Abstract
Control of swine influenza A virus (IAV) in the United States is hindered because inactivated vaccines do not provide robust cross-protection against the multiple antigenic variants cocirculating in the field. Vaccine efficacy can be limited further for vaccines administered to young pigs that possess maternally derived immunity. We previously demonstrated that a recombinant A/sw/Texas/4199-2/1998 (TX98) (H3N2) virus expressing a truncated NS1 protein is attenuated in swine and has potential for use as an intranasal live attenuated influenza virus (LAIV) vaccine. In the present study, we compared 1 dose of intranasal LAIV with 2 intramuscular doses of TX98 whole inactivated virus (WIV) with adjuvant in weanling pigs with and without TX98-specific maternally derived antibodies (MDA). Pigs were subsequently challenged with wild-type homologous TX98 H3N2 virus or with an antigenic variant, A/sw/Colorado/23619/1999 (CO99) (H3N2). In the absence of MDA, both vaccines protected against homologous TX98 and heterologous CO99 shedding, although the LAIV elicited lower hemagglutination inhibition (HI) antibody titers in serum. The efficacy of both vaccines was reduced by the presence of MDA; however, WIV vaccination of MDA-positive pigs led to dramatically enhanced pneumonia following heterologous challenge, a phenomenon known as vaccine-associated enhanced respiratory disease (VAERD). A single dose of LAIV administered to MDA-positive pigs still provided partial protection from CO99 and may be a safer vaccine for young pigs under field conditions, where dams are routinely vaccinated and diverse IAV strains are in circulation. These results have implications not only for pigs but also for other influenza virus host species.
INTRODUCTION
The speed and complexity of swine influenza A virus (IAV) evolution have increased sharply since 1998, when a new reassortant lineage with the triple-reassortant internal gene (TRIG) constellation began to circulate and eventually predominate in the North American pig population (29). As a result, many antigenic variants continue to emerge and diminish the field efficacy of IAV vaccines (11, 16, 27). Fully licensed influenza vaccines for use in swine in North America and Europe consist of whole inactivated virus (WIV), which may not be an optimal form of antigen for inducing cross-reactive cellular and mucosal immunity against antigenic variants (12). Live attenuated influenza virus (LAIV) vaccines represent an approach that could potentially prime pigs for broader cross-protective immunity. The rational design of attenuated IAV vaccine strains by molecular engineering has been explored in recent studies (14, 18, 23). One method is truncation of the NS1 gene, which encodes an immune-modulating interferon antagonist (23, 24). It was previously shown that an H3N2 IAV with a truncated NS1 protein (NS1Δ126 TX98) replicated poorly in pigs after intranasal (i.n.) inoculation but elicited neutralizing serum antibodies as well as mucosal antibodies and provided robust protection against homologous challenge in naïve pigs given a single i.n. application (26). There was a comparable level of cross-protection against a serologically distinct H3N2 strain in NS1Δ126 TX98-vaccinated pigs, which was likely mediated in part by cross-reactive mucosal IgA. The vaccine offered less but still substantial protection against challenge with an H1N1 virus, to which the antibodies failed to cross-react. T-cell priming was not analyzed but may have contributed to heterologous and heterosubtypic protection. We hypothesize that a replicating attenuated virus such as NS1Δ126 TX98 delivered i.n. primes a more robust cellular and mucosal immunity than that induced by an inactivated virus vaccine delivered intramuscularly (i.m.), therefore providing greater cross-protection against variant strains.
A concern with inactivated adjuvanted IAV vaccines is the phenomenon of vaccine-associated enhanced respiratory disease (VAERD) (4, 5, 8, 25). This phenomenon is associated with the use of vaccines containing a virus of the same hemagglutinin subtype as the subsequent challenge strain, but with substantial antigenic drift. Our group recently described VAERD in association with the use of a vaccine containing a human-like delta cluster H1N2 antigen followed by challenge with the 2009 pandemic H1N1 virus (5). A consistent predisposing factor for VAERD is the presence of IgG antibodies that cross-react with the heterologous virus but lack the ability to neutralize infectivity. Distinguishing pathological features of VAERD include severe bronchointerstitial pneumonia with necrotizing bronchiolitis, interlobular and alveolar edema, and hemorrhage (4). These pulmonary changes are accompanied by a significant elevation of proinflammatory cytokines.
Another obstacle for efficacious vaccination of pigs against IAV is interference from maternally derived immunity (MDI), particularly maternally derived antibodies (MDA) acquired through colostrum. Provided that there are still sufficient antibody titers in the serum when pigs are infected, MDA can reduce clinical disease (21), but the passive antibodies are less effective at blocking viral shedding from the upper respiratory tract (2, 10), probably because the predominant antibody isotype received in colostrum is IgG. Pigs with significant IAV-specific MDA titers typically have suppressed adaptive antibody responses to homologous infection or vaccination (21). This interference affects IgM, IgG, and hemagglutination inhibition (HI) antibody titers in serum, as well as nasal IgA titers (10). T-cell proliferation assays have indicated that the cellular immune response to IAV is less susceptible to MDA inhibition (8, 10). Analyses of pig immune responses to pseudorabies virus, an alphaherpesvirus, have shown a similar pattern, where MDA blocks the humoral but not the cellular immune response following piglet vaccination or infection (19, 28). One perceived advantage of vaccination with LAIV is that circulating MDA (mainly IgG) are less likely to interfere with intranasally delivered antigen than with inactivated antigen delivered by a parenteral route. In the present study, we tested the immunogenicity and protective efficacy of the intranasal NS1Δ126 TX98 vaccine versus an inactivated, adjuvanted TX98 vaccine administered intramuscularly. These vaccine strategies were tested in naïve and MDA-positive weanling pigs that were subsequently challenged with homologous or heterologous strains of H3N2 IAV.
MATERIALS AND METHODS
Viruses and vaccine preparation.
Antigen for the WIV vaccine was A/sw/Texas/4199-2/1998 (wild-type TX98), grown in Madin-Darby canine kidney (MDCK) cells. Clarified virus from infected culture was inactivated by UV irradiation, using the “sterilize” setting in a UV cross-linking chamber (GS Gene Linker; Bio-Rad, Hercules, CA). Inactivation of the virus was confirmed by failure of the virus to replicate in 2 serial passages on MDCK cells. A commercial adjuvant (Emulsigen D; MVP Laboratories, Inc., Ralston, NE) was added at a 1:5 ratio. Each dose of WIV contained approximately 128 hemagglutination (HA) units of virus. The attenuated virus for the LAIV was generated via reverse genetics as previously described (24). The attenuated vaccine virus contained an NS1 gene with a 3′ premature termination plus the insertion of four stop codons in the three frames after, producing a protein of 126 amino acids with a carboxy-terminal truncation (NS1Δ126 TX98). The remaining seven gene segments were from wild-type TX98. The challenge viruses included wild-type TX98 (H3N2) and the heterologous virus A/sw/Colorado/23619/99 (H3N2) (CO99). The TX98 and CO99 viruses were shown previously to have limited serologic cross-reactivity (22). Vaccine and challenge viruses were grown in MDCK cells.
Experimental design.
Eight sows obtained from a high-health herd free of IAV and porcine reproductive and respiratory syndrome virus (PRRSV) were vaccinated with the TX98 WIV. Each vaccinated sow received 3 doses at 2-week intervals beginning midgestation. Six sows from the same high-health source were not vaccinated for IAV. All sows delivered their pigs without surgical intervention, and pigs suckled their own dams. Pigs were bled for evaluation of transfer of MDA at 1 week of age and were weaned at 2 weeks of age. They were treated with ceftiofur crystalline-free acid (Pfizer, New York, NY) at weaning to reduce respiratory bacterial contaminants. Pigs were demonstrated to be free of influenza virus by nasal swab sampling, and those born to nonvaccinated sows were shown by serum HI assay to be free of anti-influenza virus antibody prior to piglet vaccination.
To evaluate both vaccines given in the presence or absence of H3N2 IAV-specific MDA, 51 pigs with MDA were divided into 7 groups and 52 pigs without MDA were divided into 7 groups (Table 1). Pigs in the LAIV groups were vaccinated with 2 ml of NS1Δ126 TX98 at 1 × 106 50% tissue culture infective doses (TCID50) per ml by slowly dripping the vaccine into the nose. LAIV was administered once at weaning, at approximately 14 days of age. Pigs in the WIV groups were vaccinated intramuscularly with 1 ml of the formulation described above, at approximately 14 and 28 days of age. At 8 weeks of age, nonvaccinated pigs with MDA were determined to have HI titers below 1:40, indicating waning of MDA prior to challenge. Pigs in each challenge group were inoculated with 2 ml (1 × 105 TCID50/ml) of the indicated virus. Challenge viruses were given intratracheally while the pigs were anesthetized following an intramuscular injection of a cocktail of ketamine (8 mg/kg of body weight), xylazine (4 mg/kg), and tiletamine-zolazepam (Telazol; 6 mg/kg) (Fort Dodge Animal Health, Fort Dodge, IA). Challenge groups were housed in individual isolation rooms and cared for in compliance with the institutional animal care and use committee of the National Animal Disease Center.
Table 1.
Study design for comparison of LAIV and WIV vaccines
| Treatment group | MDA statusa | Piglet vaccine | Challenge strainb | n |
|---|---|---|---|---|
| MDA/NV/NC | + | None | Sham | 4 |
| MDA/NV/TX | + | None | wt TX98 | 8 |
| MDA/NV/CO | + | None | wt CO99 | 8 |
| MDA/WIV/TX | + | TX98 WIV | wt TX98 | 8 |
| MDA/WIV/CO | + | TX98 WIV | wt CO99 | 8 |
| MDA/LAIV/TX | + | TX98 LAIV | wt TX98 | 8 |
| MDA/LAIV/CO | + | TX98 LAIV | wt CO99 | 7 |
| No MDA/NV/NC | − | None | Sham | 4 |
| No MDA/NV/TX | − | None | wt TX98 | 8 |
| No MDA/NV/CO | − | None | wt CO99 | 8 |
| No MDA/WIV/TX | − | TX98 WIV | wt TX98 | 8 |
| No MDA/WIV/CO | − | TX98 WIV | wt CO99 | 8 |
| No MDA/LAIV/TX | − | TX98 LAIV | wt TX98 | 8 |
| No MDA/LAIV/CO | − | TX98 LAIV | wt CO99 | 8 |
MDA-positive pigs suckled sows which were previously vaccinated with three doses of TX98 WIV. MDA-negative pigs suckled sows which were not vaccinated against IAV.
wt, wild type.
Clinical observation and sampling.
To compare the efficacies of WIV and LAIV against infection with homologous and heterologous viruses, infected pigs were observed daily for clinical signs. Nasal swabs were taken at 0, 3, and 5 days postinfection (dpi), placed in 2 ml minimal essential medium (MEM), and frozen at −80°C until study completion. All animals were euthanized humanely at 5 dpi with a lethal dose of pentobarbital (Sleepaway; Fort Dodge Animal Health, Fort Dodge, IA). After euthanasia, each lung was subjected to lavage with 50 ml MEM to obtain bronchoalveolar lavage fluid (BALF). Nasal swab specimens were filtered (0.45 mm), and a 200-μl aliquot of each was plated onto confluent phosphate-buffered saline (PBS)-washed MDCK cells in 24-well plates. After 1 h of incubation at 37°C, 200 μl serum-free MEM supplemented with 1 μg/ml tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-trypsin and antibiotics was added to each well. All wells were evaluated for cytopathic effect (CPE) between 48 and 72 h. Tenfold serial dilutions in serum-free MEM supplemented with TPCK-trypsin and antibiotics were made with each BALF sample and virus isolation-positive nasal swab filtrate sample. Each dilution was plated in triplicate in a 100-μl volume onto PBS-washed confluent MDCK cells in 96-well plates. Plates were evaluated for CPE between 48 and 72 h postinfection. At 72 h, plates were fixed with 4% phosphate-buffered formalin and stained using immunocytochemistry with an anti-influenza A virus nucleoprotein monoclonal antibody as previously described (8). A TCID50 titer was calculated for each sample by use of the standard method (20).
Pathological examination of lungs.
At necropsy, lungs were removed and evaluated for the percentage of the lung affected with purple-red consolidation typical of IAV infection. The percentage of the surface affected with pneumonia was estimated visually for each lung lobe, and the total percentage for the entire lung was calculated based on the weighted proportion of each lobe relative to the total lung volume (6). Tissue samples from the trachea and right middle or affected lung lobe were fixed in 10% buffered formalin for histopathologic examination. Tissues were processed by routine histopathologic procedures, and slides were stained with hematoxylin and eosin. Microscopic lesions were evaluated by a veterinary pathologist blinded to treatment groups. Individual scores were assigned for each of three parameters: percentage of intrapulmonary airways demonstrating epithelial necrosis or proliferation, percentage of bronchi and bronchioles demonstrating peribronchiolar lymphocytic cuffing (PBLC), and magnitude of neutrophil exudation in bronchioles and alveoli. The intrapulmonary airway epithelium was scored as follows: 0.0, no significant lesions; 1.0, a few airways affected, with bronchiolar epithelial damage; 1.5, more than a few airways affected (up to 25%); 2.0, 50% of airways affected, often with interstitial pneumonia; 2.5, approximately 75% of airways affected, usually with significant interstitial pneumonia; and 3.0, more than 75% of airways affected, usually with significant interstitial pneumonia. Peribronchiolar lymphocytic cuffing was scored as follows: 0.0, no significant lesions; 1.0, a few airways with light PBLC; 1.5, more than a few airways with PBLC (up to 25%); 2.0, 50% of airways with PBLC; 2.5, approximately 75% of airways with PBLC; and 3.0, more than 75% of airways with PBLC. Neutrophil (polymorphonuclear leukocyte [PMN]) exudation in bronchioles and alveoli was scored as follows: 0.0, no to minimal presence of neutrophils; 1.0, small clusters of PMNs present in occasional airways; and 2.0, prominent small to large aggregates of PMNs in bronchiolar lumens, with minimal aggregates in alveoli. A composite score was computed using the sum of the three individual scores. The average group composite score was used for statistical analysis.
The trachea was evaluated with a single score based on the magnitude of epithelial attenuation or necrosis. Trachea scores were defined as follows: 0.0, normal epithelium over the entire circumference; 1.0, focal epithelial attenuation; and 2.0, extensive epithelial attenuation or necrosis.
Serologic and mucosal antibody assays.
Serum samples were collected by anterior vena cava or jugular venipuncture at the following time points: preweaning (−7 days postvaccination [dpv]), primary vaccination (0 dpv), secondary WIV vaccination (14 dpv), 2 weeks post-secondary vaccination (28 dpv), challenge inoculation (49 dpv/0 dpi), and necropsy (5 dpi). For use in the HI assay, sera were heat inactivated at 56°C and treated to remove nonspecific agglutinants by use of a 20% suspension of kaolin (Sigma-Aldrich, St. Louis, MO) followed by adsorption with 0.5% turkey red blood cells (RBC). HI assays were done using TX98 and CO99 viral antigens and turkey RBC, using standard techniques and a maximum titer of 1:640 (17).
Enzyme-linked immunosorbent assays (ELISA) to detect total IgG and IgA antibodies against whole virus preparations of TX98 and CO99 present in serum and BALF were performed as previously described (5), with the following modifications. Serum samples were diluted to 1:2,000 for the IgG ELISA. BALF samples were diluted to 1:4 for IgG and IgA ELISAs. Samples were diluted in bovine serum albumin (fraction V BSA; Life Technologies) and PBS, with a final BSA concentration of 5%, to block nonspecific antibodies. Independent assays were conducted using 50 μl of whole TX98 or CO99 virus at 100 HA units per well as the ELISA antigen, and coated plates were blocked with 150 μl of a commercial blocking buffer (Starting Block; Thermo Fisher). Anti-swine IgG (Kirkegaard and Perry) and anti-swine IgA (Bethyl Laboratories) were used at a 1:1,500 dilution in blocking buffer. Each sample was analyzed in duplicate. The optical density (OD) was measured at a 405-nm wavelength with an automated ELISA reader. Antibody levels were reported as the mean OD for each sample, and the means for treatment groups were compared.
Statistical analysis.
Macroscopic and microscopic pneumonia scores, log10-transformed BALF and nasal swab virus titers, log2 transformations of HI reciprocal titers, and mean ODs for ELISAs were analyzed using analysis of variance (ANOVA), with P values of ≤0.05 considered significant (Prism software; GraphPad, La Jolla, CA). Data from treatment groups infected with different virus strains (TX98 versus CO99) were analyzed separately. Response variables shown to have significant effects by treatment group were subjected to pairwise mean comparisons using the Tukey-Kramer test.
RESULTS
Serology.
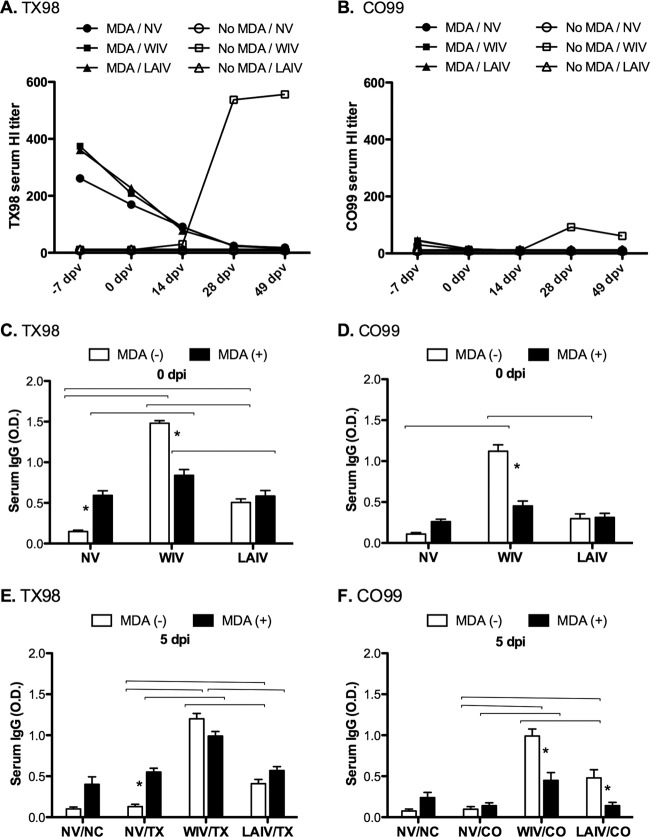
Serum antibody responses to the vaccines displayed different profiles for MDA-negative and MDA-positive pigs. For MDA-negative pigs, WIV induced high TX98-specific HI responses in sera, with a geometric mean reciprocal titer of 556 at 49 dpv, whereas LAIV induced TX98-specific HI titers that were only marginally above the limit of detection (Fig. 1A). Similarly, WIV induced higher levels of TX98-specific serum IgG in MDA-negative pigs (Fig. 1C). WIV vaccination induced a modest level of cross-reacting CO99-specific HI titers in sera from MDA-negative pigs, with a geometric mean reciprocal titer of 61 at 0 dpi (Fig. 1B), and there was a corresponding increase in CO99 serum IgG (Fig. 1D). In contrast, LAIV induced no detectable HI or total IgG cross-reactivity with CO99, even in MDA-negative pigs (Fig. 1B and D, respectively).
Fig 1.
Serum antibody levels due to maternally derived antibodies and/or responses to vaccination. Reciprocal geometric mean HI titers against TX98 H3N2 antigen (A) and CO99 antigen (B) are shown for multiple time points prior to challenge. MDA, groups with maternally derived antibodies induced by immunizing dams with the TX98 vaccine. Treatment groups were nonvaccinated (NV), vaccinated at 0 dpv and 14 dpv with TX98 WIV, or vaccinated intranasally with TX98 LAIV at 0 dpv only. Mean ODs in whole-virus ELISAs at 49 dpv (0 days postinfection) are shown for serum IgGs against TX98 antigen (C) and CO99 antigen (D). Mean ODs in whole-virus ELISAs at 5 days postchallenge are shown for IgG against TX98 antigen for groups challenged with TX98 (E) and for IgG against CO99 antigen for groups challenged with CO99 (F). Open bars designate groups without MDA, and solid bars designate groups with MDA. Statistically significant differences between MDA statuses within a vaccine group are marked with asterisks, and differences between vaccine treatment groups with matched MDA statuses are identified by connecting lines (P < 0.05).
Pigs that suckled immunized dams acquired MDA, as measured by serum HI titers against the vaccine strain TX98 7 days before vaccination (−7 dpv), with a geometric mean reciprocal titer of 312 and a titer range of 40 to ≥640; however, pigs in the MDA-positive groups did not respond to LAIV or WIV vaccination with increases in HI antibody titers to TX98 (Fig. 1A). In these MDA-positive pigs, weakly cross-reactive HI titers against CO99 were detectable at −7 dpv (Fig. 1B). Homologous HI titers in the MDA-positive pigs declined by the day of challenge (49 dpv), to levels below or near the lower limit of detection (≤40). However, prior to challenge, maternally derived serum IgG specific to TX98 was still detectable by ELISA for nonvaccinated controls (Fig. 1C), but IgG against the heterologous CO99 strain was not detectable (Fig. 1D). Although IgG levels in the WIV-vaccinated MDA-positive group were significantly higher than those in the nonvaccinated MDA-positive group, WIV vaccination resulted in significantly higher IgG levels against both the TX98 and CO99 viruses in the MDA-negative pigs (Fig. 1C and D, respectively). LAIV given to MDA-positive pigs failed to induce an increase in the preexisting total TX98- or CO99-specific IgG in serum (Fig. 1C and D), similar to the HI response. Serum IgG responses after experimental challenge with TX98 or CO99 (5 dpi) displayed a pattern similar to the prechallenge results (Fig. 1E and F), with the notable exception of a boost in the level of IgG antibodies binding to CO99 in the pigs given LAIV in the absence of MDA and challenged with CO99 (Fig. 1F).
Mucosal antibody responses.
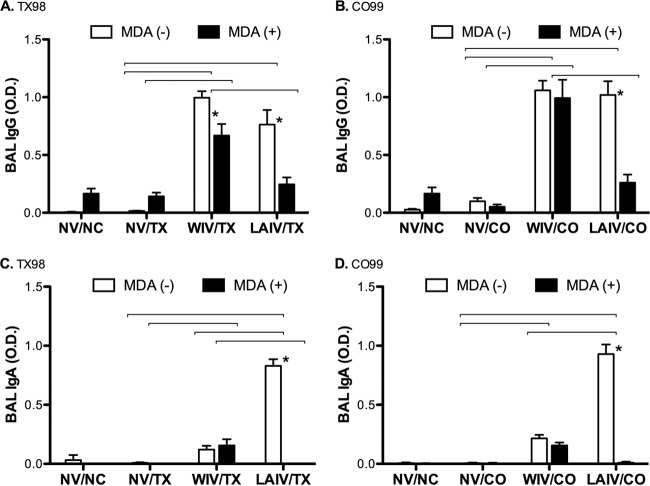
After TX98 challenge (5 dpi), there were significant levels of TX98-specific IgG in lungs of WIV vaccinates whether the animals were vaccinated in the presence or absence of MDA (Fig. 2A). LAIV vaccinates had significant levels of TX98-specific IgG in the lungs only if the vaccine was administered in the absence of MDA (Fig. 2A). This was consistent for pigs challenged with CO99 as well (Fig. 2B). In sharp contrast, statistically significant IgA levels were detected only in the lungs of pigs vaccinated with LAIV in the absence of MDA. Results were similar for pigs challenged with either virus and for both the TX98 and CO99 ELISAs (Fig. 2C and D, respectively). Thus, similar to the serum antibody profiles, mucosal antibody responses also differed between vaccine types and were affected by the presence of MDA at the time of vaccination.
Fig 2.
Antibody levels in bronchoalveolar lavage fluid at 5 days postinfection. Mean ODs in whole-virus ELISAs are shown for IgGs against TX98 antigen (A) and CO99 antigen (B) and for IgAs against TX98 antigen (C) and CO99 antigen (D). Groups challenged with TX98 are represented in panels A and C, whereas groups challenged with CO99 are represented in panels B and D. Open bars designate groups without MDA, and solid bars designate groups with MDA. Statistically significant differences between MDA statuses within a vaccine group are marked with asterisks, and differences between vaccine treatment groups with matched MDA statuses and challenge virus strains are identified by connecting lines (P < 0.05).
Replication of challenge viruses.
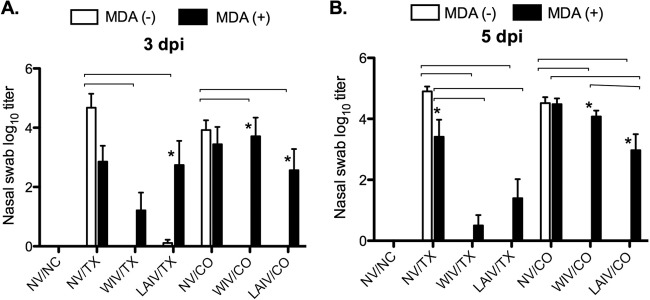
Distinct differences were detected in the replication of challenge viruses, based on both MDA and vaccine status. Nasal replication of the TX98 and CO99 challenge viruses was monitored by virus isolation and titration of virus in nasal swabs. As expected, no virus was detected in any of the pigs on the day of challenge (data not shown). In MDA-negative, nonvaccinated animals, the two viruses reached similar nasal titers, between 104 and 105 TCID50/ml, at 3 and 5 dpi (Fig. 3). Nonvaccinated pigs that received TX98-specific MDA shed 10- to 100-fold less TX98 virus at 3 and 5 dpi (Fig. 3), even though their passive serum HI titers were near the lower limit of detection at the time of challenge (Fig. 1A). In contrast, nasal shedding of CO99 was not inhibited significantly by the presence of residual TX98-specific MDA (Fig. 3B). In MDA-negative pigs vaccinated with WIV or LAIV, both vaccines provided effective protection against the nasal shedding of TX98 and CO99, as almost no virus was detected in these treatment groups at 3 or 5 dpi. Pigs that had been vaccinated with WIV in the face of MDA demonstrated reduced TX98 virus shedding at both samplings, but not complete prevention as seen in the MDA-negative WIV-vaccinated pigs. The single dose of LAIV vaccine in the face of MDA also did not prevent shedding of TX98 at 3 dpi, but it led to a statistically significant reduction in nasal titers by 5 dpi compared with those of nonvaccinated pigs with MDA. Finally, when administered in the face of MDA, both vaccines failed to protect against nasal replication of heterologous CO99, although statistically significant reductions in group mean titers were detected for the LAIV-vaccinated MDA-positive pigs at both time points.
Fig 3.
(A) Nasal shedding of challenge virus in nasal swabs at 3 (A) and 5 (B) dpi. Piglets were vaccinated in the presence or absence of circulating MDA against TX98. At vaccination, piglets received no vaccine (NV), two intramuscular doses of TX98 WIV, or one intranasal dose of TX98 LAIV. Forty-nine days after the initial vaccine dose, piglets were challenged by intratracheal inoculation with TX98 or CO99. Nasal swab specimens were collected at 3 and 5 dpi and titrated by TCID50 assay on MDCK cells. Statistically significant differences between MDA statuses within a vaccine group are marked with asterisks, and differences between vaccine treatment groups with matched MDA statuses and challenge virus strains are identified by connecting lines (P < 0.05).
Virus titers were also measured in BALF specimens collected at necropsy, at 5 dpi (Fig. 4A). BALF collected from nonvaccinated pigs had mean titers of 105 to 106 TCID50/ml. Residual MDA appeared to provide a very limited amount of protection against TX98 replication in the lung—with an approximately 10-fold reduction in titer—but not against CO99 (Fig. 4A). These results are similar to what was observed for nasal titers at 5 dpi, although reductions due to MDA were greater in the nose than in the lung. BALF samples from MDA-negative WIV and LAIV vaccinees contained no detectable TX98 or CO99, which closely followed the prevention of nasal shedding in these vaccinated groups. Even in the face of MDA, both vaccines significantly reduced the TX98 viral loads in BALF. However, CO99 viral loads in BALF were not reduced in WIV vaccinates when the vaccine was administered in the face of MDA. MDA also interfered with the efficacy of LAIV against CO99, although less dramatically than for WIV, as LAIV resulted in a significant reduction in BALF CO99 titers.
Fig 4.
Adjuvanted TX98 WIV administered to MDA-positive piglets enhances the severity of subsequent infection with heterologous H3N2 strain CO99, whereas the TX98 LAIV vaccine partially cross-protects pigs. MDA-positive pigs suckled colostrum from TX98-vaccinated sows, and MDA-negative pigs suckled colostrum from naïve sows. WIV was delivered intramuscularly at 2 and 4 weeks of age, while LAIV was delivered intranasally at 2 weeks of age only. At 8 weeks of age (49 dpv), pigs were challenged intratracheally with TX98 or CO99. At 5 dpi, pigs were euthanized, BALF samples were collected, and necropsy was conducted. (A) BALF samples were titrated by TCID50 assay on MDCK cells. (B) Macroscopic lesions were scored as the percentage of total lung surface area involved. Microscopic pneumonia (C) and tracheal damage (D) were scored as described in Materials and Methods. Statistically significant differences between MDA statuses within a vaccine group are marked with asterisks, and differences between vaccine treatment groups with matched MDA statuses and challenge virus strains are identified by connecting lines (P < 0.05).
Lung pathology.
Challenge with either H3N2 strain caused mild lung pathology in pigs lacking maternal or vaccine-induced immunity, consistent with previous reports (22, 26). MDA-positive pigs vaccinated with WIV developed enhanced macroscopic pneumonia when they were challenged with either the homologous TX98 or heterologous CO99 strain (Fig. 4B and 5C) compared to their respective MDA-negative counterparts. This WIV-associated enhancement was particularly evident after CO99 challenge, with a group mean of 35% of the lung area affected with pneumonia (Fig. 5C). Importantly, no enhancement of macroscopic pneumonia was seen with LAIV under either MDA condition with either challenge virus (Fig. 4B and 5B), and there was a general trend of a reduction in pneumonia. Nonvaccinated and LAIV groups challenged with CO99 had average percentages of pneumonia of 4.2% and 2.2%, respectively. Likewise, the LAIV vaccine significantly reduced the percentage of macroscopic lung pathology in MDA-negative animals challenged with TX98. In pigs lacking MDA, both of the vaccines were associated with significantly reduced macroscopic lung pathology following heterologous CO99 challenge. Although TX98-specific serum HI titers from MDA had largely waned in nonvaccinated pigs by the time of challenge, macroscopic pneumonia was less extensive in nonvaccinated pigs that had received MDA than in those without MDA (Fig. 4B). This difference in severity of macroscopic lesions corresponded with a similar trend in BALF viral titers at 5 dpi (Fig. 4A).
Fig 5.

Photographs of macroscopic lung pathology in pigs positive for MDA at the time of vaccination, shown 5 days after heterologous challenge with CO99. Photographs of ventral surfaces of lungs are representative of three vaccine treatment groups: nonvaccinated challenge controls (A), TX98 LAIV-vaccinated pigs (B), and TX98 WIV-vaccinated pigs (C).
When the vaccines were administered to MDA-negative animals, both showed protective effects against both challenge viruses with respect to microscopic lung lesion scores (Fig. 4C). The protective effects of LAIV against both challenge viruses, which did not reach statistical significance, appeared to be maintained when the vaccine was given to MDA-positive animals. However, as with macroscopic pneumonia, there were distinctly different outcomes when MDA-positive WIV-vaccinated pigs were challenged with homologous TX98 versus heterologous CO99. Those vaccinated with WIV in the face of MDA and then challenged with TX98 had microscopic lesion scores that were not statistically different from those of the nonvaccinated MDA-positive group. In contrast, not only did WIV vaccination of MDA-positive pigs fail to provide protection against heterologous CO99, but a significant enhancement in lesion severity was demonstrated microscopically, paralleling the dramatic difference that was seen macroscopically. Importantly, LAIV did not contribute to enhanced microscopic lesion severity with either challenge virus.
Tracheal pathology.
Regardless of MDA status, TX98 tended to induce more tracheal lesions than CO99 (Fig. 4D). In MDA-negative pigs, both vaccines provided statistically significant reductions of these TX98-induced lesions, and in MDA-positive pigs, LAIV still significantly reduced TX98-induced tracheal damage. However, the tracheal damage score was sharply higher for pigs that received the WIV vaccine in the face of MDA and then were challenged with CO99. Thus, the overall enhanced respiratory disease from heterologous infection of the MDA-positive WIV vaccinates was clearly evident in the trachea as well as in the lung.
DISCUSSION
The antigenic diversity of contemporary and emerging IAV strains is a major obstacle to effective and reliable vaccines for swine (16). IAV vaccines currently licensed around the world contain inactivated viral antigens representing H1N1, H3N2, H1N2, and 2009 pandemic H1N1 strains (12). Inactivated IAV vaccines elicit systemic neutralizing antibodies and protection against homologous challenge, but their efficacy against antigenically distinct strains is often diminished (1, 9). Intranasal vaccination with an attenuated virus is considered likely to elicit more cross-reactive T cells and mucosal antibodies against antigenically variant strains than those induced by other types of vaccination (12). Several attenuated viral constructs made by targeted genetic mutations have been tested in recent years (13, 18, 24). We previously reported the immunogenicity and protective efficacy of a TX98 virus attenuated by truncation of the NS1 gene, which encodes a type I interferon antagonist protein (23, 26). This virus (identical to the LAIV in the present report) was shown to have attenuated replication in the upper respiratory tract. When the TX98 LAIV was administered as an intranasal vaccine to young seronegative pigs, it elicited a mucosal IgA response, modest titers of serum HI antibodies, and antigen-specific T cells, while conferring protection against homologous challenge and a degree of cross-protection against variant strains, with a single- or two-dose regimen (7, 26). Our previous work indicated that two intranasal applications of the LAIV vaccine did not confer any benefit against homologous challenge compared to one dose (26), and one dose would be highly preferred for use in the swine population. Here we showed that a single dose was highly efficacious against the homologous TX98 strain and the heterologous CO99 strain in MDA-negative pigs. Although future studies are necessary to investigate whether two doses would improve efficacy in the presence of MDA, the impact of the findings of this study comes from the differences between WIV and LAIV in the face of MDA and a heterologous challenge.
One aim of the present study was to compare the efficacies of intranasal LAIV and intramuscular WIV vaccination in seronegative pigs. WIV vaccination induced high serum HI titers for antibodies against the homologous antigen, while HI responses following LAIV vaccination were weak or below the detection limits. Even before the WIV vaccinees were boosted with a second dose, they achieved higher HI titers than those of LAIV vaccinees (Fig. 1A). Cross-reactive HI antibody titers against the heterologous CO99 strain tended to be 4- to 16-fold lower than homologous titers, and these were detectable only in WIV vaccinees. Despite the marked differences in serological responses, both vaccines supplied significant protection in the absence of MDA against the replication of not only the homologous TX98 strain but also the heterologous CO99 strain. Based on these data, protective immunity induced by LAIV vaccination was likely mediated by T cells and/or mucosal antibodies, and here we demonstrated a robust IgA response in the lower respiratory tract when the LAIV was administered in the absence of MDA. Higher levels of IgG in the serum were also detected at 5 dpi, indicating a cross-reacting boost of antibody to the CO99 virus exposure. It is not clear if the higher levels of serum (and mucosal) antibodies to CO99 at 5 dpi in the MDA-negative pigs were specific to the CO99 challenge virus or if the CO99 challenge boosted the TX98-primed response, particularly against epitopes that are shared between TX98 and CO99. While there was no evidence of CO99 replication, the immune system had likely formed prior immunity against common epitopes contained in the LAIV vaccine that may have been boosted upon exposure to the CO99 virus. The protection provided by the WIV vaccine against CO99 in the non-MDA pigs was surprising, since previous studies reported limited HI cross-reactivity between TX98 and CO99 (22) and because the geometric mean cross-reactive HI titer at the time of challenge in this study was 61, about 10-fold lower than the geometric mean homologous HI titer to TX98. The cross-reacting HI antibodies and mucosal IgG antibodies detected at 5 dpi are likely to have played a role in the heterologous protection, perhaps enabled by the magnitude of the systemic antibody response to WIV. Cross-reactive T cells primed by the WIV may also have contributed to the protective effect against the heterologous CO99 infection. Consistent with this possibility, a similarly formulated H1N2 WIV vaccine was shown to prime T cells in antibody-negative young pigs (7).
IAV vaccination of sows is a widespread practice in North American swine herds (3). This presents a second practical problem concerning IAV vaccines in the swine industry: antibodies transferred in colostrum from sows to their litters can interfere with subsequent vaccination of the piglets (8) and are often poorly matched to viruses circulating on the sow farm or in downstream production stages. Colostrum-borne maternal antibodies, which are predominantly IgG antibodies, are not expected to infiltrate the nasal mucosa of the upper respiratory tract, so we hypothesized that the LAIV vaccine would be less sensitive to inhibition by MDA. Our serological results demonstrated that MDA indeed prevented pigs from mounting active HI antibody responses to the WIV vaccine (Fig. 1A). Despite this, the WIV vaccine administered in the face of MDA still provided significant protection against homologous TX98 challenge, including decreased nasal shedding (Fig. 3) and replication in the lung (Fig. 4A), but did not prevent damage to the lower respiratory tract (Fig. 4B to D). This pointed again to the possibility that the adjuvanted WIV in the face of MDA primed a cellular immune response that contributed to protection against homologous virus. Moderate T-cell priming has been demonstrated for pigs vaccinated with a similar formulation of inactivated H1N2 WIV (7).
Critically, though, there was a pronounced failure of WIV vaccination in MDA-positive pigs that were challenged with the heterologous CO99 strain. In this group, the vaccine failed to reduce viral replication in nasal passages and the viral loads in BALF (Fig. 3 and 4A). Strikingly, macroscopic lung lesions were exacerbated (Fig. 4B and 5C) in a manner similar to that in another VAERD model (4, 5, 25). Composite microscopic lung and tracheal lesions (Fig. 4C and D) were enhanced and similar in character to previously reported VAERD lesions (4). In sharp contrast, there was no enhancement of pathological lung changes in the MDA-positive group vaccinated with LAIV; in fact, the LAIV vaccine was partially protective against CO99 in terms of reducing lung damage and viral loads at 5 dpi (Fig. 4 and 5). Thus, although WIV and LAIV had similar efficacies in naïve pigs, the presence of MDA titers at the time of vaccination followed by a heterologous viral challenge produced sharply different outcomes between the two vaccines. The abrogation of the LAIV-induced IgA response in the lower respiratory tract (Fig. 2D) may explain the reduction in efficacy in the MDA-positive pigs compared to that for LAIV given to MDA-negative pigs. An evaluation of the antibody profile in the upper respiratory tract (nasal mucosa) was not conducted in this study but should be considered in future studies to understand how MDA interferes with the LAIV-induced mucosal antibody response and if the inhibition is limited to the lower respiratory tract. We speculate that the cellular immune response to LAIV in MDA-positive pigs is a key factor in cross-protection, since no lung IgA or cross-reacting serum HI antibodies were evident. It is also unclear if there was a role for the presumably nonneutralizing IgG in the lungs of MDA-positive WIV vaccinates with VAERD following heterologous challenge with CO99, as there was an absence of IgG in the lungs of MDA-positive LAIV vaccinates without VAERD.
In a previous study, MDA-positive pigs that received a bivalent IAV vaccine containing an inactivated classical H1N1 virus were primed for enhanced pneumonia upon heterologous H1N1 challenge, whereas vaccination of MDA-negative pigs provided cross-protection (8). This detrimental interaction between passive immunity and WIV vaccination parallels what we observed in the present study with H3N2 viruses. Although the earlier study did not include an LAIV treatment group for comparison, it did show evidence that intramuscular vaccination with an inactivated virus administered in the face of MDA was ineffective at priming protective T-cell memory. The mechanism(s) responsible for the enhancement of respiratory disease in our model is not completely clear. It can be hypothesized that MDA bind to vaccine antigen and that the method of antigen processing and presentation is different from that when vaccine antigen is not bound to antibody (seronegative pig). This change in antigen uptake and subsequent presentation may alter the adaptive immune response (both humoral and cell mediated) in the piglet, possibly directing it away from neutralizing epitopes to conserved, albeit nonneutralizing epitopes shared between the vaccine virus and the challenge virus. After 2 doses of WIV, HI antibodies against neither TX98 nor CO99 were detected in MDA-positive pigs, whereas HI antibodies against both were detected in MDA-negative pigs. Although there was no HI antibody response in MDA-positive pigs receiving WIV, there appeared to be a modest increase in total TX98-specific IgG in the serum prior to challenge (Fig. 1C). Total IgG was also present in the lung at 5 dpi (Fig. 2A and B), when there was still no detectable HI response in the serum for this group (data not shown). This indicates that MDA interfered with the induction of neutralizing HI antibodies by WIV. Upon challenge with the heterologous virus, immune complexes may form between nonneutralizing antibodies and challenge virus and may trigger inflammatory responses such as those implicated in vaccine-enhanced respiratory syncytial virus (RSV) infection of infants (3). The involvement of antibodies in generating immune complexes associated with severe respiratory disease and pulmonary damage has also been described for 2009 pandemic H1N1 influenza disease (15). Roles for specific IgG subclasses have not been defined clearly for the pig; thus, this type of analysis is not available for further interrogation of within-type differences in antibody responses induced by WIV vaccination in the face of MDA. However, functional or qualitative differences in the antibodies produced in response to WIV and LAIV in the presence or absence of MDA appear likely to have a critical role in the clinical outcome after infection.
Another hypothesis, though not mutually exclusive, is that differences in antigen processing and presentation of WIV vaccine antigen in MDA-positive pigs may alter the kinetics of the response or may prime a qualitatively different T-cell response, which plays a role in immunopathology. A different subset of memory T cells may develop in MDA-positive WIV-vaccinated pigs and may be activated and contribute to pathology upon heterologous challenge, when cross-neutralizing antibody is not present, possibly through granzyme release and killing of infected cells. Additional studies are needed to further characterize differences in the antibody and T-cell responses that develop following WIV vaccination of MDA-positive and MDA-negative pigs and to elucidate the immunopathogenic mechanism of enhanced disease following heterologous challenge.
Collectively, the results of this study demonstrate very distinct outcomes of IAV vaccination and infection with heterologous virus, with pivotal factors including the format and route of administration of vaccines, the presence or absence of MDA, and the antigenic similarity of the challenge virus to the vaccine strain. The differences go beyond protection versus nonprotection and point to realistic scenarios in the field where vaccinating sows and their piglets could potentiate more severe respiratory disease. This underscores the need to reevaluate the way in which efficacy studies are designed for swine influenza vaccine licensure for use in pigs in the United States. Methods of IAV vaccine evaluation that focus simply on protection against homologous challenge (or antigenically similar viruses) in seronegative pigs would be unlikely to identify this problem. Determining the immune correlates of protection versus disease exacerbation would significantly aid in the improvement of vaccine safety and efficacy under field conditions.
ACKNOWLEDGMENTS
Funding was provided by USDA-ARS to A.L.V., W.M., K.M.L., J.A.R., and C.L.L. This study was supported in part by NIAID grants R01AI046954 and U01AI070469 and by a grant from the Center for Research in Influenza Pathogenesis (CRIP) (NIAID CEIRS contract HHSN266200700010C) to A.G.-S.
We thank Michelle Harland and Gwen Nordholm for technical assistance and Brian Pottebaum and Jason Huegel for assistance with animal studies.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Footnotes
Published ahead of print 18 July 2012
REFERENCES
- 1. Bikour MH, Cornaglia E, Elazhary Y. 1996. Evaluation of a protective immunity induced by an inactivated influenza H3N2 vaccine after an intratracheal challenge of pigs. Can. J. Vet. Res. 60:312–314 [PMC free article] [PubMed] [Google Scholar]
- 2. Choi YK, Goyal SM, Joo HS. 2004. Evaluation of transmission of swine influenza type A subtype H1N2 virus in seropositive pigs. Am. J. Vet. Res. 65:303–306 [DOI] [PubMed] [Google Scholar]
- 3. Delgado MF, et al. 2009. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 15:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gauger PC, et al. 28 March 2012. Kinetics of lung lesion development and pro-inflammatory cytokine response in pigs with vaccine-associated enhanced respiratory disease induced by challenge with pandemic (2009) A/H1N1 influenza virus. Vet. Pathol. doi:10.1177/0300985812439724 [DOI] [PubMed] [Google Scholar]
- 5. Gauger PC, et al. 2011. Enhanced pneumonia and disease in pigs vaccinated with an inactivated human-like (delta-cluster) H1N2 vaccine and challenged with pandemic 2009 H1N1 influenza virus. Vaccine 29:2712–2719 [DOI] [PubMed] [Google Scholar]
- 6. Halbur PG, et al. 1995. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 32:648–660 [DOI] [PubMed] [Google Scholar]
- 7. Kappes MA, et al. 2012. Vaccination with NS1-truncated H3N2 swine influenza virus primes T cells and confers cross-protection against an H1N1 heterosubtypic challenge in pigs. Vaccine 30:280–288 [DOI] [PubMed] [Google Scholar]
- 8. Kitikoon P, et al. 2006. The immune response and maternal antibody interference to a heterologous H1N1 swine influenza virus infection following vaccination. Vet. Immunol. Immunopathol. 112:117–128 [DOI] [PubMed] [Google Scholar]
- 9. Lee JH, Gramer MR, Joo HS. 2007. Efficacy of swine influenza A virus vaccines against an H3N2 virus variant. Can. J. Vet. Res. 71:207–212 [PMC free article] [PubMed] [Google Scholar]
- 10. Loeffen WL, Heinen PP, Bianchi AT, Hunneman WA, Verheijden JH. 2003. Effect of maternally derived antibodies on the clinical signs and immune response in pigs after primary and secondary infection with an influenza H1N1 virus. Vet. Immunol. Immunopathol. 92:23–35 [DOI] [PubMed] [Google Scholar]
- 11. Lorusso A, et al. 2011. Genetic and antigenic characterization of H1 influenza viruses from United States swine from 2008. J. Gen. Virol. 92:919–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma W, Richt JA. 2010. Swine influenza vaccines: current status and future perspectives. Anim. Health Res. Rev. 11:81–96 [DOI] [PubMed] [Google Scholar]
- 13. Masic A, Booth JS, Mutwiri GK, Babiuk LA, Zhou Y. 2009. Elastase-dependent live attenuated swine influenza A viruses are immunogenic and confer protection against swine influenza A virus infection in pigs. J. Virol. 83:10198–10210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Masic A, et al. 2010. Immunogenicity and protective efficacy of an elastase-dependent live attenuated swine influenza virus vaccine administered intranasally in pigs. Vaccine 28:7098–7108 [DOI] [PubMed] [Google Scholar]
- 15. Monsalvo AC, et al. 2011. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat. Med. 17:195–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olsen CW. 2002. The emergence of novel swine influenza viruses in North America. Virus Res. 85:199–210 [DOI] [PubMed] [Google Scholar]
- 17. Palmer DF, Coleman MT, Dowdle WD, Schild GO. 1975. Advanced laboratory techniques for influenza diagnosis. Immunology series no. 6 U.S. Department of Health, Education, and Welfare, Washington, DC [Google Scholar]
- 18. Pena L, et al. 2011. Modifications in the polymerase genes of a swine-like triple-reassortant influenza virus to generate live attenuated vaccines against 2009 pandemic H1N1 viruses. J. Virol. 85:456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pomorska-Mol M, Markowska-Daniel I, Pejsak Z. 2010. Evaluation of humoral and antigen-specific T-cell responses after vaccination of pigs against pseudorabies in the presence of maternal antibodies. Vet. Microbiol. 144:450–454 [DOI] [PubMed] [Google Scholar]
- 20. Reed LJ, Muench H. 1938. A simple method of estimating fifty percent end points. Am. J. Hyg. 27:5 [Google Scholar]
- 21. Renshaw HW. 1975. Influence of antibody-mediated immune suppression on clinical, viral, and immune responses to swine influenza infection. Am. J. Vet. Res. 36:5–13 [PubMed] [Google Scholar]
- 22. Richt JA, et al. 2003. Pathogenic and antigenic properties of phylogenetically distinct reassortant H3N2 swine influenza viruses cocirculating in the United States. J. Clin. Microbiol. 41:3198–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Richt JA, et al. 2006. Vaccination of pigs against swine influenza viruses by using an NS1-truncated modified live-virus vaccine. J. Virol. 80:11009–11018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Solorzano A, et al. 2005. Mutations in the NS1 protein of swine influenza virus impair anti-interferon activity and confer attenuation in pigs. J. Virol. 79:7535–7543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vincent AL, Lager KM, Janke BH, Gramer MR, Richt JA. 2008. Failure of protection and enhanced pneumonia with a US H1N2 swine influenza virus in pigs vaccinated with an inactivated classical swine H1N1 vaccine. Vet. Microbiol. 126:310–323 [DOI] [PubMed] [Google Scholar]
- 26. Vincent AL, et al. 2007. Efficacy of intranasal administration of a truncated NS1 modified live influenza virus vaccine in swine. Vaccine 25:7999–8009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Webby RJ, Rossow K, Erickson G, Sims Y, Webster R. 2004. Multiple lineages of antigenically and genetically diverse influenza A virus co-circulate in the United States swine population. Virus Res. 103:67–73 [DOI] [PubMed] [Google Scholar]
- 28. Wittmann G, Ohlinger V. 1987. Aujeszky's disease vaccination and infection of pigs with maternal immunity: effects on cell- and antibody-mediated immunity. Arch. Virol. 92:87–101 [DOI] [PubMed] [Google Scholar]
- 29. Zhou NN, et al. 1999. Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. J. Virol. 73:8851–8856 [DOI] [PMC free article] [PubMed] [Google Scholar]