Abstract
To explore the efficacy of novel complementary prime-boost immunization regimens in a nonhuman primate model for HIV infection, rhesus monkeys primed by different DNA vaccines were boosted with virus-like particles (VLP) and then challenged by repeated low-dose rectal exposure to simian immunodeficiency virus (SIV). Characteristic of the cellular immune response after the VLP booster immunization were high numbers of SIV-specific, gamma interferon-secreting cells after stimulation with inactivated SIV particles, but not SIV peptides, and the absence of detectable levels of CD8+ T cell responses. Antibodies specific to SIV Gag and SIV Env could be induced in all animals, but, consistent with a poor neutralizing activity at the time of challenge, vaccinated monkeys were not protected from acquisition of infection and did not control viremia. Surprisingly, vaccinees with high numbers of SIV-specific, gamma interferon-secreting cells were infected fastest during the repeated low-dose exposures and the numbers of these immune cells in vaccinated macaques correlated with susceptibility to infection. Thus, in the absence of protective antibodies or cytotoxic T cell responses, vaccine-induced immune responses may increase the susceptibility to acquisition of immunodeficiency virus infection. The results are consistent with the hypothesis that virus-specific T helper cells mediate this detrimental effect and contribute to the inefficacy of past HIV vaccination attempts (e.g., STEP study).
INTRODUCTION
Immune escape mechanisms and the diversity of the circulating HIV strains pose many hurdles for the development of an effective HIV vaccine. In addition, testing the efficacy of HIV vaccines in clinical studies is time-consuming and costly. Therefore, only three vaccine strategies have been tested for efficacy in human volunteers so far. A recombinant protein vaccine based on the gp120 surface protein (AIDSVAX) did not provide protection (5, 13), although antibodies to the vaccine antigen were induced. The lack of efficacy was attributed to the absence of neutralizing antibodies. To explore the efficacy of cellular immune responses, volunteers were also immunized with adenoviral vectors carrying gag, pol, and nef of HIV. However, this did not provide protection either, and in a subgroup of volunteers with preexisting antibodies to the adenoviral vector the susceptibility to acquisition of HIV infection was increased (2). The third vaccine strategy tested for efficacy in human volunteers aimed at the induction of cellular and humoral immune responses by combining an avipox vector carrying gag, protease, and env with the AIDSVAX vaccine. The acquisition of HIV infection was reduced by approximately 30% (15), providing the first evidence that protection from HIV infection by vaccination may be possible.
Since such an efficacy is probably too low for general use, we explored the efficacy of a novel complementary prime-boost immunization in nonhuman primates. In our previous study, we compared the immunogenicities of dendritic cell (DC)-targeting DNA vaccines in rhesus macaques and could demonstrate that DNA electroporation results in robust cellular and humoral immune responses even at low doses (23). However, DNA vaccines encoding DC-targeted antigens induced lower responses than the nontargeted counterpart and nearly no response if delivered by conventional intramuscular (i.m.) injection. To further explore how these different priming strategies influenced the immunogenicity and efficacy of a complementary protein boost, rhesus monkeys were boosted with a virus-like particle (VLP) vaccine to trigger antibody responses to the Env protein of SIV in its native conformation. Although cellular and humoral immune responses were induced, vaccinated monkeys were not protected from acquisition of SIV infection in a repeated low-dose challenge. On the contrary, vaccinees with high numbers of vaccine-induced SIV-specific, gamma interferon (IFN-γ)-secreting cells were more susceptible to acquisition of challenge virus infection than poor vaccine responders.
MATERIALS AND METHODS
Production, characterization, and formulation of VLPs.
VLPs were produced by transient transfection of 293T cells by the polyethylenimine (PEI) method with plasmids Sgpsyn (26) and pcD-SIVgp140-GTM/CD, which encodes the SIV gp140 ectodomain fused with the transmembrane domain of the G protein of vesicular stomatitis virus. The transfection medium was replaced with Dulbecco's modified Eagle medium (DMEM) containing 1.5% fetal calf serum (FCS) 18 h after transfection. VLPs containing supernatants were harvested 36 h later, centrifuged at 300 × g for 10 min, and filtrated through a 0.45-μm filter to remove cellular debris. VLPs were further purified and concentrated from the conditioned medium by ultracentrifugation through a 20% sucrose cushion at 28,000 rpm for 2 h in an SW28 rotor. The pellets were resuspended in phosphate-buffered saline (PBS) at approximately 1/180 of the volume of the 293T cell supernatants. The concentration of viral proteins in the final VLP preparation was determined by an in-house enzyme-linked immunosorbent assay (ELISA) using recombinant SIVgp130 (EVA670; National Institute for Biological Standards and Control [NIBSC] Centralized Facility for AIDS Reagents) and SIVp27 (EVA643) as standards. The endotoxin level was determined to be 1.6 ng/ml by the QCL-1000 chromogenic Limulus amoebocyte lysate (LAL) endpoint assay (Cambrex, Germany). The VLP preparation was mixed with 2 mg poly-ICLC (Hiltonol; Oncovir, Washington, DC) in a final volume of 2 ml PBS. Poly-ICLC is a synthetic double-stranded RNA (dsRNA), which has been stabilized against serum nucleases of primates, binds like poly(I·C) to toll-like receptor 3 and melanoma differentiation-associated gene 5, and augments immune responses to nontargeted and CD205-targeted protein antigens (4, 21).
Immunization and immune monitoring of macaques.
The animals assigned to this study were colony-bred young adult rhesus monkeys (Macaca mulatta) of Indian origin and of either sex. These rhesus macaques were housed at the German Primate Center under conditions in accordance with the German Animal Welfare Act and in compliance with the European Union guidelines on the use of nonhuman primates for biomedical research. The study was approved by an ethics committee authorized by the Lower Saxony State Office for Consumer Protection and Food Safety. Further details on the animal specification, housing, sample collection, and approval from respective authorities are described elsewhere (23). Overall, 24 macaques were used in this study, 18 of them distributed to three vaccine groups of six animals each and another six monkeys serving as controls. Macaques were typed for the A1*01, B*08, and B*17 alleles (16). The A1*01-positive macaques were distributed evenly among the different groups.
As outlined in Table 1, all vaccinees had been immunized twice 8 weeks apart with DNA vaccines encoding SIVp27 capsid fused to either a single-chain antibody to DEC205 (scDEC-p27) or to a control single-chain antibody (scISO-p27). Eight and 16 weeks after the second DNA immunization, all SIV vaccinees were immunized with SIV VLPs containing 10 μg of SIVgp130 and 6.5 μg of SIV p27 with 2 mg poly-ICLC as the adjuvant in a total volume of 2 ml. Formulated VLPs were injected subcutaneously close to the inguinal lymph nodes. SIV-specific antibody responses to Gag and monomeric gp130 were determined as described elsewhere (23). SIVgp140 trimers were expressed by transient transfection in HEK293T cells using the pLEX vector followed by immobilized metal ion affinity chromatography (IMAC), lectin, and size exclusion chromatography purification steps. Protein purity was assessed by reducing and nonreducing SDS-PAGE. ELISA plates (Greiner Bio-One; Lumitrac 600) were coated with 25 ng of trimers/well, and the binding activity of sera was determined at a 1 to 1,000 dilution using a polyclonal rabbit anti-human IgG–horseradish peroxidase (HRP) serum at a 1 to 1,000 dilution (Dako). For the neutralization assay, 10 μl of serial dilutions of heat-inactivated serum was incubated at 37°C in duplicate with 50 μl (40 tissue culture infectious doses) of an early passage of SIVmac251 grown on rhesus monkey peripheral blood mononuclear cells (PBMCs). After 1 h, the volume was adjusted to 100 μl by adding 1 × 104 TZMbl cells and DEAE-dextran to final concentration of 10 μg/ml. After 2 days, cells were lysed in Glo lysis buffer (Promega) and luciferase activity was determined using the Bright Glo substrate (Promega). Mean luciferase activities of duplicates were used to determine the 50% neutralization titer. Background neutralization titers of preimmune sera from 17 of the 18 vaccine animals were ≤1:40. Only the preimmune serum of macaque 12149 had a background neutralization titer of 1:80. SIV p27CA- and gp130SU-specific IgA antibodies were determined as described previously (17). The IFN-γ enzyme-linked immunosorbent spot (ELISPOT) assay and tetramer staining were performed as reported previously (23). Intracellular cytokine staining was essentially carried out as detailed before (18) except that for virus-specific stimulation only the synthetic Gag peptide pool was used.
Table 1.
Efficacy of the different immunization regimens
| Group (n = 6) | DNA vaccine | Dose and route at wk −16 and −8d | VLP boost at wk 0 and 8e | Mean no. of inoc. ± SDa | Mean peak viremiab ± SD | Mean set pointc ± SD |
|---|---|---|---|---|---|---|
| A | scDEC-p27 | 0.1 mg, EP | s.c. + Adj. | 8.0 ± 4.6 | 5.6 ± 0.6 | 3.9 ± 1.3 |
| B | scISO-p27 | 0.1 mg, EP | s.c. + Adj. | 4.5 ± 2.6 | 4.3 ± 1 | 3.7 ± 1.8 |
| C | scDEC-p27 | 1 mg i.m. + Adj. | s.c. + Adj. | 8.3 ± 4.5 | 5.9 ± 0.4 | 4.5 ± 0.8 |
| D | Mock | 1 mg i.m. + Adj. | —f | 7.2 ± 5.9 | 5.4 ± 1.3 | 3.4 ± 1.3 |
Mean number of inoculations (inoc.) required for infection.
Peak log10 values of viral RNA copies per ml plasma between weeks 1 to 3 after infection.
Mean log10 values of viral RNA copy numbers per ml plasma of week 24 and 32 after infection.
EP, electroporation; i.m., intramuscular injection.
Rhesus monkeys primed by the indicated DNA vaccines as previously described (23) were injected subcutaneously (s.c.) close to the inguinal lymph nodes with SIV VLPs containing 10 μg of SIVgp130, 6.5 μg of SIV p27, and 2 mg of poly-ICLC adjuvant (Adj.).
—, no boost.
Challenge of macaques.
The repeated low-dose challenge started 8 weeks after the final immunization. The SIVmac251 stock used for challenge (SIVmac251 10/09) was prepared by one in vitro passage on monkey PBMCs of a very early passage of SIVmac251 (9), kindly provided by A. M. Aubertin (Université Louis Pasteur, Strasbourg, France) with a calculated in vitro titer of 104.6 50% tissue culture infectious doses (TCID50) per ml on C8166 cells and 2.88 × 107 viral RNA copies per ml. For virus inoculation, animals received a deep anesthesia by i.m. injection of a mixture of ketamine, xylazine, and atropine and were placed in ventral recumbency with their hips elevated for the following 20 min. For each challenge, 3 ml of virus-containing medium was administered atraumatically into the rectum using a 10-cm cylindrical human urethral catheter with a closed distal end (CH06; Urotech, Germany). The intrarectal viral exposures were performed using escalating doses, i.e., with 30 TCID50 for the first seven applications, 60 TCID50 for the following six challenges, and 120 TCID50 for the final three inoculations. Challenges were performed weekly and stopped 1 week after viral RNA became detectable in plasma with levels >100 copy equivalents/ml, indicating systemic infection (11).
Data and statistical analyses.
Differences between the experimental groups were analyzed by the Kruskal-Wallis analysis of variance (ANOVA) by ranks test. For analyzing the correlation between immune responses at the time of challenge and the number of inoculations required for infection, all 18 animals of the vaccine groups were included. Differences in the number in inoculations required for infection between monkeys of the vaccine groups with more than 200 IFN-γ-secreting cells/106 PBMCs after stimulation with AT2 SIV on the day of challenge and those with less than 200 IFN-γ-secreting cells/106 PBMCs were analyzed by the Mann-Whitney t test.
RESULTS
In a recent study, we explored whether targeting the SIV p27 capsid protein encoded by a DNA vaccine for dendritic cells (23) would increase immunogenicity in nonhuman primates. Six rhesus monkeys (group A) had been immunized twice by electroporation of a DNA vaccine (scDEC-p27) encoding a fusion protein of a single-chain antibody to CD205 (DEC205) and the SIV p27 capsid protein. As a control for the targeting of CD205, animals of group B had received a DNA vaccine (scIso-p27) encoding a fusion protein of a single-chain control antibody also fused to the SIV p27 capsid protein. Additionally, six monkeys (group C) had received scDEC-p27 with the TLR3 agonist poly-ICLC (Hiltonol) as the adjuvant twice by conventional intramuscular injection. To further explore how these different priming strategies influenced the immunogenicity of a complementary protein boost, all groups were boosted with the same VLP vaccine 8 weeks after the last DNA immunization and another 8 weeks later. The Gag and Env content of the VLP preparation was determined by ELISA. Furthermore, the envelope protein gp160 (160 kDa) and the precursor Gag protein p55 (55 kDa) and its cleavage products, including the capsid protein p27 (27 kDa), were successfully detected in Western blot analysis, indicating correct incorporation of these proteins into the VLPs (Fig. 1). The VLPs were injected together with poly-ICLC as the adjuvant. A summary of the entire immunization regimen for all groups is provided in Table 1.
Fig 1.
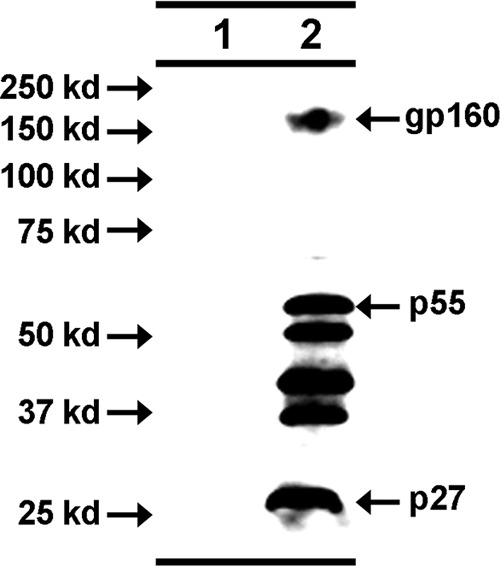
Characterization of VLPs by Western blot analysis. Purified VLPs were characterized by Western blot analysis with serum from a naive macaque (lane 1) and an SIV-infected macaque (lane 2).
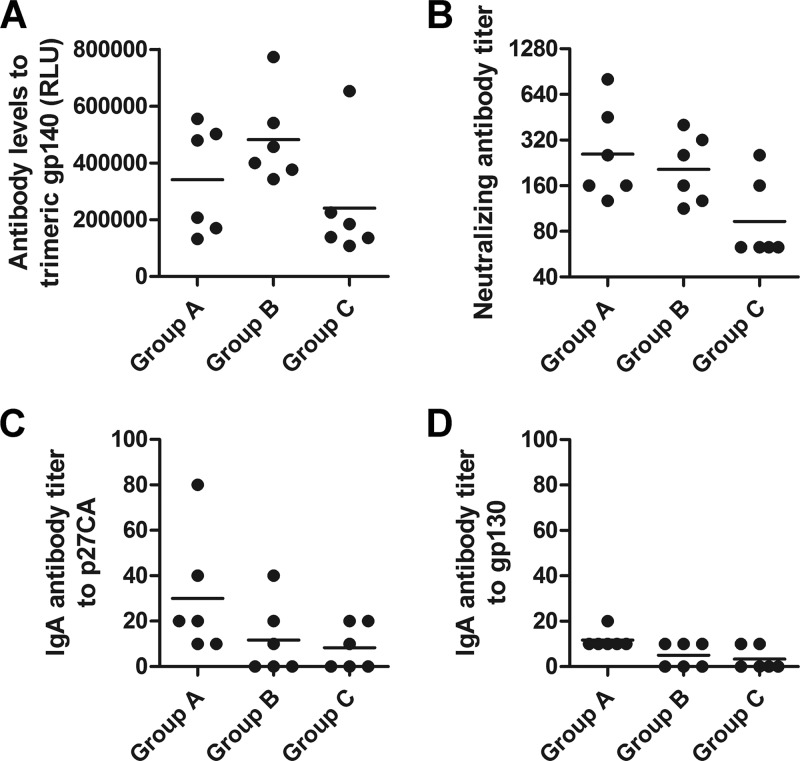
Prior to the VLP immunizations, antibodies to Gag were detectable only in groups A and B (Fig. 2A and B). The VLP immunizations induced an approximately 100-fold increase in Gag-specific antibody levels in groups A and B, while five out of six animals of group C seroconverted to Gag after the second VLP boost (Fig. 2B). Env-specific antibodies became detectable in all groups after the second VLP boost. The induced antibodies also recognized trimeric SIVmac251 gp140 (Fig. 3A), and low levels of neutralizing antibodies to the SIVmac251 isolate were also observed 2 weeks after the second VLP boost (Fig. 3B) but rapidly declined to close to background levels (data not shown). Low levels of Gag- and Env-specific IgA antibodies were also detected after the second VLP booster immunization in all animals of group A and in two or three animals of groups B and C (Fig. 3C and D).
Fig 2.
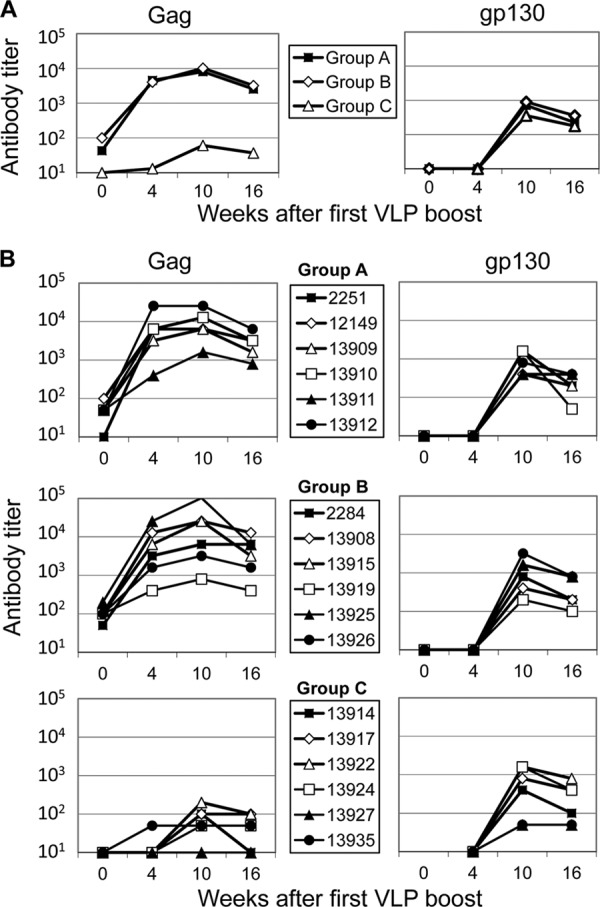
SIV-specific antibody titers in the blood after the VLP booster immunizations. Shown are antibody titers (geometric means) of vaccine groups (A) and antibody titers in individual macaques (B) in response to SIV Gag and Env at the indicated time points. Four- and five-digit numbers (B) are monkey designations.
Fig 3.
Characterization of the humoral immune response. (A) Antibody levels in response to the gp140 trimer of SIVmac251 at week 10 after the first VLP boost. RLU, relative light units. (B) Neutralizing antibody titers against SIVmac251 at week 10 after the first VLP boost. The neutralization titers of two or three independent neutralization assays are shown for all immunized macaques. The horizontal bars mark the geometric means of the groups. (C and D) IgA antibody titers in blood in response to SIVp27CA (C) and gp130 (D).
Characterization of cellular immune responses prior to the VLP booster immunization had revealed that the delivery of both DNA vaccines by electroporation induced substantial cellular immune responses, although the response to the nontargeted DNA vaccine (group B) was stronger (23). Conventional immunization with the DC-targeting DNA at a 10-fold-higher dose did not give rise to considerable cellular immune responses (23). At the time of the first VLP boost, the IFN-γ ELISPOT response after stimulation with a pool of Gag peptides was significantly higher in group B than in group A (Fig. 4). After the VLP booster immunizations a consistent increase in the IFN-γ ELISPOT response after stimulation with the Gag peptides could not be observed and the difference between groups A and B vanished (Fig. 4A and B, left). However, stimulation of the PBMCs with AT2-inactivated SIV (AT2 SIV) instead of the Gag peptides clearly revealed a strong booster effect of the VLP immunization on the IFN-γ ELISPOT response in groups A and B, but not in group C (Fig. 4A and B, right). Consistent with the poor ELISPOT response after stimulation with the SIV peptide pools, only minor responses were detected by intracellular cytokine staining of PBMCs stimulated with these peptide pools (data not shown). SIV capsid-specific CD8+ T cells were not detected in MamuA1-positive animals after the VLP boost by major histocompatibility complex class I (MHC-I) tetramer staining either (data not shown). Characteristic of the cellular immune response observed after the DNA prime-VLP boost regimen is therefore the strong IFN-γ ELISPOT response upon stimulation with AT2-inactivated SIV, but not peptide pools, and the absence of detectable levels of antigen-specific CD8+ T cells.
Fig 4.
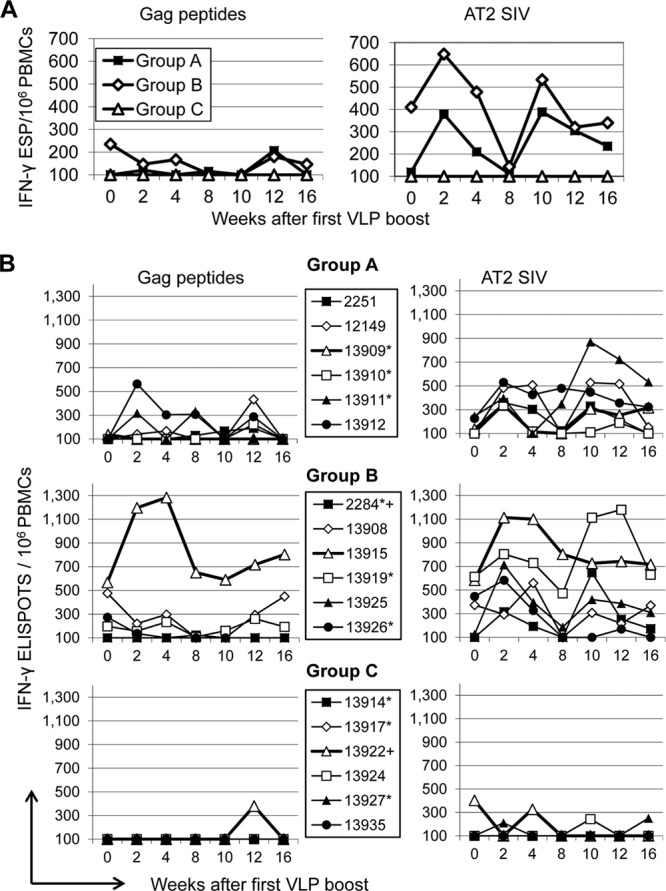
Cellular immune response in blood after the VLP booster immunizations. (A) Geometric means of numbers of IFN-γ-secreting PBMCs in the vaccine groups after stimulation with SIV Gag peptides (left) or AT2-inactivated SIV (right) at the indicated time points after the first VLP immunization. ESP, Elispots. (B) Values for each of the macaques. Four- and five-digit numbers are monkey designations. Monkeys positive for the A*01 and the B*17 MHC alleles are marked by asterisks and plus signs, respectively.
Because of the lack of validated correlates of protection, only challenge experiments can reveal whether the immune responses induced provide protection. We therefore challenged the vaccinated and naive control (group D) monkeys intrarectally with pathogenic SIVmac251. Since heterosexual transmission in HIV-infected people is frequently due to a single founder virus (6), a repeated low-dose challenge approach was used. This strategy allows assessing efficacy by (i) numbers of exposures required for infection, which should reflect rotection from acquisition of infection, (ii) peak viremia, revealing early antiviral effector mechanisms, and (iii) set point RNA levels as an indicator for persistent suppression of virus replication.
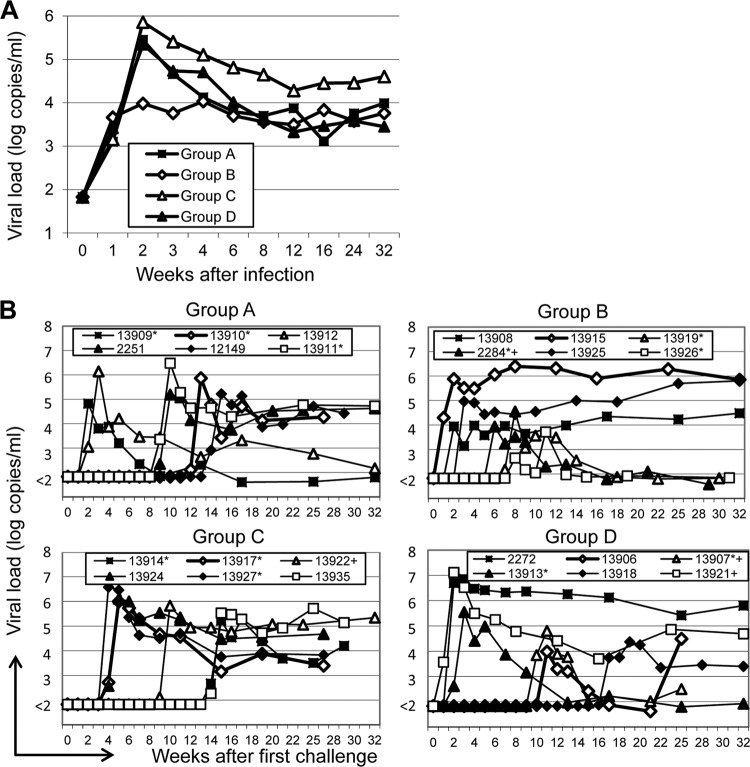
Eight weeks after the second VLP immunization, monkeys were challenged weekly after prior blood sampling to determine viral loads. Since SIV infection usually leads to detectable RNA levels 1 week after exposure, inoculations preceding the first SIV RNA-positive samples by 1 week were considered to have led to infection. Numbers of exposures leading to infection varied from 1 to 17, and viral load data for all groups are plotted against weeks after first challenge (Fig. 5B). Approximately eight inoculations were required for infection of monkeys of groups A, C, and D (naive controls), while animals of group B required only 4.5 inoculations on average (Table 1). A comparison of the means of the viral load data between groups (Fig. 5A) revealed a more than 10-fold-lower peak viremia in group B with no apparent differences in set point RNA levels (Table 1). However, neither the difference in peak viremia nor the difference in numbers of inoculations required for infection reached statistical significance.
Fig 5.
Vaccine efficacy. (A) Mean viral load levels per ml plasma for each group after synchronizing for the point in time of acquisition of infection. (B) Viral load data at the indicated time points after the first challenge. Four- and five-digit numbers are monkey designations. Monkeys positive for the A*01 and the B*17 MHC alleles are marked by asterisks and plus signs, respectively.
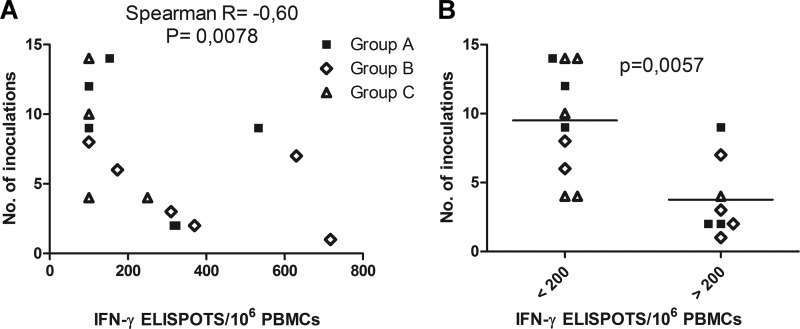
Since group B was also the group with the strongest IFN-γ ELISPOT responses after stimulation with AT2 SIV, the trend to enhanced susceptibility to infection raised the possibility that vaccine-induced cellular immune responses are responsible for this enhancement. We therefore analyzed whether there is a correlation between immune responses induced in the 18 vaccinated macaques and the number of inoculations required for infection. The magnitude of the IFN-γ ELISPOT responses after stimulation with AT2 SIV at the time of the first challenge correlated inversely with the number of inoculations required for infection (Spearman R = −0.60; P = 0.0078) (Fig. 6A). Immunized macaques with more than 200 IFN-γ-secreting cells/106 PBMCs were approximately 2-fold more susceptible to the acquisition of infection than immunized macaques with ELISPOT responses lower than 200 IFN-γ secreting cells/106 PBMCs (P = 0.0057, Mann-Whitney t test) (Fig. 6B). To explore if the initial immune response induced by the different DNA vaccines plays a role in this scenario, further correlation analyses were performed with cellular immune responses observed 2 weeks after the second DNA immunization (23). However, neither CD8+ T cell proliferation, IFN-γ ELISPOT responses to stimulation with AT2-inactivated SIV and Gag peptide pools, nor tetramer staining at 2 weeks after the second DNA immunization correlated with susceptibility to infection or viral load after challenge (data not shown). Antibody titers in response to Gag and Env at the time of the first challenge did not correlate with susceptibility to infection either. Since MHC alleles have been reported to be associated with the course of SIV infection in rhesus monkeys, all animals were typed for the A*01, B*08, and B*17 alleles. While none of the animals had the B*08 allele, neither the A*01 nor the B*17 allele was correlated with the number of inoculations required for infection, peak viremia, or set point RNA levels.
Fig 6.
Correlation of the IFN-γ ELISPOT response with susceptibility to infection. (A) Correlation of the number of inoculations required for infection and IFN-γ ELISPOTs after stimulation of PBMCs with AT2-inactivated SIV on the day of the first challenge. (B) Numbers of inoculations required for infection in macaques with more or less than 200 IFN-γ-secreting cells/106 PBMCs after stimulation with AT2-inactivated SIV at the time of the first challenge.
DISCUSSION
In this study, we boosted a cohort of rhesus macaques, which had been used previously to study the immunogenicity of DC-targeting DNA vaccines (23), with VLPs and analyzed the protective capacity of this heterologous prime-boost vaccine in a repeated low-dose challenge experiment. The VLP booster immunization increased the SIV-specific antibody levels as well as the AT2-stimulated ELISPOT responses. Significant differences in the cellular immune responses observed between the groups after the DNA priming immunization (23) faded out. Accordingly, no significant differences in susceptibility to infection and viral load after challenge between the different groups were observed. Thus, the most striking result of our study is the correlation of the magnitude of the vaccine-induced, SIV-specific IFN-γ-secreting cells with the susceptibility to acquisition of infection.
Although the possibility that the monkeys that showed a strong cellular response to vaccination were more susceptible to acquisition of infection independently of the induced immune responses cannot be excluded, it seems more likely that vaccine-induced immune responses increased their susceptibility to acquisition of infection. This is also supported by the trend to an enhanced susceptibility to infection of group B, i.e., the vaccine group with the strongest ELISPOT response. One plausible scenario is that vaccine-induced, SIV-specific CD4+ T cells in the lamina propria of the exposed mucosa and/or the draining lymph nodes were activated by exposure to SIV antigen from the viral inoculum. In the absence of protective effector mechanisms, the activated SIV-specific T helper cells may then have favored viral replication and early spread, thereby promoting the establishment of infection. This hypothesis is supported by the observation that SIV preferentially infects highly activated CD4+ T cells during primary infection (25). In addition to more efficient replication in activated T cells, HIV also preferentially infects HIV-specific CD4+ T cells (3). However, since stimulation of PBMCs obtained after the VLP booster immunizations with SIV peptide pools did not reveal a specific intracellular cytokine response, only the absence of CD8+ T cell responses as assessed by tetramer staining suggests that the cells producing IFN-γ in the ELISPOT assay after stimulation with AT2-inactivated SIV are CD4+ T helper cells.
Until now, only one study using a varicella-zoster virus-based vector vaccine expressing SIV Env has reported an increase in viral loads in macaques following vaccination (22). In this study, vaccinated monkeys were challenged intravenously with a single intermediate dose of SIV, which resulted in infection of all animals. Thus, this experimental set-up could reveal whether vaccine-induced immune responses increased viral loads once infection was established. It did not allow, however, for analysis of whether vaccination increases the susceptibility to acquisition of infection. Still, this is important since the mechanisms mediating reduction in virus replication after establishment of infection might differ from those favoring acquisition of infection. This is the notion supported by our findings, i.e., the vaccine group with the highest susceptibility to infection had the lowest peak viremia. Furthermore, the numbers of SIV-specific, IFN-γ-secreting cells in PBMCs stimulated with AT2 SIV correlated with susceptibility to infection. Thus, the vaccine-induced immune responses may on the one hand increase the susceptibility to acquisition of immunodeficiency virus infection while on the other hand they contribute to suppression of virus replication once infection has been established.
The concern that vaccine-induced immune responses may increase the susceptibility to HIV infection has also been raised previously based on the results of the STEP study (2). Vaccinated volunteers with preexisting antibody titers in response to the adenoviral vector vaccine were more likely to acquire HIV infection than nonvaccinated individuals. Since the higher rate of infection was associated with adenovirus-specific immune responses, it was hypothesized that anamnestic adenovirus-specific CD4+ T lymphocyte responses were responsible for the enhancement of HIV-1 acquisition in adenovirus-seropositive subjects (1, 19). However, subsequent studies provided evidence against this hypothesis (7, 10). A nonhuman primate study modeling the STEP study also suggested enhanced susceptibility to acquisition of SIV infection in macaques infected with adenovirus prior to adenoviral vector immunization against SIV (14). Since the trend to enhanced susceptibility to infection was not observed in macaques infected with adenovirus prior to immunization with a control adenoviral vector, SIV-specific immune responses seem to be responsible for enhanced susceptibility (14).
In the light of the present study the following hypothesis explaining the results of the STEP study gains credibility. The preexisting humoral immunity to adenoviruses may modulate the HIV-specific immune response induced by the adenoviral vector vaccine in the STEP study leading to an increased number of mucosal HIV-specific T helper cells (24). Upon exposure to HIV antigen present in the viral inoculum, these mucosal HIV-specific T helper cells get activated and thus increase the susceptibility to acquisition of HIV infection. In a case cohort follow-up analysis of the STEP study, the magnitude of numbers of antigen-specific, IFN-γ-secreting cells after vaccination was indeed greater in infected than in noninfected vaccine recipients (8). The association of the magnitude of vaccine-induced IFN-γ ELISPOT responses with susceptibility to immunodeficiency virus infection in naturally exposed human volunteers in the STEP study and nonhuman primates challenged by repeated low-dose exposure in the present study further questions the IFN-γ ELISPOT assay as a correlate of protection from HIV infection (19). It should also be noted that not all virus-specific CD4+ T cell responses seem to be detrimental. During acute HIV infection, virus-specific T cells with cytolytic activity are associated with better control of viremia (20). Depletion of CD4+ T cells during acute SIV infection also impairs reduction of viral load in the phase after peak viremia (12). It remains to be determined whether the different effects attributed to CD4+ T cells are due to differences in the quality of CD4+ T cell response. Alternatively, the same virus-specific CD4+ T cells might increase susceptibility to acquisition of infection if present at the time of exposure and contribute to control of virus replication once infection has been established.
ACKNOWLEDGMENTS
This work was supported by grants from the Wilhelm Sander Foundation (2004.107.2), the European Commission FP6 program (DEC-VAC, LSHP-CT-2005-018685, and EUROPRISE, LSHP-CT 2006-037611), the German Research Foundation (TRR60/1), and the H. W. & J. Hector Foundation. The SIV peptides (EVA7066,1-72, SIV gag overlapping peptides) were obtained from the Centre for AIDS Reagents, NIBSC HPA UK, supported by the EC FP6/7 Europrise Network of Excellence, and NGIN consortia and the Bill and Melinda Gates GHRC-CAVD Project. The AT-2 SIV was kindly provided by Jeffrey Lifson through the EU Programme EVA Centre for AIDS Reagents, NIBSC, UK (AVIP contract number LSHP-CT-2004-503487).
A.M.S. is the chief executive officer of Oncovir, Inc., which provided the poly-ICLC for the study. He was not involved in the acquisition and analysis of the data. We declare that we have no other competing interests.
Footnotes
Published ahead of print 18 July 2012
REFERENCES
- 1. Benlahrech A, et al. 2009. Adenovirus vector vaccination induces expansion of memory CD4 T cells with a mucosal homing phenotype that are readily susceptible to HIV-1. Proc. Natl. Acad. Sci. U. S. A. 106:19940–19945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buchbinder SP, et al. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the STEP study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Douek DC, et al. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95–98 [DOI] [PubMed] [Google Scholar]
- 4. Flynn BJ, et al. 2011. Immunization with HIV Gag targeted to dendritic cells followed by recombinant New York vaccinia virus induces robust T-cell immunity in nonhuman primates. Proc. Natl. Acad. Sci. U. S. A. 108:7131–7136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flynn NM, et al. 2005. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 191:654–665 [DOI] [PubMed] [Google Scholar]
- 6. Keele BF, et al. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Masek-Hammerman K, et al. 2010. Mucosal trafficking of vector-specific CD4+ T lymphocytes following vaccination of rhesus monkeys with adenovirus serotype 5. J. Virol. 84:9810–9816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McElrath MJ, et al. 2008. HIV-1 vaccine-induced immunity in the test-of-concept STEP study: a case-cohort analysis. Lancet 372:1894–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neildez O, et al. 1998. Variation in virological parameters and antibody responses in macaques after atraumatic vaginal exposure to a pathogenic primary isolate of SIVmac251. Res. Virol. 149:53–68 [DOI] [PubMed] [Google Scholar]
- 10. O'Brien KL, et al. 2009. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat. Med. 15:873–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ochieng W, et al. 2009. Susceptibility to simian immunodeficiency virus ex vivo predicts outcome of a prime-boost vaccine after SIVmac239 challenge. J. Acquir. Immune Defic. Syndr. 52:162–169 [DOI] [PubMed] [Google Scholar]
- 12. Ortiz AM, et al. 2011. Depletion of CD4+ T cells abrogates post-peak decline of viremia in SIV-infected rhesus macaques. J. Clin. Invest. 121:4433–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pitisuttithum P, et al. 2006. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 194:1661–1671 [DOI] [PubMed] [Google Scholar]
- 14. Qureshi H, et al. 2012. Low-dose penile SIVmac251 exposure of rhesus macaques infected with adenovirus type 5 (Ad5) and then immunized with a replication-defective Ad5-based SIV gag/pol/nef vaccine recapitulates the results of the phase IIb STEP trial of a similar HIV-1 vaccine. J. Virol. 86:2239–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rerks-Ngarm S, et al. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 16. Sauermann U, et al. 2008. Mhc class I haplotypes associated with survival time in simian immunodeficiency virus (SIV)-infected rhesus macaques. Genes Immun. 9:69–80 [DOI] [PubMed] [Google Scholar]
- 17. Schulte R, et al. 2009. Mucosal prior to systemic application of recombinant adenovirus boosting is more immunogenic than systemic application twice but confers similar protection against SIV-challenge in DNA vaccine-primed macaques. Virology 383:300–309 [DOI] [PubMed] [Google Scholar]
- 18. Schultheiss T, Schulte R, Sauermann U, Ibing W, Stahl-Hennig C. 2011. Strong mucosal immune responses in SIV infected macaques contribute to viral control and preserved CD4+ T-cell levels in blood and mucosal tissues. Retrovirology 8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sekaly R-P. 2008. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J. Exp. Med. 205:7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soghoian DZ, et al. 2012. HIV-specific cytolytic CD4 T cell responses during acute HIV infection predict disease outcome. Sci. Transl. Med 4:123ra25 doi:10.1126/scitranslmed.3003165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stahl-Hennig C, et al. 2009. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog. 5:e1000373 doi:10.1371/journal.ppat.1000373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Staprans SI, et al. 2004. Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein. Proc. Natl. Acad. Sci. U. S. A. 101:13026–13031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tenbusch M, et al. 2012. Immunogenicity of DNA vaccines encoding dendritic cell targeted simian immunodeficiency virus antigen in rhesus macaques. PLoS One 7:e39038 doi:10.1371/journal.pone.0039038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Überla K. 2008. HIV vaccine development in the aftermath of the STEP study: re-focus on occult HIV infection? PLoS Pathog. 4:e1000114 doi:10.1371/journal.ppat.1000114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Veazey RS, et al. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 74:57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wagner R, et al. 2000. Rev-independent expression of synthetic gag-pol genes of human immunodeficiency virus type. Hum. Gene Ther. 11:2403–2413 [DOI] [PubMed] [Google Scholar]