Abstract
An increasing number of studies suggest that individual subsets of dendritic cells (DC) exhibit distinct capabilities with regard to the generation of the adaptive immune response. In this study, we evaluated the properties of a relatively unexplored DC subset present in the lung-draining mediastinal lymph node. This subset expresses the airway dendritic cell marker CD103 together with CD8. These DC were of interest given that our previous studies using a model of respiratory infection with vaccinia virus revealed a distinct difference in the ability of CD103+ DC to prime T cells that correlated inversely with the expression of CD8, suggesting a differential role of these DC in the context of respiratory virus infection. To expand our understanding of the role of this DC population, we performed analyses to elucidate the phenotype, migratory capacity, responsiveness to innate stimuli, and priming capacity of CD8+ CD103+ DC. We found that expression of surface markers on these DC was similar to that of CD8− CD103+ DC, supporting their close relationship. Further, the two DC types were similar with regard to antigen uptake. However, although both CD103+ subsets originated from the lung, CD8-bearing CD103+ DC appeared in the lymph node with delayed kinetics following virus infection. While this subset exhibited increased responsiveness to a number of Toll-like receptor (TLR) agonists, their response to infection was virus specific, demonstrating poor responsiveness to vaccinia virus infection but robust maturation following infection with parainfluenza virus 5 or influenza virus. These findings show that CD8 marks a population of lung airway-derived DC with distinct migratory and maturation responses that likely contribute differentially to the immune response depending on the infecting pathogen.
INTRODUCTION
Dendritic cells (DC) in the lung play a critical role in the generation of the adaptive T cell response following virus infection (3, 12, 16, 20, 21). A number of studies have now established lung migrating DC as the primary initiator of T cell activation in the draining lymph nodes following respiratory infection (3, 4, 20). DC represent an increasingly complex array of distinct subsets that differ in function and phenotype. In the lung, CD103 serves as a discriminator of cells that reside at the airway versus within the parenchyma (34). However, it appears that even within this discrete tissue site, DC are not a homogeneous population. This work explores the CD8-expressing CD103+ DC subset that has been identified by a number of groups as a constituent of the lung-draining mediastinal lymph node (MLN) DC population (4, 12, 20). While the present study addresses the role of this population in the respiratory tract, the presence of a CD8+-expressing population within the CD103+ DC has been reported for the skin (1, 36), small intestine (15), and spleen (28). Thus, this population is likely to contribute to the generation of the T cell response at multiple sites.
In the absence of infection and at early times following infection, expression of CD8+ on CD103+ DC is restricted to the lung-draining mediastinal lymph node, i.e., these cells are not present in the lung (27, 34; our unpublished data). There is, however, evidence to support their presence in the lung at later times following pathogen infection (12). It has been suggested that these DC may be the result of migration from the MLN to the lung (12). In this scenario, CD8+ CD103+ DC that migrate from the lung to the MLN traffic back to the lungs at a late time postinfection (p.i.), i.e., after induction of the adaptive immune response. Whether this can account for the CD8+ CD103+ DC reported by others in the lung at late times p.i. or whether lung-resident CD103+ DC can upregulate CD8 as a result of infection associated changes in the lung environment is not yet clear. It is intriguing that CD8+ DC in the lung have been shown to support the activity and survival of CD8+ effector cells following infection through an interleukin-15 (IL-15)-dependent process (22, 23). The infection-induced presence of CD8+ CD103+ T cells in the lung may contribute to this phenomenon.
At present, the signals that regulate the presence of CD8 on CD103+ DC are unknown. There is evidence to support a role for maturation in acquisition of CD8 expression in CD103+ cells (27). However, it is clear from our studies that maturation by itself is not sufficient for the expression of CD8, as we find immature CD8+ CD103+ DC in the draining LN (4). Interestingly, a number of transcription factors known to be vital for the development of conventional CD8+ DC are also important for generation of the CD103+ DC compartment. For example, mice lacking either Batf3 or Irf8 lack both tissue-resident CD103+ DC and CD8+ DC (14, 18). Thus, the expression of CD8 by CD103+ DC may be linked by a common differentiation program in these two types of DC.
Our interest in this subset was generated by our previous studies of lung-derived DC present in the MLN following pulmonary vaccinia virus (VV) infection, wherein we noted a subpopulation of CD103+ DC that coexpressed CD8 (4). The presence of this population was selective for the MLN, since CD103+ DC bearing CD8 were absent in the lung. Although also observed by others, data assessing the role and functional capabilities of this subset are highly limited. Specifically, our studies showed that in the context of vaccinia virus infection, these DC were decreased in their ability to activate CD8+ T cells (4). To our knowledge, the only additional study to address the immunologic capability of CD8+ CD103+ DC is a recent study by Fujimoto et al. (15), which evaluated the capacity of these cells in the small intestine to elicit a T cell response. In contrast to their CD8− counterpart, CD8+ CD103+ DC at this site efficiently stimulated Th1 and CD8+ T cell responses.
Given the dearth of information regarding CD8+ CD103+ lung-derived DC, we performed analyses to better understand the inherent properties of this population, including migration from the lung, phenotype, response to Toll-like receptor (TLR) agonist, and virus stimulation and capacity for antigen uptake. We found that CD8+ CD103+ DC closely resemble the airway-resident CD8− CD103+ DC with regard to both cell surface marker expression and antigen uptake capabilities. However, they responded in a distinct manner following infection with VV. On further study, the response to infection was found to diverge depending on the infecting virus, with these DC exhibiting partial or full maturation in response to infection with influenza virus or parainfluenza virus 5 (PIV5), respectively. This differential responsiveness suggested that the role of the CD8+ CD103+ subset in generating the adaptive response differs depending on the virus present. Identification of the signals that differentially impact maturation in these two subsets may allow harnessing of their activating potential in the context of therapeutics and vaccines.
MATERIALS AND METHODS
Mice and infections.
C57BL/6 mice (Frederick Cancer Research Facility, National Cancer Institute, Frederick, MD) were used throughout this study. OT-I mice were from a colony established with breeding pairs obtained from Jackson Laboratories (Bar Harbor, ME). TLR2-deficient and MyD88-deficient mice were purchased from Jackson laboratories (Bar Harbor, ME). Mice were maintained in the Wake Forest University School of Medicine animal facilities, under specific-pathogen-free conditions and in accordance with approved Animal Care and Use Committee (ACUC) protocols. Mice for these studies were between 6 and 10 weeks of age. Mice were infected with PIV5 (106 PFU), influenza virus (1,000 50% egg infective doses [EID50]), or vaccinia virus (107 PFU) by intratracheal (i.t.) instillation.
Intratracheal instillation of CTO.
For the analysis of migration kinetics, mice were infected with vaccinia virus intratracheally on day zero, and 24 h prior to each time point of MLN analysis, mice were anesthetized and 50 μl of 1 mM Cell Tracker Orange (CTO) (Molecular Probes) was delivered i.t. For the 24-h time point, mice received CTO 2 h following i.t. infection with vaccinia virus (107 PFU).
DC isolation from the MLN.
At the indicated day postinfection, MLN were isolated and pooled within each experimental condition. The tissue was mechanically disrupted and allowed to incubate in complete media supplemented with 1 mg/ml collagenase D (Roche) for 45 min at 37°C. Cells were then passed through a 70-μm nylon cell strainer (BD Falcon). Red blood cells (RBC) were removed by treatment with ACK lysis buffer (Lonza).
Analysis of DC maturation.
Cells were obtained from the MLN by collagenase digestion. For analysis of maturation, cells were stained with a combination of anti-CD11c-APC, -CD103-PE, -CD8-Pacific Blue, and either anti-CD80 or -CD86 conjugated to PerCP-Cy5.5. Cells were also negatively gated using anti-CD90.2. For phenotype analysis, cells were incubated with CD16/32 (to bind Fc receptors on the DC), followed by staining with antibodies to CD11c, CD90.2, CD103, CD8, CD205, and CD24. Data were analyzed using FacsDiva software (BD Biosciences).
Treatment with TLR agonists.
Twenty-four hours prior to MLN isolation, 10 μg of TLR agonist poly(I·C) (TLR3), LPS (TLR4), CL097 (TLR7), or CpG (TLR9) or 100 μg of purified lipoteichoic acid (LTA) from Staphylococcus aureus (endotoxin, <0.001 endotoxin units [EU]/μg) (all from Invivogen) was instilled i.t. in a 50-μl volume.
Naïve T cell activation.
Twenty-four hours prior to MLN isolation, 10 μg of poly(I·C) was delivered i.t. Alternatively, 48 h prior to MLN isolation, mice were infected with VV.NP-S-eGFP (kind gift of J. Bennink and J. Yewdell [NIH]). This virus expresses a fusion protein containing the NP protein from influenza virus, the SIINFEKL epitope from ovalbumin (Ova), and enhanced green fluorescent protein (eGFP) (26). Following isolation, MLN cells were incubated with 10−11 M Ova peptide for 2 h at 37°C followed by washing to remove unbound peptide. CD11c-expressing cells were enriched by positive selection using the Miltenyi column system (enriched populations were routinely 45 to 65% CD11c+). The enriched population was stained with CD11c-APC, CD8-PerCP-Cy5.5, CD103-PE, and CD103-PerCP-Cy5.5, along with biotinylated CD19, CD90.2, and CD49b antibodies (all from BD BioSciences). Streptavidin 525 Qdots (Molecular Probes) were used to detect biotinylated antibodies. Cells positive for the 525 Qdots were gated out of the analysis prior to sorting. Thus, all sorted cells met the criteria of CD11c+ CD90.2− CD49b− CD19− CD11c+ CD90.2− CD49b− CD19− cells were then sorted using a BD FACsAria into CD8+ or CD8− CD103+ populations. Sorted populations were routinely 94 to 99% pure. To assess the ability of the sorted DC subsets to induce naive T cell activation, CFSE-labeled OT-I T cells were cocultured with sorted DC populations at a ratio of 1:10 (DC:OT-I) in a V-bottomed, 96-well plate. Cells were incubated for 60 h at 37°C. Following incubation, OT-I T cells were analyzed for proliferation and cytokine production. Samples were acquired using a BD FACsCalibur and analyzed with FlowJo software (Treestar, Inc.).
Pinocytosis in vivo.
Lung-resident DC were labeled by administration of Cell Tracker Orange. Mice then received either 107 PFU vaccinia virus or 10 μg poly(I·C) with or without 60 μg fluorescein isothiocyanate-ovalbumin (FITC-Ova) by intratracheal instillation Two days following vaccinia virus infection or 1 day following poly(I·C) treatment, MLN were harvested, processed, and analyzed for the presence of FITC within each DC subset.
Statistical analysis.
Data were analyzed using either analysis of variance (ANOVA) or Student's t test as appropriate to determine significance. In cases where variance was found to be significantly different, Welch's correction was applied.
RESULTS
CD8+ CD103+ DC do not express CD8β or CD3 on their surface.
In our previous analyses of the DC subsets present in the lung-draining mediastinal lymph node (MLN) following viral infection, we observed a population of cells that coexpressed CD8 and CD103 (4). The expression of CD8 is well characterized as a marker of a spleen- and LN-resident DC subset that has properties that distinguish it from other lymph node-resident (nontrafficking) populations (for a review, see reference 31). The expression of CD8 on CD103+ DC in the MLN could result either from the upregulation of this molecule on a subset of cells or from membrane exchange following contact with CD8+ T cells in the MLN. To discriminate between these two possibilities, we assessed the expression of CD8α and β on the surface of the CD103+ DC in the MLN. CD8 expression by DC differs from T cells in that it is found exclusively as an αα homodimer, as opposed to an αβ heterodimer (31). Approximately 21% of the CD103+ DC present in the MLN expressed CD8α. In contrast, we found negligible expression of CD8β or CD3 on the CD103+ DC within the MLN (Fig. 1A). Further, CD8+ CD103+ DC were readily detected in Rag-deficient mice, which lack T lymphocytes (Fig. 1B). Together, these data establish the expression of CD8 on CD103+ DC in the lung-draining LN as an αα homodimer and suggest that expression is intrinsic to the cell as opposed to being derived from a membrane-sharing event.
Fig 1.
CD8+ CD103+ DC in the MLN do not express CD8β or CD3. Expression of CD8α, CD8β, and CD3 were analyzed in the CD103+ DC present in the MLN of naïve C57BL/6 (A) and Rag−/− (B) mice. Plots are pregated on CD11c+CD90.2− cells. Data are representative of three individual experiments.
CD8+ and CD8− CD103+ DC express similar levels of CD24 and CD205.
CD103+ DC in tissue are reported to express CD205 (10, 15, 19, 30) and CD24 (5, 14). CD205 (DEC205) is a mannose receptor important in endocytosis and subsequent antigen presentation (7, 13, 32). CD24 (also known as heat-stable antigen) is a variably glycosylated membrane protein reported to have costimulatory properties (2). It has also been routinely utilized as a marker of precursors that give rise to CD8+ DC (9, 24, 25). We thus determined whether changes in CD8 expression were associated with changes in these cell surface markers. Overall, we found similar expressions of these markers in the two subsets. CD205 was expressed on approximately 57.6 ± 0.15% of CD8− cells and 63.3 ± 0.9% of CD8+ cells (Fig. 2A and B). Furthermore, the level of expression of this marker did not vary (Fig. 2B).
Fig 2.
CD8+ CD103+ DC resemble CD8− CD103+ DC in the expression of CD205 and CD24. MLN from naïve C57BL/6 mice were harvested and pooled. CD8− CD103+ and CD8+ CD103+ DC were analyzed for the expression of CD205 and CD24. (A) Representative histograms. Solid lines represent staining for CD205 or CD24 as indicated. Isotype control antibody staining is shown in the broken lines. Averaged data for mean fluorescent intensity (MFI) values (B) and percent positive for CD205 and CD24 expression (C) are shown. Data are from three individual experiments, each containing 5 mice. Error bars represent the standard error of the mean (SEM). Statistical analysis was performed using the Student t test. *, P ≤ 0.05; ns, (not significant).
Both CD103+ DC subsets expressed CD24 on nearly 100% of their cells (Fig. 2A and C). CD8+ CD103+ DC had a significant but modest increase in the level of expression of CD24 compared to CD8− CD103+ DC (Fig. 2C). The expression profiles of CD205 and CD24 on MLN DC indicate that the program that results in expression of CD8 does not result in major alterations in the expression of these molecules.
The appearance of lung-derived CD8+ CD103+ DC in the MLN is delayed compared to the CD8− counterpart.
The phenotypic similarities between the CD8+ CD103+ DC and the CD8− CD103+ DC supported the hypothesis that the CD103+ DC in the lung upregulated CD8 during migration to or following entry into the MLN. One possibility was that the DC that upregulated CD8 were a random subset of all DC that entered the MLN. If this were the case, we would expect to see a similar proportion of CD103+ DC that migrated to the MLN expressing CD8 at all times following infection. Thus, we assessed the appearance of CD8+ CD103+ DC in the MLN over the course of infection. To accurately determine when DC were migrating, we assessed the appearance of DC within discrete 24-h periods, as opposed to the cumulative migration analysis routinely performed. To accomplish this, mice received Cell Tracker Orange by intratracheal instillation at 2, 24, 48, or 72 h postinfection with vaccinia virus. Our previous studies established that CTO resulted in minimal nonspecific migration of DC from the lungs to the MLN (4). Mice were euthanized 24 h following each labeling event (Fig. 3A). In all cases, the number of CTO+ DC in each subset was compared to the number in uninfected mice treated with CTO as a reference for baseline migration.
Fig 3.
CD8+ CD103+ DC appear in the MLN with delayed kinetics compared to CD8− CD103+ DC. Mice were infected with VV. Twenty-four hours prior to analysis of MLN DC (days 1 to 4), CTO was instilled i.t. (A) Schema of the experimental approach. (B) Analysis of the total number of CTO+ CD11c+ cells that migrated into the MLN during the time periods indicated on the x axis. (C) Number of CTO+ CD8+ or CD8− CD103+ DC that migrated into the MLN during the 24-h time period indicated. Data are the means for six animals, analyzed in two independent experiments. Error bars represent the standard error of the mean (SEM). The Student t test was used in panels B and C to compare each time point to the CTO-only value. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.005.
Within the first 24 h of infection, the total number of CTO+ DC in the MLN tripled compared to that in noninfected mice (Fig. 3B). The new influx of DC from the lung continued through the 24- to 48-h and 48- to 72-h time points. At its maximum, infection-induced migration resulted in a 4-fold increase in lung-derived DC migration to the MLN. By 96 h postinfection, the migration of DC from the lung was similar to baseline. These results revealed that infection resulted in continued migration of DC from the lung over the first 3 days of infection.
We next compared the migration kinetics of CD8+ and CD8− DC, within the CD103+ DC subset. There were more CTO+ CD8− CD103+ DC than CTO+ CD8+ CD103+ DC in the MLN at all time points. With regard to infection-induced migration of the two subsets, CTO CD8− CD103+ DC were readily detectable in the MLN at 24 h postinfection (Fig. 3C). In contrast, no increase in the number of CD8+ CD103+ DC was apparent at this early time point (Fig. 3C). CD8+ CD103+ DC began migrating to the MLN between 24 and 48 h p.i. with a significant increase detected between 48 and 72 h p.i. (Fig. 3C). Infection-induced migration of both CD8+ and CD8− CD103+ DC continued through 72 h. By 96 h, migration of the CD8− subset was not significantly different from that in noninfected animals. In contrast, infection-induced increases in the appearance of CD8+ CD103+ cells remained. Of note, at this late time point CD103+ DC account for a reduced percentage of the total lung-migrating DC, with CD11b+ DC accounting for the majority of new immigrants (data not shown). This is the result of continued migration of CD11b+ DC from the lung at this later time point. These data show that lung-derived CD103+ cells that express CD8 are delayed in their appearance in the MLN compared to those that remain CD8−.
CD8− CD103+ DC and CD8+ CD103− DC differ in T cell-activating potential depending on the environmental stimulus.
Our previous studies showed that in the context of vaccinia virus infection, CD103+ DC that express CD8 were inferior to their CD8− counterpart with regard to T cell activation (4). In those analyses CD8+ and CD8− CD103+ DC from mice infected with a vaccinia virus expressing the Ova257–264 epitope were isolated by sorting and cultured with OT-I cells, which bear a T cell receptor (TCR) specific for Ova257–264. These studies revealed a significant decrease in the ability of CD8+ CD103+ DC to induce proliferation compared to their CD8− counterpart (4). This was not the result of differences in antigen access, as CD8+ and CD8− CD103+ DC exhibited similar percentages of eGFP+ cells (our unpublished data). However, whether the decreased stimulatory potential following vaccinia virus infection was due to inherent differences in the capabilities of these two subsets or specific regulation following virus infection was unknown.
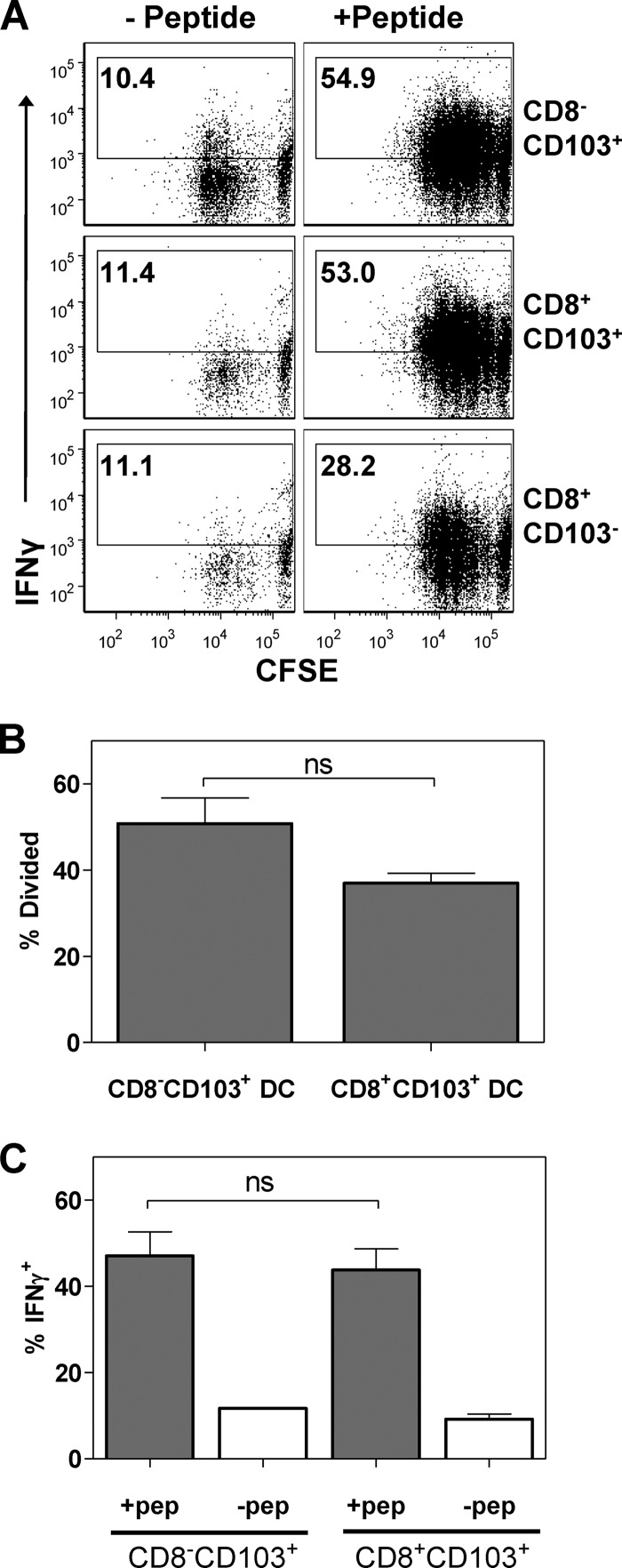
To directly address this possibility, we utilized poly(I·C) treatment as an alternative maturation stimulus based on the known expression of TLR3 on CD103+ DC (34). Twenty-four hours after poly(I·C) administration, MLN cells were isolated and pulsed with a limiting concentration, i.e., one that requires costimulatory signals for OT-I cells to become activated, of Ova257–264 peptide. CD8+ and CD8− CD103+ DC were then sorted followed by coculture with carboxyfluorescein succinimidyl ester (CFSE)-labeled OT-I T cells. Following a 3-day culture period, OT-I T cells were analyzed for CFSE dilution and the ability to produce gamma interferon (IFN-γ) following restimulation with peptide.
In contrast to what was observed with vaccinia virus infection-matured CD8+ CD103+ DC, poly(I·C)-matured CD8+ CD103+ DC stimulated division in OT-I cells as efficiently as CD8− CD103+ DC (Fig. 4A). Although there was a trend toward decreased division when CD8+ CD103+ DC were used, this did not reach statistical significance (Fig. 4B). Both DC types were also efficient inducers of effector function in responding OT-I cells. Approximately 45% of cells produced IFN-γ following restimulation with peptide (Fig. 4C). Further, there was no difference in the amount of IFN-γ produced on a per cell basis (data not shown). Thus, while the CD8+ CD103+ DC taken from mice infected with VV show a diminished capacity to stimulate naïve OT-I T cells ex vivo compared to the CD8− CD103+ DC, this does not appear to be the result of an inherent inability of CD8+ CD103+ DC to mature and subsequently activate T cells.
Fig 4.
CD8+ CD103+ DC are not inherently defective in the ability to stimulate proliferation and the acquisition of IFN-γ production in naïve CD8+ T cells. Mice received 10 μg poly(I·C) by intratracheal instillation. Twenty-four hours later, MLN cells were isolated, divided, and either pulsed with limiting 10−11 M Ova peptide (a concentration that relies on the presence of mature APC for activation) or left untreated. DC subsets of interest were isolated by sorting followed by culture with CFSE-labeled OT-I T cells. At the completion of the 3-day culture period, cells were restimulated with peptide and CFSE dilution and IFN-γ production was measured. Representative data are shown (A). Averaged data for proliferation (B) and IFN-γ production (C). The background for IFN-γ production was determined using OT-I T cells that had been cultured with non-peptide-pulsed DC. Data are derived from three independent experiments. Error bars represent the standard errors of the means (SEM). Significance was determined using Student's t test. ns, not significant.
CD8− CD103+ DC and CD8+ CD103+ DC do not differ in the capacity for antigen uptake.
An important aspect of T cell-activating potential is the capacity to take up antigen. As a measure of this capability, we assessed the uptake of FITC-conjugated ovalbumin by the two DC types. CTO administration was utilized to mark cells present in the lung at the time of FITC-Ova administration. Twenty-four hours post instillation, the percentage of CTO+ CD8− CD103+ DC and CTO+ CD8+ CD103+ DC in the MLN that were positive for the uptake of FITC-Ova was determined. When the CD8+ and CD8− subsets were compared, we found that these two cell types were comparable with regard to the percentage of FITC+ cells within the CTO+ lung migrating population (Fig. 5A). Further, there was no difference in the intensity of FITC on a per cell basis (data not shown).
Fig 5.

Antigen uptake is similar in CD8+ and CD8− CD103+ DC. Lung-resident DC were labeled by i.t. administration of CTO. Mice were subsequently infected with VV or instilled with poly(I·C). Two hours later, FITC-Ova was delivered i.t. Twenty-four hours following poly(I·C) instillation (A) or 48 h postinfection (B), CTO+ DC present in the MLN were analyzed for the presence of FITC. The presence of FITC+ cells was determined using infected mice that did not receive FITC-Ova to set the threshold for positivity. The graphs are averages of 2 independent experiments with a combined total of 6 animals. Error bars represent the SEM. Significance was assessed using Student's t test.
We then determined whether vaccinia virus infection impacted the ability of either of the DC subsets to take up FITC-Ova. Again, similar percentages of CD8+ and of CD8− CD103+ DC were positive for FITC (Fig. 5B). Thus, these two cell types did not differ in their capacity to take up antigen.
We also noted that there was no significant difference in uptake for a given subset following administration of poly(I·C) versus vaccinia virus infection, suggesting that the nature of the stimulus did not impact the capacity of the DC to take up antigen. As expected from the MLN analysis, evaluation of lung DC showed that a comparable percentage of CD103+ DC were FITC+ (approximately 30%) following treatment with poly(I·C) or infection with vaccinia virus (data not shown).
CD8+ CD103+ DC exhibit superior upregulation of CD86 and CD80 in response to TLR agonist stimulation compared to CD8− CD103+ DC.
The divergence in the stimulatory capacity of CD8+ CD103+ DC isolated from the MLN following vaccinia virus infection versus poly(I·C) treatment led us to assess the capacity of these two DC types to upregulate costimulatory markers in response to maturation stimuli. Thus, we tested the responsiveness of CD8+ and CD8− CD103+ DC to a variety of TLR agonists following delivery to the lung. We chose TLR agonists that represent viral infection, i.e., poly(I·C) (TLR3), CL097 (TLR7), and CpG (TLR9), as well as bacterial infection, i.e., lipopolysaccharide (LPS) (TLR4). TLR agonists were administered i.t., and 24 h later DC in the MLN were analyzed for expression of CD80 and CD86. Mock-treated mice that received phosphate-buffered saline (PBS) served as controls.
Compared to the PBS-treated mice, both DC subsets isolated from mice treated with poly(I·C), LPS, or CpG demonstrated a significant upregulation of CD80 and CD86 (Fig. 6). There was no significant difference in the percentages of DC expressing CD86 in the CD8− and in the CD8+ CD103+ DC following stimulation of poly(I·C), LPS, or CpG, with upwards of 94% of each subset expressing this molecule (Fig. 6B). We found no evidence for TLR7 agonist (CL097) responsiveness in CD103+ DC, regardless of CD8 expression. Although a similar percentage of DC upregulated CD86, when the level was assessed, CD8+ CD103+ DC were found to express significantly larger amounts of this molecule than did CD8− CD103+ DC (Fig. 6C).
Fig 6.
CD8+ CD103+ DC exhibit enhanced expression of costimulatory markers following TLR agonist stimulation. TLR agonists or PBS was delivered i.t., and 24 h later the DC subsets in the MLN were analyzed for expression of the costimulatory molecules CD80 and CD86. Representative data for CD86 (A) and CD80 (D) are shown. Broken lines represent the isotype control, and solid lines represent CD86 or CD80 expression, as indicated, following each treatment. Gray lines show levels of CD80 or CD86 following PBS treatment. Averaged data for the percentage of each DC type expressing CD86 (B) or CD80 (E) as well as the level of expression of each molecule on the positively expressing cells (C and F) are shown. Data are from three independent experiments. Error bars represent SEM. Statistical analysis was performed using a 2-way ANOVA with Bonferroni's adjustment posttest. **, P ≤ 0.01; ***, P ≤ 0.001; ns, not significant.
In contrast to what was observed for CD86, the percentage of DC expressing CD80 differed significantly between the subsets following treatment with the TLR agonists. A higher percentage of CD8+ CD103+ DC expressed CD80 compared to CD8− CD103+ DC (poly(I·C), 92.2% ± 1.0% and 71.4% ± 3.1%; CpG, 88.5% ± 3.2% and 61.2% ± 7.8%, respectively) following treatment with poly(I·C) or CpG (Fig. 6E). In agreement with what was observed for CD86, the level of CD80 on mature DC was significantly higher on CD8+ CD103+ than on CD8− CD103+ DC (Fig. 6F). These data show that while CD8+ and CD8− CD103+ DC respond to the TLR ligands poly(I·C), CpG, and LPS, they do so with differing intensity, with CD8+ CD103+ DC demonstrating increased maturation compared to CD8− CD103+ DC.
CD8+ CD103+ DC exhibit inferior maturation in response to vaccinia virus infection compared to CD8− CD103+ DC.
We next determined whether the maturation of CD8+ and CD8− CD103+ DC differed in vivo in response to VV infection. Mice were infected with VV, and 48 h later (a time previously shown to promote maximal levels of costimulatory molecule expression on CD103+ DC [4]), CD8+ and CD8− CD103+ DC were assessed for the expression of CD80 and CD86. Figure 7A and B shows that CD80 and CD86 were only modestly upregulated on CD8+ CD103+ compared to CD8− CD103+DC in response to infection. Thus, in contrast to what was observed following administration of the TLR agonists poly(I·C), CpG, or LPS, where CD8+ CD103+ DC showed superior maturation compared to CD8− CD103+ DC, VV-induced maturation was inferior in CD8+ CD103+ DC. Thus, the relative efficiency of maturation in the two subsets differed depending on the maturation stimulus.
Fig 7.
CD8+ CD103+ and CD8− CD103+ DC exhibit differential maturation following virus infection. Mice were infected with vaccinia virus, influenza virus, or PIV5. On day 2 postinfection, DC in the MLN were analyzed for the expression of CD80 and CD86. Averaged data for the level of CD80 following vaccinia virus (A) or PIV5 or influenza infection (C) and CD86 expression following vaccinia virus (B) or PIV5 or influenza infection (D) are shown. Error bars represent SEM. Statistical analysis was performed using Student's t test. *, P ≤ 0.05; **, P ≤ 0.001; ns, not significant.
CD8+ CD103+ DC undergo maturation in response to infection with influenza virus or PIV5.
One possibility to explain the above results was that CD8+ CD103+ DC cannot undergo maturation in response to virus infection of the respiratory tract. To test this possibility, mice were infected with parainfluenza virus 5 (PIV5) or influenza virus PR8. In contrast to what was observed with VV virus infection, CD8+ CD103+ DC exhibited robust upregulation of both CD80 and CD86 following infection with PIV5 that was similar to that observed in CD8− CD103+ DC (Fig. 7C and D). While infection with influenza virus resulted in similar responses in both subsets with regard to upregulation of CD80, CD8− cells exhibited superior upregulation of CD86 compared to CD8+ DC. Together, these data show that CD8+ CD103+ DC can undergo robust maturation following viral infection that can be equivalent to that of their CD8− counterpart, as was the case for PIV5. Further, the extent of infection-induced maturation in CD8+ CD103+ DC was dependent on the virus present.
CD8+ CD103+ DC exhibit inferior maturation compared to CD8− CD103+ DC following stimulation with the TLR2 ligand LTA.
Previous studies have reported TLR2 engagement as a critical mediator of DC maturation following intravenous (i.v.) infection with VV (38). Therefore, one possibility to explain the discrepancy in the CD8+ versus CD8− CD103+ subsets was differential responsiveness to TLR2 engagement. To test this possibility, we administered highly purified LTA to recipient mice by intratracheal instillation, similar to what was done for the TLR agonists tested above. CD80 and CD86 levels were similar on CD8+ CD103+ DC from treated and from untreated mice (Fig. 8A and B). In contrast, robust upregulation of CD80 and CD86 was observed on the CD8− subset, with CD80 levels exceeding that on CD8+ DC 2.7-fold and CD86 3.2-fold on average. The minimal responsiveness to LTA in CD8+ CD103+ DC suggested that the differential maturation between the subsets following vaccinia virus infection could be the result of differential TLR2 responsiveness in these two subsets.
Fig 8.
CD8− CD103+ DC but not CD8+ CD103+ DC are responsive to TLR2 agonists. LTA (100 μg) or PBS was delivered i.t., and 24 h later DC subsets in the MLN were analyzed for expression of CD80 (A) and CD86 (B). Data are from three independent experiments, each analyzing cells pooled from multiple mice. TLR2−/− or wild-type (WT) mice were infected with VV, and DC in the MLN were analyzed at 48 h p.i. for the expression of CD80 (C) and CD86 (D). TLR2−/− data are from two independent experiments, each analyzing cells pooled from multiple mice. Error bars represent SEM. Statistical analysis was performed using Student's t test. *, P ≤ 0.05; ns, not significant.
To directly test the possibility that CD8− CD103+ DC were maturing as a result of TLR2 engagement, we infected TLR2−/− mice and assessed maturation within the CD103+ populations 48 h later. Surprisingly, CD8− CD103+ DC from TLR2−/− mice exhibited maturation within this subset that was similar to that seen in TLR2-sufficient mice (Fig. 8C and D). This result suggested that airway-derived DC can undergo TLR2-independent maturation following infection with VV, seemingly utilizing an alternative pathway for this process.
Maturation of airway-derived DC occurs in the absence of MyD88.
The above results led us to question whether there was a requirement for MyD88-dependent signaling in CD103+ airway-derived DC following respiratory infection with VV. To address this issue, DC present in the MLN of MyD88-deficient mice were assessed at 48 h p.i. with VV. We observed some decrease in CD80 and CD86 in both DC subsets in the absence of MyD88 (Fig. 9). However, this reached significance only with regard to a decrease in the modest upregulation of CD86 that is present on CD8+ CD103+ DC following VV infection. These data show that the maturation of airway-derived DC in response to VV infection is relatively independent of MyD88 signaling pathways.
Fig 9.
Maturation of airway DC in response to respiratory infection with VV infection is largely independent of MyD88. MyD88-deficient or WT mice were intratracheally infected with vaccinia virus. On day 2 postinfection, DC in the MLN were analyzed for the expression of CD80 (A) and CD86 (B). Averaged data are shown. Error bars represent SEM from 3 independent experiments, each containing multiple mice that were pooled for analysis. Statistical analysis was performed using Student's t test. *, P ≤ 0.05; ns, not significant.
DISCUSSION
CD103+ airway DC play a critical role in the generation of an adaptive T cell response following viral infection of the respiratory tract. In this study, we provide the first functional characterization of a recently described subset (CD8+) within the CD103+ DC population that is present in lung-draining mediastinal lymph node. Our studies reveal five critical insights into this population. These cells (i) are derived from the lung and share close marker relationship with CD8− CD103+ DC; (ii) appear to be under the control of a distinct migratory program compared to their CD8− counterpart; (iii) show increased maturation in response to TLR ligand engagement by LPS, CpG, and poly(I·C) compared to CD8− CD103+ DC; (iv) are differentially responsive to TLR2 engagement; and (v) exhibit virus-specific regulation with regard to maturation following infection.
Although CD8+ CD103+ DC have been reported in several studies, there has been a lack of consistency with regard to whether the CD8+ CD103+ DC found in the MLN are grouped with CD103+ migrating DC or CD8+ lymph node-resident DC (for examples, see references 3, 10, 16, and 20). This has resulted in this distinct subset being analyzed with either the lung migratory or the LN-resident DC population. Consequently, this subset has not been carefully evaluated. Importantly, our data suggest that this is a subset with unique capabilities and should be considered independently.
CD8+ CD103+ DC appeared in the MLN with delayed kinetics compared to the CD8− population following infection with vaccinia virus. This delay was observed when migration was assessed only within the 24 h window prior to sample collection and thus does not reflect a requirement for CD103+ DC to reside in the MLN for an extended period of time for CD8 upregulation to occur. We would propose that the signal required for the expression of CD8 is delayed. The source of the signal may be the lung, received prior to migration, or it may be present in the MLN. Either tissue could experience a change in environment over the course of infection that results in the presence of the signal that triggers the differentiation program promoting CD8 expression. Alternatively, it is possible that DC differ in their ability to express CD8. If so, a population of DC with increased potential to upregulate CD8 may be recruited into the inflamed lung at later times postinfection and thus appear in the MLN with delayed kinetics.
What might induce CD8 expression in a subset of DC? In some DC populations, CD8 has been linked to maturation signals. For example, Langerhans cells upregulate CD8 expression following stimulation with CD40L (1). In humans, DC generated from peripheral blood monocytes responded to CD40L stimulation by increasing the expression of Batf3 (35), a known contributor to the differentiation of CD8+ LN-resident DC (18). While a model wherein CD40L-mediated increases in Batf3 drive a differentiation program that results in CD8 expression is attractive, our preliminary studies in CD40L−/− mice do not support a role for CD40 engagement in the acquisition of CD8 expression (our unpublished data). Further, mature CD8− CD103+ DC are clearly present in the lymph node following infection with vaccinia virus (4), demonstrating that the upregulation of CD8 is not a consequence of maturation. Thus, identification of the signals that promote expression of CD8 in CD103+ DC awaits further study.
With regard to the functional capabilities of CD8+ CD103+ DC, our data show that CD8+ and CD8− CD103+ DC can be equally potent for CD8+ T cell activation, as was the case with poly(I·C)-matured cells. The potential for CD8+ CD103+ cells to stimulate CD8+ T cell responses is in keeping with a recent study that evaluated a phenotypically similar population in the small intestine (15). Although the two DC subsets studied here are both capable of CD8+ T cell activation, it is clear that their ability to do so can be differentially regulated depending on the stimulatory environment. For example, following vaccinia virus infection, CD8+ CD103+ DC that migrate to the lung-draining lymph node are minimally stimulatory for CD8+ T cell activation (4). We show here that this reduced capacity following vaccinia virus infection is associated with reduced expression of CD80 and CD86 in these DC. In our analysis of VV-induced DC regulation, we assessed CD80 and CD86 expression as a general indicator of DC maturation. At this time, we cannot conclude that the reduced stimulatory capacity is solely the result of differences in these molecules. In fact, it is likely that other molecules fail to be efficiently upregulated in these antigen-presenting cells (APC), e.g., cytokines or additional costimulatory molecules.
Importantly, the CD8+ DC subset was not generally nonresponsive to virus-induced maturation, as infection with PIV5 virus resulted in similarly robust expression of CD80 and CD86 in the two subsets. Our findings with PIV5 suggest that CD8+ DC matured under these circumstances would be efficient activators of T cell responses. Experimental demonstration of this has proven challenging as a result of the low numbers of antigen-bearing DC in the MLN following PIV5 infection. In our preliminary studies, we have determined that the approach used to assess DC stimulatory capacity (as was used for vaccinia virus infection) lacks sufficient sensitivity to evaluate DC priming abilities in vitro following infection with PIV5, even though priming clearly occurs in vivo.
The regulation of maturation in the CD8+ CD103+ subset is complex. In contrast to the maturation patterns observed following exposure to vaccinia virus or PIV5, infection with influenza virus resulted in high levels of CD80 but more limited increases in CD86 compared to the CD8− subset. These data suggest a model wherein the two DC populations can both be effective activators of CD8+ T cells. However, they differentially sense individual pathogens, responding with an array of maturation phenotypes.
We had postulated that the suboptimal maturation in CD8+ CD103+ DC following vaccinia virus infection was the result of their poor responsiveness to TLR2 agonists, based on a previous study that reported dependence on TLR2 for VV-induced DC maturation following i.v. infection (38). However, a subsequent study reported MyD88-independent DC maturation when virus was administered i.p., suggesting TLR2 was not involved in this process (37). The latter result is in agreement with our findings that maturation of CD103+ DC, regardless of CD8 expression, occurs in the absence of TLR2 and MyD88. These data raise the possibility that the role of MyD88 in DC maturation is dependent on the route of infection. This likely reflects either intrinsic differences in the responding DC or in the environment present in the lymphoid organ in which the DC reside. The latter may include divergence in the induction of proinflammatory cytokines as a result of differences in the types of cells infected with virus. Quantitative or qualitative differences in inflammatory mediators may overcome the need for MyD88-dependent signaling.
An MyD88-independent mechanism for DC maturation is undoubtedly advantageous in the context of infection with VV, given the demonstrated ability of this virus to inhibit this pathway (6, 8, 11, 17, 29, 33). Inhibition of MyD88 occurs via interaction of A46 with TIR domain-containing proteins (8). In addition, vaccinia virus encodes a number of other proteins that can negatively impact TLR signaling. For example, B14 and N1L inhibit NF-κB activation by targeting IκB kinase β (IKKβ) (6, 11), while A52 disrupts signaling complexes by interaction with TRAF6 and IRAK2 (8, 17).
While our study is the first to compare CD103+ DC populations expressing CD8 in the lung, a recent study evaluating the expression of CD8+ CD103+ and CD8− CD103+ DC in the lamina propria identified differences in the expression of TLR molecules between these subsets, with the CD8+ subset expressing TLR3, -7, and -9 whereas the CD8− population expressed TLR5 and TLR9 (15). Interestingly, neither subset expressed TLR2. Although we also found differences in TLR responsiveness based on CD8 expression, they were distinct from those observed in gastrointestinal (GI) tract-resident DC. This finding suggests site-specific regulation of TLR agonist responsiveness in DC populations that are phenotypically similar.
In conclusion, here we provide a broad analysis of the functional significance of CD8 expression on airway-derived CD103+ DC, a critical APC in the generation of T cell responses. We found that the expression of CD8 marks a subset of airway DC that behaves differently with regard to maturation and migration following virus infection and TLR stimulation. Specifically, CD8+ and CD8− CD103+ DC exhibited strikingly different patterns of CD80 and CD86 upregulation following respiratory infection with VV versus PIV5 versus influenza virus. Further, while both DC types had the capacity to activate CD8+ T cells, the efficiency with which they did so was dependent on the nature of the maturation stimulus. Our findings suggest that both CD8+ and CD8− CD103+ DC are likely to contribute to regulation of the antiviral CD8+ T cell response. However, their role in this process will presumably differ depending on the infecting pathogen.
ACKNOWLEDGMENTS
We thank Jack Bennink and Jonathon Yewdell for provision of the VV.NP-S-eGFP, Jim Wood and Beth Holbrook for help in sorting DC populations, and Beth Hiltbold Schwartz for helpful discussion regarding the manuscript.
This work was supported by National Institutes of Health grants P01 AI060642 (to Steven Mizel, M.A.A.-M. project leader) and R01 HL071985 (to M.A.A.-M.).
Footnotes
Published ahead of print 18 July 2012
REFERENCES
- 1. Anjuere F, Martinez del Hoyo G, Martin P, Ardavin C. 2000. Langerhans cells acquire a CD8+ dendritic cell phenotype on maturation by CD40 ligation. J. Leukoc. Biol. 67:206–209 [DOI] [PubMed] [Google Scholar]
- 2. Askew D, Harding CV. 2008. Antigen processing and CD24 expression determine antigen presentation by splenic CD4+ and CD8+ dendritic cells. Immunology 123:447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ballesteros-Tato A, Leon B, Lund FE, Randall TD. 2010. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8+ T cell responses to influenza. Nat. Immunol. 11:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beauchamp NM, Busick RY, Alexander-Miller MA. 2010. Functional divergence among CD103+ dendritic cell subpopulations following pulmonary poxvirus infection. J. Virol. 84:10191–10199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belz GT, et al. 2004. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc. Natl. Acad. Sci. U. S. A. 101:8670–8675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benfield CT, et al. 2011. Mapping the IkappaB kinase beta (IKKbeta)-binding interface of the B14 protein, a vaccinia virus inhibitor of IKKbeta-mediated activation of nuclear factor kappaB. J. Biol. Chem. 286:20727–20735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonifaz LC, et al. 2004. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med. 199:815–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bowie A, et al. 2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. U. S. A. 97:10162–10167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caminschi I, et al. 2008. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood 112:3264–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. del Rio ML, Rodriguez-Barbosa JI, Kremmer E, Forster R. 2007. CD103− and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J. Immunol. 178:6861–6866 [DOI] [PubMed] [Google Scholar]
- 11. DiPerna G, et al. 2004. Poxvirus protein N1L targets the I-kappa B kinase complex, inhibits signaling to NF-kappa B by the tumor necrosis factor superfamily of receptors, and inhibits NF-kappa B and IRF3 signaling by Toll-like receptors. J. Biol. Chem. 279:36570–36578 [DOI] [PubMed] [Google Scholar]
- 12. Dunne PJ, Moran B, Cummins RC, Mills KH. 2009. CD11c+CD8alpha+ dendritic cells promote protective immunity to respiratory infection with Bordetella pertussis. J. Immunol. 183:400–410 [DOI] [PubMed] [Google Scholar]
- 13. East L, Isacke CM. 2002. The mannose receptor family. Biochim. Biophys. Acta 1572:364–386 [DOI] [PubMed] [Google Scholar]
- 14. Edelson BT, et al. 2010. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J. Exp. Med. 207:823–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujimoto K, et al. 2011. A new subset of CD103+CD8alpha+ dendritic cells in the small intestine expresses TLR3, TLR7, and TLR9 and induces Th1 response and CTL activity. J. Immunol. 186:6287–6295 [DOI] [PubMed] [Google Scholar]
- 16. GeurtsvanKessel CH, et al. 2008. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J. Exp. Med. 205:1621–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harte MT, et al. 2003. The poxvirus protein A52R targets toll-like receptor signaling complexes to suppress host defense. J. Exp. Med. 197:343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hildner K, et al. 2008. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322:1097–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jelinek I, et al. 2011. TLR3-specific double-stranded RNA oligonucleotide adjuvants induce dendritic cell cross-presentation, CTL responses, and antiviral protection. J. Immunol. 186:2422–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim TS, Braciale TJ. 2009. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One 4:e4204 doi:10.1371/journal.pone.0004204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lukens MV, Kruijsen D, Coenjaerts FEJ, Kimpen JLL, van Bleek GM. 2009. Respiratory syncytial virus-induced activation and migration of respiratory dendritic cells and subsequent antigen presentation in the lung-draining lymph node. J. Virol. 83:7235–7243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGill J, Van Rooijen N, Legge KL. 2008. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J. Exp. Med. 205:1635–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McGill J, Van Rooijen N, Legge KL. 2010. IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection. J. Exp. Med. 207:521–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Naik SH, et al. 2006. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat. Immunol. 7:663–671 [DOI] [PubMed] [Google Scholar]
- 25. Naik SH, et al. 2005. Cutting edge: generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J. Immunol. 174:6592–6597 [DOI] [PubMed] [Google Scholar]
- 26. Norbury CC, Malide D, Gibbs JS, Bennink JR, Yewdell JW. 2002. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat. Immunol. 3:265–271 [DOI] [PubMed] [Google Scholar]
- 27. Pascual DW, Wang X, Kochetkova I, Callis G, Riccardi C. 2008. The absence of lymphoid CD8+ dendritic cell maturation in L-selectin-/- respiratory compartment attenuates antiviral immunity. J. Immunol. 181:1345–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qiu CH, et al. 2009. Novel subset of CD8α+ dendritic cells localized in the marginal zone is responsible for tolerance to cell-associated antigens. J. Immunol. 182:4127–4136 [DOI] [PubMed] [Google Scholar]
- 29. Seet BT, et al. 2003. Poxviruses and immune evasion. Annu. Rev. Immunol. 21:377–423 [DOI] [PubMed] [Google Scholar]
- 30. Segura E, Villadangos JA. 2009. Antigen presentation by dendritic cells in vivo. Curr. Opin. Immunol. 21:105–110 [DOI] [PubMed] [Google Scholar]
- 31. Shortman K, Heath WR. 2010. The CD8+ dendritic cell subset. Immunol. Rev. 234:18–31 [DOI] [PubMed] [Google Scholar]
- 32. Shrimpton RE, et al. 2009. CD205 (DEC-205): a recognition receptor for apoptotic and necrotic self. Mol. Immunol. 46:1229–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stack J, et al. 2005. Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J. Exp. Med. 201:1007–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sung SS, et al. 2006. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J. Immunol. 176:2161–2172 [DOI] [PubMed] [Google Scholar]
- 35. Tureci O, et al. 2003. Cascades of transcriptional induction during dendritic cell maturation revealed by genome-wide expression analysis. FASEB J. 17:836–847 [DOI] [PubMed] [Google Scholar]
- 36. Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. 2001. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J. Exp. Med. 193:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao Y, et al. 2009. The adaptor molecule MyD88 directly promotes CD8 T cell responses to vaccinia virus. J. Immunol. 182:6278–6286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu J, Martinez J, Huang X, Yang Y. 2007. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood 109:619–625 [DOI] [PMC free article] [PubMed] [Google Scholar]