Abstract
The declining efficacy of artemisinin derivatives against Plasmodium falciparum highlights the urgent need to identify alternative highly potent compounds for the treatment of malaria. In Papua Indonesia, where multidrug resistance has been documented against both P. falciparum and P. vivax malaria, comparative ex vivo antimalarial activity against Plasmodium isolates was assessed for the artemisinin derivatives artesunate (AS) and dihydroartemisinin (DHA), the synthetic peroxides OZ277 and OZ439, the semisynthetic 10-alkylaminoartemisinin derivatives artemisone and artemiside, and the conventional antimalarial drugs chloroquine (CQ), amodiaquine (AQ), and piperaquine (PIP). Ex vivo drug susceptibility was assessed in 46 field isolates (25 P. falciparum and 21 P. vivax). The novel endoperoxide compounds exhibited potent ex vivo activity against both species, but significant differences in intrinsic activity were observed. Compared to AS and its active metabolite DHA, all the novel compounds showed lower or equal 50% inhibitory concentrations (IC50s) in both species (median IC50s between 1.9 and 3.6 nM in P. falciparum and 0.7 and 4.6 nM in P. vivax). The antiplasmodial activity of novel endoperoxides showed different cross-susceptibility patterns in the two Plasmodium species: whereas their ex vivo activity correlated positively with CQ, PIP, AS, and DHA in P. falciparum, the same was not apparent in P. vivax. The current study demonstrates for the first time potent activity of novel endoperoxides against drug-resistant P. vivax. The high activity against drug-resistant strains of both Plasmodium species confirms these compounds to be promising candidates for future artemisinin-based combination therapy (ACT) regimens in regions of coendemicity.
INTRODUCTION
Approximately 3.3 billion people (i.e., almost 50% of the world's population) are at risk of malaria with two Plasmodium species responsible for the majority of infections: P. falciparum and P. vivax (6, 7, 43). Traditionally, malaria control and research efforts have focused on P. falciparum, the dominant species in Africa. However, outside Africa, P. falciparum almost invariably coexists with P. vivax (7), with both species inflicting significant morbidity, particularly in infants and pregnant women (18, 27).
Chloroquine (CQ)-resistant P. falciparum is already well established, with emerging evidence that susceptibility to CQ in P. vivax is also declining across much of the world in which vivax is endemic. This combined threat is driving the investigation of alternative schizonticidal treatment regimens, such as artemisinin-based combination therapy (ACT), for deployment against both P. falciparum and P. vivax (29). ACTs have become the mainstay of antimalarial chemotherapy, adopted in more than 100 countries worldwide (42). This huge demand for artemisinin and its derivatives relies on isolation from the plant source Artemisia annua and is vulnerable to harvest and production costs and intermittent supply (2, 11). Of particular concern are recent reports of prolonged in vivo parasite clearance times following ACTs on the Thai-Cambodian border (4, 21), highlighting the possibility of emerging artemisinin resistance. These constraints have driven new efforts to develop novel semisynthetic artemisinins or fully synthetic peroxides, some of which are currently in phase I and II clinical trials. The fully synthetic peroxides include the 1,2,4-trioxolanes OZ277 and OZ439 (also known as ozonides) (3), and the semisynthetic artemisinins include the 10-alkylamino-artemisinin derivative artemisone and its thiomorpholine precursor, artemiside (8). OZ277 (also known as RBx11160 or arterolane) was the first ozonide to enter clinical trials, is currently evaluated in combination with piperaquine (PIP) (25), and has obtained approval for use in India. The novel ozonide OZ439 has a slower elimination than OZ277 and is able to cure Plasmodium berghei infections in mice with a single oral administration (3). Artemisone in combination with mefloquine, amodiaquine, or clindamycin cures P. falciparum infections in Aotus monkeys (24) and is effective against murine cerebral malaria (38). Preliminary phase II data demonstrated that artemisone is curative at one-third the dose level of artesunate (AS) when used in patients with nonsevere malaria (19).
Outside Africa, the high levels of malaria coendemicity and the emergence of multidrug resistance in both species have led to calls for a unified policy of ACT for both P. falciparum and P. vivax (5), an approach now endorsed by Indonesia, Papua New Guinea, the Solomon Islands, and Vanuatu. The success of such a policy is dependent upon confirmation of antimalarial activity of established and novel endoperoxide drugs against clinical isolates of both P. falciparum and P. vivax, particularly in areas of known multidrug resistance. The aim of the current study was to investigate the ex vivo drug susceptibility profiles of novel endoperoxide agents and compare these with those of established antimalarials.
MATERIALS AND METHODS
Compounds.
The antimalarial drugs chloroquine (CQ), amodiaquine (AQ; Sigma-Aldrich, Australia), piperaquine (PIP; Ranbaxy Lab. Ltd., Gurgaon, India), artesunate (AS; Guilin Pharmaceutical Co., Ltd., Guangxi, China), and dihydroartemisinin (DHA; Chongqing Holley Wuling Mountain Pharmaceutical Co., Ltd., China) and the experimental compounds OZ277, OZ439 (MMV), artemisone, and artemiside (The Hong Kong University of Science and Technology) were prepared as 10 mM stock solutions in dimethyl sulfoxide (DMSO). Drug plates were then predosed by diluting the compounds in 50% ethanol, followed by lyophilization, and stored at 4°C. Drug plates were quality controlled by assessing drug response profiles in the CQ-resistant and -sensitive laboratory strains K1 and FC27, respectively, before and after completion of the study.
Field location and sample collection.
Plasmodium species isolates were collected from patients with malaria attending outpatient clinics in Timika, Papua Province, Indonesia, a region where multidrug-resistant strains of P. vivax and P. falciparum are highly prevalent (10, 30, 31). Patients with symptomatic malaria were recruited into the study if they were singly infected with P. falciparum or P. vivax and had a parasitemia of between 2,000 μl−1 and 80,000 μl−1. After written informed consent was obtained, venous blood (5 ml) was collected by venipuncture. Host white blood cells (WBC) were removed by using CF 11 cellulose, and packed infected red blood cells (IRBC) were used for the ex vivo drug susceptibility assay.
Ex vivo drug susceptibility assay.
Plasmodium drug susceptibility was measured using a protocol modified from the WHO microtest as described previously (17, 31). Two hundred microliters of a 2% hematocrit blood medium mixture (BMM), consisting of RPMI 1640 medium plus 10% AB+ human serum (P. falciparum) or McCoy's 5A medium plus 20% AB+ human serum (P. vivax), was added to each well of predosed drug plates containing 11 serial concentrations (2-fold dilutions) of the antimalarials (maximum concentration shown in parentheses) CQ (2,992 nM), AQ (80 nM), PIP (769 nM), AS (25 nM), DHA (34 nM), OZ277 (34 nM), OZ439 (34 nM), artemisone (24 nM), and artemiside (26 nM). A candle jar was used to mature the parasites at 37.0°C for 32 to 56 h. Incubation was stopped when >40% of ring stage parasites had reached the mature schizont stage (i.e., ≥4 distinct nuclei per parasite) in the drug-free control well.
Thick blood films made from each well were stained with 5% Giemsa solution for 30 min and examined microscopically. The number of schizonts per 200 asexual stage parasites was determined for each drug concentration and normalized to that of the control well. The dose-response data were analyzed using nonlinear regression analysis (WinNonLn 4.1; Pharsight Corporation), and the 50% inhibitory concentration (IC50) was derived using an inhibitory sigmoid maximum effect (Emax) model. IC50 ex vivo data were used from predicted curves only where the Emax and E0 were within 15% of 100 and 0, respectively.
Data analysis.
Analysis was performed using STATA software (version 10.1; Stata Corp., College Station, TX). The Mann-Whitney U test, Wilcoxon signed-rank test, and Spearman rank correlation were used for nonparametric comparisons and correlations. Since previous studies have highlighted several potential confounders of ex vivo/in vitro assessment of drug susceptibility (13, 31), we used robust multiple regression analysis on log-transformed data to control for the effect of potential confounders on ex vivo drug response, including the initial parasitemia, the proportion of parasites at ring stage at the start of the assay, and the duration of the assay.
Ethical approval.
Ethical approval for this project was obtained from the Human Research Ethics Committee of the Northern Territory Department of Health & Families and Menzies School of Health Research (HREC 2010-1396), Darwin, Australia, and the Eijkman Institute Research Ethics Commission (EIREC-47), Jakarta, Indonesia, in September 2010.
RESULTS
Antimalarial susceptibility.
Ex vivo drug susceptibility was assessed in field isolates from 46 patients presenting with single-species infections of P. falciparum (n = 25) or P. vivax (n = 21). Adequate growth for harvest was achieved in 92% (23/25) of P. falciparum and 90% (19/21) of P. vivax isolates. Baseline characteristics of the isolates processed are presented in Table 1. Median IC50s for all isolates which fulfilled criteria for successful culture for the two species and for the P. falciparum laboratory strains FC27 and K1 are depicted in Table 2.
Table 1.
Baseline characteristics of isolates for which the ex vivo assay was accomplished
| Baseline characteristic | P. falciparum | P. vivax |
|---|---|---|
| No. of isolates reaching harvest/total no. of isolates (%) | 23/25 (92) | 18/21 (90) |
| Median (range) delay from venipuncture to start of culture (min) | 140 (80–246) | 102.5 (77–180) |
| Median (range) duration of assay (h) | 46 (32–56) | 47 (41–48) |
| Geometric mean (95% CIa) parasitemia (asexual parasites/μl) | 17,291 (11,212–26,666) | 11,110 (6,925–17,823) |
| Median initial % (range) of parasites at ring stage | 100 (100–100) | 85 (59–97) |
| Mean (95% CI) schizont count at harvest | 36 (16–80) | 34 (20–50) |
CI, confidence interval.
Table 2.
Overall ex vivo sensitivity for each drug according to the species tested
| Drug | Indicated values for each group of isolates |
|||||
|---|---|---|---|---|---|---|
| Median IC50 (nM) in each P. falciparum lab linea |
P. falciparum clinical field isolates |
P. vivax clinical field isolates |
||||
| FC27 (CQs) | K1 (CQr) | n (%)c | Median IC50 (range) in nM and P valueb | n (%)c | Median IC50 (range) in nM and P valueb | |
| Chloroquine | 39.9 | 168.7 | 23 (100) | 91.4 (37.3–147.7) | 18 (100)d | 48.3 (12.9–143.4) |
| Amodiaquine | 36.2 | 21.7 | 23 (100) | 11.8 (5.6–22.8) | 18 (100) | 15.9 (7.1–25.8) |
| Piperaquine | 64.2 | 50.2 | 23 (100) | 16.4 (4.0–43.6) | 18 (100) | 14.6 (4.0–28.9) |
| Artesunate | 15.4 | 10.3 | 22 (96)e | 4.5 (0.3–9.8) | 15 (83)e | 4.0 (1.6–6.3) |
| DHA | 10.0 | 9.6 | 22 (96)f | 6.4 (2.2–11.1), P = 0.013 | 16 (89)e | 4.9 (2.8–11.5), P = 0.013 |
| OZ277 | 10.9 | 20.0 | 23 (100) | 3.6 (0.1–18.3), P = 0.884 | 16 (89)e | 4.6 (1.7–16.3), P = 0.088 |
| OZ439 | 11.1 | 18.6 | 23 (100) | 2.1 (0.4–10.5), P = 0.012 | 16 (89)e | 4.5 (1.3–10.3), P = 0.306 |
| Artemisone | 1.9 | 2.0 | 22 (96)e | 1.9 (0.1–5.7), P < 0.001 | 15 (83)e | 0.7 (0.3–4.1), P < 0.001 |
| Artemiside | 4.0 | 4.3 | 22 (96)e | 2.5 (0.2–15.0), P = 0.592 | 15 (83)e | 3.0 (0.5–14.4), P = 0.460 |
Mean IC50s (derived from 2 independent experiments) were assessed by in vitro schizont maturation quantified by microscopy. CQs, chloroquine-sensitive laboratory strain; CQr, chloroquine-resistant laboratory strain.
P values are from a comparison with values for artesunate (Wilcoxon signed-rank test).
Total number of assays with acceptable IC50s (percentage of samples which fulfilled the criteria for a successful culture).
One P. vivax isolate was excluded from analysis (harvested at <30 h).
Insufficient BMM mixture to test all compounds in 3 P. vivax isolates and 1 P. falciparum isolate.
No IC50 estimate of DHA for 1 P. falciparum isolate (highly sensitive, model not possible).
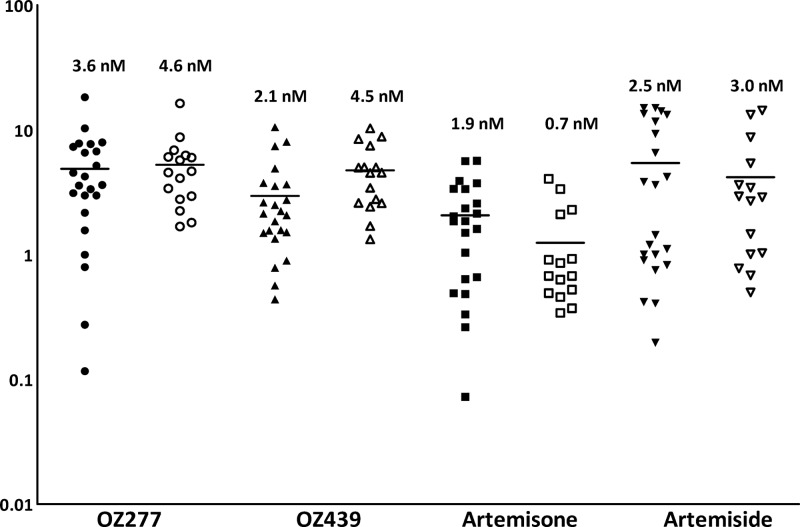
Drug susceptibility to CQ was significantly lower in P. falciparum isolates (median IC50, 91.4 nM; range, 37.3 to 147.7 nM) than in P. vivax isolates (median, 48.3 nM; range, 12.9 to 143.4 nM; P < 0.001) (Fig. 1), whereas OZ439 exhibited higher IC50s for P. vivax (4.5 nM versus 2.1 nM; P = 0.013) (Fig. 1). Previous studies have highlighted a difference in the stage-specific activities of CQ and AQ in P. vivax. Stratified analysis of P. vivax isolates with ≥80% and 60 to 80% of parasites at ring stage at the start of the assay showed a slight but significant difference in activity for AS (median IC50 of 3.5 nM in isolates with ≥80% rings and 4.9 nM in isolates with 60 to 80% rings; P = 0.037) and DHA (median IC50 of 4.8 nM in isolates with ≥80% rings and 6.0 nM in isolates with 60 to 80% rings; P = 0.051) but no stage-specific difference in ex vivo susceptibility to the other compounds (Table 3).
Fig 1.
Ex vivo drug susceptibility (median IC50s) of the synthetic ozonides OZ277 and OZ439 and the semisynthetic endoperoxides artemisone and artemiside in P. falciparum (filled symbols) and P. vivax (open symbols) clinical field isolates.
Table 3.
Overall ex vivo sensitivity for P. vivax with ≥80% and <80% of parasites at ring stage at the start of the assay
| Drug | Indicated values for P. vivax isolates with: |
|||
|---|---|---|---|---|
| ≥80% rings |
60–80% rings |
|||
| n (%)a | Median IC50 (range) in nM and P valueb | n (%)a | Median IC50 (range) in nM and P valueb | |
| Chloroquine | 11/11 (100) | 45.7 (12.9–143.4) | 7/7 (100) | 50.8 (23.6–68.6) |
| Amodiaquine | 11/11 (100) | 17.1 (7.1–25.8) | 7/7 (100) | 13.4 (7.5–17.0) |
| Piperaquine | 11/11 (100) | 13.1 (4.0–28.7) | 7/7 (100) | 21.9 (5.2–28.9) |
| Artesunate | 10/11 (91) | 3.5 (1.6–6.2) | 5/7 (71) | 4.9 (3.6–6.3) |
| DHA | 10/11 (91) | 4.8 (2.8–6.8), P = 0.029 | 6/7 (86) | 6.0 (4.3–11.5), P = 0.225 |
| OZ277 | 10/11 (91) | 5.2 (1.7–8.7), P = 0.037 | 6/7 (86) | 4.0 (1.8–16.3), P = 0.500 |
| OZ439 | 10/11 (91) | 3.7 (1.3–8.8), P = 0.285 | 6/7 (86) | 4.8 (2.6–10.3), P = 0.892 |
| Artemisone | 10/11 (91) | 0.6 (0.3–3.4), P = 0.005 | 5/7 (71) | 0.9 (0.6–4.1), P = 0.043 |
| Artemiside | 10/11 (91) | 2.8 (0.7–14.4), P = 0.386 | 5/7 (71) | 2.9 (0.5–13.3), P = 0.893 |
Total number of assays with acceptable IC50s/total number of assays (percentage).
P values are from a comparison with values for artesunate (Wilcoxon signed-rank test).
A within-species comparison with AS revealed significantly lower IC50s for artemisone in both species (median AS IC50 of 4.5 nM versus a median artemisone IC50 of 1.9 nM [P < 0.001] for P. falciparum and 4.0 nM versus 0.7 nM, respectively [P < 0.001], for P. vivax). The median IC50 for OZ439 was also significantly lower than that for AS, but this was apparent only in P. falciparum isolates (median, 2.1 nM; P = 0.012). IC50s for DHA were significantly higher than those for AS in both species (median DHA IC50 of 6.4 nM [P = 0.013] for P. falciparum and 4.9 nM [P = 0.013] for P. vivax) (Table 2). The trends in drug susceptibility compared to the AS reference drug remained after controlling for the initial stage composition of the parasite at the start of the assay.
Cross-susceptibility patterns.
Cross-susceptibility patterns were assessed using the Spearman correlation analysis (Table 4). The initial parasitemia was identified as a weak confounding factor for the ex vivo drug response to OZ277 in both species (P. falciparum, rs = 0.568, P = 0.021; P. vivax, rs = 0.394, P = 0.036) and artemisone and artemiside in P. vivax (rs = 0.617, P = 0.005, and rs = 0.481, P = 0.035, respectively), whereas this was not apparent for either the duration of assay or the initial parasite stage. In multivariate analysis, correlation patterns did not change substantially after controlling for these confounding factors (Table 4), but there was a difference in correlation patterns between the two Plasmodium species. In P. falciparum isolates, novel endoperoxide drug responses correlated significantly with those of AS, DHA, CQ, and PIP. While ex vivo drug response was correlated with the novel endoperoxides (ozonides and artemisone and artemiside) in P. vivax isolates, there was no correlation between these compounds and AS, DHA (the exception being artemiside), CQ, or PIP in this species (Table 4).
Table 4.
Correlation coefficients for ex vivo antimalarial susceptibilities in P. falciparum and P. vivax field isolates
| Novel drug and potential confounder or compared antimalarial drug | Correlation coefficient and P value for field isolates ofa: |
|||
|---|---|---|---|---|
|
P. falciparum |
P. vivax |
|||
| Coefficient | P value | Coefficient | P value | |
| OZ277 | ||||
| Parasitemia | 0.568 | 0.021 | 0.394 | 0.036 |
| Duration of assay | −0.041 | 0.667 | −0.080 | 0.537 |
| Rings at start of the assay | NA | NA | 0.053 | 0.641 |
| Chloroquine | 0.736 | 0.032 | −0.050 | 0.176 |
| Amodiaquine | 0.397 | 0.181 | −0.059 | 0.546 |
| Piperaquine | 0.718 | <0.001 | −0.027 | 0.057 |
| Artesunate | 0.291 | 0.002 | 0.282 | 0.389 |
| DHA | 0.581 | 0.014 | 0.424 | 0.172 |
| OZ439 | 0.853 | <0.001 | 0.456 | 0.325 |
| Artemisone | 0.767 | <0.001 | 0.693 | 0.001 |
| Artemiside | 0.828 | <0.001 | 0.611 | 0.059 |
| OZ439 | ||||
| Parasitemia | 0.424 | 0.104 | 0.082 | 0.487 |
| Duration of assay | −0.193 | 0.290 | −0.020 | 0.854 |
| Rings at start of the assay | NA | NA | −0.117 | 0.494 |
| Chloroquine | 0.658 | 0.018 | 0.427 | 0.014 |
| Amodiaquine | 0.563 | <0.001 | 0.100 | 0.145 |
| Piperaquine | 0.653 | 0.004 | 0.077 | 0.809 |
| Artesunate | 0.486 | 0.019 | 0.629 | 0.091 |
| DHA | 0.665 | 0.008 | 0.353 | 0.054 |
| Artemisone | 0.693 | <0.001 | 0.550 | 0.031 |
| Artemiside | 0.809 | <0.001 | 0.500 | 0.052 |
| Artemisone | ||||
| Parasitemia | 0.353 | 0.069 | 0.617 | 0.005 |
| Duration of assay | −0.258 | 0.177 | 0.078 | 0.725 |
| Rings at start of the assay | NA | NA | −0.268 | 0.504 |
| Chloroquine | 0.682 | 0.016 | 0.327 | 0.432 |
| Amodiaquine | 0.319 | 0.681 | 0.325 | 0.010 |
| Piperaquine | 0.747 | <0.001 | 0.600 | 0.528 |
| Artesunate | 0.501 | <0.001 | 0.593 | 0.452 |
| DHA | 0.573 | <0.001 | 0.621 | 0.273 |
| Artemiside | 0.461 | 0.308 | 0.454 | <0.001 |
| Artemiside | ||||
| Parasitemia | 0.393 | 0.133 | 0.481 | 0.035 |
| Duration of assay | 0.143 | 0.388 | 0.195 | 0.496 |
| Rings at start of the assay | NA | NA | 0.156 | 0.596 |
| Chloroquine | 0.660 | 0.010 | −0.021 | 0.849 |
| Amodiaquine | 0.509 | 0.060 | −0.157 | 0.216 |
| Piperaquine | 0.565 | 0.038 | 0.111 | 0.781 |
| Artesunate | 0.273 | 0.143 | 0.489 | 0.028 |
| DHA | 0.570 | 0.055 | 0.711 | 0.012 |
Correlation coefficients were obtained by the Spearman rank correlation. P values were obtained by robust multiple regression analysis. Bold values indicate significant correlations. NA, not applicable.
DISCUSSION
The synthetic peroxides OZ277 and OZ439 and the semisynthetic 10-alkylamino-artemisinin derivatives artemisone and artemiside demonstrate potent ex vivo activity against both Plasmodium species. All of the novel compounds tested in our study showed IC50s lower than or equal to those of AS and DHA, with IC50s varying between 1.9 and 3.6 nM against P. falciparum and 0.7 and 4.6 nM in P. vivax (Table 2).
Significant interspecies differences in ex vivo drug responses were observed for CQ (the median CQ IC50 is greater in P. falciparum than P. vivax) and OZ439 (the median OZ439 IC50 is greater in P. vivax than P. falciparum). The differences observed in CQ IC50s may reflect the spectrum of acquired resistance for each species prevalent within the community, but this is unlikely to explain the difference for OZ439. Although intrinsic species variation in life cycle maturation and drug susceptibility inherent to the assay cannot be ruled out, the number of isolates assessed was relatively small, with a borderline level of significance for the OZ439 comparison (P = 0.013), and thus the result may represent a chance occurrence within the context of multiple comparisons.
A comparative analysis in P. vivax between isolates with >80% rings and 60 to 80% rings at the start of the assay revealed a significant, albeit modest, difference in IC50s for AS and DHA, with isolates containing 80% rings at the start of the assay appearing more susceptible. Stage specificity of drug action in P. vivax has been highlighted in previous studies for CQ (31, 33) in which ex vivo susceptibility increased by more than 20-fold for isolates predominantly at the trophozoite stage compared to the ring stage. The variation in drug susceptibility between parasites at the ring and trophozoite stages was considerably less for AS and DHA (1.5-fold) and was not apparent for any of the novel endoperoxide compounds (Table 2).
The IC50 values for DHA were significantly higher than those for artesunate and, to various degrees, the other endoperoxides. This may represent either reduced stability of DHA in predosed drug plates (35) or differences in in vitro drug partition and metabolism (14, 16, 37). High variability and fluctuations in IC50s for artemisinin and its derivatives have been reported for both P. falciparum laboratory strains and field isolates (15, 34, 41) and reflect, at least in part, the application of nonstandardized in vitro assay systems, including differences in levels of parasitemia at the start of the assay, parasite synchrony, duration of the assay, and quantification method of the drug response (9, 12, 13). These variations were also apparent in the IC50 estimates derived from laboratory-adapted P. falciparum strains in the current study, for which drug susceptibility was assessed by the schizont maturation test as opposed to a reinvasion assay. The median IC50s ranged from 1.9 to 15.4 nM and 2.0 to 20.0 nM in the CQ-sensitive and CQ-resistant P. falciparum culture-adapted strains FC27 and K1, respectively (Table 2).
The schizont maturation test has been used to quantify P. falciparum drug susceptibility for more than 30 years, although alternative, more robust methods are now widely used (1a). However, for P. vivax, the assay options remain limited, and in view of the marked stage specificity of drug action, microscopic quantification of parasite growth remains the preferred option (12a, 17, 31). Previous studies have demonstrated that IC50 estimates derived from schizont maturation tend to be higher than those derived by in vitro reinvasion assays with growth quantified by 3H-hypoxanthine incorporation (1, 23, 36, 39).
Recent clinical, molecular, and in vitro studies have raised the specter of emerging drug resistance to the artemisinin derivatives, the focus of which appears to be on the western border of Cambodia. The hallmark of artemisinin tolerance is a delayed parasite clearance rate in vivo. However, the relationship between this delayed parasitological response and in vitro artemisinin drug susceptibility using established in vitro assays is unclear (4, 21, 22). This lack of in vivo-in vitro correlates has undermined the definition of artemisinin resistance and the ex vivo characterization of this process (20, 28, 40). As fears that artemisinin tolerance has already spread beyond Cambodia grow, there is an urgent need to develop ex vivo assay systems to characterize better the phenotypic response of Plasmodium to current and novel artemisinin derivatives. These will need to address stage-specific quantification of in vitro/ex vivo drug response and potentially the detection and quantification of dormant parasite stages.
Our study is one of the first to assess parasite susceptibility of a range of endoperoxide compounds, quantifying stage-specific growth between two Plasmodium species. In P. falciparum, the activity of the novel endoperoxides correlated with that of AS, DHA, CQ, and PIP, a phenomenon previously observed in studies of field isolates (2a, 14, 26, 31). The same trend was observed in P. vivax but did not reach statistical significance. Although this may simply reflect the small number of isolates tested and, thus, the low statistical power to detect a true difference, it may also represent species-specific differences in drug uptake and metabolism as well as the mode of action of these compounds.
The novel endoperoxides have a number of advantages, including better pharmacodynamic profiles, pharmacokinetic profiles amenable to single-dosing strategies, and feasibility of synthesis, over currently used artemisinin derivatives. Our study highlights their potent ex vivo activity against clinical field isolates of both P. falciparum and P. vivax in an area of known multidrug resistance. Together, these factors demonstrate that these novel compounds represent promising candidates for future ACT combination regimens that will have activity in areas of coendemicity. Development of ex vivo assays that robustly quantify ex vivo/in vitro growth dynamics will help to characterize the stage-specific activity of these compounds and facilitate assessment of their activity against clinical Plasmodium field isolates in areas in which the clinical efficacy of artemisinin is declining.
ACKNOWLEDGMENTS
We are grateful to Lembaga Pengembangan Masyarakat Amungme Kamoro, the staff of the Rumah Sakit Mitra Masyarakat (RSMM) Hospital, and Paulus Sugiarto for their support in conducting this study. We thank the Australian Red Cross blood transfusion service for the supply of human sera.
The study was funded by the Wellcome Trust (Senior Research Fellowship in Clinical Science 091625 to R.N.P.), the National Health and Medical Research Council (Program 496600 and Fellowship to N.M.A.), the Swiss National Science Foundation (Fellowship for Prospective Researchers to J.M.), and AusAID (infrastructure support for the Timika Translational Research Facility).
Footnotes
Published ahead of print 30 July 2012
REFERENCES
- 1. Alin MH, et al. 1995. Efficacy of oral and intravenous artesunate in male Tanzanian adults with Plasmodium falciparum malaria and in vitro susceptibility to artemisinin, chloroquine, and mefloquine. Am. J. Trop. Med. Hyg. 53:639–645 [DOI] [PubMed] [Google Scholar]
- 1a. Bacon DJ, et al. 2007. World Antimalarial Resistance Network (WARN) II: in vitro antimalarial drug susceptibility. Malar. J. 6:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barbacka K, Baer-Dubowska W. 2011. Searching for artemisinin production improvement in plants and microorganisms. Curr. Pharm. Biotechnol. 12:1743–1751 [DOI] [PubMed] [Google Scholar]
- 2a. Brockman A, et al. 2000. Plasmodium falciparum antimalarial drug susceptibility on the north-western border of Thailand during five years of extensive use of artesunate-mefloquine. Trans. R. Soc. Trop. Med. Hyg. 94:537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Charman SA, et al. 2011. Synthetic ozonide drug candidate OZ439 offers new hope for a single-dose cure of uncomplicated malaria. Proc. Natl. Acad. Sci. U. S. A. 108:4400–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dondorp AM, et al. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Douglas NM, Anstey NM, Angus BJ, Nosten F, Price RN. 2010. Artemisinin combination therapy for vivax malaria. Lancet Infect. Dis. 10:405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feachem RG, et al. 2010. Shrinking the malaria map: progress and prospects. Lancet 376:1566–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. 2004. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect. Dis. 4:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haynes RK, et al. 2006. Artemisone—a highly active antimalarial drug of the artemisinin class. Angew. Chem. Int. ed Engl. 45:2082–2088 [DOI] [PubMed] [Google Scholar]
- 9. Hofer S, et al. 2008. In vitro assessment of the pharmacodynamic properties of DB75, piperaquine, OZ277 and OZ401 in cultures of Plasmodium falciparum. J. Antimicrob. Chemother. 62:1061–1064 [DOI] [PubMed] [Google Scholar]
- 10. Karyana M, et al. 2008. Malaria morbidity in Papua Indonesia, an area with multidrug resistant Plasmodium vivax and Plasmodium falciparum. Malar. J. 7:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kindermans JM, Pilloy J, Olliaro P, Gomes M. 2007. Ensuring sustained ACT production and reliable artemisinin supply. Malar. J. 6:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kocken CH, et al. 2006. Plasmodium vivax: in vitro susceptibility of blood stages to synthetic trioxolane compounds and the diamidine DB75. Exp. Parasitol. 113:197–200 [DOI] [PubMed] [Google Scholar]
- 12a. Kosaisavee V, et al. 2006. Plasmodium vivax: isotopic, PicoGreen, and microscopic assays for measuring chloroquine sensitivity in fresh and cryopreserved isolates. Exp. Parasitol. 114:34–39 [DOI] [PubMed] [Google Scholar]
- 13. Kreidenweiss A, Kremsner PG, Dietz K, Mordmuller B. 2006. In vitro activity of ferroquine (SAR97193) is independent of chloroquine resistance in Plasmodium falciparum. Am. J. Trop. Med. Hyg. 75:1178–1181 [PubMed] [Google Scholar]
- 14. Kreidenweiss A, Mordmuller B, Krishna S, Kremsner PG. 2006. Antimalarial activity of a synthetic endoperoxide (RBx-11160/OZ277) against Plasmodium falciparum isolates from Gabon. Antimicrob. Agents Chemother. 50:1535–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lim P, et al. 2009. Pfmdr1 copy number and arteminisin derivatives combination therapy failure in falciparum malaria in Cambodia. Malar. J. 8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maerki S, et al. 2006. In vitro assessment of the pharmacodynamic properties and the partitioning of OZ277/RBx-11160 in cultures of Plasmodium falciparum. J. Antimicrob. Chemother. 58:52–58 [DOI] [PubMed] [Google Scholar]
- 17. Marfurt J, et al. 2011. Ex vivo activity of histone deacetylase inhibitors against multidrug-resistant clinical isolates of Plasmodium falciparum and P. vivax. Antimicrob. Agents Chemother. 55:961–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mendis K, Sina BJ, Marchesini P, Carter R. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64:97–106 [DOI] [PubMed] [Google Scholar]
- 19. Nagelschmitz J, et al. 2008. First assessment in humans of the safety, tolerability, pharmacokinetics, and ex vivo pharmacodynamic antimalarial activity of the new artemisinin derivative artemisone. Antimicrob. Agents Chemother. 52:3085–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noedl H. 2005. Artemisinin resistance: how can we find it? Trends Parasitol. 21:404–405 [DOI] [PubMed] [Google Scholar]
- 21. Noedl H, et al. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359:2619–2620 [DOI] [PubMed] [Google Scholar]
- 22. Noedl H, Socheat D, Satimai W. 2009. Artemisinin-resistant malaria in Asia. N. Engl. J. Med. 361:540–541 [DOI] [PubMed] [Google Scholar]
- 23. Noedl H, et al. 2001. In vivo-in vitro model for the assessment of clinically relevant antimalarial cross-resistance. Am. J. Trop. Med. Hyg. 65:696–699 [DOI] [PubMed] [Google Scholar]
- 24. Obaldia N, et al. 2009. Evaluation of artemisone combinations in Aotus monkeys infected with Plasmodium falciparum. Antimicrob. Agents Chemother. 53:3592–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olliaro P, Wells TN. 2009. The global portfolio of new antimalarial medicines under development. Clin. Pharmacol. Ther. 85:584–595 [DOI] [PubMed] [Google Scholar]
- 26. Osorio L, et al. 2007. In vitro susceptibility of P. falciparum populations from Colombia and Tanzania to a new synthetic peroxide (OZ277). Am. J. Trop. Med. Hyg. 76:1024–1026 [PubMed] [Google Scholar]
- 27. Poespoprodjo JR, et al. 2008. Adverse pregnancy outcomes in an area where multidrug-resistant Plasmodium vivax and Plasmodium falciparum infections are endemic. Clin. Infect. Dis. 46:1374–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pradines B, Bertaux L, Pomares C, Delaunay P, Marty P. 2011. Reduced in vitro susceptibility to artemisinin derivatives associated with multi-resistance in a traveller returning from South-East Asia. Malar. J. 10:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Price RN, Douglas NM, Anstey NM. 2009. New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr. Opin. Infect. Dis. 22:430–435 [DOI] [PubMed] [Google Scholar]
- 30. Ratcliff A, et al. 2007. Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine-pyrimethamine in southern Papua, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 101:351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Russell B, et al. 2008. Determinants of in vitro drug susceptibility testing of Plasmodium vivax. Antimicrob. Agents Chemother. 52:1040–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reference deleted.
- 33. Sharrock WW, et al. 2008. Plasmodium vivax trophozoites insensitive to chloroquine. Malar. J. 7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sisowath C, et al. 2009. In vivo selection of Plasmodium falciparum parasites carrying the chloroquine-susceptible pfcrt K76 allele after treatment with artemether-lumefantrine in Africa. J. Infect. Dis. 199:750–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tanariya P, Tippawangkoso P, Karbwang J, Na-Bangchang K, Wernsdorfer WH. 2000. In vitro sensitivity of Plasmodium falciparum and clinical response to lumefantrine (benflumetol) and artemether. Br. J. Clin. Pharmacol. 49:437–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thanh NV, et al. 2010. Monitoring for Plasmodium falciparum drug resistance to artemisinin and artesunate in Binh Phuoc Province, Vietnam: 1998-2009. Malar. J. 9:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vyas N, Avery BA, Avery MA, Wyandt CM. 2002. Carrier-mediated partitioning of artemisinin into Plasmodium falciparum-infected erythrocytes. Antimicrob. Agents Chemother. 46:105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Waknine-Grinberg JH, et al. 2010. Artemisone effective against murine cerebral malaria. Malar. J. 9:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wernsdorfer WH, McGregor I. 1989. Malaria: principles and practice of malariology. Churchill Livingstone, Edinburgh, Scotland [Google Scholar]
- 40. Witkowski B, et al. 2010. Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob. Agents Chemother. 54:1872–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wongsrichanalai C, et al. 2001. In vitro susceptibility of Plasmodium falciparum isolates from Myanmar to antimalarial drugs. Am. J. Trop. Med. Hyg. 65:450–455 [DOI] [PubMed] [Google Scholar]
- 42. World Health Organization 2010. Guidelines for the treatment of malaria, 2nd ed World Health Organization, Geneva, Switzerland [Google Scholar]
- 43. World Health Organization 2011. World malaria report 2011. World Health Organization, Geneva, Switzerland [Google Scholar]