing pBabe vector, wild-type LMP2A, and the LMP2A PY and ITAM mutants. phospho-FAK expression was normalized to total FAK, expressed relative to pBabe, and averages from two experiments are presented.
To determine whether LMP2A affects the phosphorylation status of FAK, Western blots were used to measure FAK phosphorylated at the serine 722 residue. It has been shown that active GSK3β can phosphorylate FAK on serine 722 and inhibit FAK signaling (4). It is thought that the activation of Akt by LMP2 results in inhibition of GSK3β as detected by phosphorylation on the Akt target serine 9 (Fig. 4C). To determine if these effects of LMP2 on GSK3β affected FAK inhibition, the phosphorylation of FAK at the putative GSK3β target serine 722 was evaluated. In HFK and HaCaT cells, the inhibitory phosphorylation of FAK at serine 722 was reduced in the presence of LMP2A (Fig. 5G). The effect of LMP2A on decreased inhibitory FAK phosphorylation was not affected by mutation of the PY or ITAM motif (Fig. 5G). The lack of effect of these mutations, which both impair activation of Akt and inhibition of GSK3β, suggests that the absence of the inhibitory phosphorylation on FAK is not specifically mediated by LMP2A and likely reflects the general activation of FAK.
LMP2A induces Syk/ITAM-dependent translocation of αV-integrin- and αV-integrin-dependent migration.
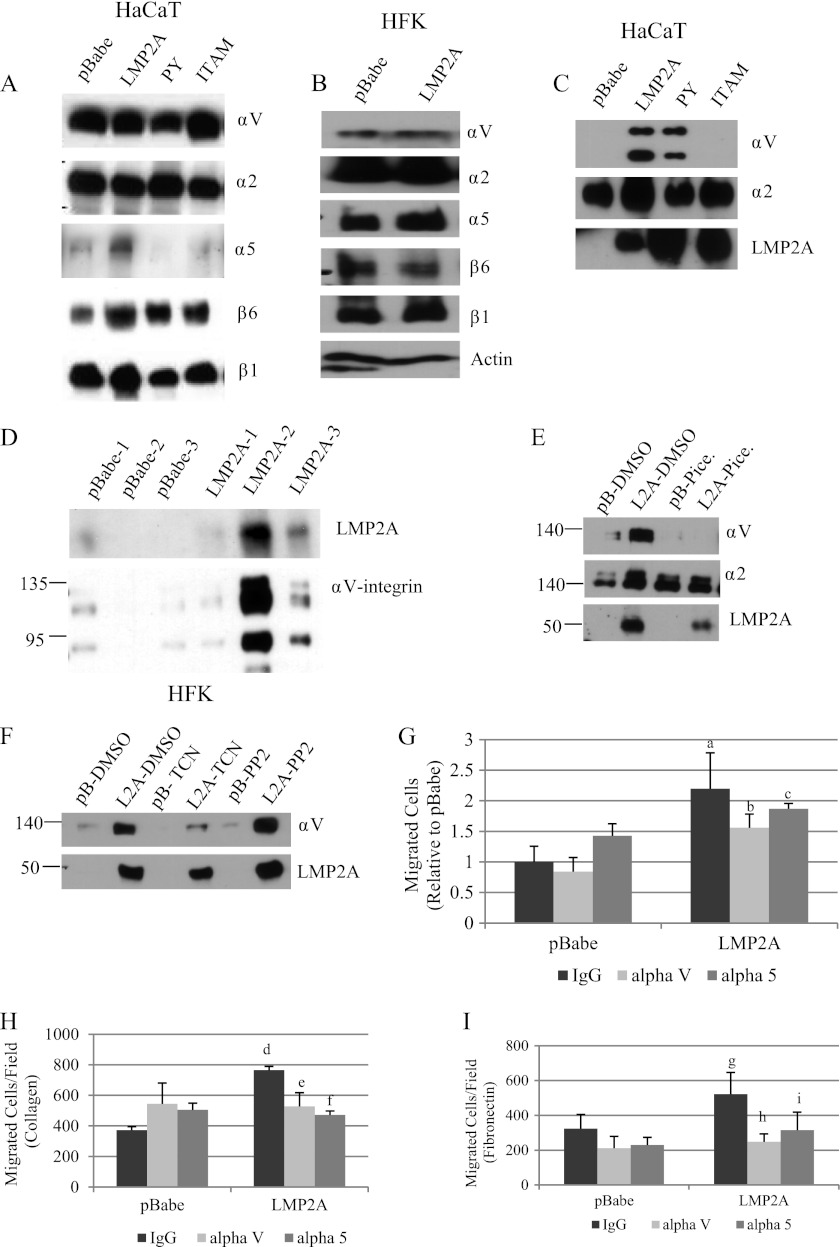
Integrin clustering and interaction with extracellular matrix ligands induces FAK activation and autophosphorylation. As FAK signaling was required for LMP2A-induced wound healing and Transwell migration to fibronectin and FBS, the effects of LMP2A on integrin expression were assessed. Immunoblot analysis of whole-cell lysates and membrane-enriched lysates detected αV-, α2-, α5-, β6-, and β1-integrins in whole-cell lysates from HaCaT cells (Fig. 6A) and HFK (Fig. 6B). Expression of α3-, αL-, and β3-integrins were not detected in HaCaT cells or HFK (data not shown). Expression of αV-, α2-, and β1-integrins was essentially unchanged in pBabe, LMP2A, and the PY and ITAM mutants in HaCaT cells. Expression of β6-integrin was modestly upregulated by LMP2A (Fig. 6A). α5-Integrin was induced by LMP2A in whole-cell lysates, and this induction required the PY and ITAM signaling domain (Fig. 6A). In HFK, LMP2A did not affect expression of any of the tested integrins (Fig. 6B). Integrin signaling is induced by ligand binding and modulated by membrane localization; therefore, membrane-enriched fractions were evaluated for integrin expression. The α2-integrin was detected in the membrane fraction of all cell lines, with a small increase being detected in LMP2A-expressing HaCaT cells (Fig. 6C). In contrast, the αV-integrin was detected only in the membrane fractions from LMP2A-expressing HaCaT cells, not the pBabe control cells (Fig. 6C). This induction of αV-integrin required the ITAM motif of LMP2A but not the PY motif (Fig. 6C). The α5-integrin was not detected in the membrane fraction (data not shown). Expression of LMP2A and the mutants was readily detected in the membrane enriched fractions. Similarly in HFK, expression of LMP2 induced the membrane translocation of αV-integrin. Analysis of three independent cell lines indicated that the translocation of αV-integrin was directly proportional to LMP2A expression (Fig. 6D). Thus, LMP2A induced membrane translocation of αV-integrin in both HaCaT cells and HFK.
Fig 6.
LMP2A induces αV-integrin membrane expression and αV-integrin-dependent migration. Integrin expression in HaCaT cells (A) and HFK (B) was determined by Western blotting from whole-cell lysates. (C) LMP2A, α2-integrin, and αV-integrin expression was measured in HaCaT cell membrane-enriched lysates by Western blotting. (D) αV-integrin and LMP2A expression was measured by Western blotting in membrane lysate preparations from three independent HFK expressing different levels of LMP2A. (E) Protein expression of αV-integrin, α2-integrin, and LMP2A was measured by Western blotting in HaCaT pBabe (pB) and LMP2A (L2A) membrane-enriched lysates following 24 h treatment with DMSO vehicle control (pB-D, L2A-D) or the Syk inhibitor piceatannol (pB-P, L2A-P). (F) Protein expression of αV-integrin and LMP2A in HaCaT cells expressing pBabe (pB) and wild-type LMP2A (L2A) was measured by Western blotting following 24 h treatment with DMSO vehicle control, the Akt inhibitor triciribine (TCN; 5 μM), or the Src inhibitor PP2 (500 nM). (G) HFK were treated for 1 h with IgG control antibody, αV-integrin, or α5-integrin neutralizing antibody prior to initiation of Transwell migration to FBS for 24 h. HaCaT cells were treated for 1 h with IgG control antibody, αV-integrin, or α5-integrin neutralizing antibody prior to initiation of Transwell migration to collagen (H) and fibronectin (I) as described in Materials and Methods. Data are expressed as averages (n = 2; experiments were performed in quadruplicate). a, P = 0.00012 versus pBabe IgG; b, P = 0.012 versus LMP2A IgG; c, P = 0.304 versus LMP2A IgG; d, P = 9.4 × 10−6 versus pBabe IgG; e, P = 0.02566 versus LMP2A IgG; f, P = 7.7 × 10−5 versus LMP2A IgG; g, P = 0.17584 versus pBabe IgG; h, P = 0.05476 versus LMP2A IgG; i, P = 0.19075 versus LMP2A IgG.
As the ITAM motif was required for membrane translocation of αV-integrin, the contribution of Syk was determined using piceatannol. HaCaT cells were treated with the Syk inhibitor piceatannol and DMSO vehicle control for 24 h prior to preparation of membrane-enriched lysates. αV- and α2-integrin expression was increased in LMP2A-expressing cells, and the LMP2A-induced membrane translocation of αV-integrin was impaired by piceatannol (Fig. 6E), indicating that the LMP2A induction of αV-integrin in the membrane fraction required the ITAM motif and Syk activation. Syk inhibition did not affect the steady-state level of α2-integrin in the membrane-enriched lysates.
As LMP2A activation of Akt also requires the ITAM motif, the contribution of Akt and Src activation to αV-integrin membrane enrichment was evaluated. Treatment of HaCaT cells with triciribine decreased αV-integrin in the membrane but not below levels detected in the vector control cells (Fig. 6F). Inhibition of Src with PP2 did not affect the translocation of αV-integrin. These findings suggest that Akt contributes to αV-integrin membrane enrichment and that activation of Src kinase family members apparently is not required for the effects of LMP2A on integrin membrane translocation.
To evaluate the contribution of αV-integrin in chemoattractant-dependent migration induced by LMP2A, Transwell migration assays were performed in the presence of neutralizing antibody to αV-integrin, α5-integrin, and an IgG isotype control. Neutralizing antibody to αV-integrin but not α5-integrin significantly impaired LMP2A-induced migration to FBS in HFK (Fig. 6G). In HaCaT cells, antibody to either αV-integrin or α5-integrin inhibited migration to both collagen and fibronectin (Fig. 6H and I).
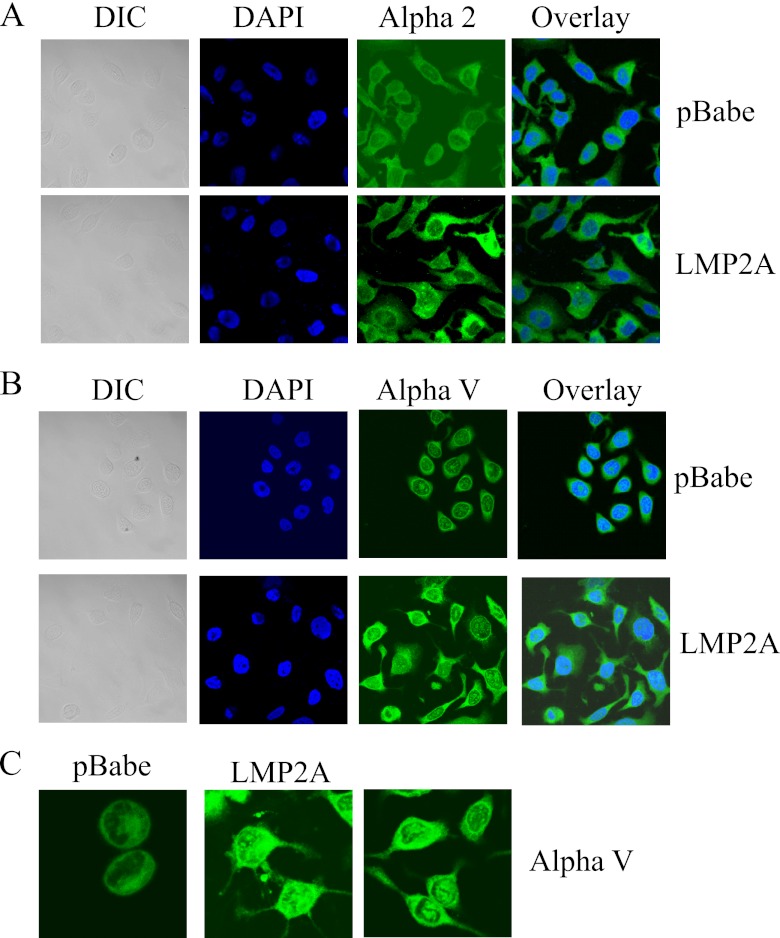
To further assess the effects of LMP2A-induced membrane localization of αV-integrin in epithelial cells, expression of αV- and α2-integrins was determined using immunofluorescent staining in HaCaT cells expressing pBabe vector control and wild-type LMP2A. Expression of α2-integrin was uniform throughout the cytoplasm, and its distribution and localization was similar in pBabe and LMP2A cells (Fig. 7A). The intensity of staining was increased in some LMP2A-expressing cells compared to that in pBabe-expressing cells. In contrast, staining of αV-integrin revealed distinct patterns of distribution and cellular localization in pBabe-expressing cells compared with those expressing wild-type LMP2A. LMP2A cells had increased cytoplasmic staining of αV-integrin extending to the cell membrane and throughout the cellular extensions, whereas pBabe-expressing cells had striking perinuclear staining and punctate staining within the nucleus and very little αV-integrin localized throughout the cytoplasm or in cell extensions (Fig. 7B and C). The different localization of αV-integrin detected by immunofluorescence in pBabe compared with LMP2A cells supports the increased immunoblot detection in membrane-enriched fractions. These findings indicate that LMP2A induces a cellular translocation of αV-integrin to the membrane and throughout cellular extensions that facilitates migration.
Fig 7.
LMP2A induces translocation of αV-integrin. Expression and cellular localization of α2-integrin (A) and αV-integrin (B) was determined by immunofluorescence assay in HaCaT cells expressing pBabe and LMP2A. (C) Higher-magnification images of pBabe and LMP2A-expressing cells stained for αV-integrin. Mouse primary antibodies were used to detect expression to αV-integrin and α2-integrin, were visualized with an anti-mouse secondary antibody conjugated to FITC, and are shown in green. Nuclei were stained with DAPI and are shown in blue. Differential interference contrast (DIC) and overlay images are also shown. All images were acquired using a Zeiss 710 confocal microscope from the UNC Microscopy Services Laboratory. Brightness and contrast were adjusted to all parts of the images using Adobe Photoshop software.
LMP2A-induces distinct signaling pathways to induce membrane translocation of αV-integrin and promote migration.
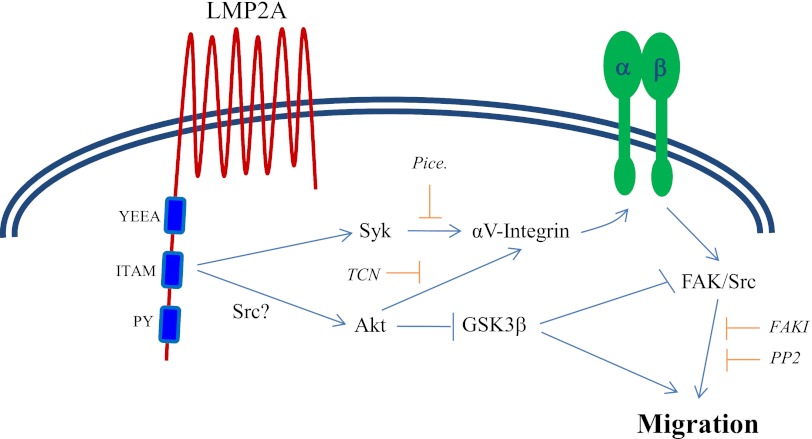
The data presented in this study show that LMP2A induces two signaling pathways that require the ITAM domain (Fig. 8). Syk and Akt are activated independently, although both converge to promote the membrane translocation of αV-integrin. FAK and Src are subsequently activated downstream of integrin clustering in a manner that has been well documented to promote migration. The activation of Akt may also be enhanced by the effects of Src family kinases on activation of the ITAM signaling domain. GSK3β has been shown to phosphorylate FAK on a residue that inhibits its activity. By inhibiting GSK3β, Akt may contribute to activation of FAK by blocking the inhibitory phosphorylation of FAK as a mechanism to control migration in addition to promoting membrane localization of αV-integrin.
Fig 8.
LMP2A induces signaling pathways that promote membrane localization of αV-integrin and epithelial migration. This schematic diagram summarizes the data on mechanisms of LMP2A-induced migration. LMP2A induced two distinct signaling pathways from the ITAM domain by activating Syk and Akt. Syk and Akt promoted translocation of αV-integrin to membrane-enriched compartments of epithelial cells and subsequent activation of FAK and Src to control migration. A separate signaling pathway initiated by Akt through inhibition of GSK3β may also relieve an inhibitory phosphorylation of FAK to promote migration. TCN, triciribine; FAKI, FAK inhibitors; PP2, Src inhibitor.
DISCUSSION
This study investigated the effects of the EBV protein LMP2A on the migratory properties of human epithelial cells and the cellular pathways that are affected by LMP2A to control migration. LMP2A induced migration in the HaCaT keratinocyte cell line and the human foreskin keratinocyte (HFK) cell line, as determined by a wound healing scratch assay and by a chemoattractant-directed Transwell assay using three different chemoattractants. The ability of LMP2A to induce migration in the scratch assay, which measures migration of cells that maintain cell-cell and cell-matrix contacts, and in the Transwell assay, which measures single-cell chemoattractant-directed migration, indicates that LMP2A broadly regulates cellular migration through effects on common pathways. All migration tested required the ITAM signaling domain of LMP2A and was inhibited by the Syk inhibitor piceatannol. The pBabe controls were also inhibited by piceatannol, indicating that activation of Syk is required for epithelial cell migration. Scratch assay migration induced by LMP2A was blocked by inhibitors of Akt, GSK3β, FAK, and Src in both cell lines. These findings indicate that the activation of ITAM/Syk, Akt, and FAK/Src by LMP2A is generally required for induced migration. The inhibition of migration by LiCl treatment was unexpected, as GSK3β is thought to be inhibited by Akt and to induce inhibitory phosphorylation of FAK. However, LiCl has also been shown to inhibit migration in studies with HeLa and CHO cells that have suggested that GSK3β promotes migration, and it has been suggested that one mechanism may be inhibition of Akt transcription (14, 20). However, as shown here, LiCl did not reduce total Akt levels but eliminated phospho-Akt. Overall, these findings suggest that the inhibitory effects of GSK3β on migration reflect the block in phosphorylation of Akt.
One difference in the LMP2A-induced signaling pathways involved in epithelial migration was the requirement of the PY domain for wound healing but not for Transwell migration. The PY domains of LMP2A interact with WW domain-containing proteins, including the Nedd4-like ubiquitin ligase Itch (13). Recently, Itch was shown to ubiquitinate and regulate the expression of angiomotin (AMOT). AMOT family members are involved in angiogenesis, integrity of tight junctions, formation of lamellipodia, migration, and epithelial-mesenchymal transition (EMT) (33). The interaction of LMP2A with Itch may affect the formation and integrity of tight junctions and promote wound healing migration. Itch has been shown to promote wound healing migration and the expression of features of EMT in the breast epithelial cell line MCF10A (26). By regulating protein expression and stability through interaction with the ubiquitin ligase Itch, LMP2A may target a group of proteins that can specifically promote wound healing migration.
LMP2A activated two distinct signaling pathways to control migration. Activation of both Syk and Akt required the ITAM motif but were apparently activated independently as phosphorylation of Akt and GSK3β was unaffected by the Syk inhibitor. Both Syk and Akt however, converged to promote membrane translocation of αV-integrin and membrane localization and integrin activation promoted migration through activation of FAK and recruitment of Src. These data suggest that Src family kinases may contribute to migration through facilitating activation of Akt and Syk through the ITAM motif and also through recruitment to the FAK signaling complex initiated by integrin clustering. Differences in signaling were identified in Transwell migration assays when migration of the two cell lines to FBS, collagen, and fibronectin was compared. Migration to FBS, fibronectin, and collagen all required the ITAM signaling domain of LMP2A and downstream Syk tyrosine kinase activity. Migration to fibronectin in HaCaT cells required a typical integrin-activated signaling pathway, involving FAK, Src, and Akt. Migration of HFK to FBS was impaired by inhibitors of FAK and Akt and by LiCl but was unaffected by the Src inhibitor PP2, suggesting that the requirement for FAK signaling was independent of Src. FAK has been shown to interact with other signaling mediators, including PI3K, at Y397, the autophosphorylation site which binds Src (37). LMP2A activation of FAK and Akt in HFK may also involve PI3K and the adaptor protein Grb7, both of which have been shown to interact with FAK in a Src-independent manner and promote migration.
Migration of HaCaT cells to collagen was distinct from migration to FBS and fibronectin, with the requirements for ITAM and Syk being the only commonalities. LMP2A-induced migration to collagen was independent of FAK and all signaling pathways targeted by LiCl and was slightly impaired by the Akt inhibitor triciribine, but this effect was not statistically significant. Collagen-mediated migration required Src activity, with PP2 completely blocking LMP2A-induced migration and partially blocking pBabe migration. Src has been shown previously to regulate migration in a FAK-independent manner through nitric oxide synthase and α9β1-integrin (12). FAK-independent, Src-dependent signaling by LMP2A suggests an alternative role for Src in controlling migration other than as part of the typical integrin/FAK/Src signaling complex. The involvement of different signaling pathways downstream of integrin activation when epithelial cells were induced to migrate to FBS, fibronectin, or collagen suggests that even with the common requirement of αV-integrin, downstream signaling is ligand dependent.
LMP2A has been previously shown to induce scratch assay migration and Matrigel invasion by upregulating the laminin-binding α6-integrin (21). Although α6-integrin was expressed in HaCaT cells and HFK, and mRNA levels were induced by LMP2A (data not shown), the limited ligand repertoire of α6-integrin implied that other integrins must be involved to account for the global effect LMP2A has on epithelial migration. HaCaT cells and HFK expressed several integrins, including αV, α2, α5, β1, and β6. Most integrin expression in whole-cell lysates was detected at comparable levels and was not induced by LMP2A, although α5-integrin and β6-integrin were slightly increased by LMP2A in HaCaT cells. While LMP2A did not affect expression of most integrins at the protein or mRNA level (data not shown), the induction of αV-integrin in the membrane-enriched fraction in a manner dependent on the ITAM motif of LMP2A and Syk and Akt activation suggests that LMP2A promotes migration by increasing translocation of αV-integrin to membrane-enriched compartments of epithelial cells. Membrane translocation of αV-integrin was unaffected by PP2 and is therefore independent of Src, suggesting that Src likely contributes to migration through integrin and FAK signaling. The membrane translocation of αV-integrin may have broader effects due to its recognition of ligands containing the RGD active site, including fibronectin, vitronectin, fibrinogen, fibrillin, and thrombospondin. The ability of αV-integrin to recognize such a large number of ligands indicates that the effect on αV may be responsible for the ability of LMP2A to globally control migration. A neutralizing antibody to αV-integrin inhibited migration to FBS, fibronectin, and collagen, supporting its role in migration to several extracellular matrix ligands. LMP2A has previously been shown to induce migration to both fibronectin and collagen and migration to collagen required Syk activation (16). Although not typically a collagen-binding integrin, αVβ3-integrin can bind denatured or proteolysed collagen (19, 34), and LMP2A was recently reported to promote invasion of NPC cells by upregulating the collagenase MMP-9 (15), suggesting the intriguing possibility that LMP2A can induce proteolysis of collagen through MMPs to expose the RGD binding site. Although undetectable in the membrane fractions by Western blotting, neutralizing antibody to α5-integrin also significantly inhibited LMP2A-induced migration to fibronectin and collagen in HaCaT cells, suggesting that α5 also contributes to migration. The contribution of α5-integrin, which has a restricted repertoire of ligands, is likely to be less important than the effects mediated by αV-integrin. The membrane translocation of αV-integrin without effects on total protein levels suggests that LMP2A controls integrin expression posttranscriptionally. Posttranscriptional regulation of proteins also contributes to LMP2A-mediated inhibition of epithelial differentiation through stabilization of ΔNp63α expression (8).
Nasopharyngeal carcinoma is a cancer that frequently metastasizes and is consistently associated with expression of LMP2A. Successful metastasis requires the tumor cell to resist cell death and to interact with its local microenvironment, migrate, invade, and induce angiogenesis at the secondary site (2). By regulating integrin localization, LMP2A may promote metastasis by participating in migration, invasion, and interaction of the tumor cell with the microenvironment. Although αV-integrin was important for all Transwell migration studied, cell type and ligand specificity may also depend on the β subunit required by each specific ligand, and subunit expression can determine the activation of downstream signaling pathways. β1-Integrin has been demonstrated to interact with protein kinase Cα (PKCα), whereas β3-integrin interacts with Src (17). Phosphorylation and signaling events activated by integrins also control their internalization, a process that plays a key role in migration by redistributing integrins during assembly and disassembly of adhesions at the leading edge and the trailing edge of a migrating cell. β-Integrin subunits interact with adapter proteins like Dab-2, Numb, Rab-5, PKD1, and ACAP1 that regulate internalization and recycling of integrins (17). Since PKC is also inhibited by the GSK3β inhibitor LiCl, and LiCl impaired LMP2A-induced migration to FBS and fibronectin, PKC activity may be involved in signaling by the β subunit. α-Integrin subunit expression may also participate in specificity of integrin signaling. Rab21 and p210RasGAP can interact with α1 and α2 subunits to mediate recycling (17). Further study of the β-integrin subunits expressed by epithelial cells and involved in migration may clarify some of the differences in signaling pathways activated by different chemoattractants.
An important finding of this study is the dramatic effects of LMP2A on the membrane translocation of the αV-integrin and its requirement for migration. Importantly, the broad specificity of αV for several extracellular ligands suggests that LMP2A would therefore exert wide-ranging effects on cellular migration. The effects on αV-integrin membrane localization and epithelial migration required ITAM activation and activation of Syk, Akt, FAK, and Src signaling pathways. Through its effects on integrin activity and signaling, LMP2A expression likely contributes to cancer metastasis by modulation of adhesion, migration, and invasion.
ACKNOWLEDGMENTS
This study was supported by NCI CA 32979 and CA 19014 to N.R.-T.
Footnotes
Published ahead of print 25 July 2012
REFERENCES
- 1. Allen MD, Young LS, Dawson CW. 2005. The Epstein-Barr virus-encoded LMP2A and LMP2B proteins promote epithelial cell spreading and motility. J. Virol. 79:1789–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bacac M, Stamenkovic I. 2008. Metastatic cancer cell. Annu. Rev. Pathol. 3:221–247 [DOI] [PubMed] [Google Scholar]
- 3. Reference deleted.
- 4. Bianchi M, et al. 2005. Regulation of FAK Ser-722 phosphorylation and kinase activity by GSK3 and PP1 during cell spreading and migration. Biochem. J. 391:359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brooks L, Yao QY, Rickinson AB, Young LS. 1992. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J. Virol. 66:2689–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Busson P, et al. 1992. Consistent transcription of the Epstein-Barr virus LMP2 gene in nasopharyngeal carcinoma. J. Virol. 66:3257–3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Desgrosellier JS, Cheresh DA. 2010. Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 10:9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fotheringham JA, Mazzucca S, Raab-Traub N. 2010. Epstein-Barr virus latent membrane protein-2A-induced DeltaNp63alpha expression is associated with impaired epithelial-cell differentiation. Oncogene 29:4287–4296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fruehling S, Longnecker R. 1997. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology 235:241–251 [DOI] [PubMed] [Google Scholar]
- 10. Fruehling S, Swart R, Dolwick KM, Kremmer E, Longnecker R. 1998. Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency. J. Virol. 72:7796–7806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fukuda M, Longnecker R. 2007. Epstein-Barr virus latent membrane protein 2A mediates transformation through constitutive activation of the Ras/PI3-K/Akt Pathway. J. Virol. 81:9299–9306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta SK, Vlahakis NE. 2009. Integrin alpha9beta1 mediates enhanced cell migration through nitric oxide synthase activity regulated by Src tyrosine kinase. J. Cell Sci. 122:2043–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ikeda M, Ikeda A, Longan LC, Longnecker R. 2000. The Epstein-Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology 268:178–191 [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi T, et al. 2006. Glycogen synthase kinase 3 and h-prune regulate cell migration by modulating focal adhesions. Mol. Cell. Biol. 26:898–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lan YY, et al. 2012. Epstein-Barr virus latent membrane protein 2A promotes invasion of nasopharyngeal carcinoma cells through ERK/Fra-1-mediated induction of matrix metalloproteinase 9. J. Virol. 86:6656–6667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu J, et al. 2006. Syk tyrosine kinase mediates Epstein-Barr virus latent membrane protein 2A-induced cell migration in epithelial cells. J. Biol. Chem. 281:8806–8814 [DOI] [PubMed] [Google Scholar]
- 17. Margadant C, Monsuur HN, Norman JC, Sonnenberg A. 2011. Mechanisms of integrin activation and trafficking. Curr. Opin. Cell Biol. 23:607–614 [DOI] [PubMed] [Google Scholar]
- 18. Miller CL, et al. 1995. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity 2:155–166 [DOI] [PubMed] [Google Scholar]
- 19. Montgomery AM, Reisfeld RA, Cheresh DA. 1994. Integrin alpha v beta 3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc. Natl. Acad. Sci. U. S. A. 91:8856–8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nemoto T, et al. 2008. Regulation of Akt mRNA and protein levels by glycogen synthase kinase-3beta in adrenal chromaffin cells: effects of LiCl and SB216763. Eur. J. Pharmacol. 586:82–89 [DOI] [PubMed] [Google Scholar]
- 21. Pegtel DM, et al. 2005. Epstein-Barr-virus-encoded LMP2A induces primary epithelial cell migration and invasion: possible role in nasopharyngeal carcinoma metastasis. J. Virol. 79:15430–15442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raab-Traub N. 1992. Epstein-Barr virus and nasopharyngeal carcinoma. Semin. Cancer Biol. 3:297–307 [PubMed] [Google Scholar]
- 23. Raab-Traub N. 2002. Epstein-Barr virus in the pathogenesis of NPC. Semin. Cancer Biol. 12:431–441 [DOI] [PubMed] [Google Scholar]
- 24. Raab-Traub N. 1992. Epstein-Barr virus infection in nasopharyngeal carcinoma. Infect. Agents Dis. 1:173–184 [PubMed] [Google Scholar]
- 25. Rickinson AB, Kieff E. 2001. Epstein-Barr virus, 4th ed Lippincott/Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 26. Salah Z, Melino G, Aqeilan RI. 2011. Negative regulation of the Hippo pathway by E3 ubiquitin ligase ITCH is sufficient to promote tumorigenicity. Cancer Res. 71:2010–2020 [DOI] [PubMed] [Google Scholar]
- 27. Scholle F, Bendt KM, Raab-Traub N. 2000. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J. Virol. 74:10681–10689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seo MD, Park SJ, Kim HJ, Lee BJ. 2007. Identification of the WW domain-interaction sites in the unstructured N-terminal domain of EBV LMP 2A. FEBS Lett. 581:65–70 [DOI] [PubMed] [Google Scholar]
- 29. Shair KH, Schnegg CI, Raab-Traub N. 2008. EBV latent membrane protein 1 effects on plakoglobin, cell growth, and migration. Cancer Res. 68:6997–7005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shair KH, Schnegg CI, Raab-Traub N. 2009. Epstein-Barr virus latent membrane protein-1 effects on junctional plakoglobin and induction of a cadherin switch. Cancer Res. 69:5734–5742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Swart R, Ruf IK, Sample J, Longnecker R. 2000. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-kinase/Akt pathway. J. Virol. 74:10838–10845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vasudevan KM, Garraway LA. 2010. AKT signaling in physiology and disease. Curr. Top. Microbiol. Immunol. 347:105–133 [DOI] [PubMed] [Google Scholar]
- 33. Wang C, et al. 2012. The Nedd4-like ubiquitin E3 ligases target angiomotin/P130 to ubiquitin-dependent degradation. Biochem. J. 444:279–289 [DOI] [PubMed] [Google Scholar]
- 34. Wilder RL. 2002. Integrin alpha V beta 3 as a target for treatment of rheumatoid arthritis and related rheumatic diseases. Ann. Rheum. Dis. 61(Suppl 2):ii96–ii99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yadav V, Denning MF. 2011. Fyn is induced by Ras/PI3K/Akt signaling and is required for enhanced invasion/migration. Mol. Carcinog. 50:346–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Young LS, Rowe M. 1992. Epstein-Barr virus, lymphomas and Hodgkin's disease. Semin. Cancer Biol. 3:273–284 [PubMed] [Google Scholar]
- 37. Zhao X, Guan JL. 2011. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 63:610–615 [DOI] [PMC free article] [PubMed] [Google Scholar]