Abstract
Artemisinin-based combination therapy for malaria has become widely available across Africa. Populations of Plasmodium falciparum that were previously dominated by chloroquine (CQ)-resistant genotypes are now under different drug selection pressures. P. malariae, P. ovale curtisi, and P. ovale wallikeri are sympatric with P. falciparum across the continent and are frequently present as coinfections. The prevalence of human Plasmodium species was determined by PCR using DNA from blood spots collected during a cross-sectional survey in northern Angola. P. falciparum was genotyped at resistance-associated loci in pfcrt and pfmdr1 by real-time PCR or by direct sequencing of amplicons. Of the 3,316 samples collected, 541 (16.3%) contained Plasmodium species infections; 477 (88.2%) of these were P. falciparum alone, 6.5% were P. falciparum and P. malariae together, and 1.1% were P. vivax alone. The majority of the remainder (3.7%) harbored P. ovale curtisi or P. ovale wallikeri alone or in combination with other species. Of 430 P. falciparum isolates genotyped for pfcrt, 61.6% carried the wild-type allele CVMNK at codons 72 to 76, either alone or in combination with the resistant allele CVIET. No other pfcrt allele was found. Wild-type alleles dominated at codons 86, 184, 1034, 1042, and 1246 of the pfmdr1 locus among the sequenced isolates. In contrast to previous studies, P. falciparum in the study area comprises an approximately equal mix of genotypes associated with CQ sensitivity and with CQ resistance, suggesting either lower drug pressure due to poor access to treatment in rural areas or a rapid impact of the policy change away from the use of standard monotherapies.
INTRODUCTION
Malaria is a major public health problem in Angola, with 3.7 million cases per year reported by the National Malaria Control Program (NMCP), two-thirds of which occur in children under 5 (7, 49). Although incidence rates of malaria have been decreasing due to control measures, according to the NMCP, malaria remains the primary cause of morbidity and mortality among children under 5 and pregnant women, accounting for 35% of the overall infant mortality, 60% of hospital admissions in this age group, and 25% of maternal deaths (7, 8, and http://www.pmi.gov/countries/profiles/angola_profile.pdf).
Five Plasmodium species cause human malaria in Africa: P. falciparum, P. malariae, P. vivax, P. ovale wallikeri, and P. ovale curtisi. The relative prevalence of each species varies across the continent, with P. falciparum being the most common and causing the most morbidity and mortality. In Angola, according to Ministry of Health estimates, P. falciparum accounts for 92% of infections, followed by P. vivax in about 7% of cases and P. malariae (3%) (7, 35, 49). In countries in which malaria is endemic, mixed-species infections are common and described at various frequencies depending on the geographic area (2, 28, 34). However, they are often underreported due to the unfamiliarity of laboratory technicians with microscopic forms of species other than P. falciparum and difficulty in detecting cases with low parasitemia (18, 19, 27, 50).
The continuous and indiscriminate use of chloroquine (CQ) as monotherapy in the 20th century created strong selection pressure and led to an increased prevalence of CQ-resistant (CQR) parasites worldwide (11). The recent introduction of artemisinin-based combination therapy (ACT) has changed this drug pressure, and the recovery of CQ-sensitive (CQS) P. falciparum populations has been reported in Malawi, China, and Kenya (24, 26, 33, 45). However, widespread ACT use may also increase the prevalence of ACT-tolerant phenotypes (46).
Mutations in the P. falciparum genomic region encompassing amino acids 72 to 76 of the CQ resistance transporter gene (pfcrt) are associated with resistance to CQ and amodiaquine (AQ) (1, 6, 21, 29, 44). The different pfcrt mutant haplotypes have a consistent geographical distribution: the CQR haplotype CVIET is predominant in southeast Asia and Africa, whereas the AQ-resistant (AQR) haplotype SagtVMNT is predominant in Asia and South America, and StctVMNT, from South America, is very rare in Africa (16).The wild-type haplotype CVMNK is widespread (6, 16, 29, 38). A recent study reported the presence of the StctVMNT haplotype of pfcrt in Angola, but this has not yet been verified by others (15, 37). Polymorphisms in the parasite protein Pgh-1, encoded by pfmdr1, are thought to modulate resistance to drugs such as quinine, mefloquine, halofantrine, artemisinin, lumefantrine, CQ, and AQ (1, 21, 32, 38). Mutations in this gene have some association with geographic areas. Considering codons 86, 184, 1034, 1042, and 1246, the pfmdr1 CQR haplotype YYSND is common in Asia and Africa, whereas NFCDY has occurred more frequently in CQ-resistant parasites in the Americas (16, 24, 29, 38).
In this cross-sectional study conducted in periurban and rural northwest Angola, we evaluated drug resistance-associated haplotypes of pfcrt and pfmdr1 among 525 P. falciparum-infected individuals, many of whom harbored additional coinfecting Plasmodium species, and discuss the implications of our findings for the treatment of malaria in the study area.
MATERIALS AND METHODS
Study area.
This study was conducted within the CISA (Health Research Center in Angola) Project's Health and Demographic Surveillance System (HDSS). The HDSS study area encompasses three communes (Caxito, Mabubas, and Úcua) of the Dande municipality, Bengo Province, in northwestern Angola. This is a largely rural area 60 km north of the capital Luanda, spanning more than 4,700 km2. The initial census, carried out between September 2009 and February 2010, registered 60,075 inhabitants in 15,643 households distributed in 69 hamlets (9). Bengo province is considered an area in which malaria is mesoendemic with stable transmission intensity (7). The main peak of malaria occurs in the rainy season, between November and May (8).
Sample collection.
The blood samples used for this study were collected between May and August of 2010 as part of a malaria baseline prevalence survey in women and their preschool- and school-aged children in the HDSS study area (40). Households with at least one preschool child (0 to 5 years old) or one school-aged child (6 to 15 years old) and a mother/caregiver (ideally from 16 to 49 years of age) were randomly selected, as described by Sousa-Figueiredo et al. (40). During the survey, finger-prick blood samples were collected and spotted onto Whatman 3MMChr filter paper and slides. Blood spots on filter paper were left to air dry and were stored at 4°C until DNA extraction. A total of 3,316 samples were analyzed, 2,632 from children (1,217 females and 1,146 males) and 952 from their mothers or caregivers.
Microscopy.
Thick and thin blood smears were prepared on the same slide, stained with 10% Giemsa for 15 min, and screened for malaria parasites by two independent microscope technicians (double blinded). Any discordance in whether or not parasites were present was resolved by a third reader.
DNA extraction.
Genomic DNA was extracted from the dried filter papers using a QIAmp DNA minikit (Qiagen, United Kingdom) by following the manufacturer's instructions. Positive-control blood spots prepared from known P. falciparum-positive samples and negative-control blood spots prepared from uninfected blood were also included and extracted in the same way as the field samples. DNA already extracted from samples of the other species was added to each PCR as further positive controls.
Nested PCR for RNA (small-subunit [SSU]-rRNA) amplification and species identification.
For the determination of Plasmodium species, we performed an optimized nested PCR (39). The first PCR was performed with primers specific for the Plasmodium genus: RPLU6 and RPLU5New (the latter primer, CYTGTTGTTGCCTTAAACTTC, was modified from the published primer, RPLU5, by the United Kingdom Malaria Reference Laboratory, London School of Hygiene and Tropical Medicine [LSHTM] [unpublished data]) (39). The second nested PCR was performed in 5 separate reactions, 4 containing Plasmodium species-specific primers as described by Snounou et al. and an additional reaction with primers for P. ovale wallikeri, rOVAv, described by Calderaro et al. (3, 39). DNA amplification was performed in a thermocycler (Gene Amp PCR System 9700; Applied Biosystems). PCR products were separated on a 2% agarose gel (Invitrogen) and stained with GelRed (Biotarget).
Real-time and nested PCR for amplification of pfcrt codons 72 to 76.
Polymorphisms at codons 72 to 76 of the pfcrt gene were successfully determined in 430 out of 525 P. falciparum PCR-positive samples by real-time quantitative PCR (qPCR) using dually labeled probes complementary to CVMNK (CQ sensitive; 3D7 type), CVIET (CQR; International Standard [IS] type), and SagtVMNT (AQR; 7G8 type) haplotypes (42). To increase sensitivity, a first-round PCR was carried out on all samples using primers P1 and P2 (21). The PCR product was then used in the qPCR procedure as described by Sutherland et al. and run on a Corbett Rotorgene 3000 thermocycler (Corbett Lifescience, Qiagen, Germany) (42). Unpublished primer sequences and cycling conditions are listed in Table 1. For the analysis, a threshold was set manually by reference to the appropriate positive and negative controls. DNA from 3D7 (CVMNK), IS (CVIET from NIBSC, United Kingdom), and 7G8 (SagtVMNT) clones were used as positive controls. As the qPCR was nested in this format, only qualitative data were obtained.
Table 1.
Unpublished primers and cycling conditions
| Gene and primer | Sequence | Product size (bp) | Nested PCR cycling conditions |
|---|---|---|---|
| SSU-rRNA | |||
| RPLU5New | 5′-CYTGTTGTTGCC TTAAAC TTC-3′ | 1,200 | 95°C for 5 min; 25 cycles of 58°C for 2 min, 72°C for 2 min, and 94°C for 1 min; 58°C for 2 min; 72°C for 5 min |
| pfcrt, nested | |||
| Nest 2 forward D1 | 5′-TGTGCTCATGTGTTTAAACTT-3′ | 145 | 94°C for 30 s, 30 cycles of 56°C for 30 s, and 65°C for 1 min; 65°C for 5 min; 15°C for 5 min |
| Nest 2 reverse D2 (12) | 5′-CAAAACTATAGTTACCAATTTTG-3′ | ||
| pfmdr1, fragment 3, seminested | |||
| Nest 1 forward MDRFR3N1 | 5′-GCATTTTATAATATGCATACTG-3′ | 234 | 94°C for 1 min; 30 cycles of 94°C for 30 s, 55°C for 30 s, and 68°C for 45 s; 68°C for 2 min; 15°C for 2 min |
| Nest 1 reverse MDRFR3R1 | 5′-GGATTTCATAAAGTCATCAAC-3′ | ||
| Nest 2 forward MDRFR3N2 | 5′-GGTTTAGAAGATTATTTCTGTAA-3′ | 201 | |
| Nest 2 reverse MDRFR3R1 | 5′-GGATTTCATAAAGTCATCAAC-3′ | ||
| pfmdr1, fragment 4, seminested | |||
| Nest 1 forward MDRFR4N1 | 5′-CAAACCAATCTGGATCTGCAGAAG-3′ | 194 | |
| Nest 1 reverse MDRFR4R1 | 5′-CAATGTTGCATCTTCTCTTCC-3′ | ||
| Nest 2 forward MDRFR4N2 | 5′-GATCTGCAGAAGATTATACTG-3′ | 182 | |
| Nest 2 reverse MDRFR4R1 | 5′-CAATGTTGCATCTTCTCTTCC-3′ |
To check for the possible presence of additional genotypes, such as StctVMNT, that may not be recognized by our three qPCR probes, all 525 samples positive for P. falciparum, including those with apparent failure to amplify in the qPCR, were also processed using the nested PCR protocols of Djimde et al. and run on 2% agarose gels (12). A selection of 42 samples was sequenced (see below).
Nested and seminested PCR for pfmdr1 amplification.
To identify pfmdr1 haplotypes, we performed nested PCR on a subset of 184 samples for fragment 1 (codons 86 and 184) and 177 for fragments 3 (codons 1034 and 1042) and 4 (codon 1246) of the 525 P. falciparum-positive samples corresponding to the first two plates of extracted DNA (Fig. 1). Amplicons for sequencing were generated by nested and seminested PCR as described in reference 21 and in Table 1.
Fig 1.
Sample selection process.
Sequencing protocol for pfcrt and pfmdr1.
Polymorphisms in the pfmdr1 gene were determined by direct sequencing of the amplicons resulting from the nested PCR described above using a BigDye Terminator v3.1 cycle sequencing kit in an ABI 3730 sequencer (Applied Biosystems). Data were analyzed using Geneious 5.4.4 Pro.
To solve discrepancies between nested and real-time PCR for pfcrt, in particular where a band was present in the nested PCR but no qPCR signal was obtained, 42 of the 51 discordant samples were sequenced on both strands to confirm single-nucleotide polymorphisms (SNPs) at codons 72 to 76 (Fig. 1).
Statistical analysis.
Database entry methods utilized in the baseline prevalence survey have been previously described in detail by Sousa-Figueiredo et al. (40). Plasmodium mixed infections and resistance gene haplotype prevalences were determined through tabulations in STATA v11.0 software.
Ethical approval.
Ethical approval for the study was obtained from the Angolan Ministry of Health Ethics Committee. Written informed consent was obtained before inclusion in the study, and antimalarial treatment was provided when appropriate.
RESULTS
Plasmodium species identification.
Of the 3,316 blood smears analyzed after Giemsa staining, 525 samples were found to be infected with P. falciparum (15.8%) (40). No other Plasmodium species, either in single or mixed infections, were detected by the microscopists.
The same 3,316 samples were screened by SSU-rRNA-based nested PCR, with 541 samples (16.3%) detected as having a Plasmodium infection (Table 2). Of those infected, 493 individuals (91.1%) carried a single species only. The majority of the Plasmodium infections were due to P. falciparum single infections (88.2%), whereas P. vivax single infections were seen in 6 (1.1%) of the infected samples. P. ovale curtisi, P. ovale wallikeri, and P. malariae single infections were observed in less than 2% of the infected samples: 4 (0.7%), 3 (0.6%), and 3 (0.6%), respectively. The 48 mixed-species infections included 41 individuals (7.6%) infected with 2 species, 5 (0.9%) with triple infections, and 2 (0.4%) with quadruple infections (Table 2). The most commonly found double species infection was the combination of P. falciparum and P. malariae, which was observed in 35 (6.5%) individuals.
Table 2.
Prevalence of Plasmodium species and mixed infections by PCR
| Type of infection (n = 3,316) | Presence of: |
No. positive (% of total) | % of positive samples | ||||
|---|---|---|---|---|---|---|---|
| P. falciparum | P. malariae | P. vivax | P. ovale curtisi | P. ovale wallikeri | |||
| P. falciparum | + | − | − | − | − | 477 (14.4) | 88.2 |
| P. malariae | − | + | − | − | − | 3 (0.1) | 0.6 |
| P. vivax | − | − | + | − | − | 6 (0.2) | 1.1 |
| P. ovale curtisi | − | − | − | + | − | 4 (0.1) | 0.7 |
| P. ovale wallikeri | − | − | − | − | + | 3 (0.1) | 0.6 |
| P. falciparum + P. malariae | + | + | − | − | − | 35 (1.1) | 6.5 |
| P. falciparum + P. ovale curtisi | + | − | − | + | − | 2 (0.1) | 0.4 |
| P. falciparum + P. ovale wallikeri | + | − | − | − | + | 4 (0.1) | 0.7 |
| P. falciparum + P. ovale curtisi + P. ovale wallikeri | + | − | − | + | + | 1 (0.0) | 0.2 |
| P. falciparum + P. malariae + P. ovale curtisi | + | + | − | + | − | 2 (0.1) | 0.4 |
| P. falciparum + P. malariae + P. ovale wallikeri | + | + | − | − | + | 2 (0.1) | 0.4 |
| P. falciparum + P. malariae + P. ovale curtisi + P. ovale wallikeri | + | + | − | + | + | 2 (0.1) | 0.4 |
| No. positive (% of total) | 525 (15.8) | 44 (1.3) | 6 (0.2) | 11 (0.3) | 12 (0.4) | 541 (16.3) | 100 |
| % of positive samples | 97.0 | 8.1 | 1.1 | 2.0 | 2.2 | 100 | |
Characterization of pfcrt alleles.
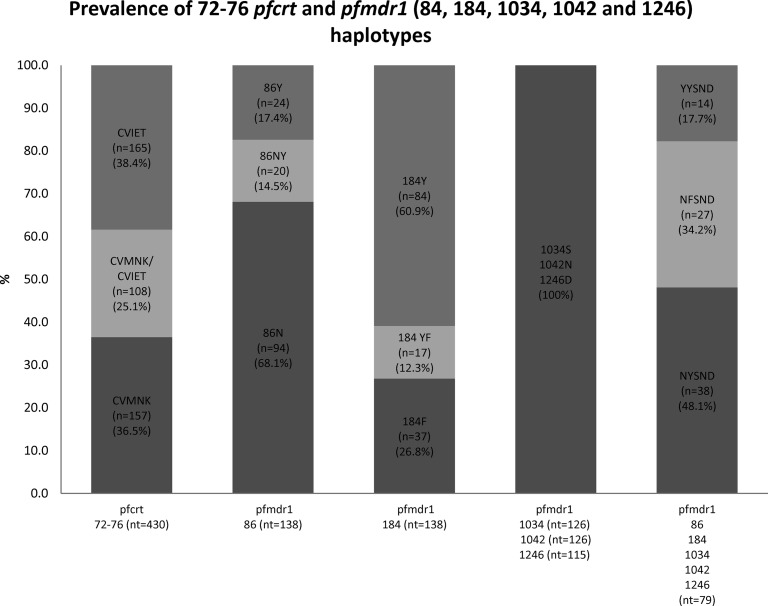
The 525 samples with P. falciparum (single or mixed) infections were further investigated by qPCR to determine each pfcrt haplotype. Alleles at pfcrt codons 72 to 76 were successfully identified in 430 out of 525 samples. A proportion of 63.5% of the samples harbored the CVIET (CQ-resistant) haplotype, whereas 61.6% harbored the CVMNK (wild-type) haplotype, including the ones present in mixed infections (25.1%) (Fig. 2). No other haplotype occurred among our isolates, including those which failed to generate a signal in the qPCR, as sequencing at codons 72 to 76 in 42 samples failed to identify SVMNT or any other alleles.
Fig 2.
Prevalence of haplotypes of pfcrt codons 72 to 76 and pfmdr1 codons 84, 184, 1034, 1042, and 1246. The prevalence of each haplotype was calculated with n/nt, where n is the number of positive samples and nt is the number of successfully sequenced samples. The pfmdr1 (codons 86, 184, 1034, 1042, and 1246) mixed haplotypes NY/FSND (7 samples), N/YYSND (8 samples), and N/YY/FSND (8 samples) were removed from the analyses.
Characterization of pfmdr1 alleles.
Out of 184 samples sequenced for fragment 1 (codons 86 and 184), 138 produced readable sequences. Regarding codon 86, the wild-type N86 allele was the most prevalent (114/138; 82.6%), while 44/138 (31.9%) of the samples carried the tyrosine mutation (Y86) associated with CQ and AQ resistance (Fig. 2). At codon 184, the wild-type tyrosine (Y) was also the most prevalent (101/138; 73.2%), whereas 54/138 (39.1%) carried the F184 polymorphism whether alone or in mixed infections.
Out of 177 samples sequenced for fragments 3 and 4 of pfmdr1, 126 produced readable sequences for fragment 3 (codons 1034 and 1042) and 115 produced readable sequences for fragment 4 (codon 1246). There were no mutations found at any of these codons, as all samples were of the wild-type haplotype 1034S, 1042N, or 1246D (Fig. 2).
In samples where a single nucleotide was detected at all codons (i.e., no mixed-haplotype infections), the most prevalent pfmdr1 haplotype at codons 86, 184, 1034, 1042, and 1246 was the wild-type NYSND (3D7 type) allele (38/79; 48.1%), followed by the NFSND haplotype (27/79; 34.2%) and then the YYSND allele (14/79, 17.7%) (Fig. 2). The YYSNY allele, associated with AQ resistance in east Africa, was not found.
DISCUSSION
The literature regarding malaria species other than Plasmodium falciparum in Angola is limited. Here, we describe the prevalence of the different Plasmodium species using molecular techniques. P. falciparum was the dominant species found in almost all infected individuals (96.2%) regardless of the presence of other species. P. vivax was less prevalent (1.1%) than previously reported in Angola but was more abundant than reported for the wider geographic area, where it has appeared to be almost absent (8, 10, 36). Furthermore, we are the first to report the existence of the two P. ovale species (P. ovale curtisi and P. ovale wallikeri) in Angola, confirming their presence in this region of Africa (34, 43) and providing the first documented cases of individuals simultaneously infected with both P. ovale species. P. malariae and P. ovale species occurred almost exclusively in mixed infections with P. falciparum, and together they represented almost 9% of the total infections.
Despite being the standard method for Plasmodium detection, we could not identify malaria parasites other than P. falciparum by microscopy in our mostly asymptomatic subjects. Parasite density of non-falciparum Plasmodium infections is usually low and therefore easily missed in microscopic observation, particularly in the absence of symptoms. Likewise, in mixed infections, other species become difficult to differentiate from a background of large numbers of P. falciparum parasites.
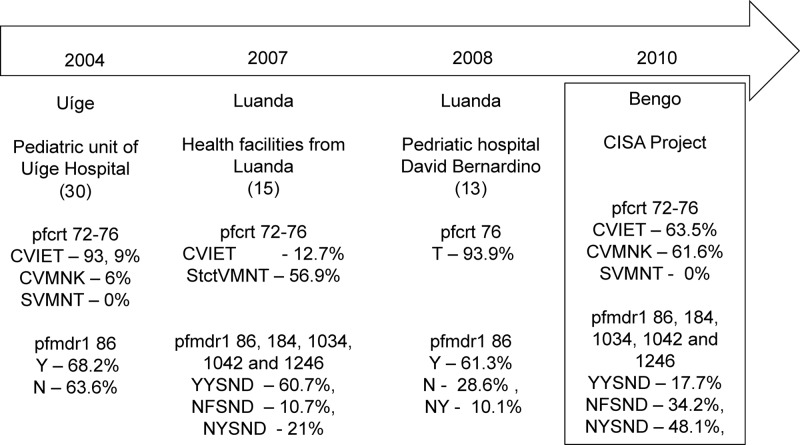
The national guidelines for malaria treatment changed from CQ to artemether-lumefantrine (AL) in 2004, following WHO 2001 guidelines and high treatment failure rates observed in bordering countries (4, 17, 23, 25, 31, 48). However, due to difficulties in the wide-scale distribution of AL to health facilities throughout the country, other drugs may have been used until 2007 to 2008, when AL became broadly available (fightingmalaria.gov/technical/acts/angola_acts.html). The heterogeneous access to drugs across health facilities has likely led to different drug pressures, which may have influenced the prevalence of drug-resistant and -sensitive haplotypes circulating within the country. This could explain the fact that, in this cross-sectional community study, the prevalence of the CQS haplotype CVMNK was high (61.6%), contrasting with studies conducted in 2004 and 2008 in health facilities in the Angolan provinces of Uíge and Luanda, where a very high prevalence of the pfcrt 76T haplotype was found (Fig. 2) (13, 30).
Alternatively, this result suggests that, as CQ was withdrawn, a loss of the survival advantage of CQR parasites occurred in the absence of drug pressure, leading to a resurgence in the relative abundance of wild-type CVMNK (20, 26). A gradual decrease in the prevalence of CQR haplotypes has also been observed in China and Kenya (33, 45). Although the antimalarial activity of CQ would likely still be poor in our study area (as the prevalence of pfcrt codons 72 to 76 [CVIET] is close to 64%), reintroduction of CQ as a partner drug in ACTs or in intermittent presumptive treatment for adults (IPTa) may become possible in Angola should the CVMNK haplotype become predominant, as occurred in Malawi (22, 24, 26). Nevertheless, we should be aware that the decline in rates of CQR mutations depends on geographic, epidemiologic, and parasite genetic factors (26, 38). Additionally, efficacy trials would have to be undertaken to support in vivo the molecular findings described here.
The pfcrt SVMNT haplotype is associated with AQ resistance; it is postulated to carry a lower fitness cost than the CVIET haplotype and to persist among the parasite population even after the removal of drug pressure, potentially jeopardizing the effectiveness of artesunate-AQ, which is widely used in west Africa (37, 38). Surprisingly, this haplotype, which is usually found in South America and parts of Asia, was detected in adult samples from Luanda in 2007 (15). Despite the proximity of Luanda to our study site, we have not found this haplotype among our samples, suggesting that AQ would still be effective in this area. Further studies are needed to determine which provinces harbor the StctVMNT haplotype and whether it compromises the efficacy of artesunate-AQ combination therapy.
Our pfmdr1 results (82.6% of the wild-type N86 allele) differ from those found in Luanda in 2007/2008, where Y86 was the most prevalent, and from Uíge in 2004, where similar proportions of Y86 and N86 were observed (Fig. 3) (13, 15, 30). There were no mutations at codons 1034, 1042, and 1246, i.e., 100% of the haplotypes were SND, in agreement with what has been reported in other parts of west Africa, including in Angola (13, 20, 29). We expect that the high prevalence of pfcrt CQR and high intensity of transmission in Uíge favor the accumulation of pfmdr1 Y86 (13, 15). This suggests that parasite populations with different pfmdr1 allele frequencies exist within the country. It would be interesting to investigate the role of endemicity on the evolution and spread of the studied haplotypes, as Luanda, Uíge, and Bengo are areas of low, hyper-, and mesoendemic stable transmission, respectively (7, 8). Alternatively, access to other drugs such as AQ, known to select strongly for the Y86 allele (44), is more likely in urban areas, leading to qualitative differences in drug pressure compared to rural districts.
Fig 3.
Drug resistance and efficacy studies in Angola.
Given these results, we postulate that a reversion process is also occurring regarding pfmdr1 (codons 86 and 184), consistent with in vitro and in vivo observations reporting a modest decrease in the frequency of N86Y mutations in the absence of CQ pressure (20) and the known selective advantage of the pfmdr1 haplotype NFSND under AL treatment (1, 14, 44). Given the limitations of clinical diagnosis and the limited laboratory confirmation of suspected cases (such that many febrile diseases are wrongly diagnosed as malaria), it is possible that AL is being overprescribed, exacerbating this selection upon pfmdr1. The presence of the NFSND haplotype of pfmdr1 among our samples thus raises concern and suggests that efficient monitoring of ACT efficacy in Angola is needed. On the other hand, it has been postulated that limited reintroduction of CQ, along with the use of AQ in combination therapy, could help to prevent resistance to ACTs, as both CQ and AQ have well-described antagonistic interactions with artemisinins in vitro and in vivo (5, 21, 41, 46).
ACKNOWLEDGMENTS
We thank the researchers and technicians whose support was fundamental to this study, namely, David Simão from CISA, Ismael Teles from the Molecular Biology Laboratory of the Pediatric Hospital David Bernardino, Luanda, Angola, Debbie Nolder (Malaria Centre, LSHTM) for validation of nested PCR results, and Khalid Beshir, Gisela Henriques, and Sumi Britton (LSHTM) for support in processing the samples. We also acknowledge Luis Bernardino for the molecular biology laboratory facilities, the CDC and LSHTM Malaria Center for positive controls, Elizabeth Benito Garcia for guiding the data analysis, and João Pires for critical reading of the manuscript. We also thank the local administration and all the members of the community who were part of this study.
This work was funded by the promoters of the CISA Project (Portuguese Institute for Development Assistance, Calouste Gulbenkian Foundation, Bengo Provincial Government, and the Ministry of Health of Angola), the EC FP7 MALACTRES project, and the United Kingdom Health Protection Agency.
Footnotes
Published ahead of print 30 July 2012
REFERENCES
- 1. Beshir K, et al. 2010. Amodiaquine resistance in Plasmodium falciparum malaria in Afghanistan is associated with the pfcrt SVMNT allele at codons 72 to 76. Antimicrob. Agents Chemother. 54:3714–3716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bruce MC, et al. 2000. Cross-species interactions between malaria parasites in humans. Science 287:845–848 [DOI] [PubMed] [Google Scholar]
- 3. Calderaro A, et al. 2007. Genetic polymorphisms influence Plasmodium ovale PCR detection accuracy. J. Clin. Microbiol. 45:1624–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carme B, Mbitsi A, Moudzeo H, Ndinga M, Eozenou P. 1987. Drug resistance of Plasmodium falciparum in the Congo. 2. Comparative study in vivo of chloroquine and amodiaquine in Brazzaville schoolchildren (November 1986). Bull. Soc. Pathol. Exot. Filiales 80:426–433 [PubMed] [Google Scholar]
- 5. Chawira AN, Warhurst DC. 1987. The effect of artemisinin combined with standard antimalarials against chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum in vitro. J. Trop. Med. Hyg. 90:1–8 [PubMed] [Google Scholar]
- 6. Chen N, et al. 2005. Origin and dissemination of chloroquine-resistant Plasmodium falciparum with mutant pfcrt alleles in the Philippines. Antimicrob. Agents Chemother. 49:2102–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Consultoria de Serviços e Pesquisas–COSEP Lda., Consultadoria de Gestão e Administração em Saúde-Consaúde Lda, and Macro International Inc 2011. Inquérito de indicadores de malária em Angola 2011. [Google Scholar]
- 8. Consultoria de Serviços e Pesquisas–COSEP Lda., Consultadoria de Gestão e Administração em Saúde-Consaúde Lda, and Macro International Inc 2007. Inquérito de Indicadores da Malária em Angola 2006/2007. [Google Scholar]
- 9. Costa MJ, Langa REA, António G, Bendriss A. Setting up a demographic surveillance system in the Dande Municipality, Angola. Afr. Popul. Stud., in press [Google Scholar]
- 10. Culleton RL, et al. 2008. Failure to detect Plasmodium vivax in west and central Africa by PCR species typing. Malar J. 7:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D'Alessandro U, Buttiens H. 2001. History and importance of antimalarial drug resistance. Trop. Med. Int. Health 6:845–848 [DOI] [PubMed] [Google Scholar]
- 12. Djimde A, et al. 2001. A molecular marker for chloroquine-resistant falciparum malaria. N. Engl. J. Med. 344:257–263 [DOI] [PubMed] [Google Scholar]
- 13. Figueiredo P, et al. 2008. Prevalence of pfmdr1, pfcrt, pfdhfr and pfdhps mutations associated with drug resistance, in Luanda, Angola. Malar J. 7:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gadalla NB, et al. 2011. Increased pfmdr1 copy number and sequence polymorphisms in Plasmodium falciparum isolates from Sudanese malaria patients treated with artemether-lumefantrine. Antimicrob. Agents Chemother. 55:5408–5411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gama BE, et al. 2010. Plasmodium falciparum isolates from Angola show the StctVMNT haplotype in the pfcrt gene. Malar J. 9:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gbotosho GO, et al. 2012. Different patterns of pfcrt and pfmdr1 polymorphisms in P. falciparum isolates from Nigeria and Brazil: the potential role of antimalarial drug selection pressure. Am. J. Trop. Med. Hyg. 86:211–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guthmann JP, et al. 2005. Antimalarial efficacy of chloroquine, amodiaquine, sulfadoxine-pyrimethamine, and the combinations of amodiaquine + artesunate and sulfadoxine-pyrimethamine + artesunate in Huambo and Bie provinces, central Angola. Trans. R. Soc. Trop. Med. Hyg. 99:485–492 [DOI] [PubMed] [Google Scholar]
- 18. Hanscheid T. 2003. Current strategies to avoid misdiagnosis of malaria. Clin. Microbiol. Infect. 9:497–504 [DOI] [PubMed] [Google Scholar]
- 19. Hanscheid T. 1999. Diagnosis of malaria: a review of alternatives to conventional microscopy. Clin. Lab. Haematol. 21:235–245 [DOI] [PubMed] [Google Scholar]
- 20. Hayward R, Saliba KJ, Kirk K. 2005. pfmdr1 mutations associated with chloroquine resistance incur a fitness cost in Plasmodium falciparum. Mol. Microbiol. 55:1285–1295 [DOI] [PubMed] [Google Scholar]
- 21. Humphreys GS, et al. 2007. Amodiaquine and artemether-lumefantrine select distinct alleles of the Plasmodium falciparum mdr1 gene in Tanzanian children treated for uncomplicated malaria. Antimicrob. Agents Chemother. 51:991–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kamugisha E, et al. 2012. Large differences in prevalence of Pfcrt and Pfmdr1 mutations between Mwanza, Tanzania and Iganga, Uganda–a reflection of differences in policies regarding withdrawal of chloroquine? Acta Trop. 121:148–151 [DOI] [PubMed] [Google Scholar]
- 23. Khan AA, Maguire MJ. 1978. Relative chloroquine resistance of P. falciparum in Zambia. Br. Med. J. 1:1669–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kublin JG, et al. 2003. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J. Infect. Dis. 187:1870–1875 [DOI] [PubMed] [Google Scholar]
- 25. Kyronseppa H, Lumio J, Ukkonen R, Pettersson T. 1984. Chloroquine-resistant malaria from Angola. Lancet i:1244. [DOI] [PubMed] [Google Scholar]
- 26. Laufer MK, et al. 2006. Return of chloroquine antimalarial efficacy in Malawi. N. Engl. J. Med. 355:1959–1966 [DOI] [PubMed] [Google Scholar]
- 27. McKenzie FE, Bossert WH. 1997. Mixed-species Plasmodium infections of humans. J. Parasitol. 83:593–600 [PMC free article] [PubMed] [Google Scholar]
- 28. Mehlotra RK, et al. 2000. Random distribution of mixed species malaria infections in Papua New Guinea. Am. J. Trop. Med. Hyg. 62:225–231 [DOI] [PubMed] [Google Scholar]
- 29. Mehlotra RK, et al. 2008. Discordant patterns of genetic variation at two chloroquine resistance loci in worldwide populations of the malaria parasite Plasmodium falciparum. Antimicrob. Agents Chemother. 52:2212–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Menegon M, et al. 2009. Monitoring for multidrug-resistant Plasmodium falciparum isolates and analysis of pyrimethamine resistance evolution in Uige province, Angola. Trop. Med. Int. Health 14:1251–1257 [DOI] [PubMed] [Google Scholar]
- 31. Ministry of Health and Social Services 2005. National Drug Policy for Namíbia. Ministry of Health and Social Services, Windhoek, Namibia [Google Scholar]
- 32. Mobula L, Lilley B, Tshefu AK, Rosenthal PJ. 2009. Resistance-mediating polymorphisms in Plasmodium falciparum infections in Kinshasa, Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 80:555–558 [PubMed] [Google Scholar]
- 33. Mwai L, et al. 2009. Chloroquine resistance before and after its withdrawal in Kenya. Malar J. 8:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oguike MC, et al. 2011. Plasmodium ovale curtisi and Plasmodium ovale wallikeri circulate simultaneously in African communities. Int. J. Parasitol. 41:677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Programa Nacional de Controlo da Malária (PNCM), Fundo das Nações Unidas para a Infância (UNICEF), Consultoria de Gestão e Administração em Saúde–Consaúde Lda. Malária em Angola 1998–2005. 2006 Relatório. [Google Scholar]
- 36. Rosenberg R. 2007. Plasmodium vivax in Africa: hidden in plain sight? Trends Parasitol. 23:193–196 [DOI] [PubMed] [Google Scholar]
- 37. Sa JM, Twu O. 2010. Protecting the malaria drug arsenal: halting the rise and spread of amodiaquine resistance by monitoring the PfCRT SVMNT type. Malar. J. 9:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sa JM, et al. 2009. Geographic patterns of Plasmodium falciparum drug resistance distinguished by differential responses to amodiaquine and chloroquine. Proc. Natl. Acad. Sci. U. S. A. 106:18883–18889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Snounou G, et al. 1993. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 61:315–320 [DOI] [PubMed] [Google Scholar]
- 40. Sousa-Figueiredo JC, et al. 2012. Epidemiology of malaria, schistosomiasis, geohelminths, anemia and malnutrition in the context of a demographic surveillance system in northern Angola. PLoS One. 7:e33189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sutherland CJ, Babiker H, Mackinnon MJ, Ranford-Cartwright L, Sayed BB. 2011. Rational deployment of antimalarial drugs in Africa: should first-line combination drugs be reserved for paediatric malaria cases? Parasitology 138:1459–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sutherland CJ, et al. 2007. Chloroquine-resistant Plasmodium falciparum infections among UK travellers returning with malaria after chloroquine prophylaxis. J. Antimicrob. Chemother. 59:1197–1199 [DOI] [PubMed] [Google Scholar]
- 43. Sutherland CJ, et al. 2010. Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J. Infect. Dis. 201:1544–1550 [DOI] [PubMed] [Google Scholar]
- 44. Ursing J, Kofoed PE, Rodrigues A, Rombo L, Gil JP. 2007. Plasmodium falciparum genotypes associated with chloroquine and amodiaquine resistance in Guinea-Bissau. Am. J. Trop. Med. Hyg. 76:844–848 [PubMed] [Google Scholar]
- 45. Wang X, et al. 2005. Decreased prevalence of the Plasmodium falciparum chloroquine resistance transporter 76T marker associated with cessation of chloroquine use against P. falciparum malaria in Hainan, People's Republic of China. Am. J. Trop. Med. Hyg. 72:410–414 [PubMed] [Google Scholar]
- 46. Warhurst DC, Duraisingh MT. 2001. Rational use of drugs against Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 95:345–346 [DOI] [PubMed] [Google Scholar]
- 47. Reference deleted.
- 48. WHO 2001. Antimalarial drug combination therapy: report of a WHO technical consultation. WHO, Geneva, Switzerland [Google Scholar]
- 49. WHO 2010. World malaria report 2010. WHO, Geneva, Switzerland [Google Scholar]
- 50. Zimmerman PA, Mehlotra RK, Kasehagen LJ, Kazura JW. 2004. Why do we need to know more about mixed Plasmodium species infections in humans? Trends Parasitol. 20:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]