Abstract
Regulatory T cells (Tregs) play a pivotal role in the maintenance of tolerance as well as in the control of immune activation, particularly during chronic infections. In the setting of HIV infection, the majority of studies have reported an increase in Treg frequency but a decrease in absolute number in all immune compartments of HIV-infected individuals. Several nonexclusive mechanisms have been postulated to explain this preferential Treg accumulation, including peripheral survival, increased proliferation, increased peripheral conversion, and tissue redistribution. The role played by Tregs during HIV infection is still poorly understood, as two opposing hypotheses have been proposed. A detrimental role of Tregs during HIV infection was suggested based on the evidence that Tregs suppress virus-specific immune responses. Conversely, Tregs could be beneficial by limiting immune activation, thus controlling the availability of HIV targets as well as preventing immune-based pathologies. Despite the technical difficulties, getting a better understanding of the mechanisms regulating Treg dynamics remains important, as it will help determine whether we can successfully manipulate Treg function or number to the advantage of the infected host. The aim of this review is thus to discuss the recent findings on Treg homeostasis and function in the setting of HIV infection.
INTRODUCTION
Human immunodeficiency virus (HIV) causes a gradual loss of immune competence, leading to AIDS. HIV-associated defects in cell-mediated immunity (CMI) are of particular importance, as these impairments lead to poor control of HIV replication and of other pathogens whose clearance depends on CMI. Importantly, a number of immune deficits caused by HIV infection can be partially restored in vitro, suggesting the existence of active regulatory mechanisms. Several mechanisms are involved in the regulation of the immune system; among these, regulatory T cells (Tregs), a subset of CD4+ T cells, play a pivotal role that has been recognized for several years. In addition to their role in controlling peripheral tolerance, Tregs promote the establishment of multiple persistent infections (viral, bacterial, parasitic, and fungal) (reviewed in reference 10). In most studies, Treg frequency was found to be substantially increased during the chronic phase of HIV infection (4, 7, 12, 19, 28, 63, 65, 71, 80, 82, 96). Thus, Tregs may contribute substantially to inefficient CMI during HIV infection. However, Tregs may also play a beneficial role, as their role in controlling immune activation may limit cellular targets available for HIV infection, as well as decrease the pathology associated with immune activation in HIV infection. To date, the mechanism(s) underlying the relative accumulation of Tregs during HIV/simian immunodeficiency virus (SIV) infection is poorly understood, and its role in the setting of HIV infection is still under debate. The aim of this review is to discuss the recent findings on Treg origin and function in the setting of HIV infection.
Treg PHENOTYPE AND SUPPRESSIVE MECHANISMS
Tregs are a subset of CD4+ T cells, constituting approximately 1 to 10% of circulating CD4+ T cells (6). Initially characterized in the mouse by the constitutive expression of the interleukin-2Rα (IL-2Rα) chain (CD25) (76), Tregs were shown in further studies to express low levels of the IL-7Rα chain (CD127) (52, 81). In 2003, Fontenot and colleagues reported that the transcriptional factor forkhead box P3 (FOXP3) plays a crucial role in Treg differentiation, function, and biology in both mice and humans (32). Lack of functional FOXP3 is associated with the development of immunodysregulation polyendocrinopathy enteropathy X-linked syndrome (IPEX), a severe autoimmune disease that leads to death in the first year of life in humans (11, 20, 97). Additional markers are frequently associated with human Treg function, including cytotoxic-T-lymphocyte-associated antigen (CTLA-4) (74), L-selectin (CD62L) (31), αE-integrin (CD103) (15, 79), the glucocorticoid-induced tumor necrosis factor receptor (GITR) (84), and the glycoprotein A repetitions predominant (GARP) receptor (91, 95). However, none of the above-mentioned markers (including FOXP3) are exclusively expressed by Tregs, as they are also transiently upregulated in activated effector T cells, making identification of Tregs difficult in the setting of chronic immune activation induced by uncontrolled infections (34, 37, 77, 84).
Deficits in Treg number and function are frequently described for autoimmune and inflammatory diseases (reviewed in references 53 and 57). Moreover, Tregs regulate immune activation in the setting of chronic infections, including HIV infection (10, 49, 75). In addition to the so-called “natural” Tregs that arise and mature in the thymus, a growing body of evidence demonstrates that human Tregs can be induced in the periphery from both naïve (23) and memory (43) conventional CD4+ T cells (the so-called “induced” Tregs).
Tregs can suppress immune activation through a vast array of mechanisms, which include both contact-independent and -dependent mechanisms (94). Contact-dependent mechanisms are critical in vitro, since physical separation between Tregs and conventional T cells abrogates Treg suppression (70). Within these contact-dependent mechanisms, CTLA-4 is a major mediator of suppression (61, 85). Recently, Tregs were also shown to inhibit activation of conventional T cells by mechanisms involving cyclic AMP (cAMP), a known inhibitor of T cell growth, differentiation, and proliferation (reviewed in reference 94). Additionally, the ectonucleotidases CD39 and CD73 are expressed on human Tregs, and their activity contributes to Treg suppressive functions (24, 45). Transforming growth factor β (TGF-β), IL-10, and IL-35 are the cytokines most often described as involved in Treg function (reviewed in reference 94), although the role of IL-35 in human Tregs remains controversial (8, 21). Additionally, Treg suppressive activity can be exerted by interaction with antigen-presenting cells that in turn lead to the production of indoleamine 2,3-dioxygenase (IDO), a key immunomodulatory enzyme that promotes peripheral immune tolerance by inhibiting T cell activation and proliferation through tryptophan catabolism (reviewed in reference 41). However, whether all these mechanisms act individually or synergistically, as well as whether their efficacy is different in different compartments, is not yet clear.
RELATIVE ACCUMULATION OF Tregs DURING HIV/SIV INFECTION
Several studies have shown that the frequency of Treg increased in patients with chronic progressive HIV infection in the circulating blood (12, 19, 64, 71, 80, 96) as well as in lymphoid tissues (gut, tonsils, and rectal mucosa) and the thymus of HIV-infected individuals (4, 7, 28, 65, 82). However, it is important to note that, in these patients, the absolute numbers of circulating Tregs were reduced (4, 12, 26, 36, 40, 44, 71, 80, 86, 89, 93), suggesting a relative resistance of Tregs to the cell-depleting effects of HIV infection. This relative increase in Treg frequency is controlled to a certain extent by suppressive highly active antiretroviral therapy (HAART) (28, 71, 96), although two studies reported opposite results (19, 63). The majority of studies with patients who were HIV-infected long-term nonprogressors (LTNP) or elite controllers reported decreases in Treg frequencies in peripheral blood (68, 80, 82) and rectal mucosa (82) in these individuals compared to that in progressors. However, Chase et al. observed an increase in the proportion of FOXP3+ cells in the peripheral blood of elite-controller subjects compared to that in HAART-treated patients (19), but a different gating strategy used to define Tregs could explain the latter finding. Importantly, LTNP patients exhibit absolute numbers of Tregs similar to those of progressors but lower numbers than healthy controls (80, 82). As expected, absolute numbers of Tregs are similar in elite controllers and healthy controls (80).
Little is known about Treg accumulation during the acute phase. Ndhlovu et al. reported an expansion of CD4+ CD127lo FOXP3+ T cells during the acute phase (62), and the levels of these cells inversely correlated with CD8 activation. Kared et al. reported that CD4+ CD25hi T cell frequency was lower during primary HIV infection than in chronically infected patients but did not compare these patients to healthy subjects (41). Using the African green monkey model of nonpathogenic infection, Kornfeld et al. observed a very early (24 h postinfection) and strong induction of FOXP3 expression, which correlated with increased levels of CD4+ CD25+ T cells in the blood (46). In the pathogenic model of SIV infection of rhesus macaques, two groups showed relative accumulations of Tregs in the first 2 weeks postinfection in both mucosal tissues and peripheral lymph nodes (2, 38). However, important differences exist depending on the model employed, as a rapid loss of Tregs was found in the highly pathogenic model of infection of pigtailed macaques with immunosuppressive SIV (18, 73), suggesting that the effect of HIV/SIV on Treg frequency is dependent on multiple factors.
POTENTIAL MECHANISMS THAT EXPLAIN SELECTIVE ACCUMULATION OF Tregs DURING HIV INFECTION
(i) Preferential survival.
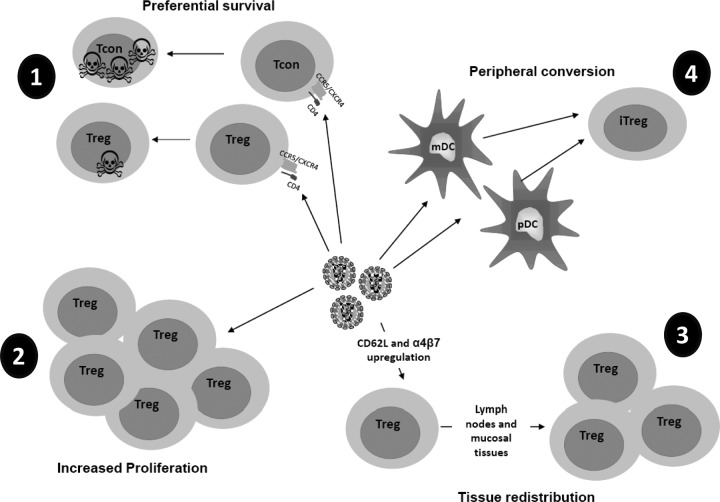
Tregs are susceptible to HIV infection both in vitro (33, 60, 66, 67) and in vivo (18, 25). However, mucosal Tregs appear less susceptible to productive SIV infection than non-Tregs and could thus be selectively spared from SIV/HIV-mediated cell death (2). In agreement with this hypothesis, exposure of Tregs to HIV selectively promoted their survival via a CD4-gp120-dependent pathway (9, 65). Of note, HIV DNA harboring cells appeared more abundant in the Treg subset than in non-Tregs in patients on prolonged HAART (92). Similar results were found in mucosal Tregs from SIV-infected macaques (2), suggesting that Tregs could constitute a viral reservoir. The different mechanisms of Treg accumulation are summarized in Fig. 1.
Fig 1.
Potential mechanisms that may explain selective accumulation of Tregs during HIV infection. Treg accumulation could be due to different, as well as synergistic, mechanisms. (1) Preferential survival. HIV infects both Tregs and conventional CD4+ T cells (Tcon), but Tregs could be less susceptible to cell death than Tconvs. (2) Increased proliferation. HIV could increase the expansion of Tregs by inducing these cells to enter into the cell cycle. (3) Tissue redistribution. HIV infection could induce upregulation of molecules, such as CD62L and integrin-α4β7, that are involved in T cell chemotaxis, leading the migration of Tregs from the blood to the lymphoid tissues, where these cells accumulate. (4) Peripheral conversion. Interaction of HIV-exposed DCs with non-Tregs could lead to conversion into induced Tregs (iTreg).
(ii) Increased proliferation.
Treg expansion under circumstances of antigen persistence has been described for infections with other pathogens, such as Helicobacter pylori (54), Leishmania major (10), and hepatitis C virus (87). Increased expression of Ki67, a marker of the cell cycle, was found in mucosal Tregs after SIV infection, suggesting that local expansion of Tregs could be an underlying mechanism of increased Treg frequency in lymphoid tissues (2). In agreement with this hypothesis, we and other groups have reported increased Ki67 expression in circulating Tregs from untreated, chronically infected patients. This increase was controlled by HAART (12, 50, 71, 98). Of note, a direct effect of HIV on Treg expansion has been hypothesized, as direct interaction of HIV with Tregs induced their expansion and promoted the expression of FOXP3, CD25, and CTLA-4 (3). Accordingly, our lab and others observed that in vitro exposure of CD4+ T cells to HIV increased Treg frequency (65; also unpublished data).
(iii) Tissue redistribution.
Some researchers have hypothesized that redistribution of Tregs from blood to lymphoid tissues could explain the increased frequencies in tissues. Ji and Cloyd showed that HIV-1 binding induced the upregulated expression of the homing receptors CD62L and integrin-α4β7 by CD4+ CD25+ Tregs, which in turn could result in more rapid Treg migration to peripheral and mucosal lymphoid tissues where HIV replication occurs (38). In agreement with this hypothesis, Treg prevalence correlated better with viral load in tissues than with plasma viremia (14). However, using characterization of Tregs by flow cytometry, we and others have also found increased Treg frequencies in the peripheral blood of infected subjects compared to those in the peripheral blood of uninfected subjects (12, 19, 63, 71, 80, 96), which does not strongly support increased Treg homing as a mechanism for their accumulation in tissue.
(iv) Increased peripheral conversion.
Several groups reported data suggesting increased peripheral conversion driven by HIV/SIV infection. First, HIV-exposed plasmacytoid dendritic cells (pDCs) convert allogeneic non-Tregs into CD4+ CD25+ FOXP3+ Tregs, while unexposed pDCs do not (56). Second, lymph node DCs from untreated HIV-infected subjects induced a phenotype of Tregs in normal allogeneic T cells, an ability that was lost after antiretroviral therapy (ART) (47). Third, our lab recently demonstrated that tissue myeloid dendritic cells (mDCs) from chronically SIV-infected rhesus macaques were more efficient at inducing expression of CD25 and FOXP3 in autologous FOXP3− CD25− CD4+ T cells than mDCs of uninfected macaques (72). However, the contribution of mDC-mediated Treg conversion to the increased frequency of Tregs may depend on the stage of infection and/or the body compartment, as we recently found that blood mDCs infected in vitro with HIV were not able to convert non-Tregs into Tregs (P. Presicce, submitted for publication).
Mechanisms that could be involved in increased DC-mediated conversion during HIV infection are not well characterized. HIV-exposed pDCs acted through IDO (56), and IDO expression is increased in many organs of SIV-infected rhesus macaques with high viral loads compared to that in macaques with low viral loads (14). Another molecule involved in Treg conversion is TGF-β (reviewed in reference 83). TGF-β levels are high during HIV infection (4, 30) and could thus promote increased conversion. Interestingly, although CD103 mDC+ has been reported to be critical for Treg conversion in gut-associated lymphoid tissue (GALT) (22, 88), our recent data showed that the increased capacity of mDCs from SIV-infected macaques to induce FOXP3 was not directly associated with the presence of CD103+ DCs, suggesting that other molecules may be involved in this conversion (72). Potential candidates are PD-L1, whose expression is increased on mDCs during HIV/SIV infection (13), and IDO, also increased in HIV-exposed mDCs (78).
THE DARK SIDE OF Tregs IN HIV INFECTION: THE SUPPRESSION OF HIV-SPECIFIC EFFECTOR RESPONSES
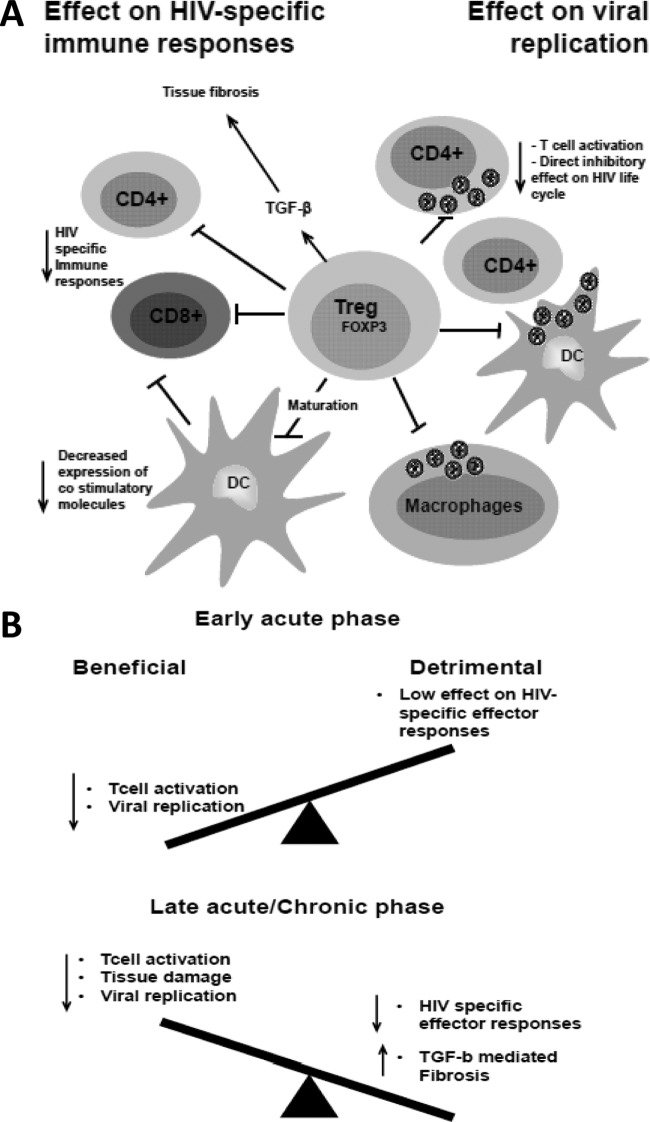
Ex vivo depletion of Tregs from peripheral blood mononuclear cells (PBMCs) or lymphoid cell suspensions enhances T cell responses to HIV or SIV antigens (1, 35, 41, 44, 63, 96). (Fig. 2). In addition, several correlative studies suggest that Tregs dampen HIV/SIV responses. During the acute phase of pathogenic SIV infection, increased Treg frequency correlated with inefficient SIV-specific CD8+ responses (29). Furthermore, a high-perforin-to-FOXP3 ratio was associated with nonprogressive disease, suggesting that the immune control of virus replication represents a balance between CMI and Treg-mediated counterregulation of such responses (65). Confirming this hypothesis, an important study recently showed that HIV-specific CD8+ T cells bearing protective HLA alleles (HLA B*27 and B*57) are able to evade Treg suppression (27), suggesting that Treg suppressive activity is a major mechanism that impairs development of protective effector responses.
Fig 2.
Potential role of Tregs during HIV infection. (A) Increased Treg frequency during HIV infection can lead directly to decreased T cell activation, thus decreasing the availability of target cells for HIV replication. Tregs can also decrease the capacity of DCs to transmit the virus to CD4+ T cells as well as decrease viral replication in macrophages. Importantly, Tregs can decrease viral replication by directly affecting the HIV replication cycle. On the other hand, Tregs can decrease CD4 and CD8 HIV-specific responses either directly or indirectly by decreasing the maturation of DCs, thus impairing DC capacity to induce immune responses. Tregs also contribute to immune dysfunction by secreting large amounts of TGF-β, thus contributing to lymphoid tissue fibrosis. (B) Based on the literature, a potential model for Tregs' role is that they may have an overall beneficial effect during early acute HIV infection, before HIV-specific immune responses are fully activated, by controlling T cell activation and decreasing the availability of target cells for HIV replication and transmission. In contrast, during the late acute and chronic phases, increased Treg frequency may have an overall-negative role, as the Treg suppressive effect on HIV antiviral immune responses predominates.
All the above-mentioned data also suggest that Tregs retain their suppressive function during HIV infection. This is in agreement with in vitro data showing that HIV- or gp120-exposed Tregs have maintained, or even enhanced, suppressive activity (9, 60). Interestingly, Thorborn et al. demonstrated increased sensitivity of effector cells to Treg-mediated suppression in asymptomatic HIV-1-infected patients (90). Recently, Angin et al. observed that the suppressive function of purified Tregs (from the blood or GALT) from chronically infected subjects was as strong as that of purified Tregs from elite controllers or uninfected controls (5).
Tregs could decrease HIV-specific responses via several mechanisms. The role of CTLA-4 is suggested by the fact that in vivo CTLA-4 blockade increased CD4+ and CD8+ T cell effector function during the chronic phase when given in combination with ART (35). However, the results of this study are not easy to interpret, as anti-CTLA-4 antibody does not specifically act on Tregs. Furthermore, the same antibody did not increase effector immune responses during the acute phase (17). Recently, we and others found an upregulation of CD39 expression by Tregs of HIV-infected subjects (63, 71, 80). Blockade of CD39 relieved, although not completely, the suppressive effect of Tregs on effector T cells (63). Of note, a CD39 gene polymorphism that leads to low levels of CD39 expression is associated with relative protection against the development of AIDS (63), although the contribution of low CD39 expression levels specifically by Tregs in this resistance is not clearly established, as CD39 is also expressed by other cells, such as B cells and platelets.
Another detrimental facet of Treg biology is their involvement in the exacerbated collagen deposition within T cell zones that destroys the lymphatic tissue architecture and hampers CD4 reconstitution in the lymphoid tissues of infected hosts. Estes et al. showed that Tregs were a major source of TGF-β in SIV-infected rhesus macaques (30) and thus contributed heavily to the development of fibrosis in these animals.
THE BRIGHT SIDE OF Tregs IN HIV INFECTION: THE ANTIVIRAL EFFECT
In contrast to the studies reviewed above, Tregs have been associated with a favorable outcome of HIV infection (Fig. 2). The observation that Treg frequency is higher in mucosal tissues than in the peripheral blood of untreated HIV-infected individuals suggests that Tregs could reduce the availability of HIV target cells in these tissues (28, 65). Several experimental observations support this hypothesis. The timing of Treg accumulation could play a crucial role in the early events of HIV infection. Tregs accumulate in both nonpathogenic and pathogenic SIV infections. However, Treg accumulation in the nonpathogenic model occurred within 24 h postinfection, while this accumulation was not observed before 2 weeks after infection in the pathogenic model (46, 55). Conversely, early interference with the Treg suppressive function using an anti-CTLA antibody worsened infection in rhesus macaques (17). Importantly, low Treg frequency during chronic HIV infection is associated with increased immune activation (26, 67). Interestingly, chronically infected individuals with strong Treg function in vitro had significantly lower levels of plasma viremia than individuals with weak Treg function (44). Finally, data from exposed uninfected cohorts also suggest that increased Treg frequency correlates with decreased T cell activation and decreased HIV infection (16, 48).
In vitro, Tregs were shown to directly control HIV infection in susceptible targets, such as macrophages infected by a pseudotyped HIV (51) or infected conventional T cells (58), thus providing a potential mechanism for the correlative studies described above. In particular, we showed that cAMP was critical for this antiviral effect, acting either by direct transfer of cAMP through gap junctions or by the extracellular pathway, which involves CD39 activity (58). Notably, our data also suggest that Tregs may restrain HIV infection and replication at several levels, including mechanisms independent of their effect on CD4+ proliferation. As cAMP can restrain HIV replication at both pre- and postintegration levels (reviewed in reference 59), Tregs may control HIV replication in T cells through direct interference with one or several steps of the HIV life cycle.
In vivo studies directly evaluating the role of Tregs in HIV/SIV infection are scarce. To date, only one paper describes the effect of denileukin diftitox (Ontak), a fusion protein that combines a recombinant IL-2 and a cytocidal diphtheria toxin moiety that depletes CD25+ cells in SIV-infected African green monkeys. Animals treated during the chronic phase showed significantly higher levels of immune activation and a significant increase in viral replication compared to control animals (69), suggesting that Tregs can control virus infection in vivo. However, it is important to note that the data from this study are difficult to interpret, as the frequency of FOXP3+ cells was not significantly decreased in the denileukin diftitox-treated animals, suggesting that denileukin diftitox may have acted through another mechanism. Notably, the higher level of immune activation induced by denileukin diftitox treatment may have increased the numbers of available cellular targets for HIV infection. In another study, denileukin diftitox treatment of humanized Rag2−/− gammaC−/− mice infected with a highly pathogenic HIV isolate reduced levels of productively infected cells in lymphoid organs. However, these results are difficult to generalize, as FOXP3+ Tregs are preferentially infected and depleted in this model (39).
Interestingly, in addition to their effect on viral replication, Tregs may protect the host against immune-mediated damage. This aspect of Treg biology is not well studied but is suggested by one study in which Tregs were shown to attenuate HIV-associated neurodegeneration (51).
CONCLUSION
In recent years, our knowledge of Treg biology during HIV infection has progressed considerably. Taken together, data from multiple studies suggest that Tregs act as a double-edged sword during HIV infection. A Treg-mediated decrease in HIV infection could play a beneficial role during early HIV infection, when effector immune cells are not yet activated, suggesting that the Treg antiviral effect during acute infection could be beneficial to the host, as the earliest events in acute infection affect the subsequent viral set point and predict disease progression (55). During the chronic phase, Tregs appear to maintain some protective role by limiting HIV infection; however, the overall “antiviral” effect of Tregs during the chronic phase may be offset by their dampening effect on HIV/SIV-specific CD8 effector cells, as well as their production of fibrosis-inducing TGF-β1. Further in vivo studies will be needed to ascertain the role of Tregs during the different phases of infection, but the paucity of reagents suitable for Treg depletion in vivo makes these studies difficult to perform. Further studies are also needed to elucidate Treg origin in the setting of HIV/SIV infections. Despite the technical difficulties, gaining a better understanding of the mechanisms regulating Treg dynamics and their role during HIV infection remains an important question, as this knowledge will help in determining whether we can successfully manipulate Treg function or number to the advantage of the infected host.
ACKNOWLEDGMENTS
This work was supported by NIH RO1 grant AI068524.
We thank Barbara Shacklett and Gene Shearer for reviews of the manuscript.
Footnotes
Published ahead of print 18 July 2012
REFERENCES
- 1. Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. 2004. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 78:2454–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allers K, et al. 2010. Gut mucosal FOXP3+ regulatory CD4+ T cells and nonregulatory CD4+ T cells are differentially affected by simian immunodeficiency virus infection in rhesus macaques. J. Virol. 84:3259–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amarnath S, Dong L, Li J, Wu Y, Chen W. 2007. Endogenous TGF-β activation by reactive oxygen species is key to Foxp3 induction in TCR-stimulated and HIV-1-infected human CD4+CD25− T cells. Retrovirology 4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andersson J, et al. 2005. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J. Immunol. 174:3143–3147 [DOI] [PubMed] [Google Scholar]
- 5. Angin M, et al. 2012. Preserved function of regulatory T cells in chronic HIV-1 infection despite decreased numbers in blood and tissue. J. Infect. Dis. 205:1495–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245–1253 [DOI] [PubMed] [Google Scholar]
- 7. Bandera A, et al. 2010. CD4+ T cell depletion, immune activation and increased production of regulatory T cells in the thymus of HIV-infected individuals. PLoS One 5:e10788 doi:10.1371/journal.pone.0010788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bardel E, Larousserie F, Charlot-Rabiega P, Coulomb-L'Hermine A, Devergne O. 2008. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J. Immunol. 181:6898–6905 [DOI] [PubMed] [Google Scholar]
- 9. Becker C, et al. 2009. Protection from graft-versus-host disease by HIV-1 envelope protein gp120-mediated activation of human CD4+CD25+ regulatory T cells. Blood 114:1263–1269 [DOI] [PubMed] [Google Scholar]
- 10. Belkaid Y, Rouse BT. 2005. Natural regulatory T cells in infectious disease. Nat. Immunol. 6:353–360 [DOI] [PubMed] [Google Scholar]
- 11. Bennett R. 2001. Antenatal genetic testing and the right to remain in ignorance. Theor. Med. Bioeth. 22:461–471 [DOI] [PubMed] [Google Scholar]
- 12. Bi X, Suzuki Y, Gatanaga H, Oka S. 2009. High frequency and proliferation of CD4+ FOXP3+ Treg in HIV-1-infected patients with low CD4 counts. Eur. J. Immunol. 39:301–309 [DOI] [PubMed] [Google Scholar]
- 13. Boasso A, et al. 2008. PDL-1 upregulation on monocytes and T cells by HIV via type I interferon: restricted expression of type I interferon receptor by CCR5-expressing leukocytes. Clin. Immunol. 129:132–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boasso A, et al. 2007. Regulatory T-cell markers, indoleamine 2,3-dioxygenase, and virus levels in spleen and gut during progressive simian immunodeficiency virus infection. J. Virol. 81:11593–11603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Campbell DJ, Ziegler SF. 2007. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat. Rev. 7:305–310 [DOI] [PubMed] [Google Scholar]
- 16. Card CM, et al. 2009. Decreased immune activation in resistance to HIV-1 infection is associated with an elevated frequency of CD4+CD25+FOXP3+ regulatory T cells. J. Infect. Dis. 199:1318–1322 [DOI] [PubMed] [Google Scholar]
- 17. Cecchinato V, et al. 2008. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J. Immunol. 180:5439–5447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chase AJ, et al. 2007. Severe depletion of CD4+ CD25+ regulatory T cells from the intestinal lamina propria but not peripheral blood or lymph nodes during acute simian immunodeficiency virus infection. J. Virol. 81:12748–12757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chase AJ, Yang HC, Zhang H, Blankson JN, Siliciano RF. 2008. Preservation of FoxP3+ regulatory T cells in the peripheral blood of human immunodeficiency virus type 1-infected elite suppressors correlates with low CD4+ T-cell activation. J. Virol. 82:8307–8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chatila TA, et al. 2000. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J. Clin. Invest. 106:R75–R81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chaturvedi V, Collison LW, Guy CS, Workman CJ, Vignali DA. 2011. Cutting edge: human regulatory T cells require IL-35 to mediate suppression and infectious tolerance. J. Immunol. 186:6661–6666 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Coombes JL, et al. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β- and retinoic acid-dependent mechanism. J. Exp. Med. 204:1757–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Curotto de Lafaille MA, Lafaille JJ. 2009. Natural and adaptive Foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 30:626–635 [DOI] [PubMed] [Google Scholar]
- 24. Deaglio S, et al. 2007. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204:1257–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dunham RM, et al. 2008. CD127 and CD25 expression defines CD4+ T cell subsets that are differentially depleted during HIV infection. J. Immunol. 180:5582–5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eggena MP, et al. 2005. Depletion of regulatory T cells in HIV infection is associated with immune activation. J. Immunol. 174:4407–4414 [DOI] [PubMed] [Google Scholar]
- 27. Elahi S, et al. 2011. Protective HIV-specific CD8+ T cells evade Treg cell suppression. Nat. Med. 17:989–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Epple HJ, et al. 2006. Mucosal but not peripheral FOXP3+ regulatory T cells are highly increased in untreated HIV infection and normalize after suppressive HAART. Blood 108:3072–3078 [DOI] [PubMed] [Google Scholar]
- 29. Estes JD, et al. 2006. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J. Infect. Dis. 193:703–712 [DOI] [PubMed] [Google Scholar]
- 30. Estes JD, et al. 2007. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J. Infect. Dis. 195:551–561 [DOI] [PubMed] [Google Scholar]
- 31. Fisson S, et al. 2003. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J. Exp. Med. 198:737–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fontenot JD, Gavin MA, Rudensky AY. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336 [DOI] [PubMed] [Google Scholar]
- 33. Holmes D, Knudsen G, Mackey-Cushman S, Su L. 2007. FoxP3 enhances HIV-1 gene expression by modulating NFκB occupancy at the long terminal repeat in human T cells. J. Biol. Chem. 282:15973–15980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hori S, Nomura T, Sakaguchi S. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057–1061 [DOI] [PubMed] [Google Scholar]
- 35. Hryniewicz A, et al. 2006. CTLA-4 blockade decreases TGF-β, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood 108:3834–3842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hunt PW, et al. 2011. A low T regulatory cell response may contribute to both viral control and generalized immune activation in HIV controllers. PLoS One 6:e15924 doi:10.1371/journal.pone.0015924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jago CB, Yates J, Camara NO, Lechler RI, Lombardi G. 2004. Differential expression of CTLA-4 among T cell subsets. Clin. Exp. Immunol. 136:463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ji J, Cloyd MW. 2009. HIV-1 binding to CD4 on CD4+CD25+ regulatory T cells enhances their suppressive function and induces them to home to, and accumulate in, peripheral and mucosal lymphoid tissues: an additional mechanism of immunosuppression. Int. Immunol. 21:283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jiang Q, et al. 2008. FoxP3+CD4+ regulatory T cells play an important role in acute HIV-1 infection in humanized Rag2−/−γC−/− mice in vivo. Blood 112:2858–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiao Y, et al. 2009. The decrease of regulatory T cells correlates with excessive activation and apoptosis of CD8+ T cells in HIV-1-infected typical progressors, but not in long-term non-progressors. Immunology 128(Suppl 1):e366–e375 doi:10.1111/j.1365–2567.2008.02978.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kared H, et al. 2008. HIV-specific regulatory T cells are associated with higher CD4 cell counts in primary infection. AIDS 22:2451–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reference deleted.
- 43. Kim BS, et al. 2010. Conversion of Th2 memory cells into Foxp3+ regulatory T cells suppressing Th2-mediated allergic asthma. Proc. Natl. Acad. Sci. U. S. A. 107:8742–8747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kinter AL, et al. 2004. CD25+CD4+ regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4+ and CD8+ HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J. Exp. Med. 200:331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kobie JJ, et al. 2006. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J. Immunol. 177:6780–6786 [DOI] [PubMed] [Google Scholar]
- 46. Kornfeld C, et al. 2005. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J. Clin. Invest. 115:1082–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Krathwohl MD, Schacker TW, Anderson JL. 2006. Abnormal presence of semimature dendritic cells that induce regulatory T cells in HIV-infected subjects. J. Infect. Dis. 193:494–504 [DOI] [PubMed] [Google Scholar]
- 48. Legrand FA, et al. 2006. Strong HIV-1-specific T cell responses in HIV-1-exposed uninfected infants and neonates revealed after regulatory T cell removal. PLoS One 1:e102 doi:10.1371/journal.pone.0000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li L, Wu CY. 2008. CD4+ CD25+ Treg cells inhibit human memory γδ T cells to produce IFN-γ in response to M tuberculosis antigen ESAT-6. Blood 111:5629–5636 [DOI] [PubMed] [Google Scholar]
- 50. Lim A, French MA, Price P. 2009. CD4+ and CD8+ T cells expressing FoxP3 in HIV-infected patients are phenotypically distinct and influenced by disease severity and antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 51:248–257 [DOI] [PubMed] [Google Scholar]
- 51. Liu J, et al. 2009. Neuromodulatory activities of CD4+CD25+ regulatory T cells in a murine model of HIV-1-associated neurodegeneration. J. Immunol. 182:3855–3865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu W, et al. 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 203:1701–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Long SA, Buckner JH. 2011. CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J. Immunol. 187:2061–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lundgren A, et al. 2005. Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect. Immun. 73:523–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lyles RH, et al. 2000. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J. Infect. Dis. 181:872–880 [DOI] [PubMed] [Google Scholar]
- 56. Manches O, et al. 2008. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J. Clin. Invest. 118:3431–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Miyara M, et al. 2011. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun. Rev. 10:744–755 [DOI] [PubMed] [Google Scholar]
- 58. Moreno-Fernandez ME, Rueda CM, Rusie LK, Chougnet CA. 2011. Regulatory T cells control HIV replication in activated T cells through a cAMP-dependent mechanism. Blood 117:5372–5380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moreno-Fernandez ME, Rueda CM, Velilla PA, Rugeles MT, Chougnet CA. 2012. cAMP during HIV infection: friend or foe? AIDS Res. Hum. Retroviruses 28:49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Moreno-Fernandez ME, Zapata W, Blackard JT, Franchini G, Chougnet CA. 2009. Human regulatory T cells are targets for human immunodeficiency virus (HIV) infection, and their susceptibility differs depending on the HIV type 1 strain. J. Virol. 83:12925–12933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nakamura K, Kitani A, Strober W. 2001. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J. Exp. Med. 194:629–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ndhlovu LC, Loo CP, Spotts G, Nixon DF, Hecht FM. 2008. FOXP3 expressing CD127lo CD4+ T cells inversely correlate with CD38+ CD8+ T cell activation levels in primary HIV-1 infection. J. Leukoc. Biol. 83:254–262 [DOI] [PubMed] [Google Scholar]
- 63. Nikolova M, et al. 2011. CD39/adenosine pathway is involved in AIDS progression. PLoS Pathog. 7:e1002110 doi:10.1371/journal.ppat.1002110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nikolova M, Lelievre JD, Carriere M, Bensussan A, Levy Y. 2009. Regulatory T cells differentially modulate the maturation and apoptosis of human CD8+ T-cell subsets. Blood 113:4556–4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nilsson J, et al. 2006. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood 108:3808–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Oh U, et al. 2006. Reduced Foxp3 protein expression is associated with inflammatory disease during human T lymphotropic virus type 1 infection. J. Infect. Dis. 193:1557–1566 [DOI] [PubMed] [Google Scholar]
- 67. Oswald-Richter K, Grill SM, Leelawong M, Unutmaz D. 2004. HIV infection of primary human T cells is determined by tunable thresholds of T cell activation. Eur. J. Immunol. 34:1705–1714 [DOI] [PubMed] [Google Scholar]
- 68. Owen RE, et al. 2010. HIV+ elite controllers have low HIV-specific T-cell activation yet maintain strong, polyfunctional T-cell responses. AIDS 24:1095–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pandrea I, et al. 2008. Cutting edge: experimentally induced immune activation in natural hosts of simian immunodeficiency virus induces significant increases in viral replication and CD4+ T cell depletion. J. Immunol. 181:6687–6691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Piccirillo CA, et al. 2002. CD4+CD25+ regulatory T cells can mediate suppressor function in the absence of transforming growth factor β1 production and responsiveness. J. Exp. Med. 196:237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Presicce P, et al. 2011. Frequency of circulating regulatory T cells increases during chronic HIV infection and is largely controlled by highly active antiretroviral therapy. PLoS One 6:e28118 doi:10.1371/journal.pone.0028118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Presicce P, Shaw JM, Miller CJ, Shacklett BL, Chougnet CA. 2012. Myeloid dendritic cells isolated from tissues of SIV-infected rhesus macaques promote the induction of regulatory T cells. AIDS 26:263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Qin S, et al. 2008. Chemokine and cytokine mediated loss of regulatory T cells in lymph nodes during pathogenic simian immunodeficiency virus infection. J. Immunol. 180:5530–5536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Read S, Malmstrom V, Powrie F. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rouse BT, Suvas S. 2007. Regulatory T cells and immunity to pathogens. Expert Opin. Biol. Ther. 7:1301–1309 [DOI] [PubMed] [Google Scholar]
- 76. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164 [PubMed] [Google Scholar]
- 77. Salomon B, Bluestone JA. 2001. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 19:225–252 [DOI] [PubMed] [Google Scholar]
- 78. Samikkannu T, Rao KV, Gandhi N, Saxena SK, Nair MP. 2010. Human immunodeficiency virus type 1 clade B and C Tat differentially induce indoleamine 2,3-dioxygenase and serotonin in immature dendritic cells: implications for neuroAIDS. J. Neurovirol. 16:255–263 [DOI] [PubMed] [Google Scholar]
- 79. Sather BD, et al. 2007. Altering the distribution of Foxp3+ regulatory T cells results in tissue-specific inflammatory disease. J. Exp. Med. 204:1335–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schulze zur Wiesch J, et al. 2011. Comprehensive analysis of frequency and phenotype of T regulatory cells in HIV infection: CD39 expression of FoxP3+ T regulatory cells correlates with progressive disease. J. Virol. 85:1287–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Seddiki N, et al. 2006. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203:1693–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shaw JM, et al. 2011. Increased frequency of regulatory T cells accompanies increased immune activation in rectal mucosae of HIV-positive noncontrollers. J. Virol. 85:11422–11434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shevach EM, Tran DQ, Davidson TS, Andersson J. 2008. The critical contribution of TGF-β to the induction of Foxp3 expression and regulatory T cell function. Eur. J. Immunol. 38:915–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. 2002. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 3:135–142 [DOI] [PubMed] [Google Scholar]
- 85. Sojka DK, Hughson A, Fowell DJ. 2009. CTLA-4 is required by CD4+CD25+ Treg to control CD4+ T-cell lymphopenia-induced proliferation. Eur. J. Immunol. 39:1544–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Suchard MS, et al. 2010. FOXP3 expression is upregulated in CD4+ T cells in progressive HIV-1 infection and is a marker of disease severity. PLoS One 5:e11762 doi:10.1371/journal.pone.0011762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sugimoto K, et al. 2003. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology 38:1437–1448 [DOI] [PubMed] [Google Scholar]
- 88. Sun CM, et al. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204:1775–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Thorborn G, et al. 2010. Increased sensitivity of CD4+ T-effector cells to CD4+CD25+ Treg suppression compensates for reduced Treg number in asymptomatic HIV-1 infection. PLoS One 5:e9254 doi:10.1371/journal.pone.0009254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Thorborn GS, Pomeroy L, Ishohanni H, Peters BS, Vyakarnam A. 2012. Elevated effector cell sensitivity to Treg-cell suppression that is not associated with reduced Th17-cell expression distinguishes HIV+ asymptomatic subjects from progressors. Eur. J. Immunol. 42:138–146 [DOI] [PubMed] [Google Scholar]
- 91. Tran DQ, et al. 2009. GARP (LRRC32) is essential for the surface expression of latent TGF-β on platelets and activated FOXP3+ regulatory T cells. Proc. Natl. Acad. Sci. U. S. A. 106:13445–13450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tran TA, et al. 2008. Resting regulatory CD4 T cells: a site of HIV persistence in patients on long-term effective antiretroviral therapy. PLoS One 3:e3305 doi:10.1371/journal.pone.0003305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tsunemi S, et al. 2005. Relationship of CD4+CD25+ regulatory T cells to immune status in HIV-infected patients. AIDS 19:879–886 [DOI] [PubMed] [Google Scholar]
- 94. Vignali DA, Collison LW, Workman CJ. 2008. How regulatory T cells work. Nat. Rev. 8:523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang R, et al. 2009. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc. Natl. Acad. Sci. U. S. A. 106:13439–13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Weiss L, et al. 2004. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood 104:3249–3256 [DOI] [PubMed] [Google Scholar]
- 97. Wildin RS, et al. 2001. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 27:18–20 [DOI] [PubMed] [Google Scholar]
- 98. Xing S, et al. 2010. Increased turnover of FoxP3high regulatory T cells is associated with hyperactivation and disease progression of chronic HIV-1 infection. J. Acquir. Immune Defic. Syndr. 54:455–462 [DOI] [PubMed] [Google Scholar]