Abstract
Loss of β1 integrin expression inhibits renal collecting-system development. Two highly conserved NPXY motifs in the distal β1 tail regulate integrin function by associating with phosphtyrosine binding (PTB) proteins, such as talin and kindlin. Here, we define the roles of these two tyrosines in collecting-system development and delineate the structural determinants of the distal β1 tail using nuclear magnetic resonance (NMR). Mice carrying alanine mutations have moderate renal collecting-system developmental abnormalities relative to β1-null mice. Phenylalanine mutations did not affect renal collecting-system development but increased susceptibility to renal injury. NMR spectra in bicelles showed the distal β1 tail is disordered and does not interact with the model membrane surface. Alanine or phenylalanine mutations did not alter β1 structure or interactions between α and β1 subunit transmembrane/cytoplasmic domains; however, they did decrease talin and kindlin binding. Thus, these studies highlight the fact that the functional roles of the NPXY motifs are organ dependent. Moreover, the β1 cytoplasmic tail, in the context of the adjacent transmembrane domain in bicelles, is significantly different from the more ordered, membrane-associated β3 integrin tail. Finally, tyrosine mutations of β1 NPXY motifs induce phenotypes by disrupting their interactions with critical integrin binding proteins like talins and kindlins.
INTRODUCTION
The multibranched kidney collecting system develops from the ureteric bud (UB), which undergoes iterative branching morphogenesis following its interactions with the metanephric mesenchyme (MM). This process depends on growth factor-mediated cell signaling and integrin-dependent cell-extracellular matrix (ECM) interactions. β1, the most abundantly expressed β integrin subunit, is a component of at least 12 integrin heterodimers. They include the laminin-binding integrins, α3β1 and α6β1, and the collagen-binding integrins, α1β1 and α2β1, which are the principal integrins expressed by the kidney (24). The importance of β1 integrins in UB development was verified in mice, where the β1 integrin gene was selectively deleted in the UB at embryonic day 10.5 (E10.5), when it invades the MM. These mice develop a severe UB branching morphogenesis defect with decreased nephron formation, a major proliferation defect, and decreased activation of multiple integrin-activated signaling pathways (43, 46).
The β1 integrin cytoplasmic tail plays key roles in integrin functions, many of which are mediated by two well-defined NPXY motifs found in the distal region of the tail (29). Mutating Y783/Y795 to F783/F795 (YY/FF) in keratinocytes and fibroblasts moderately impaired cell adhesion, spreading, chemotaxis, and integrin signaling (11, 32–35), while Y783/Y795-to-A783/A795 mutations (YY/AA) in keratinocytes resulted in abnormalities indistinguishable from β1-null keratinocytes (11). Consistent with the in vitro data, mice with the YY/AA mutation constitutively or selectively knocked into keratinocytes resembled β1-null phenotypes (8, 11). Surprisingly, mice with the YY/FF knock-in mutation displayed no developmental abnormalities (8, 11), although they developed fewer tumors than wild-type (WT) mice in a skin carcinogenesis model (26). The YY/AA and YY/FF mouse phenotypes were shown to be caused by decreased affinity for the integrin tail binding proteins talin and kindlin. Consistent with the difference in severity between the genotypes, the affinity of talin and kindlin is higher for β1 with NPXF motifs than for those with NPXA motifs. Except those in skin, no organ-specific studies have been conducted to define the role of the NPXY motifs in development or injury models. In addition, it is unclear whether these mutations induce the phenotypes by altering binding affinities of integrin binding proteins or by inducing a major structural change in the β1 integrin tail.
The NPXY motifs of the β1 tail are critical for the binding of cytosolic proteins, including talins, kindlins, and integrin cytoplasmic domain-associated protein 1 (ICAP-1) (3, 4, 21, 29), which are required for integrin affinity regulation and outside-in signaling. YY/AA mutations inhibit binding of and abrogate signaling by these proteins (6, 27, 30). Although developmental studies in YY/FF mice suggest that these tyrosine residues (or at least the phenolic —OH) are not critical in the mechanisms regulating integrin function (8, 11), there is evidence suggesting that β integrin tyrosine phosphorylation is a conserved mechanism for turning off talin-induced integrin activation (2). Indeed, it was shown that a Y/F783 or Y/A783 mutation in the membrane-proximal NPXY motif of the β1 tail results in a dramatic reduction in the binding affinities of both talin1 and talin2 for the two major splice variant forms (β1A and β1D) of the cytoplasmic domain (CTD) of the integrin (3). These data suggested an essential role for unphosphorylated Tyr783 in activation of β1 by talin-induced disruption of the interface between the transmembrane and cytoplasmic domains (TM/CTD) of β1 and its α integrin subunit partners, while phosphorylation of Y783 favors the inactive state by both reducing the affinity of β1 for talin and enhancing the affinity of the CTD for Dok1. Similarly, kindlins have been shown to bind to the membrane-distal NPXY motif of integrins (14, 27), and this binding is disrupted by a Y/A795 mutation. Phosphorylation of the corresponding membrane-distal NITY759 motif in the β3 CTD disrupts kindlin-2 recognition and the ability of kindlin-2 to coactivate the αIIβ3 integrin (14).
The TM and CTD of the β3 integrin have been subjected to numerous structural studies (1, 12, 13, 16, 18, 19, 22, 23, 25, 40, 41, 44). However, only recently have experimental advances for β1 (predominantly the β1D isoform) been made, and they have focused on analyzing its CTD free in solution or in complex with talin (2, 4). These studies showed that the isolated β1 CTD is structurally disordered in the absence of a binding partner. Upon complex formation with the talin F3 domain, the membrane-proximal portion of the CTD (residues 752 to 773) adopts an α-helical structure that makes several important contacts with talin. Part of the membrane-distal portion of the CTD in the complex adopts ordered (but irregular) structure in which the NPIY motif adopts a β turn conformation and Y783 makes the initial contacts with talin. Interestingly, the local conformation and orientation of the corresponding NPLY747 motif of the β3 CTD in complex with talin differs considerably, although the orientation of the Tyr side chain in the talin contact interface is essentially the same. The more membrane-distal NPKY795 motif of β1 appears to be in a disordered segment both before and after binding of talin, indicating that its roles in integrin recognition by cytosolic effectors must involve proteins other than talin. This motif is the primary binding site for the kindlins (14, 27, 28, 39). Taken together, these studies of the β1 CTD indicate an induced fit or conformational selection mechanism whereby the disordered domain becomes ordered upon binding with talin. However, it could be that the conformational properties of the CTD are different when they are associated with the intact β1 TM domain within the plasma membrane.
To address this question, we conducted structural studies of the intact β1 TM/CTD domains under conditions in which the protein is embedded in bilayered model membranes (i.e., bicelles). Furthermore, we also defined the effect of YY/AA and YY/FF mutations of the β1 integrin NPXY motifs on the collecting system of the kidney and examined whether the double-mutant forms exhibit any perturbations in the ability of their TM/CTD domains to heterodimerize with the TM/CTD domain of the α2 integrin. We demonstrate that, unlike the constitutive and skin-specific YY/AA β1 integrin knock-ins, which resembled the β1 loss-of-function phenotype (8, 11), the YY/AA mutation resulted in a significantly less severe UB branching phenotype and that inducing the YY/FF mutations rendered mice highly susceptible to stress injury by unilateral ureteric obstruction. Mechanistically, YY/AA and, to a lesser extent, YY/FF mutations induced adhesion defects and signaling abnormalities in response to growth factors. These defects and the structural findings are discussed below.
MATERIALS AND METHODS
Generation of YY/AA and YY/FF mice.
All experiments were approved by the Vanderbilt University Institutional Animal Use and Care Committee. The YY/FF mice, which were generated as previously described (11), were an F7 to F10 generation toward the C56/Black6 background. Aged-matched WT and homozygote littermates were obtained from heterozygote-by-heterozygote crosses. The YY/AA mice were generated as previously described (11). All mice were an F7 to F10 generation toward the C56/Black6 background. Because YY/AA mutants were embryonic lethal, they were intercrossed with mice carrying a floxed β1 integrin gene and mice carrying the hoxB7 promoter-driven Cre recombinase transgene (17). Littermates carrying the floxed and the YY/AA mutated β1 integrin allele without the Cre recombinase transgene served as controls for experiments performed with the YY/AA mutant.
Generation of integrin β1 cell lines.
β1-null collecting-duct (CD) cells described previously (46) were transfected with either full-length human integrin β1 or β1 integrins carrying YY/AA and YY/FF mutations. To ensure equal surface expression of the WT or mutant β1 integrin subunits, they were selected by fluorescence-activated cell sorter (FACS) sorting using antibody AIIB2, a monoclonal antibody directed against the extracellular domain of human integrin β1 (primary) and an anti-rat phycoerythrin (PE) (secondary).
Cell adhesion.
Cell adhesion assays were performed in 96-well plates as previously described (7). Cells (1 × 105) were seeded in serum-free medium onto plates containing different concentrations of ECM for 60 min. Adherent cells were fixed, stained with crystal violet, and solubilized, and the optical densities of the cell lysates were read at 570 nm (OD570).
Cell migration.
Cell migration was assayed as previously described (7). Transwells with 8-μm pores were coated with different ECM components, and 1 × 105 cells were added to the upper well in serum-free medium. Cells that migrated through the filter after 4 h were counted.
Cell proliferation.
Cell proliferation was determined on 96-well plates coated with different ECM proteins, as previously described (46). Cells (5 × 103) were seeded in each well and maintained in 10% serum for 4 h. The cells were then incubated in 1% serum medium for 12 h, pulsed with 1 μCi/well [3H]thymidine for 12 h, and solubilized. Radioactivity was measured using a scintillation counter.
Tubule formation.
CD cells were grown in collagen/matrigel gels as previously described (7). CD cells (5 × 103) were seeded into the gels, which were overlaid with 100 μl of medium and allowed to grow for 5 to 7 days. The gels were stained with rhodamine-phalloidin, and the tubules were photographed using a Zeiss Axio 510 confocal microscope (400×).
Growth factor-dependent cell signaling was analyzed as previously described (46). The CD cells were seeded onto collagen-coated plates (10 μg/ml) in serum-free medium and allowed to attach for 1 h. The cells were exposed to glial cell-derived neurotrophic factor (GDNF), fibroblast growth factor 10 (FGF-10), or epidermal growth factor (EGF) for different time periods, and signaling was examined by immunoblotting.
Unilateral ureteric obstruction.
The right ureters of 6-week-old WT and YY/FF mice were ligated, and mice were sacrificed 5 and 10 days after the surgery, as described previously (46). Tubular injury was scored by calculating the percentage of tubules with cell necrosis, loss of brush border, cast formation, or tubular dilatation as follows. The degree of injury was scored as 0 (none), 1 (1 to 10%), 2 (11 to 25%), 3 (26 to 45%), 4 (46 to 75%), or 5 (76 to 100%). The degrees of interstitial fibrosis and inflammatory infiltrate were judged using the same scale. At least 10 fields were analyzed for each slide.
Statistics.
Student's t test was used for comparisons between two groups, and analysis of variance using Sigma Stat software was used for statistical differences between multiple groups. A P value of <0.05 was considered statistically significant.
Expression purification of integrin β1 TM/CTD proteins.
The N-terminally 6×His-tagged integrin β1 TM/CTD (residues 719 to 798 of the full-length protein) was cloned into a pET16b vector, which was then transformed into Escherichia coli BL21(DE3) CodonPlus-RP cells. These bacteria were grown in 1 liter of M9 medium at room temperature, which was rotary shaken until the OD600 reached 0.8, when protein expression was induced by 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), followed by continued rotary shaking for ∼16 h at room temperature. 15NH4Cl M9 medium was used for 15N labeling. [13C]glucose and D2O were used for 13C and 2H labeling. Cells were harvested by centrifugation, and the His6-tagged protein was purified as follows. Cells were suspended in lysis buffer (75 mM Tris-HCl, pH 7.7, 300 mM NaCl, 0.2 mM EDTA, 0.2 mg/ml phenylmethylsulfonyl fluoride [PMSF], lysozyme-DNase-RNase at 0.2/0.02/0.02 mg/ml, and 5 mM magnesium acetate) and tumbled at room temperature for ∼30 min. The cells were probe sonicated for 5 min with cycles of 5 s on and 5 s off. Empigen (30% solution) was added to the cell lysate (1 ml per 10 ml lysate) to a final concentration of 3% (vol/vol) and mixed at 4°C for 30 min. The solubilized cell lysate was centrifuged at 20,000 × g for 20 min. The supernatant was mixed with Ni(II)-nitrilotriacetic acid (NTA) resin for 45 min at 4°C. The resin was then collected by centrifugation at 3,400 × g for 10 min. The Ni resin was washed five times with 1 column volumes of ice-cold Emp/A (3% Empigen in 40 mM HEPES, 300 mM NaCl, pH 7.5) and further washed with wash buffer (1.5% Empigen, 40 mM imidazole, 40 mM HEPES, 300 mM NaCl, pH 7.8) until all non-His-tagged proteins were eluted. The resin was reequilibrated using 8 column volumes of preequilibration buffer (20 mM imidazole plus 100 mM NaCl, pH 6.5, plus 1% diheptanoylphosphatidylcholine [D7PC] and 0.5 mM dithiothreitol [DTT]) and further with 2 column volumes of column equilibration buffer (20 mM imidazole, 100 mM NaCl, pH 6.5, 2% bicelles, 0.5 mM DTT, 10% D2O). The bicelles were composed of 1:3.3 (mol/mol) dimyristoylphosphatidylcholine-diheptanoylphosphatidylcholine (DMPC/D7PC) (q = 0.3, where q is the lipid-to-detergent mole ratio). The proteins were then eluted using elution buffer (250 mM imidazole plus 2% bicelles, 0.5 mM DTT, and 10% D2O, pH 7.4). The pH of the eluted protein solution was adjusted to 6.5 after the addition of 1 mM EDTA and concentrated 10 times using centrifugal ultrafiltration with 10-kDa cutoff filters. The sample was then used for nuclear magnetic resonance (NMR) spectroscopy.
Backbone NMR assignments.
The NMR sample used for backbone assignment contained 0.7 mM uniformly 2H-, 13C-, and 15N-labeled β1 TM/CTD in bicelle solution, which was comprised of 20% (wt/vol) DMPC/D7PC (1:3.3 [mol/mol]), 250 mM imidazole, pH 4.0, and 10% D2O. A set of transverse relaxation optimized spectroscopy (TROSY)-based triple-resonance NMR experiments with deuterium decoupling, including HNCA, HNCACB, HN(CO)CA, HN(CO)CACB and HNCO, were carried out on a Bruker 800-MHz spectrometer equipped with cryoprobe at 25°C. NMR data were processed using NMRpipe and analyzed with NMRview. Peaks were initially assigned at pH 4.0 and then used to assign the pH 6.5 amide 1H-15N TROSY peaks by collecting a series of spectra between pH 4.0 and pH 6.5, allowing correlation of the assigned pH 4.0 peaks with their pH 6.5 counterparts.
Protein secondary structure and predicted backbone torsion angles were estimated from the backbone chemical shift data using chemical shift index (CSI) analysis (42) and the Talos+ program (36).
Probing membrane topology using paramagnet-induced relaxation enhancement.
U-15N-β1-TM/CTD (1.0 mM) in bicelles was titrated with a lipophilic paramagnetic probe, 16-doxyl stearic acid (16-DSA), or with a hydrophilic paramagnetic probe, gadolinium(III) complex with diethyltriaminepentaacetic acid [Gd(III)DTPA]. The impact of the paramagnet on the NMR spectrum of β1-TM/CTD was monitored using the 15N TROSY heteronuclear single quantum coherence (HSQC) experiment, which was carried out on a 600-MHz Bruker spectrometer equipped with a cryoprobe. Gd(III)DTPA was titrated over 0 to 10 mM from a stock concentration of 250 mM Gd(III)DTPA, 250 mM imidazole, 250 mM EDTA, pH 6.5. 16-DSA was titrated over a concentration range of 0 to 0.8 mM. During the course of the titration, the samples were diluted by a factor of 4%. Peak intensities were measured as an indicator of line broadening by proximal paramagnetic probes.
Stable isotope labeling by amino acids in cell culture (SILAC)-based peptide pulldowns.
Pulldowns were performed as described previously (10). Briefly, desthiobiotinylated peptides corresponding to amino acids 758 to 798 of the β1 integrin were synthesized and immobilized on Dynabeads MyOne Streptavidine C1 (10 mg per ml; Invitrogen). Cell lysates were generated from immortalized WT mouse keratinocytes cultured in the presence of natural (light-labeled) or heavy-labeled arginine (l-[13C6, 15N4]arginine) and lysine (l-[13C6, 15N2]lysine). The lysates were sonicated briefly and cleared by centrifugation. One to 2 mg of light- and heavy-labeled supernatant was incubated with either control or experimental peptide overnight at 4°C. After washing with lysis buffer, beads of corresponding peptide pairs were combined. For the crossover experiment, the labeling was reversed. Protein was eluted by incubating the beads in 16 mM biotin (Sigma) subsequently precipitated by acetone. The protein was separated by SDS-PAGE, and bands were analyzed by liquid chromatography-mass spectrometry (LC-MS). LC-MS and data analysis were performed as previously described (26).
Integrin activation assay.
β1 integrin activation was determined using a state-specific antibody that specifically binds to the active conformation of integrin β1 (15). The amount of bound antibody was determined by flow cytometry. The total surface expression of the β1 integrin was determined using the AIIB2 antibody. The activation index of the β1 integrin was calculated as the percentage of cells that bound to the 12G10 antibody relative to the total surface expression of β1 integrin.
RESULTS
YY/AA mutations of β1 integrin cytoplasmic tails cause moderate developmental defects of the kidney collecting system.
To define the roles of the two NPXY motifs of the β1 integrin cytoplasmic domain in UB development, we studied the phenotypes of mice constitutively expressing YY/FF mutations or selectively expressing YY/AA mutations in the UB. The generation of the YY/FF mice was previously described (11), and they survive as homozygous animals. Due to the embryonic lethality of the YY/AA mice, we generated mice with YY/AA mutations only in the UB by intercrossing heterozygous YY/AA mutant mice with floxed β1 integrin mice and transgenic mice expressing Cre recombinase under the hoxB7 promoter (17), resulting in Cre-mediated deletion of the floxed β1 allele in the UB and expression of only the YY/AA mutant allele.
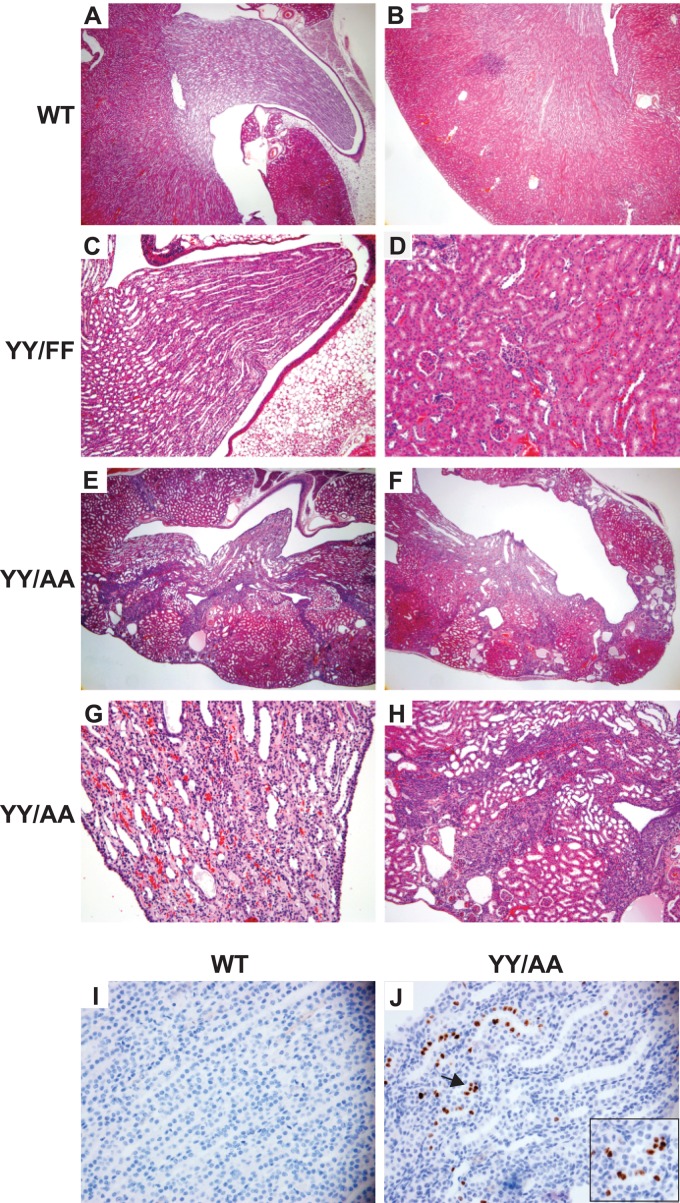
Both the YY/FF and YY/AA mice were born in the normal Mendelian ratio. The YY/FF animals lived a normal life span, and their kidneys were normal at all ages (Fig. 1A to D). In contrast, the YY/AA mice died between 4 and 6 months of age due to renal failure, and their kidneys were, on average, 50% smaller than those of WT mice at the time of death. The principal lesion seen in the kidneys of the YY/AA mice between 1 month of age and death was papilla hypoplasia, which resulted in obstruction and ultimately destruction of the cortex and the medulla (Fig. 1A, B, and E to H). The number of tubules found within the renal papilla of the YY/AA mice was moderately decreased compared to WT controls, suggesting the mice had a branching morphogenesis defect (Fig. 1A versus E). When the collecting ducts in both the medulla and cortex were observed under higher power, severe abnormalities of the tubular architecture, characterized by increased luminal cellularity and tubular obstruction, were noted (Fig. 1G and H). There was also evidence of tubulointerstitial fibrosis. Interestingly, by performing Ki67 staining, we found continued cellular proliferation in the collecting ducts of YY/AA mutants in mice older than 3 weeks, when renal development should be complete, while there was very little proliferation in the WT controls (Fig. 1I and J), suggesting the increased luminal cellularity in the YY/AA mice was due to continued collecting-duct proliferation.
Fig 1.
YY/AA mice, but not YY/FF mice, develop end stage renal failure. (A and B) Microscopy of periodic acid-Schiff (PAS)-stained kidney slides showing the medulla (A) and cortex (B) of 6-week-old WT (β1flox/flox) kidneys (magnification, ×40). (C and D) Microscopy of PAS-stained kidney slides showing the medulla (C) (magnification, ×100) and cortex (D) (magnification, ×100) of 6-week-old YY/FF kidneys. (E to H) There is a mild to moderate branching defect in some YY/AA kidneys (E) (magnification, ×40) and destruction of the medulla and corticomedullary junction in others (F) (magnification, ×40). The collecting ducts in the papilla (G), as well as in the medulla and cortex (magnification, ×200) (H), of the YY/AA mice are dilated, disorganized, and fibrosed (magnification, ×200). (I and J) Ki67 staining of 3-week-old WT papillae does not show any cell proliferation (I), while there is proliferation in YY/AA mouse papillae (magnification, ×400) (J). The proliferation was noted to be within some tubules (indicated by the arrow), as shown in the highly magnified inset.
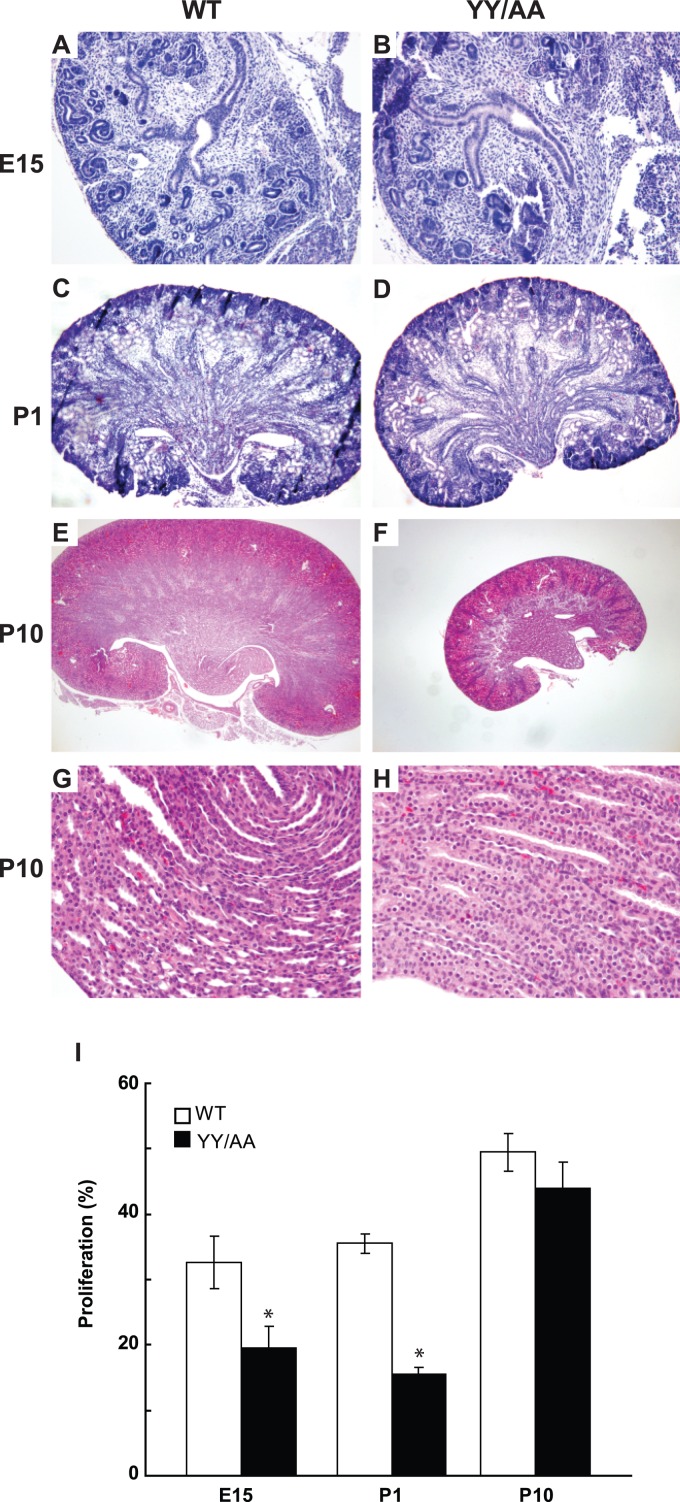
We previously reported (46), and verified in this study (data not shown), that the key feature observed upon deletion of β1 integrin from the developing UB was an early and severe branching morphogenesis defect with the formation of severely hypoplastic kidneys. As this feature was not prominent in the YY/AA kidneys postdevelopment, we performed histological analysis of kidneys from embryos at different time points. Consistent with our observations in the adult mice, there was only a mild to moderate branching defect in the YY/AA mice at E15.5 (Fig. 2A and B), and this was confirmed at postnatal day 1 (P1) (Fig. 2C and D). The kidneys of the YY/AA mutant were only slightly smaller than those of controls at both time points. At P10, the kidneys still demonstrated a moderate branching phenotype; however, the mutant kidneys were approximately 40% smaller than those of WT mice (3.5 ± 0.5 cm versus 5 ± 0.2 cm) (Fig. 2E and F). Consistent with the smaller size of the YY/AA kidneys, there was decreased papillary tubular cell proliferation in mice from E15 to P1, which are the developmental stages characterized by high proliferation; however the difference was less obvious at P10 (Fig. 2I). By P10, the intratubular cellularity in the papilla of the YY/AA mice became evident (Fig. 2G and H).
Fig 2.
YY/AA kidneys have a moderate branching morphogenesis defect and intraluminal hypercellularity. (A to H) Kidneys were isolated from embryos of WT and YY/AA mice at E15 (A and B), P1 (C and D), and P10 (E and F). A moderate UB branching phenotype with decreased size was observed in YY/AA mice (magnification, ×100). Intraluminal cellularity was noted in the YY/AA, but not the WT, mice at P10 (magnification, ×400) (H). (I) Ki67 staining was performed on at least three WT and YY/AA kidneys. The number of Ki67-positive cells in the UB (E15.5) or collecting ducts (P1 and P10) of the mice was quantified and expressed as the mean ± standard deviation (SD) of five high-power fields for five different mice. *, statistically significant differences (P < 0.05) between WT and YY/AA mutant mice.
Taken together, these data demonstrate that introducing the β1 YY/AA mutation in the UB of the kidney resulted in a moderate branching UB defect and decreased cell proliferation during the highly proliferative stages of renal development, which are followed by abnormal tubular architecture postdevelopment characterized by intratubular cellularity and tubulointerstitial fibrosis.
β1 YY/FF and YY/AA mutations differentially regulate collecting-duct cell function and signaling.
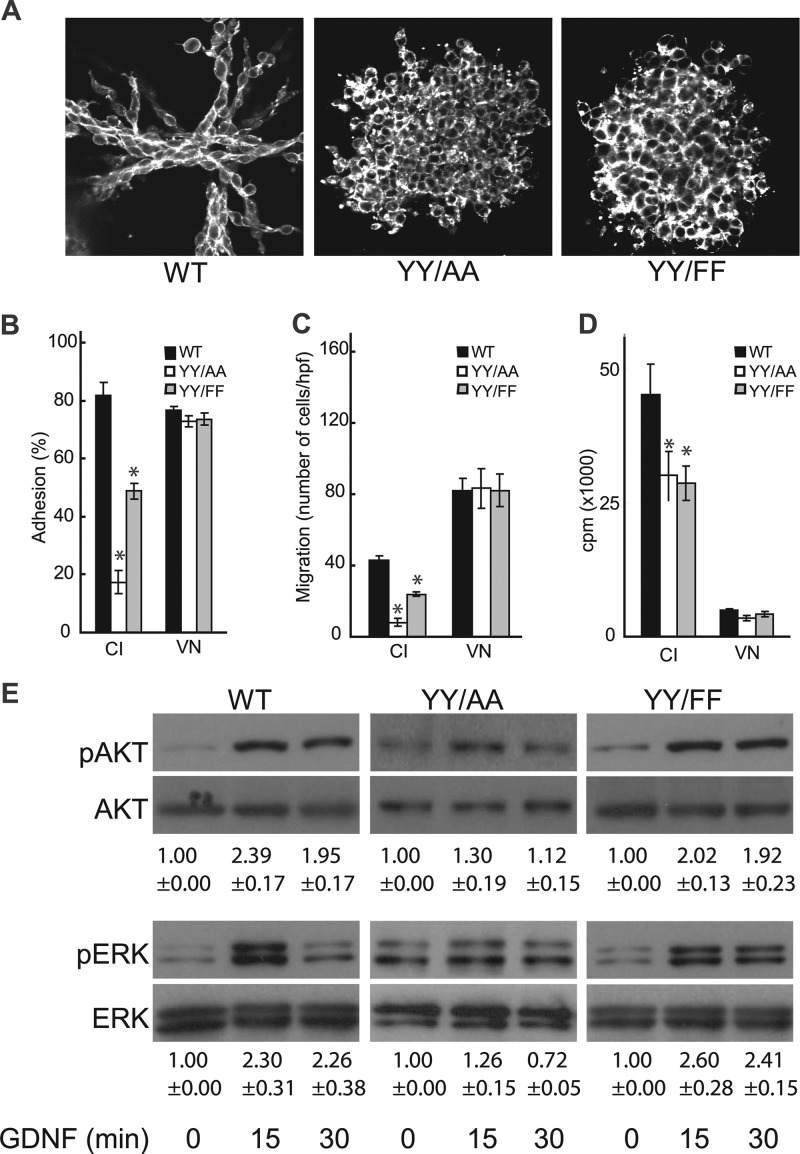
To define the mechanisms whereby the NPXY mutations induced the in vivo phenotypes, we expressed either the human β1 integrin (WT) or YY/AA or YY/FF mutants in β1-null CD cells (46). CD cells were sorted for equal levels of expression by flow cytometry (data not shown). Tubulogenesis in 3-dimensional (3D) collagen I and Matrigel gels revealed that WT CD cells were able to make well-defined tubules (Fig. 3A); however, the YY/AA and YY/FF CD cells made cysts that were indistinguishable from each other.
Fig 3.
NPXY mutations in the β1 integrin tail result in severe defects in cell function. (A) WT and NPXY mutant CD cells placed in 3D collagen and Matrigel gels were allowed to undergo tubulogenesis over 7 days in the presence of 5% fetal bovine serum (FBS). They were stained with rhodamine-phalloidin and visualized by confocal microscopy. (B) CD cell populations were allowed to adhere to collagen I (CI) or vitronectin (VN), and cell adhesion was evaluated 1 h after plating. The values are the means ± SD of three experiments performed in triplicate. *, statistically significant difference (P < 0.05) between cells expressing WT and mutant integrins. (C) CD cells were plated on transwells coated with collagen I (10 μg/ml) or vitronectin, and migration was evaluated after 4 h. The values are the means ± SD of three experiments performed in triplicate. *, statistically significant difference (P < 0.05) between cells expressing WT and mutant integrins. (D) CD cell populations were plated on collagen I or vitronectin. After 24 h, the cells were treated with [3H]thymidine and incubated for a further 24 h. [3H]thymidine incorporation was then determined as described in Materials and Methods. The values are the means ± SD of three experiments performed in triplicate. *, statistically significant difference (P < 0.05) between cells expressing WT and mutant integrins. (E) WT and mutant CD cells were allowed to adhere to collagen I for 1 h, after which they were treated with GDNF for various times. The cells were then lysed, and 20 μg of total cell lysates was analyzed by Western blotting for levels of phospho-AKT (pAKT) and pERK. Immunoblots of total AKT and ERK are shown to verify equal protein loading. A representative blot from 5 independent experiments is shown. The increases in intensity of phosphorylated proteins were quantified using Image J software and expressed as the fold increase relative to untreated cells.
Since cell adhesion, migration, and proliferation are key components of tubule formation, we tested the effects of the mutations on these cell functions on collagen I. The YY/AA and YY/FF CD cells adhered to and migrated on collagen I approximately 25% and 60% as well as WT CD cells, respectively (Fig. 3B and C). Similar results were seen with laminin 511 (data not shown); however, all cells adhered equally well to and migrated on the β3 integrin-dependent ligand vitronectin. The YY/AA and YY/FF CD cells proliferated significantly less on collagen I than WT CD cells (Fig. 3D). As with adhesion and migration, similar results were seen with laminin 511 (data not shown), while all cells proliferated equally well on vitronectin (Fig. 3D). Thus, consistent with the severe phenotype in the YY/AA mouse, there were major abnormalities in CD cells expressing this mutation. More interestingly, despite the lack of a developmental phenotype in the YY/FF mice, the mutation resulted in abnormalities in functional assays of CD cells in vitro, although always to a lesser extent than the YY/AA mutations.
The growth factors GDNF and the FGFs play a critical role in UB branching morphogenesis, and β1 integrin expression is required for GDNF- and FGF-dependent signaling both in vivo and in vitro (46). We therefore tested whether mutating the β1 NPXY motifs alters the ability of these growth factors to mediate well-defined cell-signaling pathways in CD cells. When the YY/AA-CD cells were plated on collagen I matrices in the presence of either GDNF or FGF-10, there was a marked attenuation of AKT and extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) activation (Fig. 3E; see Fig. S1 in the supplemental material); however, it was not as severe as that seen in β1-null CD cells (46). In contrast, the YY/FF mutants transduced these signals to the same extent as the WT cells. No differences in p38MAPK activation between the WT and either mutant in response to these growth factors were seen (data not shown). Thus, the YY/AA mutant demonstrated markedly decreased GDNF- and FGF-mediated AKT and ERK MAPK signaling, while signaling was normal in the YY/FF mutant.
YY/FF mutations of the β1 integrin tail render mice more susceptible to renal injury.
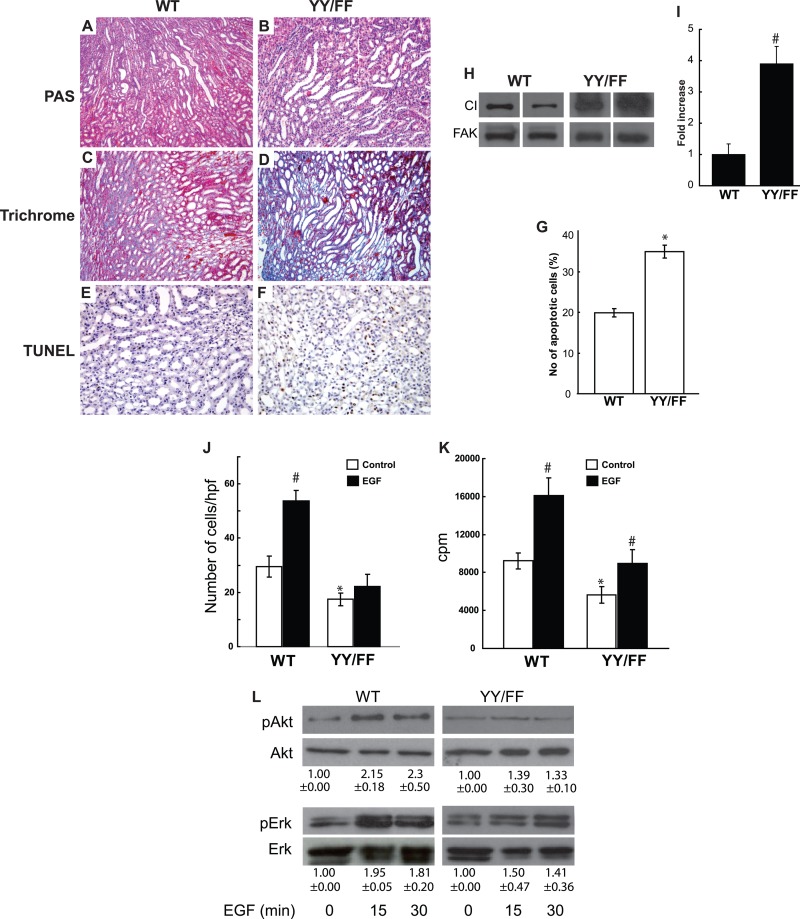
Although the YY/FF mice developed normally, it was clear that YY/FF CD cells had abnormal cell adhesion, migration, and proliferation. These in vitro data led us to hypothesize that the YY/FF mice are more susceptible to stress-induced injury. Therefore, we tested stress-induced injury by subjecting 6-week-old YY/FF and WT mice to unilateral ureter obstruction (UUO), a well-established model of tubular injury. There was markedly increased tubular dilatation and flattening of tubular epithelial cells in the YY/FF mice compared to WT controls at 5 (Fig. 4A and B) and 10 (data not shown) days following injury. The increased renal injury was verified by scoring at day 5 (4.7 ± 0.5 versus 1.9 ± 0.4; P < 0.01). Although there was no increase in matrix production in the contralateral kidney of either genotype (see Fig. S2 in the supplemental material), the YY/FF mice had more matrix deposition, as shown by Trichome Blue staining (Fig. 4C and D), and excessive collagen I production, as verified by immunoblotting of the obstructed kidneys (Fig. 4H and I). More tubular apoptosis, as shown by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining, was present in the injured YY/FF mice (Fig. 4E to G). Thus, although YY/FF mice developed normally, they were more susceptible to obstructive renal injury.
Fig 4.
YY/FF mice develop severe injury following unilateral ureteric obstruction. (A and B) Kidneys of 6-week-old YY/FF mice show more severe tubular dilatation and injury 5 days after unilateral ureteric obstruction than WT mice (magnification, ×200). (C and D) More intense and abundant Trichrome Blue staining was evident in 5-day-injured YY/FF than in WT mice. (E to G) Increased TUNEL staining was evident in 5-day-injured YY/FF compared to WT mice. Apoptosis was quantified and expressed as the mean number of apoptotic cells per microscopic field ± SD (10 fields of 10 kidneys from either genotype were analyzed). Differences between YY/FF and WT mice were significant (*), with P values of <0.05. (H) Immunoblotting with an antibody directed against collagen I was performed on individual medullas of 5-day-injured YY/FF and WT mice. FAK, focal adhesion kinase. (I) Intensities of collagen bands were quantified using Image J software. Collagen expression in YY/FF mice was expressed as the fold change relative to that seen in WT controls (5 animals per group). #, differences between YY/FF and WT mice were significant; P < 0.001. (J) YY/FF and WT CD cells were plated on transwells coated with collagen I (10 μg/ml), and migration was evaluated after 4 h. The values are the means ± SD of three experiments performed in triplicate. *, statistically significant difference (P < 0.01) between WT and YY/FF mutants; #, statistically significant difference (P < 0.01) between EGF-treated and untreated cells. hpf, high-power field.(K) YY/FF and WT CD cells were plated on collagen I. After 24 h, the cells were treated with EGF and [3H]thymidine and incubated for a further 24 h. [3H]thymidine incorporation was then determined as described in Materials and Methods. The values are the means ± SD of three experiments performed in triplicate. *, statistically significant difference (P < 0.01) between WT and YY/FF mutant proteins; #, statistically significant difference (P < 0.01) between EGF-treated and untreated cells. (L) WT and mutant CD cells were allowed to adhere to collagen I for 1 h, after which they were treated with EGF at various times. The cells were then lysed and analyzed by Western blotting for levels of pAKT and pERK. Immunoblots of total AKT and ERK are shown to verify equal protein loading. A representative blot from 3 independent experiments is shown. The intensities of phosphorylated proteins were quantified using Image J software and expressed as the fold increase relative to untreated cells.
β1 integrin is a fundamental regulator of EGF receptor signaling, which is a key controller of renal tubule responses to injury (45). We therefore investigated whether the YY/FF mutations affected EGF-dependent signaling and CD function. When we analyzed the abilities of WT and YY/FF CD cells to migrate toward EGF on collagen I-coated transwells, there was a significant increase in WT cell migration toward the ligand that was not seen with the YY/FF CD cells (Fig. 4J). When proliferation of cells plated on collagen I in response to EGF was determined, both the WT and YY/FF CD cells increased proliferation significantly, but the effect of EGF was more profound on WT CD cells (Fig. 4K). Consistent with these abnormalities in migration and proliferation, the YY/FF CD cells plated on collagen I activated AKT and ERK MAPK less robustly than WT cells following EGF treatment (Fig. 4L). Thus, in contrast to GDNF and FGF-10, where YY/FF CD cells signaled like WT CD cells with respect to AKT and ERK MAPK pathways, YY/FF CD cells had reduced signaling following EGF stimulation and YY/FF cells migrated less toward EGF than WT cells plated on a collagen I matrix.
The β1 integrin tyrosine mutations did not alter the membrane interactions of the distal cytoplasmic tail region.
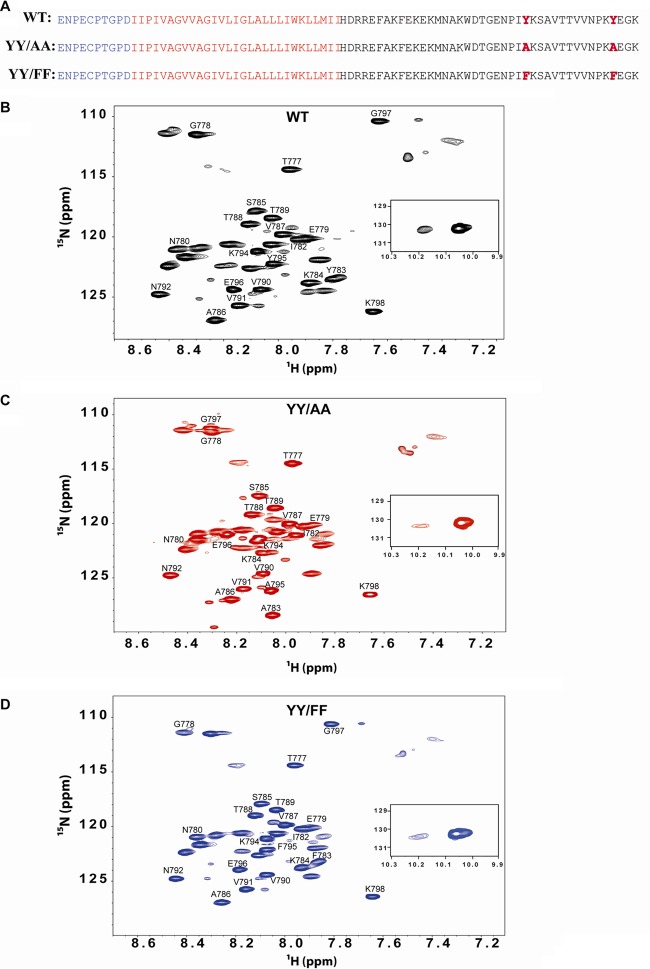
In an effort to define a structural basis for the differences in the in vivo and in vitro phenotypes of the YY/AA and YY/FF mutants, we undertook studies using NMR spectroscopy of the combined β1 transmembrane/cytoplasmic tail domain (TM/CTD) in bicelles (residues 719 to 798). We initially investigated the interactions between the WT, YY/AA, and YY/FF CTDs and the adjacent membrane (i.e., bicelle) surface. The proteins investigated correspond to the TM/CTD of integrin β1 (residues 719 to 798) (Fig. 5A). Hexa-His-tagged WT, YY/AA, and YY/FF mutant proteins were expressed in E. coli as uniformly 15N-labeled proteins and purified, with final NMR samples being composed of the TM/CTD in bicelles comprised of 20% DMPC/D7PC at 1:3.3 (mol-to-mol), pH 6.5, and 298 K. Backbone NMR resonance assignment of the protein spectrum was carried out by using 3D experiments with uniformly 13C- and 15N-labeled protein (see Fig. S3 and Table S1 in the supplemental material). Resonance assignments revealed that the majority of the observable peaks and all of those that could be assigned using the 3D data were from the membrane-distal cytosolic domain (residues 777 to 798) (Fig. 5B to D). The vast majority of the peaks from the TM and membrane-proximal CTD (residues 719 to 776) are too broad to observe at 298 K. The visible distal CTD peaks correspond to a segment that further analysis (below) indicated to be disordered. To gain insight into the conformations of the spectroscopically invisible TM and membrane-proximal CTD, a far-UV circular dichroism spectrum of the protein was collected under the same sample conditions as used for NMR. The spectrum (see Fig. S4 in the supplemental material) exhibited a typical pattern for a highly helical protein. The analysis of the spectrum indicated 85% α-helical content, suggesting that virtually all of the TM/CTD is helical and that the TM and the membrane-proximal CTD very likely form a single extended helix. The absence of NMRs for most of this helix at 298 K is likely due to the high molecular weight and slow tumbling of the bicelle-protein complex. This hypothesis is supported by observation of TM peaks at 313 K, where tumbling is more rapid (data not shown).
Fig 5.
TM/CTD of the integrin β1 recombinant shows differences in TROSY NMR spectra due to mutation of the tyrosine residues. (A) Sequence of the TM/CTD of integrin WT and YY/AA and YY/FF mutant proteins expressed and purified in E. coli. Blue, extracellular domain; red, transmembrane domain; black, cytosolic tail; bold, mutations. (B to D) 1H,15N-TROSY spectra of the integrin U-15N-TM/CTD integrin were collected using a 600-MHz Bruker NMR Avance-III spectrometer at 298 K, pH 6.5, in DMPC/D7PC bicelles (q = 0.3), 20% total amphiphile, and a protein concentration of 0.6 to 0.8 mM. Shown are WT (B), YY/AA (C), and YY/FF (D). The spectra in panels B to D exhibit only modest differences.
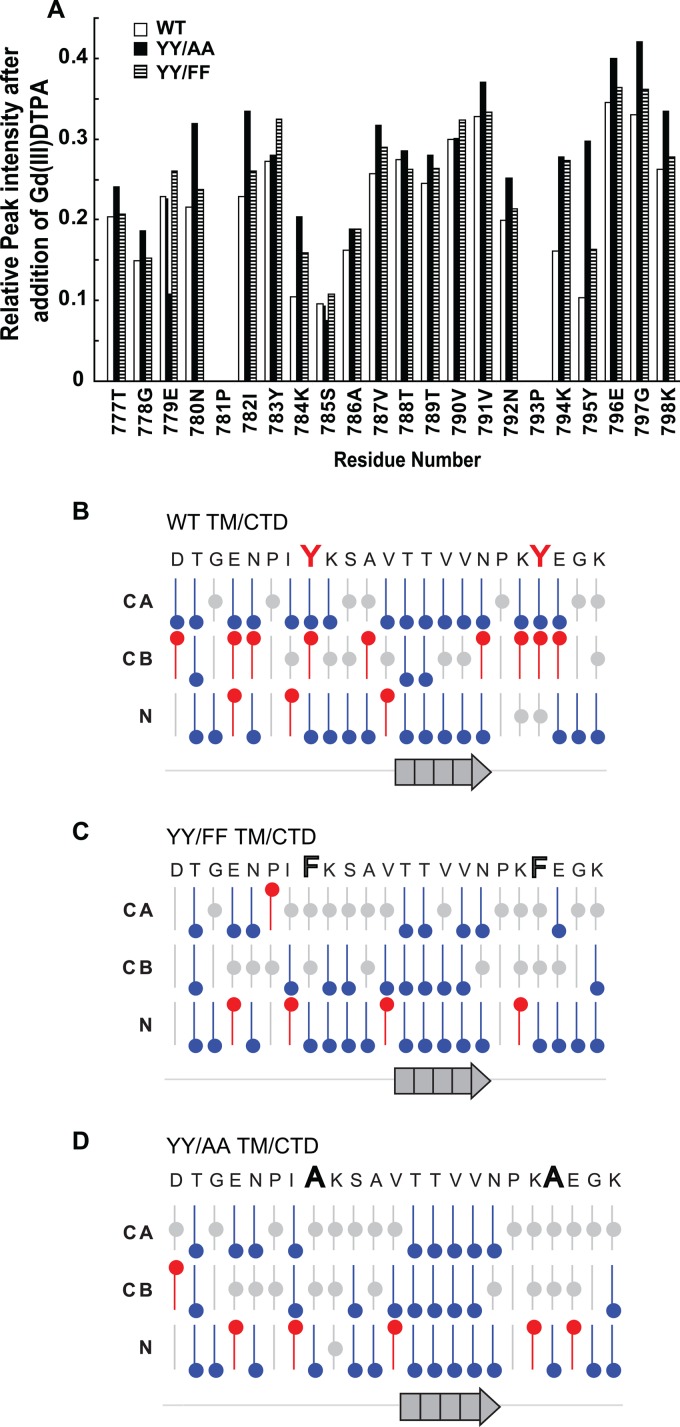
When TROSY-HSQC spectra were obtained from the WT, YY/AA, and YY/FF mutant proteins (Fig. 5B to D) and overlaid (see Fig. S5 in the supplemental material), we observed that the two mutants exhibited spectra similar to that of the WT, with the only major changes being for peaks from residues at or near the mutation sites (Fig. 5; see Fig. S5 in the supplemental material). This suggests that the three peptides maintain similar structures, despite the mutations. To determine whether the mutations change the association of the CTD of the protein with the bicelle surface, these 15N-labeled proteins were titrated with a water-soluble paramagnetic compound, Gd(III)DTPA. The addition of this compound led to a >50% decrease in intensity for all peaks (Fig. 6A), consistent with the above-mentioned notion that all three peptides were largely unstructured and water exposed. The residue-by-residue intensity reductions were very similar for the WT protein and both mutants, pointing to very similar structures and a lack of avid interactions with the membrane surface [so that residue-specific Gd(III)DTPA accessibility patterns were similar]. Titration of the WT and mutant proteins with the hydrophobic 16-DSA failed to induce significant reductions in peak intensities and thus corroborated the absence of a significant interaction of the cytosolic domain of the integrin β1-TM/CTD protein with the bicellar surface. Together, these results indicate that most residues in the membrane-distal CTD are located in the aqueous phase with no observable surface association of the domain in either the WT or mutant protein. Furthermore, the paramagnetic probe experiments failed to provide strong evidence for more than transient higher-order structure, although the modest variations in residue-to-residue accessibility (Fig. 5A) are supportive of some preferences in the ensemble of interconverting conformations. These preferences, however, were very similar for the WT and mutant proteins.
Fig 6.
YY/AA and YY/FF mutations did not alter membrane interactions or the secondary structure of the integrin β1 distal cytoplasmic domain. (A) Membrane interactions of the β1 integrin CTD were examined using 1H,15N-TROSY NMR titration in which 15N-labeled WT and mutant recombinant proteins (1.0 mM) in bicelles were titrated with a water-soluble paramagnetic probe, Gd(III)DTPA (5 mM). Paramagnetism-induced decreases in peak intensity relative to untreated samples were measured. The uncertainty associated with the intensities of values was 5%. (B to D) The secondary structure of the β1 tail region was assessed from the observed backbone chemical shifts using chemical shift index analysis. WT (B), YY/FF (C), and YY/AA (D) proteins all showed similar chemical shifts and subsequent predicted secondary-structure patterns. Red, gray, and blue symbols indicate that the chemical shifts for backbone alpha carbon-13 (CA), beta carbon-13 (CB), or backbone nitrogen-15 (N) predict that the associated residue position is part of an α-helix, random coil segment, or β-strand, respectively. The arrow at the bottom of each panel indicates a consistent prediction of β-strand secondary structure for the 5 residues indicated immediately above the arrow. The remainder of segments (indicated by the grey line) are not consistently predicted by the chemical shifts to adopt α-helical or β-strand structure.
Substitutions of the β1 tail tyrosines maintain the structure of the cytosolic domain.
The measured backbone chemical shifts for the CTD of bicelle-associated β1-TM/CTD (see Table S1 in the supplemental material) can be interpreted in terms of protein secondary structure using CSI analysis and in terms of backbone torsion angles using Talos+. Deviations of observed backbone shifts from the corresponding amino acid-specific random-coil values are summarized in Fig. 6B to D. Comparison of the data patterns of the WT with the two mutant proteins revealed high similarity, indicating the WT structure remained unperturbed, or at most little perturbed, by the YY/AA or YY/FF mutation, respectively. Moreover, both CSI and Talos+ analyses were consistent with the distal CTD of the β1-TM/CTD being primarily a random coil with a single extended strand that spans residues T788 to N792. Neither of the tyrosine residues appeared to be part of a segment containing regular secondary structure or a beta turn. This was further confirmed by measuring backbone amide-amide 1H-1H nuclear Overhauser effects (NOEs) (data not shown), which did not show the expected patterns for helices or for well-defined turns associated with the tyrosines.
The β1 tail tyrosine substitutions maintain avidity with the α2 TM/CTDs.
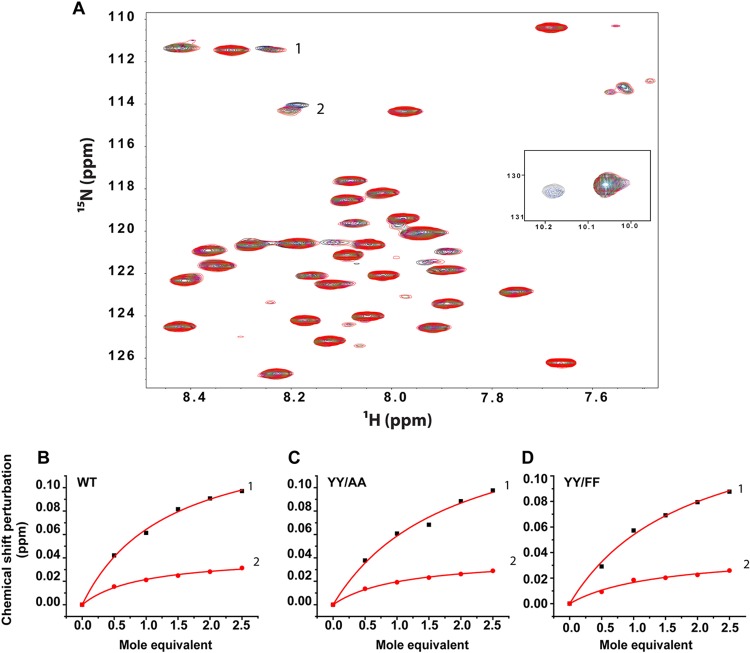
Correct integrin function requires association of the α and β subunits in the resting state, which involves adhesive contacts between the TM and membrane-proximal CTD. To determine whether the tyrosine substitutions in the β1 tail alter the heterodimerization propensity of the β1 TM/CTD with the corresponding α2 TM/CTD, we carried out titrations of 15N-labeled WT, YY/AA, and YY/FF proteins with unlabeled α2 TM/CTD in bicelles (Fig. 7) and monitored formation of the heterodimeric complex by NMR spectroscopy. The TROSY NMR spectra for the WT titration are shown in Fig. 7A. At least two peaks (labeled 1 and 2) shifted significantly in response to the titration of the α2 subunit. When the shifts of these two peaks were plotted versus the α2-to-β1 (mol/mol) ratio, the data for both peaks fitted well with a 1:1 binding model in which the dissociation constant of heterodimers was in the range of 0.5 mM (Fig. 7B to D). Titration of the YY/AA and YY/FF peptides produced similar titration spectra (see Fig. S5 in the supplemental material). The chemical shift changes for the same two peaks observed to shift for the WT case exhibited very similar magnitudes of shifts and dependency on the α2-to-β1 (mol/mol) ratio (Fig. 7C and D). This result indicates that the tyrosine substitutions do not significantly affect the avidity between the α2 and β1 TM/CTDs.
Fig 7.
YY/AA and YY/FF mutations of the β1 integrin TM/CTD did not alter the avidity of complex formation with the α2 integrin TM/CTD. (A) Titration of the U-15N-β1 TM/CTD with the unlabeled α2 TM/CTD was carried out in DMPC/D7PC bicelles (q = 0.3l; pH 6.5; 298 K) and monitored using 1H,15N-TROSY NMR. The protein concentrations were 1.0 mM for the WT and mutants. Changes in the chemical shifts of peaks were interpreted as reporting on complex formation. (B) Two of the WT β1 peaks that were observed to shift in response to α2 titration were selected, and the changes in chemical shifts of these two peaks were plotted versus the α2-to-β1 (mol/mol) ratio. (C and D) Corresponding plots are presented for titrations involving the two β1 mutants, YY/AA (C) and YY/FF (D). It can be qualitatively seen that the mutations resulted in little change in avidity between the TM/CTD domains of the two integrin subunits.
β1 YY/FF and YY/AA mutations alter binding of cytosolic proteins and integrin affinity.
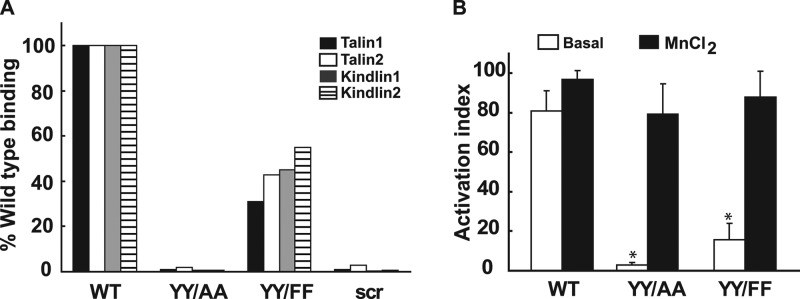
The structural data show that the YY/AA and the YY/FF mutations do not affect the β1 secondary structure, its interactions with the membrane, or heterodimerization with the α2-TM/CTD. We therefore investigated whether the mutants exhibited differential binding to cytosolic proteins and whether they altered integrin activation. To assess the impact of YY/FF and YY/AA mutations on talin and kindlin binding, which are known to bind to the membrane-proximal and membrane-distal NPXY motifs, respectively (29), we synthesized peptides corresponding to amino acids 758 to 798 of the wild-type, YY/FF, and YY/AA forms of the β1 integrin. Binding of talin and kindlin to these peptides was assessed by a SILAC-based quantitative-proteomics approach (26), and SILAC ratios were used to calculate the binding strength as a percentage of that of the WT (Fig. 8A). While YY/FF-mutated integrin tails showed only a moderate reduction in binding to talins and kindlins, binding was almost completely inhibited by the less conservative YY/AA mutations. We next defined the consequences of these mutations for β1 integrin activation in CD cells by defining the affinity of β1 integrin for the 12G10 monoclonal antibody (a gift from Martin Humphries), which binds only to the active integrin. This was done using flow cytometry and is expressed as an activation index (the percentage of 12G10 monoclonal antibody binding relative to total β1 integrin expression). CD cells expressing WT β1 exhibited an activation index of 80%, while the YY/FF and YY/AA mutants had activation indices of approximately 20% and 5%, respectively (Fig. 8B). Thus, both pairs of mutations severely attenuated integrin activation; however, this effect was markedly more severe for the YY/AA mutant. In addition, the activation index directly correlated with the binding of kindlins and talins to the β1 cytoplasmic tail, consistent with the notion that NPXY motifs regulate integrin function by altering the CTD binding affinities for critical integrin binding proteins.
Fig 8.
YY/AA and YY/FF mutations of the β1 CTD alter talin and kindlin binding and activation of integrin β1. (A) The binding affinities of β1 integrin with cytoplasmic proteins talin and kindlin were determined using the SILAC assay performed with WT, YY/AA, YY/FF, and scrambled (scr) β1 integrin peptides. (B) The activation of the β1 integrin was determined by quantifying the amount of antibody 12G10 that bound to the active conformation of human β1 integrin using a FACS assay. The percentage of binding was in each case normalized with the total surface expression of integrin and expressed as an activation index. Total surface expression was quantified by using the AIIB2 antibody in the FACS assay. The error bars indicate SD.
DISCUSSION
UB development requires both regulated growth factor signaling and cell-ECM interactions, and loss of β1 integrin expression in the UB causes hypoplastic and dysplastic kidneys due to abnormalities in both integrin adhesive functions and growth factor-dependent signaling (46). Here, we defined the roles of the two tyrosines in the canonical integrin β1 cytoplasmic tail NPXY motifs in UB development. YY/AA mutations cause a moderate branching and proliferation phenotype during development, followed by postnatal dysregulated epithelial cell proliferation that results in intratubular obstruction and renal fibrosis. In contrast, although YY/FF kidneys developed normally, they were more susceptible than those of WT mice to UUO-mediated injury. These in vivo data suggest that divergent signals transduced by the β1 integrin tail NPXY motifs differentially regulate UB development and the response of the collecting system of the kidney to injury.
We further demonstrated that both YY/AA and YY/FF CD cells had adhesion, migration, and proliferation abnormalities, indicating a crucial role for Tyr phosphorylation and/or for the phenolic —OH moiety in supporting these integrin-dependent functions. A similar conclusion could be reached for EGF responsiveness, as YY/FF CD cells were unable to respond to EGF, one of the mediators of renal repair following injury (45). In contrast, only YY/AA CD cells had signaling deficiencies in response to growth factors important for UB development, suggesting that both Tyr phosphorylation and the phenolic —OH groups are dispensable for the integrin/growth factor cross talk involved in normal UB development. Utilizing solution NMR, we confirmed that the distal β1 integrin tail is mainly unstructured and showed that the YY/AA and YY/FF mutations did not alter α2β1 heterodimerization, membrane association, or the secondary structure of the β1 integrin tail. Together, these data suggest that NPXY motif-dependent actions are not determined by their intrinsic structural conformation but rather by their interactions with critical integrin binding proteins. Furthermore, specific (and often divergent) functions are likely regulated in a cell-type-specific manner by different combinations of NPXY-binding proteins, which might be impacted by the phosphorylation status of each NPXY motif.
The YY/FF kidneys were normal, and the YY/AA kidneys were very different than the β1-null kidneys (46). Normal development in the YY/FF kidneys was the same as that seen in the constitutive (8) and skin-specific (11) knock-ins and indicates that neither tyrosine phosphorylation nor the phenolic —OH of Tyr is required for the β1 integrin to support development. The β1-null mice died significantly earlier than the YY/AA mice (12 weeks versus 24 weeks) due to renal failure caused by a severe branching abnormality (46), while the YY/AA mice had a moderate branching morphogenesis phenotype and died from obstruction and/or severe renal fibrosis. This major discrepancy in phenotype between β1-null and YY/AA mice was unexpected because constitutive (8) or skin-specific (11) expression of the YY/AA mutation resulted in phenotypes indistinguishable from that of β1-null mice. An interesting feature in the YY/AA mice not present in the β1-null mice was dysregulated proliferation after development, resulting in cell accumulation within the tubules, causing destructive obstruction of the collecting system. This characteristic was similar to that of ILK-null mice, where it was ascribed to a defect in P38 MAPK-dependent contact inhibition of CD cells (37); however, this was not the case in the YY/AA mutants, as they undergo contact inhibition and have no defect in P38 MAPK signaling (data not shown). Another possible explanation for the increased luminal cell number in the lumens of the adult YY/AA mice is that defective cell adhesion leads to a failure of newly divided cells to reconnect to the extracellular matrix. This potential mechanism has not been experimentally tested.
We utilized CD cells to better define the mechanisms underlying the YY/AA kidney phenotype. Like β1-null CD cells, YY/AA cells had serious adhesion and migration defects; however, the proliferation defect was less severe (46). Interestingly, although there was decreased activation of GDNF- and FGF-10-dependent signaling pathways in the YY/AA CD cells compared to WT cells, it was not as marked as in β1-null CD cells. As the phenotype of the β1-null kidneys was previously attributed to both adhesion- and growth factor-dependent signaling abnormalities (46), the increased growth factor-dependent signaling could explain the less severe developmental phenotype in the YY/AA relative to the β1-null kidneys. In this regard, the ERK pathway regulates UB branching morphogenesis (31).
Although the YY/FF mice developed normally and the YY/FF CD cells signaled like WT CD cells in response to GDNF and FGF-10, YY/FF CD cells exhibit abnormalities in tubulogenesis, adhesion, migration, and proliferation. In addition, they have a decreased ability to migrate toward EGF and an attenuated signaling response to EGF. Furthermore, YY/FF mice developed significantly worse stress-induced injury than WT controls. Thus, YY/FF mutations inhibited some integrin β1-dependent functions, but not others. These functions, which are dependent on normal interactions with crucial binding partners, are evidently dependent on Tyr phosphorylation and/or the side chain phenolic —OH group. The conservative Y/F mutations, which are sufficient to decrease but not totally abolish the binding of integrin binding proteins (Fig. 8) to β1 tails, result in alterations in inside-out and outside integrin signaling that cause abnormalities only when the mice are placed in stress situations, such as the UUO model. Consistent with our data, YY/FF mice developed fewer tumors than WT mice in a skin cancer model (26). Although our structural studies do not offer direct insight into whether Tyr phosphorylation plays a role in supporting native functions, it is known that Y783 phosphorylation reduces the affinity of β1 for talin and enhances affinity for Dok1 (2). In addition, Y/A795 mutations of β1integrin abrogate kindlin binding (14, 27), and it is known that phosphorylation of the corresponding membrane-distal NITY759 motif in the β3 CTD disrupts kindlin-2 recognition and the ability of kindlin-2 to coactivate the αIIβ3 integrin (14). Although specific mechanisms whereby the YY/AA and YY/FF mutations differentially altered signaling pathways in UB development and after injury were not defined, our structural and binding studies suggest it is due to the altered ability of the mutant tails to bind critical cytoplasmic integrin binding proteins, which are spatially and temporally expressed in a cell-type-specific manner.
Our NMR spectra of the β1 TM/CTD are the first for any intact integrin TM/CTD in a bicellar model membrane environment. Bicelles represent a more native-like membrane environment than conventional detergent micelles or organic solvent mixtures and have previously been used in structural studies of integrin TMs (16, 19, 20). Previous studies of the β1 and other integrin CTDs did not investigate the possibility that interactions of the CTD with the model membrane surface could perturb the structure. We found that the distal CTD of the β1 TM/CTD was disordered even when inserted into membranes, confirming previous reports that the membrane-distal CTD of β1 is disordered until it binds talin, at which point its structure becomes ordered (although irregular in terms of secondary structure) (3). Moreover, we showed that this domain does not interact with the bicelle surface. On the other hand, while the membrane-proximal domain of the CTD in the absence of an intact TM was shown to be mostly disordered (3), we observed that in the context of an intact TM in a bicelle environment, the domain is helical. Similarly, interactions between the α2 and β1 TM/CTDs were not altered by YY/FF or YY/AA mutations of the β1 tail. Our results contrast with NMR studies of the full-length β3 integrin CTD in micelles (25), where a disulfide-stabilized complex consisting of portions of the TMs and the full CTDs of αIIbβ3 were studied. In that work, the β3 CTD was shown to consist of a stable membrane-proximal helix contiguous with the TM helix and two distal amphiphilic helices. The two distal helices of β3 were shown to associate with lipid bilayers but to undergo fluctuations that would allow rapid binding of cytoplasmic proteins regulating integrin activation. Two other NMR studies performed under aqueous (38) or detergent (44) micelle conditions verified that the membrane-proximal NPXY motif of the β3 CTD is structured. In addition, phosphorylation of the NXXY motifs was shown to affect the structure and association of the β3 CTD with the membrane and suggested that the biphosphorylation of the NXXY motifs of the β3 CTD favors disruption of the intersubunit clasp that keeps αIIβ3 integrin in a low-affinity state (12). In contrast, the isolated β2 cytoplasmic tail in aqueous or micelles was previously shown to also be largely unstructured, except for the N-terminal residues Leu6-Arg14 (5, 9). Thus, it is clear that major differences in the structures of the diverse β integrin tails exist.
We also demonstrated that YY/FF and YY/AA mutations result in decreased talin and kindlin binding to the β1 tails and that this correlates with decreased integrin activation, despite the lack of alteration in integrin structure. In the case of Tyr783, this mutational effect appears to be unrelated to Tyr phosphorylation, since Tyr783 phosphorylation was previously shown to reduce binding to talin, and direct interactions of the Y783 side chain with talin were shown to be critical for the avidity of the complex (2). Similarly, phosphorylation of the membrane-distal NITY759 motif in the β3 CTD disrupts kindlin-2 recognition and binding to the integrin and its consequent activation (14).
In conclusion, we show that mutations in the integrin β1 tail NPXY motifs in the kidney produce phenotypes different from those seen in constitutive and skin-specific knock-ins, demonstrating that these highly conserved motifs differentially regulate integrin-dependent processes in a cell-specific manner. Our data also confirm that the structure and membrane association of the distal integrin tails that contain these highly conserved domains are different in β1 and β3 integrins. Thus, our studies highlight the importance of defining the binding partners and phosphorylation patterns for the highly conserved NPXY motifs of integrin β subunits in different organs, because they likely regulate markedly different integrin-dependent functions. In addition, they highlight the importance of studying the structure of the integrin CTDs in complex with their effector proteins, since induced fit and/or conformational selection is clearly a major factor in the binding of some CTD-effector protein complexes.
Supplementary Material
ACKNOWLEDGMENTS
S.M. is a fellow of the American Heart Association. This work was supported by DK083187 (R.Z. and C.R.S.), DK075594 (R.Z.), DK069221 (R.Z.), the O'Brien Center DK79341-01 (A.P. and R.Z.), P01 DK065123 (R.Z. and A.P.), and an American Heart Association Established Investigator Award (R.Z.). L.S.G. has a Career Development Award and R.Z. and A.P. have Merit Awards from the Department of Veterans Affairs.
Footnotes
Published ahead of print 6 August 2012
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1. Anthis NJ, Campbell ID. 2011. The tail of integrin activation. Trends Biochem. Sci. 36:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anthis NJ, et al. 2009. Beta integrin tyrosine phosphorylation is a conserved mechanism for regulating talin-induced integrin activation. J. Biol. Chem. 284:36700–36710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anthis NJ, Wegener KL, Critchley DR, Campbell ID. 2010. Structural diversity in integrin/talin interactions. Structure 18:1654–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anthis NJ, et al. 2009. The structure of an integrin/talin complex reveals the basis of inside-out signal transduction. EMBO J. 28:3623–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhunia A, Tang XY, Mohanram H, Tan SM, Bhattacharjya S. 2009. NMR solution conformations and interactions of integrin alphaLbeta2 cytoplasmic tails. J. Biol. Chem. 284:3873–3884 [DOI] [PubMed] [Google Scholar]
- 6. Calderwood DA, et al. 1999. The talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 274:28071–28074 [DOI] [PubMed] [Google Scholar]
- 7. Chen D, et al. 2004. Differential expression of collagen- and laminin-binding integrins mediates ureteric bud and inner medullary collecting duct cell tubulogenesis. Am. J. Physiol. Renal Physiol. 287:F602–F611 [DOI] [PubMed] [Google Scholar]
- 8. Chen H, et al. 2006. In vivo beta1 integrin function requires phosphorylation-independent regulation by cytoplasmic tyrosines. Genes Dev. 20:927–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chua GL, Tang XY, Amalraj M, Tan SM, Bhattacharjya S. 2011. Structures and interaction analyses of integrin alphaMbeta2 cytoplasmic tails. J. Biol. Chem. 286:43842–43854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cox J, Mann M. 2008. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26:1367–1372 [DOI] [PubMed] [Google Scholar]
- 11. Czuchra A, Meyer H, Legate KR, Brakebusch C, Fassler R. 2006. Genetic analysis of beta1 integrin “activation motifs” in mice. J. Cell Biol. 174:889–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deshmukh L, Meller N, Alder N, Byzova T, Vinogradova O. 2011. Tyrosine phosphorylation as a conformational switch: a case study of integrin beta3 cytoplasmic tail. J. Biol. Chem. 286:40943–40953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia-Alvarez B, et al. 2003. Structural determinants of integrin recognition by talin. Mol. Cell 11:49–58 [DOI] [PubMed] [Google Scholar]
- 14. Harburger DS, Bouaouina M, Calderwood DA. 2009. Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects. J. Biol. Chem. 284:11485–11497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Humphries JD, et al. 2005. Dual functionality of the anti-beta1 integrin antibody, 12G10, exemplifies agonistic signalling from the ligand binding pocket of integrin adhesion receptors. J. Biol. Chem. 280:10234–10243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim C, et al. 2012. Basic amino-acid side chains regulate transmembrane integrin signalling. Nature 481:209–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kobayashi A, et al. 2005. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132:2809–2823 [DOI] [PubMed] [Google Scholar]
- 18. Lau TL, Dua V, Ulmer TS. 2008. Structure of the integrin alphaIIb transmembrane segment. J. Biol. Chem. 283:16162–16168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lau TL, Kim C, Ginsberg MH, Ulmer TS. 2009. The structure of the integrin alphaIIbbeta3 transmembrane complex explains integrin transmembrane signalling. EMBO J. 28:1351–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lau TL, Partridge AW, Ginsberg MH, Ulmer TS. 2008. Structure of the integrin beta3 transmembrane segment in phospholipid bicelles and detergent micelles. Biochemistry 47:4008–4016 [DOI] [PubMed] [Google Scholar]
- 21. Legate KR, Wickstrom SA, Fassler R. 2009. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 23:397–418 [DOI] [PubMed] [Google Scholar]
- 22. Li R, et al. 2003. Activation of integrin alphaIIbbeta3 by modulation of transmembrane helix associations. Science 300:795–798 [DOI] [PubMed] [Google Scholar]
- 23. Li W, et al. 2005. A push-pull mechanism for regulating integrin function. Proc. Natl. Acad. Sci. U. S. A. 102:1424–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mathew S, Chen X, Pozzi A, Zent R. 2012. Integrins in renal development. Pediatr. Nephrol. 27:891–900 [DOI] [PubMed] [Google Scholar]
- 25. Metcalf DG, et al. 2010. NMR analysis of the alphaIIb beta3 cytoplasmic interaction suggests a mechanism for integrin regulation. Proc. Natl. Acad. Sci. U. S. A. 107:22481–22486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meves A, et al. 2011. Beta1 integrin cytoplasmic tyrosines promote skin tumorigenesis independent of their phosphorylation. Proc. Natl. Acad. Sci. U. S. A. 108:15213–15218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Montanez E, et al. 2008. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 22:1325–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moser M, et al. 2009. Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat. Med. 15:300–305 [DOI] [PubMed] [Google Scholar]
- 29. Moser M, Legate KR, Zent R, Fassler R. 2009. The tail of integrins, talin, and kindlins. Science 324:895–899 [DOI] [PubMed] [Google Scholar]
- 30. Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. 2008. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat. Med. 14:325–330 [DOI] [PubMed] [Google Scholar]
- 31. Pozzi A, et al. 2006. H-Ras, R-Ras, and TC21 differentially regulate ureteric bud cell branching morphogenesis. Mol. Biol. Cell 17:2046–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sakai T, de la Pena JM, Mosher DF. 1999. Synergism among lysophosphatidic acid, beta1A integrins, and epidermal growth factor or platelet-derived growth factor in mediation of cell migration. J. Biol. Chem. 274:15480–15486 [DOI] [PubMed] [Google Scholar]
- 33. Sakai T, Jove R, Fassler R, Mosher DF. 2001. Role of the cytoplasmic tyrosines of beta 1A integrins in transformation by v-src. Proc. Natl. Acad. Sci. U. S. A. 98:3808–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sakai T, Peyruchaud O, Fassler R, Mosher DF. 1998. Restoration of beta1A integrins is required for lysophosphatidic acid-induced migration of beta1-null mouse fibroblastic cells. J. Biol. Chem. 273:19378–19382 [DOI] [PubMed] [Google Scholar]
- 35. Sakai T, Zhang Q, Fassler R, Mosher DF. 1998. Modulation of beta1A integrin functions by tyrosine residues in the beta1 cytoplasmic domain. J. Cell Biol. 141:527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shen Y, Delaglio F, Cornilescu G, Bax A. 2009. TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J. Biomol. NMR 44:213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smeeton J, et al. 2010. Integrin-linked kinase regulates p38 MAPK-dependent cell cycle arrest in ureteric bud development. Development 137:3233–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ulmer TS, Yaspan B, Ginsberg MH, Campbell ID. 2001. NMR analysis of structure and dynamics of the cytosolic tails of integrin alpha IIb beta 3 in aqueous solution. Biochemistry 40:7498–7508 [DOI] [PubMed] [Google Scholar]
- 39. Ussar S, et al. 2008. Loss of Kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet. 4:e1000289 doi:10.1371/journal.pgen.1000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vinogradova O, et al. 2002. A structural mechanism of integrin alpha(IIb)beta(3) “inside-out” activation as regulated by its cytoplasmic face. Cell 110:587–597 [DOI] [PubMed] [Google Scholar]
- 41. Wegener KL, et al. 2007. Structural basis of integrin activation by talin. Cell 128:171–182 [DOI] [PubMed] [Google Scholar]
- 42. Wishart DS, Sykes BD. 1994. The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J. Biomol. NMR 4:171–180 [DOI] [PubMed] [Google Scholar]
- 43. Wu W, et al. 2009. {beta}1-Integrin is required for kidney collecting duct morphogenesis and maintenance of renal function. Am. J. Physiol. Renal Physiol. 297:F210–F217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang J, et al. 2009. Structure of an integrin alphaIIb beta3 transmembrane-cytoplasmic heterocomplex provides insight into integrin activation. Proc. Natl. Acad. Sci. U. S. A. 106:17729–17734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zeng F, Singh AB, Harris RC. 2009. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp. Cell Res. 315:602–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang X, et al. 2009. Beta1 integrin is necessary for ureteric bud branching morphogenesis and maintenance of collecting duct structural integrity. Development 136:3357–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.