Abstract
HIV controllers are rare individuals who spontaneously control HIV replication in the absence of antiretroviral therapy. To identify parameters of the CD4 response that may contribute to viral control rather than merely reflect a persistently low viremia, we compared the T helper profiles in two groups of patients with more than 10 years of viral suppression: HIV controllers from the Agence Nationale de Recherche sur le SIDA et les Hépatites Virales (ANRS) CO18 cohort (n = 26) and efficiently treated patients (n = 16). Cells specific for immunodominant Gag and cytomegalovirus (CMV) peptides were evaluated for the production of 10 cytokines and cytotoxicity markers and were also directly quantified ex vivo by major histocompatibility complex (MHC) class II tetramer staining. HIV controller CD4+ T cells were characterized by a higher frequency of gamma interferon (IFN-γ) production, perforin+/CD107a+ expression, and polyfunctionality in response to Gag peptides. While interleukin 4 (IL-4), IL-17, and IL-21 production did not differ between groups, the cells of treated patients produced more IL-10 in response to Gag and CMV peptides, pointing to persistent negative immunoregulation after long-term antiretroviral therapy. Gag293 tetramer-positive cells were detected at a high frequency (0.12%) and correlated positively with IFN-γ-producing CD4+ T cells in the controller group (R = 0.73; P = 0.003). Tetramer-positive cells were fewer in the highly active antiretroviral therapy (HAART) group (0.04%) and did not correlate with IFN-γ production, supporting the notion of a persistent immune dysfunction in HIV-specific CD4+ T cells of treated patients. In conclusion, HIV controllers maintained a population of highly efficient Th1 effectors directed against Gag in spite of a persistently low antigenemia, while patients treated in the long term showed a loss of CD4 effector functions.
INTRODUCTION
HIV controllers are rare individuals who spontaneously control HIV replication in the absence of antiretroviral therapy. They can maintain a viral load below the detection threshold of standard assays (<50 copies HIV RNA/ml plasma) for decades and show a very low risk of progression to AIDS (4, 23). Converging evidence indicates that HIV controllers have developed particularly efficient cellular antiviral responses, which can maintain an active control of the infected target cell population in the long term. HIV controller CD8+ T cells have the capacity to efficiently suppress HIV replication in autologous CD4+ T cells in vitro (48, 64) and are thought to play a key role in HIV control (reviewed in references 1, 3, 15, and 28).
The contribution of CD4+ T cells to HIV control remains more debated (9, 34, 45, 56). In response to HIV antigens, HIV controllers maintain a central memory CD4+ T cell population with a remarkable proliferative capacity, which is associated with an efficient secretion of interleukin 2 (IL-2) (19, 26, 58, 81) and with the inactivation of proapoptotic molecules (73). A high proliferative capacity of HIV-specific CD4+ T cells can also persist in patients who were treated very early in the primary infection stage (62). However, most of the treated patients with good CD4 proliferation and IL-2 secretion failed to control HIV replication upon therapy interruption (34, 40), indicating that IL-2 responses alone were insufficient to mediate HIV control. Several studies have since indicated that the CD4 response pattern in HIV controllers is qualitatively distinct from that of efficiently treated patients and is not just the consequence of a very low antigenemia. Controller CD4+ T cells have proven to be more “polyfunctional” than those of treated patients, indicating a greater capacity to produce the cytokines/chemokines IL-2, gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α) and macrophage inflammatory protein 1 beta (MIP-1β) simultaneously in the circulation (16, 19, 36, 52, 58), as well as in mucosal tissues (20). The expression of the negative costimulatory molecule cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4) is lower on HIV-specific CD4+ T cells of controllers than on those of treated patients, suggesting that T cell receptor (TCR)-dependent activation may be more efficient (39). In addition, we recently reported the presence of a CD4+ T cell population with a high avidity for immunodominant Gag peptides in HIV controllers, indicating the capacity to respond to minimal amounts of virus (74). All these factors may promote efficient CD4 helper function, which could then sustain a high-quality CD8 response. Mouse models of chronic viral infections indicate that CD4+ T cell help is needed for efficient antiviral CD8 responses and that CD4+ T cell depletion causes virus-specific CD8+ T cells to adopt an exhausted phenotype similar to that seen in progressive HIV infection (78). A key issue that remains is to pinpoint, among the spectrum of possible CD4+ T cell functions, those that may contribute to HIV control.
The T helper differentiation of HIV-specific CD4+ T cells, defined by the pattern of cytokines produced upon antigenic stimulation, has been characterized in viremic and treated patients but has not been fully explored in HIV controllers. The expanding diversity of T helper types, including Th1, Th2, and Th17 and follicular helper T cells (Tfh), as well as the recent recognition of plasticity in T helper differentiation (reviewed in references 22, 82, and 85) warrant further investigation of this issue. Production of the Th1 cytokine IFN-γ has been consistently detected in specific CD4+ T cells from all categories of HIV-infected patients, but its contribution to the suppression of HIV replication remains unclear, with high IFN-γ responses found at both extremities of the progression spectrum, in HIV controllers as well as in highly viremic patients (16, 20, 55, 58). HIV-specific CD4+ T cells producing IL-17 have been detected in acute HIV infection, but were few or absent afterwards in viremic and treated patients (5, 84). There have been no reports so far of the presence of HIV-specific Th17 cells in HIV controllers. However, HIV infection is known to have a clear impact on the total Th17 population, which is preferentially depleted in the blood and intestinal mucosa of progressor patients (5, 42, 46, 49, 59), but not of HIV controllers (12, 67). Th17 depletion in progressor patients may play a role in the chronic immune activation characteristic of HIV disease through a loss of cytokines that promote the regeneration of epithelial barriers, leading to compromised mucosal integrity and allowing the crossing of inflammatory bacterial components (16).
The cytokine IL-21, which is produced almost exclusively by CD4+ T cells, promotes B cell memory through the induction of B cell isotype class switching and survival (75). Models of chronic viral infection in mice have revealed the importance of IL-21 in promoting the antiviral functions of CD8+ T cells, in addition to its role in humoral responses (18, 79). Recent studies have highlighted the possible contribution of this cytokine to HIV control, with the findings that IL-21 in plasma correlates positively with CD4 counts (33) and that IL-21 secreted by HIV-specific CD4+ T cells could induce a cytotoxic differentiation program with perforin induction in cognate CD8+ T cells (11). Whether the frequencies of IL-21-producing specific CD4+ T cells differ in HIV controllers and other patient groups remains debated, with one study showing an increase in the controller group (11) and two studies showing equivalent or lower levels (77, 83). However, in the population of patients with detectable viremia, the persistence of IL-21-producing CD4+ T cells is clearly associated with a beneficial outcome, as indicated by an inverse correlation with viral load (11, 83) and a positive correlation with the frequency of IL-2+-specific CD8+ T cells (77).
We set out to characterize the T helper effector profile of HIV controller CD4+ T cells by measuring the expression of 10 cytokines, chemokines, or cytotoxicity markers (perforin, CD107a) by intracellular staining. In addition, we implemented the direct detection of HIV-specific CD4+ T cells by major histocompatibility complex (MHC) class II tetramer staining ex vivo to obtain information on their frequency and phenotype without the perturbations associated with in vitro activation. While the use of MHC class I tetramers for identifying HIV-specific CD8+ T cells is widespread, few studies have made use of the MHC class II tetramer reagents, mostly due to the lower frequency of HIV-specific CD4+ T cells and to the complexity involved in producing stable recombinant HLA-DR molecules. These hurdles are being overcome through the use of magnetic concentration or high-speed acquisition of CD4+ T cells, as well as progress in HLA class II expression strategies (13, 43, 47). Two pioneer studies found that CD4+ T cells specific for Gag p24 epitopes could be detected by class II tetramer staining in HIV-infected patients under therapy, at low but detectable frequencies (between 10−4 and 10−5), and that short-term therapy interruption led to a rebound in the frequency of tetramer-positive (Tet+) CD4+ T cells (68, 69). In contrast, prolonged viremia ultimately led to the loss of the Tet+ population, suggesting the physical depletion of Gag-specific CD4+ T cells in progressor patients (69). We measured the frequency of Tet+ CD4+ T cells in HIV controllers to test the notion that these rare patients could maintain a large population of HIV-specific CD4+ T cells in spite of low antigenemia. The Gag p24 protein has been shown to harbor immunodominant peptides that can be recognized by up to half of HIV-infected patients, due in part to the promiscuous binding of these peptides to diverse HLA-DR and DQ molecules (35, 38, 60). By amplifying p24 Gag-specific CD4+ T cells in primary cell lines, we showed previously that the peptide spanning amino acids 293 to 312 (Gag293) induced a memory response in >90% of HIV controllers and treated patients who expressed at least one of 7 common HLA-DR alleles (74). This broad recognition gave us the opportunity to compare the frequencies and phenotypes of Gag293-specific cells by MHC class II tetramer staining in a cohort of HIV controllers and in patients who had received efficient antiretroviral therapy in the long term. Both groups were characterized by viral suppression (<50 copies of HIV-1 RNA/ml) for over 10 years, allowing a comparative analysis of CD4 responses in two situations of persistently low antigenemia.
Comparison of these two groups of patients showed that the HIV controller status was associated with a higher frequency of Th1 effectors, but not of Th2, Th17, or IL-21+ effectors. The only cytokine with a higher production in the treated patient group was IL-10, suggesting the presence of a negative immunoregulatory state in the CD4+ T cell compartment in spite of pharmacological viral suppression. MHC class II tetramer analysis showed that Gag293-specific CD4+ T cells persisted at a high frequency (10−3) in HIV controllers but also in a subset of treated patients. The frequency of Tet+ cells correlated positively with IFN-γ production in the controller group but not in the treated patient group, indicating high CD4+ T cell functionality in HIV controllers and persistent immune dysfunction of the HIV-specific CD4+ T cell compartment in patients treated in the long term.
MATERIALS AND METHODS
Patients.
The HIV controller (HIC) group (n = 26) was recruited through the CO18 cohort set up by the Agence Nationale de Recherche sur le SIDA et les Hépatites Virales (ANRS). In the present study, HIV controllers were defined as HIV-1-infected patients who had been seropositive for >10 years, had received no antiretroviral treatment, and for whom >90% of plasma viral load measurements were undetectable by standard assays. All HIV controllers included in the present study had current viral loads of <40 copies/ml. The group of efficiently treated patients (highly active antiretroviral therapy [HAART] group; n = 16) had received antiretroviral therapy for a minimum of 10 years and showed long-term viral suppression with viral loads of <40 copies of HIV RNA/ml. Treated patients were recruited through the ANRS Seroco-Hemoco cohort, the Raymond Poincaré Hospital (Garches, France), and the Bicêtre Hospital (Le Kremlin-Bicêtre, France). The healthy donor (HD) group (n = 12) consisted of anonymous healthy individuals who donated blood at the Etablissement Français du Sang (EFS) (Paris, France). The study was promoted by the ANRS under number EP36 and approved by the Comité de Protection des Personnes IDF VII under number 05-22. All participants gave written informed consent prior to blood sampling.
Whole-blood phenotyping.
Expression of the activation markers HLA-DR and CD38 were evaluated in CD4+ T cell subsets by phenotyping in 100 μl whole blood. Red blood cells were lysed with Lyse/Fix buffer (BD Biosciences, San Jose, CA) and remaining leukocytes were labeled with the following antibodies: anti-CD4-phycoerythrin (PE)-Texas Red (Beckman Coulter, Fullerton, CA), anti-CD3-allophycocyanin (APC)-eFluor780, anti-CD38-PerCP-eFluor710, anti-HLA-DR-PE-Cy7 (eBioscience, San Diego, CA), anti-CD45RA-Horizon V450 and anti-CCR7-Alexa Fluor 647 (AF647, BD Biosciences). Fluorescence was collected on a 9 color CyAn flow cytometer (Beckman Coulter) using Summit version 4.3 software.
Intracellular cytokine staining assay.
HIV-specific stimulation was performed by adding a 20-mer Gag peptide (Gag293-312, Gag263-282, or Gag161-180) at 5 μg/ml to 2 × 106 freshly isolated peripheral blood mononuclear cells (PBMC), in the presence of antibodies to the costimulatory molecules CD28 (0.5 μg/ml) and CD49d (0.5 μg/ml). CMV-specific stimulation was performed by adding an optimal pp65 20-mer peptide chosen in the function of the HLA-DR genotype of the patient as previously described (74). Negative controls consisted of 2 × 106 unstimulated PBMC. Positive controls were obtained by stimulating 2 × 106 PBMC with 1 μg/ml of the superantigen Staphylococcal Enterotoxin B (Toxin Technology, Sarasota, FL). Cells were stimulated for 14 h in the presence of the secretion inhibitors monensin (2 μM) and brefeldin A (3 μg/ml). Cells were harvested, split in three, and stained for surface antigens with the following antibodies: anti-CD107a-fluorescein isothiocyanate (FITC) (BD Biosciences), anti-CD3-APC-eFluor780 (eBioscience), and anti-CD4-PE-Texas Red (Beckman Coulter). The Aqua Live/Dead viability dye (Invitrogen) was added to exclude dead cells. Cells were fixed and permeabilized using a fix/perm kit (eBioscience) according to the manufacturer's instructions. Intracellular cytokine staining was detected with anti-IL-21-PE, anti-IL-4-PE, anti-MIP-1β-PE, anti-IFN-γ-PE-Cy7, anti-IL-2-APC (all from BD Biosciences), anti-IL-17-AF488, anti-IL-10-eFluor450, anti-TNF-α-eFluor450 (all from eBioscience), and anti-Perforin-PerCP clone B-D48 (Gen-PROBE). Fluorescence was collected on a CyAn flow cytometer, with a minimum of 250,000 events collected in the lymphocyte gate for each sample.
Flow cytometry data analysis.
Flow cytometry data were analyzed using FlowJo software (version 8.8.7; Tree Star, Ashland, OR). Intracellular cytokine production was evaluated in the live (Aqua−) CD3+ CD4+ lymphocyte gate. The percentage of cytokine-producing CD4+ T cells was determined after subtracting the percentage of cytokine-positive events in unstimulated controls. Responses below the level of 0.01% cytokine-producing cells after background subtraction were considered negative.
Polyfunctionality was assessed for a first panel of 5 functions that included the production of IFN-γ, IL-2, MIP-1β, TNF-α, and CD107a. The second panel included 4 functions, corresponding to the production of IFN-γ, IL-10, IL-17, and IL-21. For each panel, the combinations of all Boolean gates were generated in Flowjo, and the data were converted in Microsoft Excel to the matrix symmetric positive definite (SPD) format. The data were then analyzed and graphically represented using SPICE software version 5.21, downloaded from http://exon.niaid.nih.gov. The threshold for cytokine positivity was 0.01%. Comparison of distributions was performed using Student's t test and a partial permutation test as described previously (61).
MHC class II tetramer labeling.
At initiation of the study, HIV controllers and treated patients were genotyped for HLA-DRB1. Patients were included in the tetramer study if their genotype matched at least one of the 6 HLA-DRB1 alleles available for MHC class II tetramer studies. This panel allowed the analysis of ≥70% of Caucasian patients (10). PE-labeled tetramers for the DRB1*0101, DRB1*0301, DRB1*1501 and DRB5*0101 alleles were obtained through the NIH Tetramer Facility at Emory University. HLA-DRB1*0401, DRB1*0701, and DRB1*1101 biotinylated monomers were produced in insect cell cultures as previously described (44, 51). Monomers were loaded with 0.2 mg/ml peptide by incubation at 37°C for 72 h in the presence of 2.5 mg/ml n-octyl-β-d-glucopyranoside and 1 mM Pefabloc SC (Sigma-Aldrich). Peptide-loaded monomers were tetramerized using APC- or PE-conjugated streptavidin (eBioscience). To each tetramer loaded with an HIV-1 Gag or a CMV pp65 peptide corresponded a control tetramer loaded with the class II-associated invariant chain peptide (CLIP).
CD4+ T cells specific for the Gag293 peptide FRDYVDRFYKTLRAEQASQE (spanning amino acids 293 to 312 in HIV-1 Gag) were detected by ex vivo MHC class II tetramer labeling for all the patients included in the tetramer study. CD4+ T cells specific for CMV pp65 were detected with tetramers loaded with an optimal peptide chosen in the function of the HLA-DR genotype of the patient, as previously described (74). The CMV peptides used corresponded to amino acids 41 to 55, 177 to 191, 281 to 295, 489 to 507, and 509 to 523 within the pp65 protein.
For each MHC class II tetramer labeling, 6 × 106 PBMC were incubated with 4 μg/ml PE-conjugated tetramer for 90 min at +4°C in phosphate-buffered saline (PBS) with 1% bovine serum albumin (BSA) and 10 mM sodium azide. Surface marker antibodies CD3-AF780-APC and CD38-PerCP-eFluor710 (from eBioscience), CD45RA-V450 Horizon, CD8-FITC, CD14-FITC, CD19-FITC, and CCR7-AF647 (all from BD Biosciences), CD4-PE-Texas Red (Beckman Coulter), and the Aqua Live/Dead viability dye were added for the last 20 min of labeling. When APC-conjugated tetramers were used, CCR7-PE (BD Biosciences) was used instead of CCR7-AF647. All events were acquired on a CyAn flow cytometer at an average speed of 1,000 events/s and were analyzed using the Flowjo software. The percentage of tetramer-positive (Tet+) cells was measured in the live (Aqua−) CD3+ CD4+ CD8− CD14− CD19− gate. Negative controls consisted of identical samples labeled with HLA-DR-matched tetramers loaded with the CLIP. The percentage of Gag293-specific Tet+ cells was computed by subtracting the percentage of CLIP Tet+ cells from that of Gag293 Tet+ cells. We verified that CD4+ T cells from healthy donors matched for HLA-DRB1 gave minimal staining (≤0.01%) with Gag293 tetramers. Due to limiting blood samples, not all patients could be tested by both tetramer and intracellular cytokine staining (ICS) assays.
Statistical analysis.
Statistical analyses were performed with the GraphPad Prism 5.0 software, using nonparametric statistical tests in all cases. Differences in variables between groups were analyzed with the Mann-Whitney U test. Correlations were analyzed with Spearman's coefficient R. All significant differences between groups (P < 0.05) are reported on data plots.
RESULTS
Immune activation of CD4+ T cells in HIV controllers and treated patients.
We set out to compare CD4+ T cell phenotypes and functions in two groups of HIV-1-infected patients characterized by long-term control of HIV infection (Table 1). The HIV controllers (HIC group, n = 26) had shown a spontaneous control of HIV replication for over 10 years, while treated patients (HAART group, n = 16) had received efficient antiviral therapy that led to pharmacological control of HIV for over 10 years. Patients in both groups had an undetectable viral load by standard assays (<40 copies HIV-1 RNA/ml plasma). Both groups were also comparable in terms of patient age (median, 52 years in the HIC group versus 49 years in the HAART group), of the estimated duration of HIV-1 infection (median of 18 years in both groups), of CD4+ T cell counts (median, 754/mm3 in the HIC group versus 765/mm3 in the HAART group), and of CD4+ T cell percentages (median, 53.6% in the HIC group versus 49.9% in the HAART group). The treated patients had a median CD4 nadir of 227 cells/mm3, which was slightly higher than that reported for historical controls in observational cohorts (17, 41) and may have been a factor in the efficient immune reconstitution in these patients (80). The similarly high CD4+ T cell counts in the HIC and HAART groups at the time of study ensured that immune differences due to the homeostatic response to CD4 lymphopenia were minimal.
Table 1.
Epidemiologic and virologic characteristics of patientsa
| Characteristic | HIV controllers (HIC, n = 26) | Treated patients (HAART, n = 16) | P value |
|---|---|---|---|
| Age (yr) | 52 (37–69) | 49 (35–60) | NS |
| Duration of HIV-1 infection (yr) | 18 (10–28) | 18 (10–22) | NS |
| Duration of antiretroviral treatment (yr) | NA | 10 (10–14) | |
| Virus load (HIV-1 RNA copies/ml plasma) | <40 | <40 | NS |
| CD4+ T cells (per mm3) | 754 (315–1976) | 765 (422–1534) | NS |
| % of CD4+ T cells in CD3+ T cells | 53.6 (31.4–72.1) | 49.9 (15.4–79.1) | NS |
| Nadir of CD4+ T cells (per mm3) | NA | 227 (6–589) | |
| % with HLA B57 or B27 (no. positive/no. tested) | 65.4 (17/26) | 10.0 (1/10) | 0.0036 |
Median (range) values are listed unless otherwise noted. P values were estimated with the nonparametric Mann-Whitney U test. NS, not significant (P ≥ 0.05); NA, not applicable.
Immune activation of CD4+ T cells, as measured by the combined expression of HLA-DR and CD38, did not differ significantly between the HIC and HAART groups (Table 2). Analyses of the percentages of HLA-DR+ CD38+ cells in the naive (Nv), central memory (CM), and effector memory (EM) CD4+ T cell subsets defined by the CD45RA and CCR7 markers did not reveal significant differences either (Table 2). However, both HIC and HAART CD4+ T cells showed signs of immune activation compared to those of healthy blood donors (HD). In particular, expression of HLA-DR and CD38 was significantly increased in the CD4+ EM compartment of both patients groups compared to the HD group (P = 0.01 for HIC versus HD; P = 0.0005 for HAART versus HD) (Table 2). Thus, there were signs of persisting immune activation in HIV controllers and treated patients in spite of the long duration of viral suppression and the relatively high CD4+ T cell counts in both groups.
Table 2.
Immune activation in CD4+ T cellsa
| Cells | % of HLA-DR+ CD38+ cells in: |
P value, HIC vs HAART | P value, HIC vs HD | P value, HAART vs HD | ||
|---|---|---|---|---|---|---|
| HIV controllers (HIC, n = 16) | Treated patients (HAART, n = 16) | Healthy donors (HD, n = 12) | ||||
| Total T CD4+ cells | 2.5 (1.3–11.9) | 3.5 (0.5–18.2) | 1.5 (0.9–2.2) | NS | 0.01 | 0.002 |
| Naive CD45RA+ CCR7+ T CD4+ cells | 1.2 (0.6–11.0) | 1.9 (0.0–6.3) | 1.1 (0.3–3.1) | NS | NS | NS |
| CM CD45RA− CCR7+ T CD4+ cells | 1.9 (0.8–5.9) | 2.2 (0.4–20.7) | 1.4 (0.7–2.9) | NS | NS | 0.03 |
| EM CD45RA− CCR7− T CD4+ cells | 3.8 (1.1–16.4) | 5.5 (0.5–11.9) | 1.9 (0.7–2.5) | NS | 0.01 | 0.0005 |
Median (range) values are listed. P values were estimated with the nonparametric Mann-Whitney U test. CM, central memory; EM, effector memory; NS, not significant.
Th1 differentiation of Gag-specific CD4+ T cells in HIV controllers.
To determine the T helper phenotype of Gag-specific CD4+ T cells, we analyzed their cytokine production pattern by intracellular staining. Responses were measured to three peptides (Gag293, Gag263, and Gag161) that induce immunodominant CD4 responses in a majority of HIV-1 infected patients (35, 38, 60, 74). The panel of cytokines analyzed included those typical of Th1 cells (IFN-γ, TNF-α, MIP-1β), Th2 cells (IL-4), and Th17 cells (IL-17). IL-21 was included as a marker of Th17 and recirculating Tfh cells. IL-2 was included to detect cells with good proliferative capacity and IL-10 to detect cells with immunoregulatory function. The combination of total perforin expression (clone D48) and of the degranulation marker CD107a was used to detect CD4+ T cells with cytotoxic potential. Negative controls consisting of healthy donor cells stimulated with Gag peptides are reported in Fig. S1 in the supplemental material. As seen in representative examples (Fig. 1A), CD4+ T cells that produced both IFN-γ and TNF-α, or IFN-γ and IL-2, could be detected in HIV controllers, while IFN-γ and IL-4, or IFN-γ and IL-17, stemmed from separate producer cell populations. Examples of unstimulated controls are reported in Fig. S2 in the supplemental material.
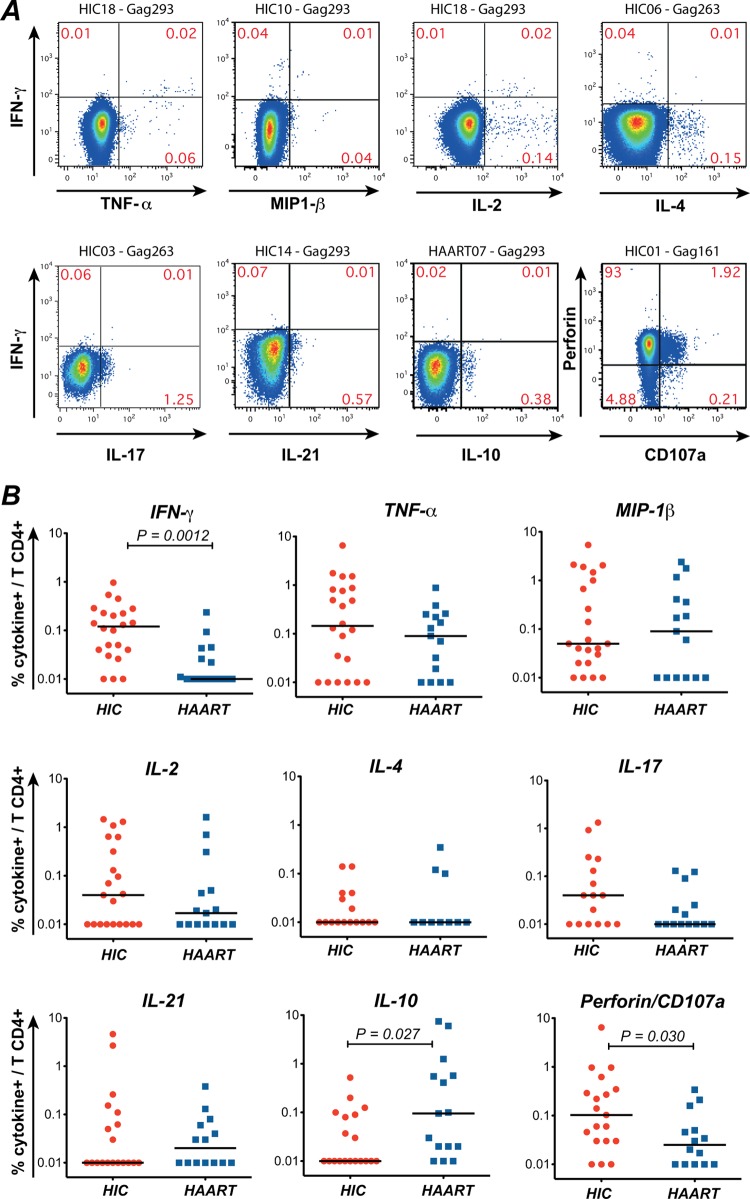
Fig 1.
Intracellular cytokine production in patients' CD4+ T cells stimulated with Gag peptides. Intracellular cytokine staining (ICS) was done on PBMC stimulated overnight with the immunodominant peptides Gag161, Gag263, and Gag293. Cytokine production was evaluated in the live CD3+ CD4+ population. (A) Examples of ICS in CD4+ T cells from patients stimulated with Gag293, Gag263, or Gag161. The first 7 plots show the production of TNF-α, MIP-1β, IL-2, IL-4, IL-17, IL-21, and IL-10 combined with that of IFN-γ. The last plot on the right, second row, shows the combined expression of total intracellular perforin and of the degranulation marker CD107a, which was used to determine the cytotoxic potential of CD4+ T cells. The patient identification number and the peptide used for stimulation are indicated above each plot. (B) Quantitation of the percentage of cytokine-producing CD4+ T cells detected in HIV controllers (HIC) and treated (HAART) patients. The summed percentages of cytokine-positive cells in response to Gag161, Gag263, and Gag293 are listed. Horizontal bars indicate the median values. Significant P values (<0.05) obtained with the nonparametric Mann-Whitney U test are shown.
Quantitation of intracellular cytokine production in responses to the 3 immunodominant Gag peptides showed that only IFN-γ production and perforin/CD107a expression were significantly increased in the HIC group compared to the HAART group (P = 0.0012 and P = 0.03, respectively) (Fig. 1B). The trends for higher IL-2 and IL-17 production in the HIC group did not reach statistical significance. It was, however, interesting that IL-17-secreting HIV-specific CD4+ T cells could be detected at high levels in a subset of HIV controllers, given that these cells appear exceedingly rare in progressor patients (5, 84). IL-4 remained undetectable in the majority of patients of both groups, indicating that HIV-1 infection did not generally promote a Th2 differentiation pattern. However, IL-4 could be detected at significant levels in a few patients (Fig. 1A, upper right plot). IL-21 production could be high in a few patients but did not differ significantly between the two groups. Intriguingly, IL-10 was the only cytokine that showed a higher expression in the HAART group than in the HIC group (P = 0.027), suggesting a dampening of effector responses by this immunoregulatory cytokine in treated patients. Taken together, these findings indicated that HIV-specific CD4+ T cells from controllers were characterized by a clear Th1 differentiation profile, the presence of effector cells with cytotoxic potential, and the occasional occurrence of Th17 differentiation.
Since certain MHC class I alleles are strongly associated with HIV control (1, 3, 15, 28), we examined the possible influence of the HLA B genotype on the level of Gag CD4 responses. HLA B typing was performed for all HIV controllers and 10 of the treated patients (Table 1). The percentages of patients carrying the protective HLA alleles B57 and B27 differed significantly between the two groups (HIC, 65% B57+ or B27+; HAART, 10% B57+ or B27+; P = 0.0036), as expected. For further analysis, HIV controllers were subdivided in two subgroups (“B57+ or B27+” versus “B57− and B27−”), which were compared for cytokine responses to Gag peptides. We did not detect significant differences for the 8 cytokines analyzed or for perforin/CD107a expression, suggesting that the HLA B genotype was not a major determinant of the HIV-specific CD4 response.
Immunoregulatory profile of Gag-specific and CMV-specific CD4+ T cells in long-term-treatment patients.
Analysis of CD4 responses at the single peptide level provided the opportunity to compare patient responses to the same epitope. The three immunodominant Gag peptides analyzed were all able to induce diverse cytokine responses in HIV-infected patients (Fig. 2A and C). Gag293 induced significantly more IFN-γ in the HIC than in the HAART group (P = 0.02). Gag161 showed a trend for increased IFN-γ and TNF-α induction (P = 0.052 and P = 0.061) and caused increased perforin/CD107a expression in the HIC group (P = 0.03). In contrast, the Gag263 peptide did not appear to be associated with the induction of Th1 effector responses (IFN-γ or perforin/CD107a), but rather showed nonsignificant trends for increased IL-17 induction in the HIC group and increased IL-10 induction in the HAART group. These observations suggested that the T helper profile of Gag-specific CD4+ T cells depended on the type of patient but also, possibly, on the nature of the recognized epitope.
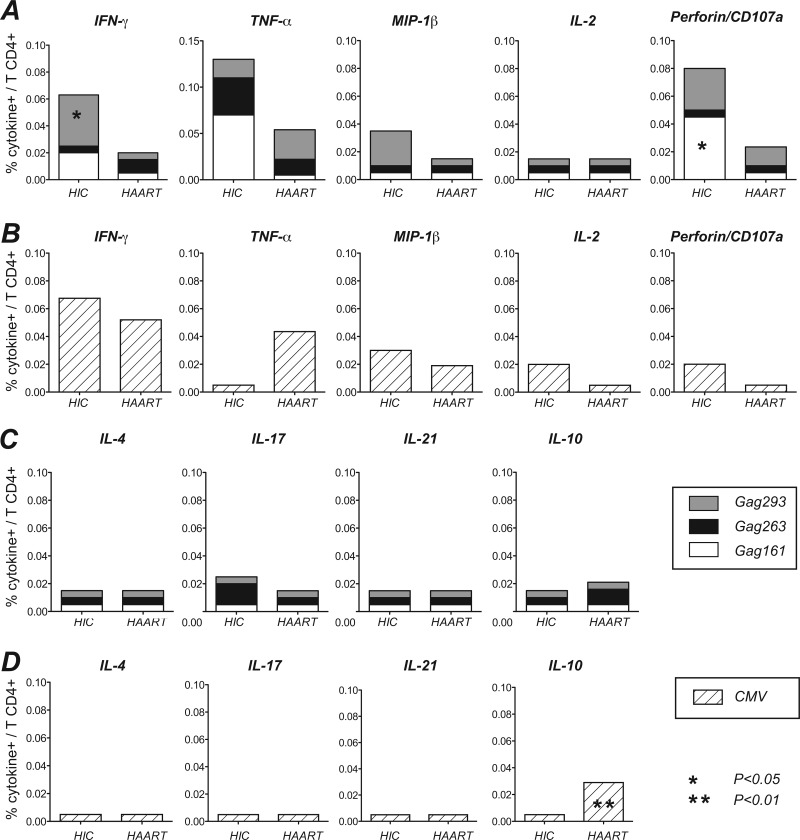
Fig 2.
Analysis of intracellular cytokine production to individual Gag and CMV peptides. (A and C) The median percentage of cytokine-producing cells induced by each of the three immunodominant Gag peptides is represented in stacked columns (Gag293, gray; Gag263, black; Gag161, white). (B and D) Cytokine production induced by optimal CMV peptides chosen in the function of patients' HLA-DR alleles are represented by the hatched bars. Median cytokine responses that did not reach the detection threshold are represented by the midpoint between the threshold value of 0.01% and zero (0.005%). Significant differences obtained in the Mann-Whitney U test are indicated by the asterisks.
As controls, responses to optimal peptides derived from the CMV matrix phosphoprotein pp65 were tested with the same cytokine panel (Fig. 2B and D). The T helper pattern of pp65 responses was mostly Th1, with a predominant production of IFN-γ, TNF-α, and MIP-1β in both the HIC and HAART groups, and equivalent cytokine production levels in both groups of patients. The one significant difference concerned IL-10 production, which again proved to be higher in the HAART group (P = 0.003). Thus, patients treated in the long term showed signs of a dampened CD4 response to both HIV and CMV through the synthesis of IL-10. Treated patients also showed a concomitant decrease of Th1 effectors directed against HIV but not CMV. In contrast, HIV controllers maintained efficient Th1 effector responses to both viruses.
Higher polyfunctionality of Gag-specific CD4+ Th1 cells in HIV controllers.
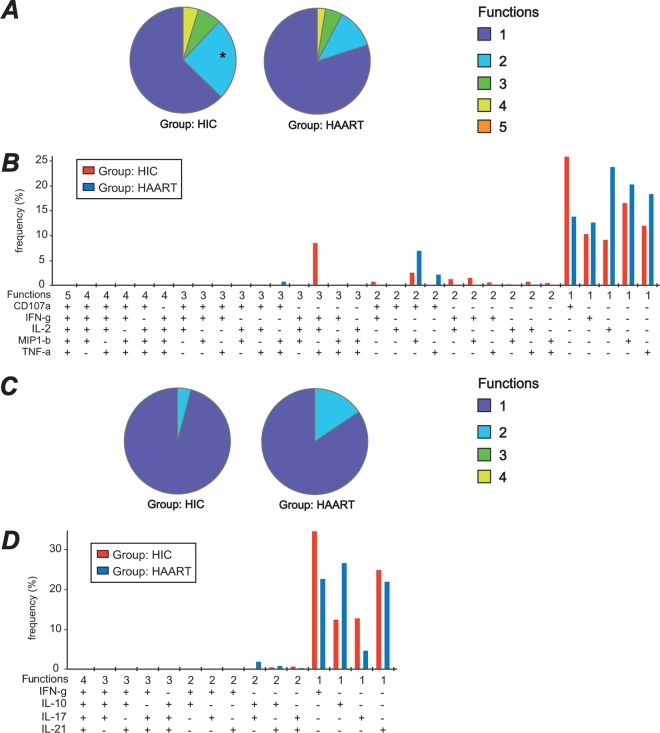
Polyfunctionality, i.e., the capacity to produce multiple cytokines simultaneously, was first analyzed for a combination of 5 markers (CD107a, IFN-γ, IL-2, MIP-1β, and TNF-α) expressed predominantly in Th1 cells. This analysis was carried out for Gag293-specific cells, since these could be detected in the majority of patients. Among Gag293-specific CD4+ T cells, monofunctional cells were the most abundant, both in the HIC and HAART group (Fig. 3A). However, the fraction of polyfunctional cells expressing 2 to 4 markers was higher in the HIC group than in the HAART group, the difference being significant for bifunctional cells (P < 0.05). Gag293-specific cells expressing all of the 5 markers together were not detected. Detailed analysis of the different marker combinations (Fig. 3B) highlighted the presence of a peak of tri-functional cells coexpressing IFN-γ, IL-2, and TNF-α that appeared characteristic of HIV controllers.
Fig 3.
Polyfunctionality of Gag293-specific CD4+ T cells. (A and B) Polyfunctionality analysis for the CD107a/IFN-γ/IL-2/MIP-1β/TNF-α panel. The colors in the pie charts represent the proportions of monofunctional cells (dark blue), bifunctional cells (light blue), trifunctional cells (green), quadrifunctional cells (yellow), and pentafunctional cells (orange) within the population of Gag293-specific CD4+ T cells, as determined by ICS. The bar chart shows the frequency of Gag293-specific cells in each functional category, with red bars corresponding to HIV controllers (HIC) and dark blue bars corresponding to treated patients (HAART). *, significant difference in bifunctional cells between the HIC and HAART groups (P < 0.05). (C and D) Polyfunctionality analysis for the IFN-γ/IL-10/IL-17/IL-21 panel. The colors in the pie charts represent the proportions of monofunctional cells (dark blue), bifunctional cells (light blue), trifunctional cells (green), and quadrifunctional cells (yellow) within the population of Gag293-specific CD4+ T cells. The bar chart shows the frequency of Gag293-specific cells in each functional category. These analyses were carried out with the SPICE 5.21 program.
Polyfunctionality of Gag293-specific CD4+ T cells was analyzed for a second panel comprising the 4 cytokines IFN-γ, IL-10, IL-17, and IL-21 (Fig. 3C). The production of these 4 cytokines was almost mutually exclusive, with a low frequency of bifunctional cells, and an absence of tri- and quadrifunctional cells. The slightly higher fraction of bifunctional cells in the HAART group appeared to be due to the cosynthesis of IL-10 and IL-17 in a subpopulation of Gag293-specific cells (Fig. 3D). Among monofunctional cells, the proportion of IFN-γ- and IL-17-producing cells was higher in the HIC group, while that of IL-10-producing cells was higher in the HAART group. Thus, analysis of the Gag293-specific response supported the notion of persistent CD4 effectors in HIV controllers, and of negative CD4 immunoregulation in treated patients. The fact that IFN-γ and IL-17 were produced by distinct cells in HIV controllers indicated that Gag293-specific cells had either a Th1 or a Th17 differentiation, but were not of the mixed Th1/Th17 phenotype. Similarly, few of the Th17 cells simultaneously produced IL-21, a cytokine that has been associated with the reinforcement of Th17 differentiation (76). Thus, a significant fraction of Th1 cells from HIV controllers were polyfunctional, but their Th17 cells appeared monofunctional.
Higher frequency of Gag293-specific cells in HIV controllers as determined by MHC class II tetramer staining.
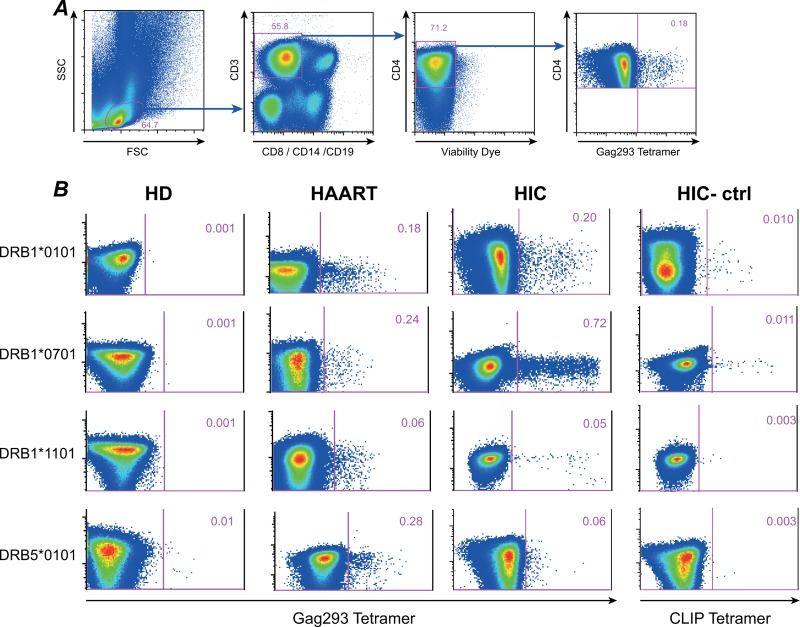
We characterized Gag293-specific CD4+ T cells by MHC class II tetramer staining, in order to directly measure their frequency ex vivo. We focused the analysis on the Gag293 peptide, which could be loaded onto 6 different tetramers (HLA DRB1*0101, DRB1*0401, DRB1*0701, DRB1*1101, DRB1*1501, and DRB5*0101) due to its promiscuous binding to HLA-DR molecules (38). A minimum of 6 × 106 PBMC was used per tetramer labeling, in order to ensure the detection of low-frequency-specific CD4+ T cells with a sensitivity threshold of 0.001%. The percentage of Gag293 tetramer-positive (Tet+) cells was minimal in CD4+ T cells of healthy blood donors (≤0.01%), but could reach up to 0.5 to 1% in both treated patients and HIV controllers, as shown on representative examples (Fig. 4B). Thus, CD4 responses to this single immunodominant Gag peptide could reach high frequencies, even though the amount of Gag antigen present in patients was minimal (viral load [VL] < 40 copies in both groups).
Fig 4.
Detection of Gag293-specific CD4+ T cells by MHC class II tetramer staining. (A) Gating strategy to detect Gag293-specific cells in CD4+ T cells. Tetramer staining was analyzed in viable CD3+ CD8− CD14− CD19− CD4+ lymphocytes, starting from a minimum of 6 × 106 PBMC. (B) Examples of detection of Gag293-specific cells with 4 different tetramers based on the DRB1*0101, DRB1*0701, DRB1*1101, and DRB5*0101 molecules. For each tetramer, the percentage of tetramer-positive (Tet+) cells in CD4+ T cells is indicated for one healthy donor (HD), one treated patient (HAART), and one HIV controller (HIC). A negative control consisting of CD4+ T cells labeled with a CLIP-loaded tetramer is shown for each of the HIV controllers tested (HIC-ctrl, right column).
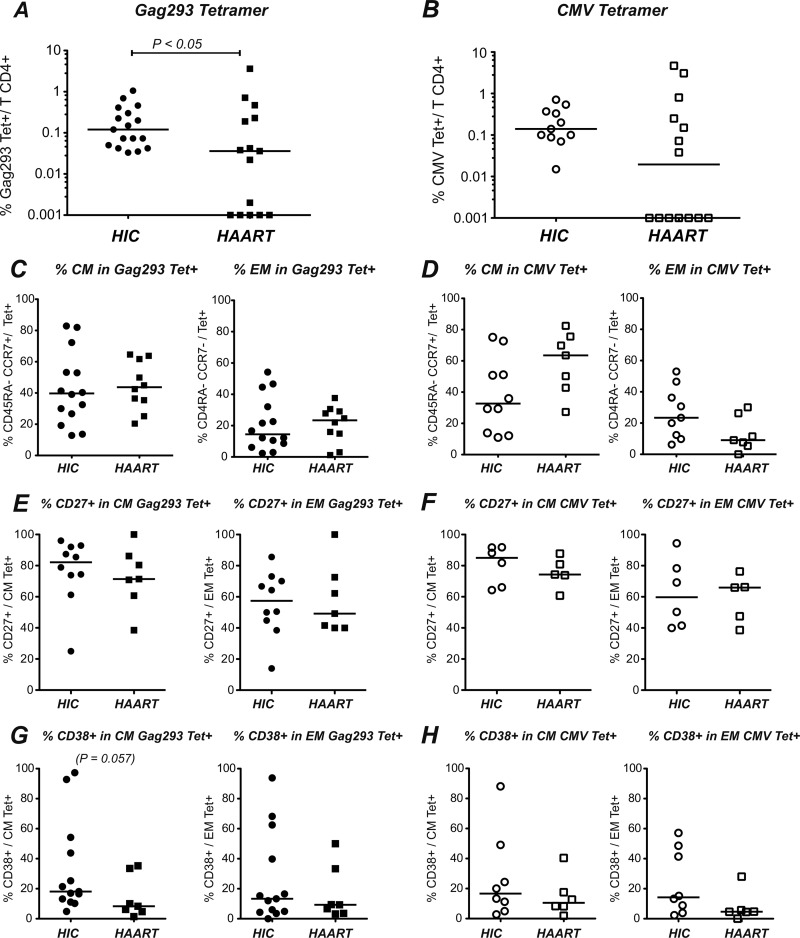
Comparison of both patient groups (Fig. 5A) showed a higher frequency of Gag293 Tet+ cells in HIV controllers, with a median value of 0.12% of CD4+ T cells in the HIC group versus 0.04% in the HAART group (P < 0.05). However, we noted a wide variation in Tet+ frequencies in the HAART group, with some of the treated patients showing as many Gag293-specific cells as the best responders in the controller group. A similar phenomenon was observed when testing tetramers loaded with optimal CMV pp65 peptides, with a trend toward lower responses in the HAART group (P = 0.1), but with a high individual variability (Fig. 5B).
Fig 5.
Frequency and phenotype of Gag293-specific tetramer-positive CD4+ T cells in HIV controllers (HIC) and treated patients (HAART). (A) The percentages of tetramer-positive (Tet+) CD4+ T cells specific for Gag293 were compared in the HIC and HAART groups. (B) The percentages of Tet+ cells specific for optimal CMV pp65 peptides were compared in the HIC and HAART groups. (C and D) The percentages of central memory (CM) (CD45RA− CCR7+) and effector memory (EM) (CD45RA− CCR7−) CD4+ T cells were analyzed in the Gag293 Tet+ population (C) and the CMV Tet+ population (D). (E and F) The percentages of CD27+ cells were analyzed in the CM and EM Gag293 Tet+ populations (E) and in the CM and EM CMV Tet+ populations (F). (G and H) The percentages of CD38+ cells were analyzed in the CM and EM Gag293 Tet+ populations (G) and in the CM and EM CMV Tet+ populations (H). The horizontal bars indicate the median values. Significant P values (<0.05) obtained with the nonparametric Mann-Whitney U test are shown.
The distribution of CD4+ T cell subsets based on the CD45RA and CCR7 markers was analyzed in the Tet+ populations (Fig. 5C and D). The Gag293 Tet+ population had a mixed phenotype, with a predominant central memory subset (CM, CD45RA− CCR7+) in the HIC as well as in the HAART groups and a lower but detectable effector memory subset (EM, CD45RA− CCR7−). Highly differentiated effectors (CD45RA+ CCR7−) were too few to be quantified, while a fraction of Tet+ cells appeared phenotypically naive (CD4R5A+ CCR7+) (not shown). These cells also predominantly expressed CD27, raising the possibility of a reversion of Gag-specific CD4+ T cells to a fully naive phenotype. The phenotype of the CMV Tet+ population was also mixed, with a trend toward more CM and fewer EM cells in the HAART group than in the HIC group, though the differences did not reach statistical significance (P = 0.13 and P = 0.07, respectively). Expression of the costimulatory marker CD27 was high in the CM Tet+ population specific for Gag293, both in the HIC and in the HAART group (medians of 82.2% and 71.4%, respectively). CD27 expression was lower in the EM Tet+ population (medians of 57.4% and of 49.2%), as expected, but still relatively high, indicating that approximately half of the EM Tet+ cells corresponded to a transitional memory population (Fig. 5E). Expression of CD27 in the CMV Tet+ population was comparable to that seen in the Gag293-specific population (Fig. 5F). Thus, CD27 expression was maintained in specific CD4+ T cells of both HIV controllers and patients treated in the long term, which is indicative of preserved costimulatory capacity and lack of immune exhaustion.
The activation status of the Tet+ populations could not be evaluated with the HLA-DR marker, because anti-HLA-DR antibodies cross-reacted with some of the tetramer reagents. Interestingly, the CD38 activation marker showed a trend for higher expression in Gag293 Tet+ cells of HIV controllers than in those of treated patients, with a P value close to significance in the CM Tet+ subset (P = 0.057) (Fig. 5G). CD38 expression did not differ significantly in the CMV Tet+ populations between the two groups, though again high CD38 expression was noted for a few HIV controllers (Fig. 5H). These findings suggested a possible increase of specific CD4+ T cell activation for a subset of HIV controllers. However, there were no signs of massive activation or of immune exhaustion in the Tet+ populations.
Functional impairment of Gag293-specific cells in treated patients.
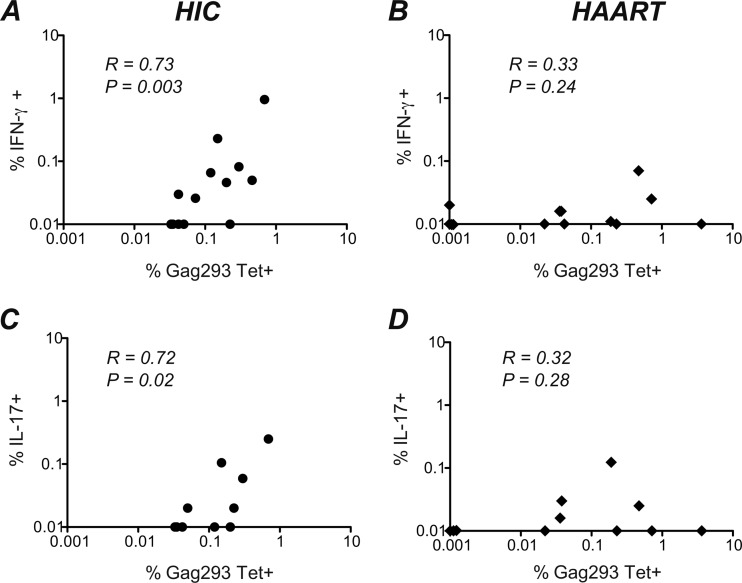
We then compared the frequencies of Gag293-specific CD4+ T cells detected physically (by class II tetramer binding) and functionally (by intracellular cytokine staining). Interestingly, the percentage of Tet+ cells and that of IFN-γ+ cells correlated positively in the HIC group (R = 0.73, P = 0.003) but not in the HAART group (R = 0.33, P ≥ 0.05) (Fig. 6A and B). The median ratio (interquartile range) of IFN-γ-producing cells to Tet+ cells was 0.28 (0.21 to 0.63) in the HIC group and 0.06 (0.00 to 0.43) in the HAART group, indicating that the functional response of Tet+ cells from HAART patients was minimal. Similar analyses for other cytokines did not yield significant correlations, with the exception of a positive association between Gag293 Tet+ and IL-17-producing cells observed in the HIC group (R = 0.72, P = 0.02) but not in the HAART group (Fig. 6C and D). Taken together, these findings suggest that the vast majority of Gag293-specific cells present in long-term-treatment patients fail to produce IFN-γ upon antigenic restimulation, pointing to an impaired Th1 response. In contrast, Gag293-specific cells from HIV controllers maintain an efficient Th1 effector function in spite of the very low antigenemia present in these individuals.
Fig 6.
Correlations between the percentages of Gag293-specific cells detected by tetramer staining and by intracellular cytokine staining (ICS). (A) Correlation between the percentage of Gag293 tetramer-positive CD4+ T cells (%Gag293 Tet+) (x axis) and the percentage of IFN-γ secreting CD4+ T cells detected by ICS after Gag293 stimulation (%IFN-γ+) (y axis) in the HIV controller (HIC) group. (B) Correlation between the percentages of Gag293 Tet+ cells and Gag293-specific IFN-γ secreting CD4+ T cells in the HAART group. (C) Correlation between the percentages of Gag293 Tet+ cells and Gag293-specific IL-17-γ-secreting CD4+ T cells in the HIC group. (D) Correlation between the percentages of Gag293 Tet+ cells and Gag293-specific IL-17-γ-secreting CD4+ T cells in the HAART group. The nonparametric Spearman correlation coefficient R and the P value are shown for each correlation.
DISCUSSION
HIV controller responses to immunodominant Gag peptides were characterized by the persistence of highly differentiated Th1 cells with effector functions, as indicated by a higher frequency of IFN-γ-secreting cells and of CD4+ T cells with degranulation capacity. The correlation between Gag293-specific cells detected by MHC class II tetramer staining and IFN-γ secretion indicated that about one-fourth of Gag-specific CD4+ T cells maintained effector function, even though the amount of Gag antigen driving effector responses was minimal in vivo. In contrast, patients treated in the long term, who had equally low antigenemia, showed a downregulation of Gag-specific CD4 effector responses, as indicated by a lower frequency of cells with IFN-γ secretion or degranulation capacity and a lower frequency of Gag293-specific cells detected by class II tetramer staining. In support of this notion, Gag-specific CD4+ T cells that persisted in treated patients showed signs of functional downregulation, as indicated by increased IL-10 secretion and the failure of Gag293-specific cells to produce IFN-γ upon restimulation. Thus, the Gag-specific response was maintained in a persistent effector state in HIV controllers, while it appeared to be subjected to negative immunoregulation in treated patients.
The differentiation of Gag-specific CD4+ T cells from HIV controllers proved to be predominantly Th1, rather than Th2, Th17, or Tfh. A bias toward a Th1 effector response fits with the expected pattern for an efficient response against an intracellular pathogen such as HIV (85), but it should be noted that Th1 differentiation alone cannot account for viral control, since IFN-γ+ CD107a+ Th1 effectors are also abundant in viremic patients (16, 25, 55). Rather, it is the capacity to maintain a functional Th1 effector population in a situation of very low antigenemia that appears characteristic of HIV controllers. The comparison with a group of patients treated efficiently in the very long term (more than 10 years) was instrumental in highlighting this difference, since other studies indicate that patients treated for a shorter period of time maintain a degree of Th1 effector function, as indicated by detectable IFN-γ+-specific CD4+ T cells with degranulation capacity (2, 50). The long duration of treatment in the present study may also account for a degree of recovery of IL-2 responses in the HAART group and the lack of a significant difference in IL-2 production with the controller group. Consistent with this notion, a recent comparison of patients treated efficiently for less than 3 years and more than 10 years showed that prolonged therapy further improved CD4 proliferative responses, while IFN-γ production by CD4+ T cells remained minimal (24). Thus, patients treated in the very long term show the expected signature of a resolved viral infection with antigen clearance, with a reappearance of IL-2-secreting memory CD4+ T cells with proliferative capacity and a loss of effector functions (25). HIV controllers provide an exception to this pattern, as they appear to maintain both IL-2 secretion and effector functions in the long term in spite of minimal antigenemia. Early studies have provided evidence for a preserved central memory CD4+ T cell compartment with remarkable proliferative and IL-2 secretion capacities in HIV controllers (19, 26, 58, 81). The notion of advanced effector differentiation has emerged in studies of CD8+ T cell responses in HIV controllers, with the realization that these rare patients harbored HIV-specific CD8+ T cell with highly efficient antiviral functions, associated with an increased loading of perforin and granzyme B within cytotoxic granules (27, 29, 48, 64). The present study suggests that advanced effector differentiation also persists in the CD4+ T cell compartment of HIV controllers. The presence of a CD107+ perforin+ CD4+ T cell population raises the possibility that Gag-specific CD4+ T cells may have direct cytotoxic potential and may contribute to the elimination of infected cells, a property that has been documented in certain chronic viral infections such as CMV (2, 8) and that characterizes CD4+ T cell clones from rhesus macaques able to control simian immunodeficiency virus infection (63). A recent study suggests that cytolytic CD4+ T cells may well play a role in the early control of HIV replication, with the finding that expansion of HIV-specific CD107a+ CD4+ T cells in the acute stage is associated with a lower viral set point (70).
It was interesting that IL-17 production could be induced by Gag peptides in about half of the HIV controllers, even though the difference with the HAART group did not reach significance. Previous studies have shown that HIV-specific Th17 cells are exceedingly rare in viremic and treated patients, except during the acute infection stage (5, 84). The detection of Gag-specific Th17 cells in HIV controllers is consistent with the preservation of the total Th17 population in the blood and the intestinal mucosa of these patients (12, 67), while Th17 cells are preferentially depleted in other patient groups (5, 42, 46, 49, 59). The presence of Gag-specific Th17 effectors may have beneficial effects, by contributing either directly or indirectly to HIV control and by helping maintain the integrity of mucosal surfaces. On the other hand, the activation of Th17 effectors may also foster the low-grade inflammatory condition that persists in HIV controllers (6, 30, 58), with possible deleterious consequences such as increased susceptibility to cardiovascular disease in the long term (14). Analysis of polyfunctionality showed that most of the IL-17+ CD4+ T cells in HIV controllers did not produce IFN-γ, indicating the presence of typical Th17 cells, rather than cells of the mixed Th1 Th17 phenotype. We cannot rule out that Th17 cells produced other cytokines that were not tested in the same panel as IL-17, such as IL-2 or TNF-α, but we noted that these cells did not produce IL-21, suggesting low polyfunctionality. The γc family cytokine IL-21, when produced by Th17 cells, acts in an autocrine fashion to amplify and stabilize IL-17 production via the activation of the transcription factor STAT3 (76). The lack of bifunctional IL-17+ IL-21+ cells suggests that Th17 differentiation may be only transient, reinforcing the notion that the predominant helper differentiation pattern is, rather, Th1 in HIV controllers.
The MHC class II tetramer analysis showed that the population of HIV-specific CD4+ T cells persisted at relatively high levels in peripheral blood in the HIV controller group, with a median of 0.12% of CD4+ T cells directed against the single Gag293 peptide. Of note, this number is a minimal estimate, given that Gag293 can be presented by other HLA-DR alleles than those studied and also by HLA-DQ molecules (35, 38). The finding of a high frequency of Tet+ cells in HIV controllers supports the notions that HIV-specific CD4+ T cells are spared from depletion in situations of controlled viremia and that a persistently active cellular response sustains the pool of specific cells. The frequency of Gag293-specific cells was significantly lower in treated patients, though it was intriguing that it could remain as high as in controllers for a few individuals. Two studies have shown that the frequency of Tet+ Gag-specific CD4+ T cells is generally low in treated patients and can rebound upon short-term-therapy interruption, with a shift toward a more effector differentiation pattern accompanying the resumption of viral replication (68, 69). We cannot rule out that some of the treated patients may have undergone blips in viral replication that would have escaped detection but would have been sufficient to stimulate the expansion of specific cells. However, we did not detect a higher proportion of Tet+ cells with an effector memory phenotype in patients with a higher frequency of Tet+ cells (not shown). Rather, central memory cells predominated in the Tet+ populations, both in the HAART and in the HIC group, consistent with a low antigenemia. A possibility is that HIV-specific central memory cells may expand in some of the patients treated in the long term, which would be consistent with a partial recovery of proliferative responses (24, 71). Importantly, increased Tet+ frequency did not correlate with increased IFN-γ secretion frequency in the HAART group, while it did so in the HIC group. Thus, Gag293-specific cells appeared to differ functionally, if not phenotypically, between the two groups, with a persistence of Th1 effector function in HIV controllers. A caveat of this interpretation is that we did not directly detect cytokine production within the Tet+ population, due to a reduced sensitivity of Tet+ cell detection after intracellular cytokine staining. However, since the frequency of IFN-γ producer cells was minimal in treated patients, it is reasonable to assume that in these patients the vast majority of Tet+ cells would fail to produce IFN-γ upon restimulation with the Gag293 peptide.
Several reasons may account for the persistence of an effector function in HIV controllers but not in patients treated in the long term. A straightforward explanation could be the capacity of HIV controller memory CD4+ T cells to reactivate upon encountering very small amounts of viral antigens. Indeed, we recently reported that a subset of CD4+ T cells with a high avidity for the Gag293 peptide could be detected exclusively in primary CD4+ T cell lines derived from HIV controllers and not in those from treated patients (74). The presence of a pool of high-avidity cells could explain the persistence of Th1 effectors that would remain activated in the presence of very low viremia. Such cells may play a key role in viral control by sensing local blips in viral replication and by triggering a rapid antiviral recall response that would ensure a return to baseline viremia. The secretion of cytokines by high-avidity cells may also help explain the low-grade chronic immune activation that persists in HIV controllers (6, 30, 58), a phenomenon that could lead to deleterious consequences in the very long term (14). Future studies should help determine whether high-avidity CD4+ T cells can be detected directly in the peripheral blood of HIV controllers, and whether such cells have a phenotype indicative of chronic reactivation. Another possible explanation for the persistence of Th1 responses in the controller group may be the presence of slightly higher levels of residual viral replication in HIV controllers than in patients treated in the long term. However, analyses based on high-sensitivity viral load assays have not revealed so far a clearly higher viral burden in the HIV controller population. Circulating HIV-1 RNA can be detected in the plasma of most HIV controllers, but median viral RNA levels appear extremely low (2 copies/ml) (54). Patients treated in the long term harbor a residual viremia of the same order of magnitude (1.5 copies/ml) (53). On the other hand, correlations between viral load and both humoral and cellular responses have been reported in HIV controllers (31, 54), suggesting that the availability of viral antigens may influence the level of recall responses. Thus, it will be important in future studies to determine the respective roles of antigen levels and antigen sensitivity of T cells in the persistence of Th1 responses in HIV controllers.
Negative immunoregulation may also contribute to the lack of Th1 cytokine production by CD4+ T cells from treated patients. Prior to therapy initiation, these patients experienced years of active HIV replication, which inflicted multiple insults on the immune system, including the deletion of key populations of CD4+ T cells but also the overstimulation and exhaustion of the remaining pool of HIV-specific cells. The expression of inhibitory receptors such as programmed death 1 (PD-1), CTLA-4, and T cell immunoglobulin mucin 3 (TIM-3) is markedly increased on HIV-specific CD4+ T cells of viremic patients and is not entirely normalized after therapy, which may limit the capacity of specific CD4+ T cells to reactivate upon stimulation with HIV antigens (37, 39, 57). It is also noteworthy that IL-10 was the only cytokine that was produced by CD4+ T cells more in the HAART group than in the HIC group. The difference was significant for Gag peptides but also, intriguingly, for responses to CMV peptides. IL-10 production can be viewed as a physiological response contributing to the containment of excessive Th1 responses. For instance, Th1 cells directly convert to IL-10-producing regulatory T cells in models of tolerance induction (21). In viremic HIV infection, IL-10 is abundantly produced by multiple cell types, including monocytes, B cells, and T cells, with monocytes being major contributors (7, 65). IL-10 causes a reversible impairment of CD4+ T cell functions such as proliferation and secretion of other cytokines (7, 65, 72). Our findings suggest that IL-10 production still contributes to the dysfunction of HIV-specific cells in treated HIV infection and that negative immunoregulation by IL-10 may also extend to responses against other pathogens such as CMV. In contrast, HIV controllers may harbor persistently high levels of T cell effectors directed against other intracellular pathogens, as suggested by studies documenting higher CMV-specific CD4 responses (32) and a possibly higher clearance rate of hepatitis C virus (HCV) infection (66) in HIV controllers than in uninfected individuals. The persistence of Th1 effectors directed against HIV may prime the immune system of controllers for a more efficient response to other viruses, for instance through a chronic release of IFN-γ that would facilitate the differentiation of Th1 cells of other specificities through the bystander effect. Alternatively, HIV controllers may harbor genetic traits that promote Th1 differentiation, which may be explored in large-scale genetic studies.
In conclusion, spontaneous HIV control is associated with the persistence of highly functional Th1 effectors, whereas pharmacological HIV control leads to a dampening of CD4 effector functions. The fact that CD4 responses are not entirely lost but, rather, dysfunctional in treated patients raises the possibility of reawakening these responses through immunotherapeutic intervention.
Supplementary Material
ACKNOWLEDGMENTS
This study was carried out in the framework of the ANRS EP36 HIV Controllers Study Group. We are grateful to patients who participated in the study. We thank Laurence Meyer for advice on methodology and collaboration with the Seroco/Hemoco cohort. We also thank the clinicians who recruited patients for this study: François Boué, Alain Krivitzky, Jean-Claude Melchior, Huguette Berthé, Gilles Pialoux, Thomas L'Yavanc, Dominique Salmon-Ceron, Marie-Pierre Piétri, Laurence Weiss, and David Zucman. We thank the Flow Cytometry Platform (PFC) team of the Pasteur Institute for its help with the cytometers. MHC class II tetramer reagents for alleles DRB1*0101, DRB1*0301, DRB1*1501, and DRB5*0101 were obtained through the NIH Tetramer Core facility at Emory University.
This work was supported by the Agence Nationale de Recherche sur le SIDA et les Hépatites Virales (ANRS, study EP36-3) and the Pasteur Institute, Paris, France. B.V. was the recipient of a Sidaction fellowship. D.B. and M.G. are the recipients of ANRS fellowships.
The authors declare no competing financial interests.
Footnotes
Published ahead of print 25 July 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Appay V, Iglesias MC. 2011. Antigen sensitivity and T-cell receptor avidity as critical determinants of HIV control. Curr. Opin. HIV AIDS 6:157–162 [DOI] [PubMed] [Google Scholar]
- 2. Appay V, et al. 2002. Characterization of CD4(+) CTLs ex vivo. J. Immunol. 168:5954–5958 [DOI] [PubMed] [Google Scholar]
- 3. Blankson JN. 2010. Effector mechanisms in HIV-1 infected elite controllers: highly active immune responses? Antiviral Res. 85:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boufassa F, et al. 2011. CD4 dynamics over a 15 year-period among HIV controllers enrolled in the ANRS French observatory. PLoS One 6:e18726 doi:10.1371/journal.pone.0018726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brenchley JM, et al. 2008. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 112:2826–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brenchley JM, et al. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365–1371 [DOI] [PubMed] [Google Scholar]
- 7. Brockman MA, et al. 2009. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 114:346–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casazza JP, et al. 2006. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J. Exp. Med. 203:2865–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chakrabarti LA, Simon V. 2010. Immune mechanisms of HIV control. Curr. Opin. Immunol. 22:488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Charron D. 1997. HLA, vol. 2. Genetic diversity of HLA, functional and medical implications. Proceedings of the 12th International Histocompatibility Workshop and Conference EDK, Sèvres, Paris [Google Scholar]
- 11. Chevalier MF, et al. 2011. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J. Virol. 85:733–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ciccone EJ, et al. 2011. CD4+ T cells, including Th17 and cycling subsets, are intact in the gut mucosa of HIV-1-infected long-term nonprogressors. J. Virol. 85:5880–5888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davis MM, Altman JD, Newell EW. 2011. Interrogating the repertoire: broadening the scope of peptide-MHC multimer analysis. Nat. Rev. Immunol. 11:551–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deeks SG. 2011. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 62:141–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deeks SG, Walker BD. 2007. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 27:406–416 [DOI] [PubMed] [Google Scholar]
- 16. Douek DC, Roederer M, Koup RA. 2009. Emerging concepts in the immunopathogenesis of AIDS. Annu. Rev. Med. 60:471–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dragsted UB, et al. 2004. Predictors of immunological failure after initial response to highly active antiretroviral therapy in HIV-1-infected adults: a EuroSIDA study. J. Infect. Dis. 190:148–155 [DOI] [PubMed] [Google Scholar]
- 18. Elsaesser H, Sauer K, Brooks DG. 2009. IL-21 is required to control chronic viral infection. Science 324:1569–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Emu B, et al. 2005. Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J. Virol. 79:14169–14178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferre AL, et al. 2010. HIV controllers with HLA-DRB1*13 and HLA-DQB1*06 alleles have strong, polyfunctional mucosal CD4+ T-cell responses. J. Virol. 84:11020–11029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gabrysova L, et al. 2009. Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10-secreting Th1 cells. J. Exp. Med. 206:1755–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghoreschi K, Laurence A, Yang XP, Hirahara K, O'Shea JJ. 2011. T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends Immunol. 32:395–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grabar S, et al. 2009. Prevalence and comparative characteristics of long-term nonprogressors and HIV controller patients in the French Hospital Database on HIV. AIDS 23:1163–1169 [DOI] [PubMed] [Google Scholar]
- 24. Guihot A, et al. 2010. Immune and virological benefits of 10 years of permanent viral control with antiretroviral therapy. AIDS 24:614–617 [DOI] [PubMed] [Google Scholar]
- 25. Harari A, et al. 2006. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol. Rev. 211:236–254 [DOI] [PubMed] [Google Scholar]
- 26. Harari A, Petitpierre S, Vallelian F, Pantaleo G. 2004. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood 103:966–972 [DOI] [PubMed] [Google Scholar]
- 27. Hersperger AR, et al. 2011. Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood 117:3799–3808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hersperger AR, Migueles SA, Betts MR, Connors M. 2011. Qualitative features of the HIV-specific CD8+ T-cell response associated with immunologic control. Curr. Opin. HIV AIDS 6:169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hersperger AR, et al. 2010. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog. 6:e1000917 doi:10.1371/journal.ppat.1000917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hunt PW, et al. 2008. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J. Infect. Dis. 197:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hunt PW, et al. 2011. HIV-specific CD4+ T cells may contribute to viral persistence in HIV controllers. Clin. Infect. Dis. 52:681–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hunt PW, et al. 2011. A low T regulatory cell response may contribute to both viral control and generalized immune activation in HIV controllers. PLoS One 6:e15924 doi:10.1371/journal.pone.0015924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iannello A, et al. 2010. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J. Immunol. 184:114–126 [DOI] [PubMed] [Google Scholar]
- 34. Jansen CA, van Baarle D, Miedema F. 2006. HIV-specific CD4+ T cells and viremia: who's in control? Trends Immunol. 27:119–124 [DOI] [PubMed] [Google Scholar]
- 35. Jones RB, et al. 2009. HIV-1 escapes from IL-2-producing CD4+ T cell responses without high-frequency fixation of mutations. J. Virol. 83:8722–8732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kannanganat S, et al. 2007. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. J. Virol. 81:12071–12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kassu A, et al. 2010. Regulation of virus-specific CD4+ T cell function by multiple costimulatory receptors during chronic HIV infection. J. Immunol. 185:3007–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaufmann DE, et al. 2004. Comprehensive analysis of human immunodeficiency virus type 1-specific CD4 responses reveals marked immunodominance of gag and nef and the presence of broadly recognized peptides. J. Virol. 78:4463–4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaufmann DE, et al. 2007. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 8:1246–1254 [DOI] [PubMed] [Google Scholar]
- 40. Kaufmann DE, et al. 2004. Limited durability of viral control following treated acute HIV infection. PLoS Med. 1:e36 doi:10.1371/journal.pmed.0010036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaufmann GR, et al. 2005. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin. Infect. Dis. 41:361–372 [DOI] [PubMed] [Google Scholar]
- 42. Klatt NR, Brenchley JM. 2010. Th17 cell dynamics in HIV infection. Curr. Opin. HIV AIDS 5:135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kwok WW. 2003. Challenges in staining T cells using HLA class II tetramers. Clin. Immunol. 106:23–28 [DOI] [PubMed] [Google Scholar]
- 44. Lemaitre F, et al. 2004. Detection of low-frequency human antigen-specific CD4(+) T cells using MHC class II multimer bead sorting and immunoscope analysis. Eur. J. Immunol. 34:2941–2949 [DOI] [PubMed] [Google Scholar]
- 45. Lichterfeld M, Pantaleo G, Altfeld M. 2005. Loss of HIV-1-specific T cell proliferation in chronic HIV-1 infection: cause or consequence of viral replication? AIDS 19:1225–1227 [DOI] [PubMed] [Google Scholar]
- 46. Macal M, et al. 2008. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 1:475–488 [DOI] [PubMed] [Google Scholar]
- 47. Mallone R, Nepom GT. 2004. MHC class II tetramers and the pursuit of antigen-specific T cells: define, deviate, delete. Clin. Immunol. 110:232–242 [DOI] [PubMed] [Google Scholar]
- 48. Migueles SA, et al. 2008. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29:1009–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ndhlovu LC, et al. 2008. Suppression of HIV-1 plasma viral load below detection preserves IL-17 producing T cells in HIV-1 infection. AIDS 22:990–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nemes E, et al. 2010. Cytotoxic granule release dominates gag-specific CD4+ T-cell response in different phases of HIV infection. AIDS 24:947–957 [DOI] [PubMed] [Google Scholar]
- 51. Novak EJ, Liu AW, Nepom GT, Kwok WW. 1999. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J. Clin. Invest. 104:R63–R67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Owen RE, et al. 2010. HIV+ elite controllers have low HIV-specific T-cell activation yet maintain strong, polyfunctional T-cell responses. AIDS 24:1095–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Palmer S, et al. 2008. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. U. S. A. 105:3879–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pereyra F, et al. 2009. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J. Infect. Dis. 200:984–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pitcher CJ, et al. 1999. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 5:518–525 [DOI] [PubMed] [Google Scholar]
- 56. Porichis F, Kaufmann DE. 2011. HIV-specific CD4 T cells and immune control of viral replication. Curr. Opin. HIV AIDS 6:174–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Porichis F, et al. 2011. Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood 118:965–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Potter SJ, et al. 2007. Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: an ANRS EP36 study. J. Virol. 81:13904–13915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Prendergast A, et al. 2010. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. AIDS 24:491–502 [DOI] [PubMed] [Google Scholar]
- 60. Ranasinghe S, et al. 2012. HIV-specific CD4 T cell responses to different viral proteins have discordant associations with viral load and clinical outcome. J. Virol. 86:277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Roederer M, Nozzi JL, Nason MC. 2011. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 79:167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rosenberg ES, et al. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447–1450 [DOI] [PubMed] [Google Scholar]
- 63. Sacha JB, et al. 2009. Gag- and Nef-specific CD4+ T cells recognize and inhibit SIV replication in infected macrophages early after infection. Proc. Natl. Acad. Sci. U. S. A. 106:9791–9796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Saez-Cirion A, et al. 2007. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar CTL activation phenotype. Proc. Natl. Acad. Sci. U. S. A. 104:6776–6781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Said EA, et al. 2010. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat. Med. 16:452–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sajadi MM, Shakeri N, Talwani R, Redfield RR. 2010. Hepatitis C infection in HIV-1 natural viral suppressors. AIDS 24:1689–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Salgado M, et al. 2011. Long-term non-progressors display a greater number of Th17 cells than HIV-infected typical progressors. Clin. Immunol. 139:110–114 [DOI] [PubMed] [Google Scholar]
- 68. Scriba TJ, et al. 2005. HIV-1-specific CD4+ T lymphocyte turnover and activation increase upon viral rebound. J. Clin. Invest. 115:443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Seth N, Kaufmann D, Lahey T, Rosenberg ES, Wucherpfennig KW. 2005. Expansion and contraction of HIV-specific CD4 T cells with short bursts of viremia, but physical loss of the majority of these cells with sustained viral replication. J. Immunol. 175:6948–6958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Soghoian DZ, et al. 2012. HIV-specific cytolytic CD4 T cell responses during acute HIV infection predict disease outcome. Sci. Transl. Med. 4:123ra125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tilton JC, et al. 2007. Changes in paracrine interleukin-2 requirement, CCR7 expression, frequency, and cytokine secretion of human immunodeficiency virus-specific CD4+ T cells are a consequence of antigen load. J. Virol. 81:2713–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Torheim EA, et al. 2009. Interleukin-10-secreting T cells define a suppressive subset within the HIV-1-specific T-cell population. Eur. J. Immunol. 39:1280–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. van Grevenynghe J, et al. 2008. Transcription factor FOXO3a controls the persistence of memory CD4(+) T cells during HIV infection. Nat. Med. 14:266–274 [DOI] [PubMed] [Google Scholar]
- 74. Vingert B, et al. 2010. HIV controller CD4+ T cells respond to minimal amounts of Gag antigen due to high TCR avidity. PLoS Pathog. 6:e1000780 doi:10.1371/journal.ppat.1000780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vogelzang A, et al. 2008. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 29:127–137 [DOI] [PubMed] [Google Scholar]
- 76. Wei L, Laurence A, Elias KM, O'Shea JJ. 2007. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J. Biol. Chem. 282:34605–34610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Williams LD, et al. 2011. Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. J. Virol. 85:2316–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yi JS, Cox MA, Zajac AJ. 2010. T-cell exhaustion: characteristics, causes and conversion. Immunology 129:474–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yi JS, Du M, Zajac AJ. 2009. A vital role for interleukin-21 in the control of a chronic viral infection. Science 324:1572–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Younes SA, et al. 2007. The duration of exposure to HIV modulates the breadth and the magnitude of HIV-specific memory CD4+ T cells. J. Immunol. 178:788–797 [DOI] [PubMed] [Google Scholar]
- 81. Younes SA, et al. 2003. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 198:1909–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yu D, Vinuesa CG. 2010. The elusive identity of T follicular helper cells. Trends Immunol. 31:377–383 [DOI] [PubMed] [Google Scholar]
- 83. Yue FY, et al. 2010. HIV-specific IL-21 producing CD4+ T cells are induced in acute and chronic progressive HIV infection and are associated with relative viral control. J. Immunol. 185:498–506 [DOI] [PubMed] [Google Scholar]
- 84. Yue FY, et al. 2008. Virus-specific interleukin-17-producing CD4+ T cells are detectable in early human immunodeficiency virus type 1 infection. J. Virol. 82:6767–6771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhou L, Chong MM, Littman DR. 2009. Plasticity of CD4+ T cell lineage differentiation. Immunity 30:646–655 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.