Abstract
Highly active antiretroviral therapy (HAART) consists of a combination of drugs to achieve maximal virological response and reduce the potential for the emergence of antiviral resistance. Despite being the first antivirals described to be effective against HIV, reverse transcriptase inhibitors remain the cornerstone of HAART. There are two broad classes of reverse transcriptase inhibitor, the nucleoside reverse transcriptase inhibitors (NRTIs) and nonnucleoside reverse transcriptase inhibitors (NNRTIs). Since the first such compounds were developed, viral resistance to them has inevitably been described; this necessitates the continuous development of novel compounds within each class. In this review, we consider the NRTIs and NNRTIs currently in both preclinical and clinical development or approved for second-line therapy and describe the patterns of resistance associated with their use as well as the underlying mechanisms that have been described. Due to reasons of both affordability and availability, some reverse transcriptase inhibitors with a low genetic barrier are more commonly used in resource-limited settings. Their use results in the emergence of specific patterns of antiviral resistance and so may require specific actions to preserve therapeutic options for patients in such settings.
INTRODUCTION
The standard treatment for patients infected with human immunodeficiency virus (HIV), referred to as highly active antiretroviral therapy (HAART), consists of three or more HIV drugs, most commonly two nucleoside reverse transcriptase inhibitors (NRTIs) in combination with either a nonnucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor (PI), or more recently, an integrase inhibitor (INI) (65). The goal of HAART is to optimally suppress HIV replication during long-term therapy and to maintain immune function (92). Rational drug selection is essential to maximize potency, minimize side effects and cross-resistance, preserve future treatment options, and increase overall duration of viral suppression (reviewed in reference 23). Although numerous antiretroviral (ARV) combinations may provide potent suppression of viral replication, therapeutic choices necessitate careful consideration of the potential impact of viral resistance on subsequent treatment options.
Advances in antiretroviral therapy have improved HIV management and the control of the spread of regional epidemics (64). However, resistance to antiretroviral drugs is largely unavoidable due to the error-prone nature of HIV reverse transcriptase (RT) and its lack of a proofreading function (76). In addition, the sheer number of replication cycles occurring in an infected individual and the high rate of RT-mediated recombination events facilitate the selection of drug-resistant mutant strains of HIV (13, 28). Furthermore, certain tissue compartments seem able to select for resistance mutations due to the presence of low drug concentrations (33). These mutations are located in the genes that encode antiretroviral targets such as RT, resulting in the production of RT that is different from its wild-type (wt) counterpart in both structure and function. Although this protein is still able to play its role in HIV replication, it is not inhibited as effectively as wt protein by the ARV drugs.
The number of mutations required for resistance to occur varies from drug to drug. Many factors determine the relative rate of resistance selection with different drugs and drug combinations, and this is reflected in the “genetic barrier” to resistance, which refers to the number of mutations that must occur within a given target in order for resistance to be present against a particular drug. Interactions between mutations, the effects of individual resistance mutations on viral replication capacity, and viral fitness all influence mutational pathways and the overall impact of resistance mutations on viral phenotype. Many different mechanisms through which HIV-1 escapes from drug pressure have been described; these mechanisms differ from one drug class to another and can even differ between drugs of the same class.
RT INHIBITORS
Two classes of RT inhibitors exist: the nucleoside reverse transcriptase inhibitors (NRTIs) and the nonnucleoside reverse transcriptase inhibitors (NNRTIs). NRTIs incorporate into nascent viral DNA, resulting in DNA chain termination and blocking further extension of DNA. The NNRTIs stop HIV-1 replication by binding to the hydrophobic pocket within the p66 subunit of the RT enzyme, thus preventing it from converting viral RNA into DNA (19, 73). NNRTIs are noncompetitive inhibitors of HIV-1 RT and do not require activation. The low fidelity of HIV-1 RT, the high level of HIV-1 replication, and the high rate of RT-mediated recombination collectively contribute to the emergence of resistance to RT inhibitors (10, 28).
EARLY NRTIs
HIV can become resistant to NRTIs via two distinct mechanisms. The first is discrimination, whereby the mutated viral RT can selectively avoid incorporating NRTIs in favor of natural deoxynucleoside triphosphates (dNTPs); this mechanism is typified by such mutations as K65R, L74V, Q151M, and M184V (37). The second mechanism of resistance allows a mutated RT to enact the phosphorolytic excision of NRTIs from the 3′ end of the viral DNA chain that extends from the primer, a process referred to as “primer unblocking.” Examples of mutations involved in this process are those selected by zidovudine (ZDV) and stavudine (d4T) that are termed thymidine analogue mutations (TAMs), e.g., M41L, D67N, K70R, L210W, T215Y/F, and K219Q/E (48, 68). TAMs confer resistance to all NRTIs except lamivudine (3TC) and emtricitabine (FTC). Although there is a degree of cross-resistance associated with TAMs, ultimate levels of resistance depend on the specific NRTI and the number of TAM mutations found in the viral RT (reviewed in reference 48).
The two NRTI resistance mechanisms of discrimination and excision can also influence each other. For example, the M184V/I mutation that is selected by 3TC and FTC is a discrimination mutation, but viruses that contain M184V/I are less likely to quickly develop TAMs under selective pressure with such drugs as ZDV. Viruses containing M184V/I are also more susceptible to ZDV and d4T than are wild-type viruses (reviewed in reference 48).
Antiretroviral therapy with abacavir (ABC) leads to the selection of such mutations as K65R, L74V, Y115F, and M184V (53), and the combination of L74V and M184V is commonly observed. The K65R mutation is also selected by tenofovir (TFV) and reduces susceptibility by 3- to 6-fold against this drug (8, 67). Usually, the selection of K65R precludes the occurrence of TAMs, while the presence of the latter mutations prevents the selection of K65R due to the fact that viruses that contain both K65R and TAMs are not viable. The standard in patients initiating therapy involves a combination of TFV/FTC or ABC/3TC together with an NNRTI (reviewed in reference 74).
EARLY NNRTIs
Nevirapine (NVP) and efavirenz (EFV) are FDA-approved NNRTIs and have become the cornerstone of therapy within both developed and underdeveloped countries. However, the low genetic barrier to resistance of these earlier NNRTIs serves as a major limitation for prolonged antiretroviral therapy and sequential use of inhibitors of this class (2, 7, 42). Notably, a single amino acid substitution in the RT enzyme is often adequate to yield high-level clinically relevant resistance. Additionally, high-level cross-resistance among early NNRTIs has been reported (7), and this can also have an impact by decreasing virologic response in patients with transmitted resistance (44). The prevalence rate of transmitted antiretroviral drug resistance in treatment-naïve patients with HIV-1 has been estimated to be 5 to 15% for resistance mutations to at least one antiretroviral class (6). In this U.S. drug resistance survey, the NNRTI class showed the highest prevalence at a rate of 6.9%, compared to NRTIs and PIs at 3.6% and 2.4%, respectively. The increasing use of NNRTIs in clinical practice and the fact that NNRTI resistance mutations do not severely impair viral replication capacity but remain part of the dominant viral variant may explain the high prevalence of NNRTI resistance (reviewed in reference 20).
The NNRTI binding pocket is located largely in the p66 subunit of RT and consists of the following residues: 95, 100, 101, 103, 106, 108, 179, 181, 188, 190, 227, 229, 234, 236, and 318 (36, 73). Some residues from p51, such as 138, also contribute to the NNRTI binding. NNRTI mutations confer resistance by disrupting the interactions between the inhibitor and enzyme. This can occur through three mechanisms: (i) they can block the entry of inhibitor into the NNRTI binding pocket (e.g., K103N), (ii) they can affect contacts between the inhibitor and residues that line the NNRTI binding pocket (e.g., Y181C), or (iii) they can alter the conformation or size of the NNRTI binding pocket so that it becomes less specific for the inhibitor (e.g., Y188L) (20). Some resistance mutations can affect the binding of NNRTIs through more than one mechanism.
NEWER NRTIs
Elvucitabine.
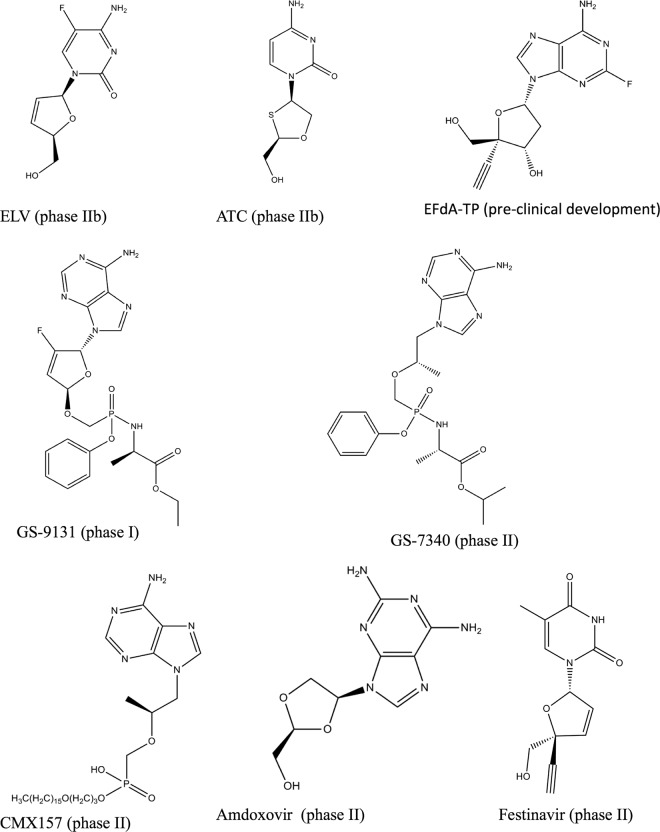
Elvucitabine (ELV) (Fig. 1) is a novel NRTI currently in late phase II study and was developed by Achillion Pharmaceuticals. In an in vitro selection study, only two amino acid substitutions, M184I and D237E, were identified in the resultant variant (21). The double mutation conferred moderate resistance to elvucitabine (about 10-fold) and cross-resistance to lamivudine but not to other nucleoside inhibitors tested. Elvucitabine has also demonstrated potent antiviral activity in HIV-infected patients with resistance to 3TC and other NRTIs. The drug has good oral bioavailability and an intracellular half-life of >24 h (15).
Fig 1.
New NRTIs undergoing preclinical or clinical development. The phase of development is indicated in parentheses.
Apricitabine.
Apricitabine (ATC) (Fig. 1) is a novel deoxycytidine NRTI currently in clinical development for the treatment of HIV infection. In vitro selection for resistance with ATC selected for M184V, V75I, and K65R (25). The resulting mutants from this selection conferred low-level resistance of less than 4-fold. Others showed that continuous passage of HIV-1 already containing M184V, K65R, or combinations of M41L, M184V, and T215Y did not result in any additional mutations (62). In vitro ATC has shown favorable antiviral activity against HIV-1 wt strains and clinical isolates containing NRTI mutations, including M184V, L74V, and TAMs (11). ATC yielded a virological response in treatment-naïve and treatment-experienced HIV-1-infected patients whose viruses contained M184V and up to 5 TAMs. Resistance to ATC was reported to be slow to develop in vitro, and there is little evidence of the development of resistance to this drug in patients (11, 25, 62).
4′-Ethynyl-2-fluoro-2′-dATP.
4′-Ethynyl-2-fluoro-2′-dATP (EF-dATP) (Fig. 1) is a new NRTI, now in preclinical development, that retains the 3′ OH group and has excellent antiviral properties, i.e., 50% effective concentration (EC50) of 0.07 nM against wt virus (31, 35), compared to approved NRTIs that range in EC50 between 17 and 89 nM (38). This robust antiviral activity is due to a mechanism of action that is different from those of approved NRTIs. Notably, EF-dATP acts by binding through its 3′ primer terminus to RT; the addition of subsequent nucleotides is then prevented by blocking the translocation of the primer strand on the viral polymerase (52). Thus, EF-dATP is termed a translocation-defective reverse transcriptase inhibitor (TDRTI). Modeling studies have confirmed the binding of EF-dATP to a hydrophobic pocket of RT residues consisting of A114, Y115, F160, and M184 and the aliphatic chain of D185 (52).
In an in vitro pre-steady-state kinetics study to determine toxicity, it was shown that EF-dATP is a poor human mitochondrial DNA polymerase γ (Pol γ) substrate, suggesting that Pol γ-mediated toxicity might be minimal (82). Resistant variants, including those containing the K65R/L74V/Q151M complex, did not affect the susceptibility of this compound, while the HIV-1 clone containing M184V alone conferred low- to moderate-level resistance to EF-dATP (31). In vitro, a parental compound of EF-dATP, 2′-deoxy-4′-C-ethynyl-adenosine (EdA), selected for resistant variants after 58 passages with a novel combination of mutations, I142V/T165R/M184V (31). Site-directed mutagenesis of clones containing the mutations showed that either I142V or T165R alone did not affect the antiviral activity of EF-dATP, while M184V alone or in combination with I142V or T165R demonstrated moderate resistance to EF-dATP. The triple mutant I142V/T165R/M184V had the highest resistance among clones tested (31).
GS-9131.
GS-9131 (Fig. 1) is a prodrug of the nucleotide reverse transcriptase analogue GS-9148, which belongs to the same family as TFV. GS-9131 demonstrated potent antiviral activity against a variety of HIV-1 subtypes, i.e., EC50 of 37 nM (12). In vitro, the parent drug (GS-9148) caused only low-level cytotoxicity compared to that of TFV (12). GS-9131 also demonstrated synergy in combination with other antiretrovirals as well as potent antiviral activity against multi-NRTI-resistant strains, including K65R, M184V, and L74V, and only a minimal increase in EC50 in regard to viruses carrying four or more TAMs (12). The use of GS-9131 was shown to result in 76 to 290 times more of the diphosphorylated form of GS-9148 than of GS-9148 itself (72).
GS-7340.
GS-7340 (Fig. 1) is a prodrug of TFV that exhibits anti-HIV activity and that possesses a favorable resistance profile. The EC50 of GS-7340 against HIV-1 in MT-2 cells was 0.005 μM, compared to 5 μM for the parent drug TFV (40). In HIV-1-infected patients, GS-7340 demonstrated enhanced antiviral activity, with no TFV mutations identified, and yielded higher intracellular concentrations of TFV-diphosphate than did TFV itself (77).
CMX157.
CMX157 (Fig. 1) is a lipid moiety prodrug of TFV that has activity against wt viruses of major HIV-1 subtypes, with EC50s ranging from 0.2 to 7.2 nM (38). In contrast, TFV has EC50s against HIV-1 groups M and O that range between 500 and 2,200 nM in peripheral blood mononuclear cells (PBMCs) (24). In an in vitro study, CMX157 demonstrated potent activity against NRTI-resistant strains, including multidrug-resistant viruses, against which it showed >300-fold the activity of TFV (38). The higher potency and lower EC50 of CMX157 are due to better cellular uptake than that for TFV and the fact that it is not a substrate for organic anion transporters. This permits it to maintain high concentrations inside cells, in contrast to TFV, which is actively metabolized by organic anion transporters, leading to decreased intracellular concentrations (71). In vitro and preclinical studies in rats showed that CMX157 also has a favorable cytotoxicity profile in PBMCs and that it does not lead to nephrotoxicity, as has been reported for TFV (38, 63). No information is available on the selection of resistance mutations with CMX157.
Amdoxovir.
Amdoxovir (AMDX) is a prodrug of β-d-dioxolane guanosine (DXG) that is currently in phase II clinical trials (Fig. 1). In vitro phenotypic analyses have shown that DXG is effective against HIV-1 variants that are resistant to 3TC and FTC (M184V/I) as well as viruses that contain TAMs, while selection studies in MT-2 cells resulted in the appearance of K65R and L74V (5, 51). The combination of AMDX together with ZDV in HIV-1-infected patients was shown to be synergistic, resulting in reduced viral loads (57). AMDX thus represents a new NRTI that possesses potent antiviral activity against NRTI-resistant viruses.
Festinavir (OBP-601).
Festinavir (OBP-601) (Fig. 1) is a new NRTI in the same family as stavudine (d4T), but it has an improved safety profile. In vitro, it shows potent antiviral activity against wt HIV-1 of multiple subtypes, with EC50s ranging from 0.76 to 5.8 μM, compared to 1.57 to 6.06 μM for d4T (90). In a phenotypic susceptibility assay, viruses carrying the K65R and Q151M resistance mutations were shown to be hypersusceptible to this compound. In contrast, a slightly decreased antiviral response was observed against viruses carrying either TAMs or TAMs together with K103N and M184V (90). More importantly, a strong synergistic effect of OBP-601 with several approved NRTIs and NNRTIs was observed against wt and resistant viruses (90).
NEWER NNRTIs
Etravirine.
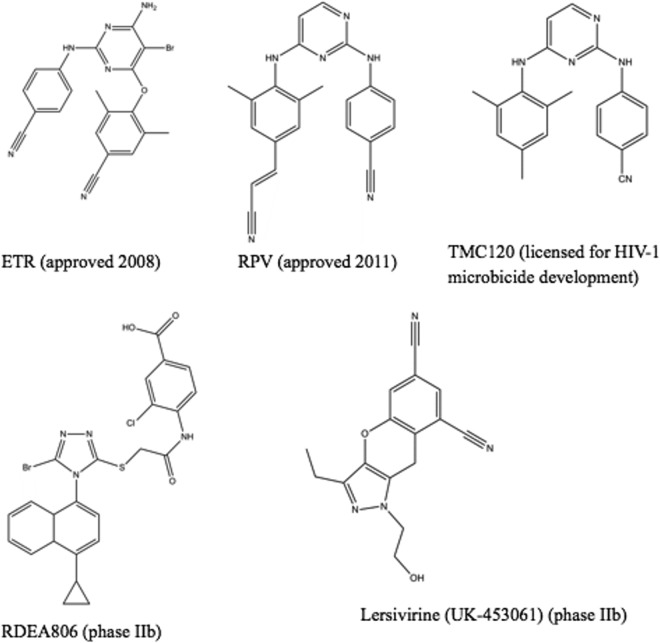
Etravirine (ETR) (Fig. 2), formerly known as TMC125, is a diarylpyrimidine (DAPY)-based NNRTI that possesses potent antiviral activity against both wt HIV-1 of multiple subtypes and some viruses containing NNRTI resistance mutations (1, 88). Specifically, ETR retains full activity against viruses containing the most prevalent NNRTI mutation, K103N (1). In vitro, ETR is more difficult to generate resistance against than are initial NNRTIs (88). In clinical studies of ETR in combination with potent background regimens that included NRTIs and integrase and protease inhibitors, it was observed that viral loads became significantly decreased in patients with resistance to older NNRTIs and some PIs (9, 45, 58). In vitro and clinical studies have described 20 ETR resistance-associated mutations (RAMs) (V90I, A98G, L100I, K101E/H/P, V106I, E138A/K/G/Q, V179D/F/T, Y181C/I/V, G190A/S, and M230L) and have allowed a weighted score to be assigned to each mutation (3, 83, 89). Of these ETR RAMs, three or more are required for high-level resistance to occur, thus demonstrating a high genetic barrier to resistance compared to those of older NNRTIs. The structure of ETR allows it to bind to the RT enzyme such that mutations in the NNRTI binding pocket do not compromise binding, and thus, activity is maintained. ETR can rotate within the pocket, allowing multiple interactions despite the presence of mutations in the binding pocket (18). Because of its unique characteristics, ETR is the only NNRTI approved for treatment of NNRTI-experienced patients.
Fig 2.
New NNRTIs that are approved or are undergoing clinical development. The phase of development is indicated in parentheses.
Only a few studies have prospectively studied the efficacy of ETR in combination with other background regimens in NNRTI-experienced patients (30, 49). In the phase III DUET-1 and DUET-2 studies, 57% of patients in the ETR arm and 36% in the placebo had a viral load of <50 copies/ml after 96 weeks of treatment (30).
In the DUET-1 and DUET-2 clinical studies, it was found that patients who experienced virologic failure had greater numbers of ETR resistance mutations at baseline than patients with treatment successes. Second, patients who experienced virologic failure were often found to have received background regimens that were less potent than the drugs given to patients who did not fail therapy (84). In this study, the V179F, V179I, and Y181C mutations in RT were commonly associated with treatment failure alongside changes at positions K101 and E138 (84). The authors concluded that these mutations usually emerge in a background of other multiple NNRTI mutations and were, in most cases, associated with a decrease in phenotypic sensitivity to ETR.
Another subanalysis of the DUET trial studied the impact of background regimen on virologic response to ETR, and the authors further confirmed that a higher virologic response rate was observed in patients who demonstrated an increased activity of the background regimen, with the highest responses being achieved in patients who used more than two active agents in addition to ETR (85). In the TMC125-C227 (ETR) trial, ETR was inferior to a protease inhibitor (PI) in PI-naïve patients with a history of previous NNRTI failure (78). In a post hoc analysis of baseline resistance data for the TMC125-C227 trial, a diminished virological response was observed in patients who possessed the following characteristics at baseline: the presence of Y181C, a baseline ETR fold change of ≥10, and a higher number of ETR resistance mutations (78). In another study, the authors studied 42 NNRTI treatment-experienced patients for 6 months on an ETR-containing regimen (49). At failure, 12 of 42 patients developed at least one new NNRTI mutation. The most frequently selected mutations included V179I, Y181C, and V179F.
Several studies have researched the theoretical potential of ETR based on the resistance patterns of patients who previously failed NNRTI therapy and accumulated ETR RAMs. These studies have observed a prevalence of more than three ETR RAMs among viral isolates from patients experiencing NNRTI treatment failure ranging from 4.6% to 10%, while the prevalence of isolates with a single ETR RAM was 17.4% to 35.9% (41, 61, 69, 70). These studies concluded that there is a low prevalence of ETR resistance at baseline and that patients with prior failure to NNRTIs may potentially benefit from ETR rescue therapy. However, these analyses focused on patients in developed countries that have full access to the most-potent antiretroviral drugs and that are constantly monitored for viral load and the development of resistance.
In contrast, patients in countries with limited resources developed resistance faster due to a lack of potent antiretroviral drugs and drug resistance testing. Some studies in resource-limited settings have observed a high prevalence of NNRTI resistance mutations associated with ETR resistance among patients failing an NNRTI-containing regimen (32, 34, 39). Using NVP in the failing regimen was associated with intermediate and reduced responses to ETR, while use of EFV and coadministration of 3TC reduced the risk of ETR resistance (39). The authors concluded that the frequent occurrence of NNRTI mutations in resource-limited settings in which drug resistance testing is rare might compromise the continuous use of ETR and also its use in second-line therapy. The Y181C mutation, associated with NVP therapy, has been reported with a high prevalence in NNRTI-experienced patients and shown to decrease susceptibility to ETR (39, 41, 46, 47). This demonstrates cross-resistance of both NVP and ETR, as confirmed by our group (3). Thus, the widespread use of NVP in resource-limited settings without resistance testing casts doubt as to whether ETR can be effective in NNRTI-experienced patients in poorer countries. One of these studies suggested that ETR should be avoided in salvage regimens in the setting of first-line NVP failure in which drug resistance testing is not performed (47). Another study analyzed the prevalence of minority variants in treatment-naïve and NNRTI-experienced patients by ultradeep pyrosequencing: while such variants were not identified in any of 13 drug-naïve patients, it was shown that 7 of 20 patients who had failed an NNRTI-containing regimen possessed minority variants as well as ETR-associated NNRTI resistance mutations (86). This suggests that minority variants in NNRTI-experienced patients may lead to decreased ETR activity and to virologic failure (66).
In vitro selection and clinical trial results with ETR have identified amino acid substitutions at position 138 in RT (3, 83). Mutations at position 138 are not associated with resistance to older NNRTIs. Although these mutations conferred only low-level resistance to ETR, E138K is also associated with resistance to most newer NNRTIs (Fig. 2); its emergence may also facilitate the development of additional ETR mutations (3). The connection domain mutation N348I has been identified and implicated in reduced susceptibility to NVP and EFV as well as to the nucleoside analogue ZDV (26). Two independent studies have assessed and confirmed that this mutation also decreases susceptibility to ETR, either alone or in combination (27, 50). This effect was reversed when M184V was coexpressed with N348I. Additional connection domain mutations found to be associated with impaired susceptibility to ETR were T369I and E399G.
Two independent genotypic scores to predict ETR resistance have been established. The first, developed by Janssen, has been correlated with treatment response in the DUET studies and, when combined with in vitro selection studies, identified 17 (recently updated to 20) ETR RAMs (83, 89). In this analysis, any three or more of these mutations were required to cause resistance to ETR. A second score by Monogram is based on a correlation of phenotype and genotype results of 4,923 samples containing at least one NNRTI mutation (54). The Monogram genotypic score identified 30 mutations associated with ETR resistance, with the weighted score for each mutation being slightly higher than the 20-mutation-based Janssen score. However, the final interpretations of the two scores seem to be similar.
Rilpivirine (TMC278).
Rilpivirine (RPV) (Fig. 2), also known as TMC278, is another DAPY compound that was recently approved for treatment of NNRTI-naïve patients. The structure and binding of RPV in the NNRTI binding pocket are similar to those of ETR, which allows reorientation of both compounds within RT. In vitro, RPV possesses subnanomolar activity against wt HIV-1 of multiple subtypes and shows antiviral activity against viruses containing many NNRTI resistance-associated mutations (4). NNRTI RAMs emerging in culture under RPV selective pressure included combinations of V90I, L100I, K101E, V106A/I, V108I, E138G/K/Q/R, V179F/I, Y181C/I, V189I, G190E, H221Y, F227C, and M230I/L. The resistance profile and genetic barrier to the development of resistance of RPV are comparable to those of ETR. High-resolution crystal structures of RPV in complex with HIV-1 RT reveal that the cyanovinyl group of TMC278 is positioned in a hydrophobic tunnel connecting the NNRTI binding pocket to the nucleic acid binding cleft (17). RPV and ETR exhibit similar flexibility in adapting to resistance mutations. In the ECHO and THRIVE phase 3 trials (14, 55), resistance analysis showed a slightly higher proportion of treatment failures in the RPV arm than in the EFV arm. The most frequent NNRTI mutation in the RPV arm was E138K, in addition to mutations such as Y181C, K101E, H221Y, V901, E138Q, and V189I (75). Also, the proportion of NRTI mutations that emerged in the study was higher in the RPV arm than in the EFV arm. The NRTI mutations selected included M184I/V, K65R, K219E, and Y115F.
The International Antiviral Society (IAS)-USA recently published a total of 15 mutations (K101E/P, E138A/G/K/Q/R, V179L, Y181C/I/V, H221Y, F227C, and M230I/L) associated with decreased susceptibility to RPV (29). These mutations have been described based on in vitro studies and in patients in whom RPV was failing. The quantitative impact of each of these mutations on RPV resistance differs.
Dapivirine (TMC120).
Dapivirine (TMC120) (Fig. 2) is another DAPY compound that can accommodate some mutations within the NNRTI binding site without significant loss of activity (42, 43). TMC120 has shown potent antiviral activity against both wt and NNRTI-resistant HIV-1 strains (22, 79). In 2004, Janssen officially licensed the further development of TMC120 for use as a vaginal microbicide to the International Partnership for Microbicides (IPM) to help prevent sexual transmission of HIV-1.
The results of both phase I and II studies have shown that TMC120 was widely distributed through the lower genital tract with low systemic absorption when administered as a vaginal gel formulation for up to 42 days (59, 60). The gel was safe and well tolerated. In vitro selection studies have identified drug resistance mutations in the presence of TMC120, notably L100I, K101E, V108I, E138K/Q, V179M/E, Y181C, and F227Y (80, 81). Most of these TMC120 resistance-associated mutations occur at exactly the same position as many of the mutations associated with ETR and RPV resistance (3, 4, 89). However, in one of these studies, it was shown that suboptimal concentrations of TMC120 alone facilitated the emergence of common NNRTI resistance mutations while suboptimal concentrations of TMC120 plus tenofovir (TFV) gave rise to fewer mutations (80). Due to the likelihood of transmitted resistant strains in HIV-1-infected individuals, resistance mutations might impact the ability of a single drug to prevent HIV-1 as a microbicide. Using a combination of antiviral drugs of different classes may be useful. Another study showed in an in vitro model that using TMC120 in combination with TFV as a microbicide exhibited synergy and was more potent than either drug alone (79).
Lersivirine.
Lersivirine (Fig. 2) is a new NNRTI belonging to the pyrazole family and is being developed by Pfizer. In an in vitro resistance study, lersivirine selected for the amino acid substitutions V108I, E138K, V179D, F227L, and M230I (16). In a phase IIb trial, Pozniak and colleagues reported better responses in patients with EFV than in patients with lersivirine, i.e., 86% versus 79%, respectively (87). Among patients who failed lersivirine, the mutations identified included K101E, V106M, V108I, H221Y, Y188H, F227C/L, and L234I.
RDEA806.
RDEA806 (Fig. 2) is a novel NNRTI being developed by Ardea Biosciences. In genotypic and phenotypic analyses of mutant viruses selected by RDEA806, the K104E, E138K, T240I, V179D, and F227L substitutions were identified (91). Phenotypic analysis of these mutations demonstrated that RDEA806 requires at least 3 mutations for a >10-fold loss of susceptibility. In a phase 2a trial, RDEA806 was well tolerated and exhibited robust antiviral activity with no genotypic or phenotypic changes (56).
CONCLUSION
An ideal new HIV inhibitor should possess a high genetic barrier in regard to potential development of resistance for this same drug class as well as a unique resistance profile in both B and HIV-1 non-B subtypes. NRTI resistance mutations are widespread, and there is currently no approved NRTI compound that possesses activity against all NRTI mutations. However, a number of new NRTIs with potent activity against NRTI-resistant viruses are now in preclinical and clinical development. In some cases, these compounds possess the ability to maintain high concentrations inside cells (e.g., CMX157, GS-9131, and GS-7340) or may have a different mechanism of action than older NRTIs, as seems to be the case for EF-dATP. Although M184V is a common NRTI mutation that confers high-level resistance to 3TC and FTC and cross-resistance to other NRTIs, the levels of resistance conferred against newer NRTIs, such as ELV, ATC, and EF-dATP, are low, suggesting that these compounds may be useful against M184V-containing viruses. It will be important to determine whether selection of M184V in patients receiving ELV, ATC, or EF-dATP may result in elevated viral loads and treatment failure. Hopefully, their potential to synergize either together or with currently approved drugs will make them important components of future antiretroviral strategies.
ETR is the only NNRTI approved for treatment of HIV-1-infected, NNRTI-experienced patients. In a limited number of studies, ETR has proven to be effective in patients with treatment failure who harbor viruses that are resistant to initial NNRTIs and that carry several resistance mutations. A number of studies have shown that patients who fail NVP therapy are more prone to developing ETR resistance mutations than those who fail EFV therapy. Considering the widespread use of NVP in developing countries, the use of ETR in NNRTI-experienced patients in these settings is under debate. RPV that is now approved for treatment-naïve patients shows high-level cross-resistance with ETR. Therefore, the use of RPV in first-line therapy may jeopardize the future of ETR as a second-line NNRTI, and the sequential use of these drugs is not recommended. The emergence of E138K as a signature mutation for almost all new NNRTIs (both approved and under clinical development) is another limitation of these compounds.
TMC120, lersivirine, and RDEA806 are still in phase II clinical trials, and large-scale phase III trials are required to exploit their potential use in the clinics. Some mutations have been selected in the presence of these three compounds together with ETR and RPV. The fact that TMC120 in combination with TFV as a microbicide is more potent and decreases the possibility of selecting for resistance mutations more than either compound alone shows potential for such an antiviral-based microbicide in preventing HIV-1 infection. The search for novel NNRTIs should now focus on compounds with different resistance profiles so as to broaden treatment options for patients who have experienced NNRTI therapy failure. Overall, new NRTI and NNRTI agents can provide a welcome therapy option for patients with existing NRTI or NNRTI resistance and also for patients who are naïve to therapy.
ACKNOWLEDGMENTS
Research in our laboratories is supported by grants from the Canadian Institutes of Health Research and the Réseau FRSQ-SIDAMI. E.L.A. is the recipient of a Departmental Scholarship from the Département de Microbiologie et d'Immunologie, Université de Montréal.
C.L.T. is the Pfizer/Université de Montréal Chair on HIV Translational Research.
We thank Peter Quashie for helping to generate the structures of the compounds.
Footnotes
Published ahead of print 25 June 2012
REFERENCES
- 1. Andries K, et al. 2004. TMC125, a novel next-generation nonnucleoside reverse transcriptase inhibitor active against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 48:4680–4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Antinori A, et al. 2002. Cross-resistance among nonnucleoside reverse transcriptase inhibitors limits recycling efavirenz after nevirapine failure. AIDS Res. Hum. Retroviruses 18:835–838 [DOI] [PubMed] [Google Scholar]
- 3. Asahchop EL, et al. 2011. Characterization of the E138K resistance mutation in HIV-1 reverse transcriptase conferring susceptibility to etravirine in B and non-B HIV-1 subtypes. Antimicrob. Agents Chemother. 55:600–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Azijn H, et al. 2010. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob. Agents Chemother. 54:718–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bazmi HZ, et al. 2000. In vitro selection of mutations in the human immunodeficiency virus type 1 reverse transcriptase that decrease susceptibility to (−)-β-d-dioxolane-guanosine and suppress resistance to 3′-azido-3′-deoxythymidine. Antimicrob. Agents Chemother. 44:1783–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bennett D, et al. 2005. U.S. surveillance of HIV drug resistance at diagnosis using HIV diagnostic sera, abstr 674. Abstr. 12th Conf. Retrovir. Oppor. Infect., Boston, MA [Google Scholar]
- 7. Brenner B, et al. 2003. A V106M mutation in HIV-1 clade C viruses exposed to efavirenz confers cross-resistance to non-nucleoside reverse transcriptase inhibitors. AIDS 17:F1–F5 [DOI] [PubMed] [Google Scholar]
- 8. Brenner BG, et al. 2006. HIV-1 subtype C viruses rapidly develop K65R resistance to tenofovir in cell culture. AIDS 20:F9–F13 [DOI] [PubMed] [Google Scholar]
- 9. Briz V, et al. 2009. Raltegravir and etravirine are active against HIV type 1 group O. AIDS Res. Hum. Retroviruses 25:225–227 [DOI] [PubMed] [Google Scholar]
- 10. Bushman FD, Fujiwara T, Craigie R. 1990. Retroviral DNA integration directed by HIV integration protein in vitro. Science 249:1555–1558 [DOI] [PubMed] [Google Scholar]
- 11. Cahn P, Wainberg MA. 2010. Resistance profile of the new nucleoside reverse transcriptase inhibitor apricitabine. J. Antimicrob. Chemother. 65:213–217 [DOI] [PubMed] [Google Scholar]
- 12. Cihlar T, et al. 2008. Design and profiling of GS-9148, a novel nucleotide analog active against nucleoside-resistant variants of human immunodeficiency virus type 1, and its orally bioavailable phosphonoamidate prodrug, GS-9131. Antimicrob. Agents Chemother. 52:655–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coffin JM. 1995. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science 267:483–489 [DOI] [PubMed] [Google Scholar]
- 14. Cohen CJ, et al. 2011. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet 378:229–237 [DOI] [PubMed] [Google Scholar]
- 15. Colucci P, et al. 2009. Multiple-dose pharmacokinetic behavior of elvucitabine, a nucleoside reverse transcriptase inhibitor, administered over 21 days with lopinavir-ritonavir in human immunodeficiency virus type 1-infected subjects. Antimicrob. Agents Chemother. 53:662–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corbau R, et al. 2010. Lersivirine, a nonnucleoside reverse transcriptase inhibitor with activity against drug-resistant human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 54:4451–4463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Das K, et al. 2008. High-resolution structures of HIV-1 reverse transcriptase/TMC278 complexes: strategic flexibility explains potency against resistance mutations. Proc. Natl. Acad. Sci. U. S. A. 105:1466–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Das K, et al. 2004. Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants. J. Med. Chem. 47:2550–2560 [DOI] [PubMed] [Google Scholar]
- 19. D'Cruz OJ, Uckun FM. 2006. Novel tight binding PETT, HEPT and DABO-based non-nucleoside inhibitors of HIV-1 reverse transcriptase. J. Enzyme Inhib. Med. Chem. 21:329–350 [DOI] [PubMed] [Google Scholar]
- 20. Domaoal RA, Demeter LM. 2004. Structural and biochemical effects of human immunodeficiency virus mutants resistant to non-nucleoside reverse transcriptase inhibitors. Int. J. Biochem. Cell Biol. 36:1735–1751 [DOI] [PubMed] [Google Scholar]
- 21. Fabrycki J, et al. 2003. In vitro introduction of HIV variants with reduced susceptibility to elvucitabine (AHC-126,443, β-L-FD4C), abstr 5. Abstr. 12th Int. HIV Drug Resist. Workshop, Los Cabos, Mexico [Google Scholar]
- 22. Fletcher P, et al. 2009. Inhibition of human immunodeficiency virus type 1 infection by the candidate microbicide dapivirine, a nonnucleoside reverse transcriptase inhibitor. Antimicrob. Agents Chemother. 53:487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gallant JE, et al. 2003. Nucleoside and nucleotide analogue reverse transcriptase inhibitors: a clinical review of antiretroviral resistance. Antivir. Ther. 8:489–506 [PubMed] [Google Scholar]
- 24. Gilead Sciences 2012. Viread (tenofovir disoproxyl fumurate) tablets prescribing information. Gilead Sciences, Foster City, CA: http://www.gilead.com/pdf/viread_pi.pdf [Google Scholar]
- 25. Gu Z, et al. 2001. BCH-10618, a new heterosubstituted nucleoside analogue against HIV-1 infection. Antivir. Ther. 6(Suppl 1):8 [Google Scholar]
- 26. Gupta S, et al. 2010. Combinations of mutations in the connection domain of human immunodeficiency virus type 1 reverse transcriptase: assessing the impact on nucleoside and nonnucleoside reverse transcriptase inhibitor resistance. Antimicrob. Agents Chemother. 54:1973–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gupta S, et al. 2011. Connection domain mutations in HIV-1 reverse transcriptase do not impact etravirine susceptibility and virologic responses to etravirine-containing regimens. Antimicrob. Agents Chemother. 55:2872–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ho DD, et al. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123–126 [DOI] [PubMed] [Google Scholar]
- 29. Johnson VA, et al. 2011. 2011 update of the drug resistance mutations in HIV-1. Top. Antivir. Med. 19:156–164 [PMC free article] [PubMed] [Google Scholar]
- 30. Katlama C, et al. 2010. Efficacy and safety of etravirine at week 96 in treatment-experienced HIV type-1-infected patients in the DUET-1 and DUET-2 trials. Antivir. Ther. 15:1045–1052 [DOI] [PubMed] [Google Scholar]
- 31. Kawamoto A, et al. 2008. 2′-deoxy-4′-C-ethynyl-2-halo-adenosines active against drug-resistant human immunodeficiency virus type 1 variants. Int. J. Biochem. Cell Biol. 40:2410–2420 [DOI] [PubMed] [Google Scholar]
- 32. Kekitiinwa A, Friedman D, Coakley E, Lie Y, Granziano F. 2010. Profiling etravirine resistance in Ugandan children with extended failure of a NNRTI-inclusive regimen as first-line ART, abstr 891. Abstr. 16th Conf. Retrovir. Oppor. Infect., San Francisco, CA [Google Scholar]
- 33. Kepler TB, Perelson AS. 1998. Drug concentration heterogeneity facilitates the evolution of drug resistance. Proc. Natl. Acad. Sci. U. S. A. 95:11514–11519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kiertiburanakul S, Wiboonchutikul S, Sukasem C, Chantratita W, Sungkanuparph S. 2010. Using of nevirapine is associated with intermediate and reduced response to etravirine among HIV-infected patients who experienced virologic failure in a resource-limited setting. J. Clin. Virol. 47:330–334 [DOI] [PubMed] [Google Scholar]
- 35. Kodama EI, et al. 2001. 4′-Ethynyl nucleoside analogs: potent inhibitors of multidrug-resistant human immunodeficiency virus variants in vitro. Antimicrob. Agents Chemother. 45:1539–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kohlstaedt LA, Wang J, Friedman JM, Rice PA, Steitz TA. 1992. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science 256:1783–1790 [DOI] [PubMed] [Google Scholar]
- 37. Krebs R, Immendorfer U, Thrall SH, Wohrl BM, Goody RS. 1997. Single-step kinetics of HIV-1 reverse transcriptase mutants responsible for virus resistance to nucleoside inhibitors zidovudine and 3-TC. Biochemistry 36:10292–10300 [DOI] [PubMed] [Google Scholar]
- 38. Lanier ER, et al. 2010. Development of hexadecyloxypropyl tenofovir (CMX157) for treatment of infection caused by wild-type and nucleoside/nucleotide-resistant HIV. Antimicrob. Agents Chemother. 54:2901–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lapadula G, et al. 2008. Prevalence and risk factors for etravirine resistance among patients failing on non-nucleoside reverse transcriptase inhibitors. Antivir. Ther. 13:601–605 [PubMed] [Google Scholar]
- 40. Lee WA, et al. 2005. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother. 49:1898–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Llibre JM, et al. 2008. Prevalence of etravirine-associated mutations in clinical samples with resistance to nevirapine and efavirenz. J. Antimicrob. Chemother. 62:909–913 [DOI] [PubMed] [Google Scholar]
- 42. Ludovici DW, et al. 2001. Evolution of anti-HIV drug candidates. Part 3. Diarylpyrimidine (DAPY) analogues. Bioorg. Med. Chem. Lett. 11:2235–2239 [DOI] [PubMed] [Google Scholar]
- 43. Ludovici DW, et al. 2001. Evolution of anti-HIV drug candidates. Part 2. Diaryltriazine (DATA) analogues. Bioorg. Med. Chem. Lett. 11:2229–2234 [DOI] [PubMed] [Google Scholar]
- 44. Ludovici DW, et al. 2001. Evolution of anti-HIV drug candidates. Part 1. From alpha-anilinophenylacetamide (alpha-APA) to imidoyl thiourea (ITU). Bioorg. Med. Chem. Lett. 11:2225–2228 [DOI] [PubMed] [Google Scholar]
- 45. Madruga JV, et al. 2007. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 370:29–38 [DOI] [PubMed] [Google Scholar]
- 46. Maiga AI, et al. 2010. Resistance-associated mutations to etravirine (TMC-125) in antiretroviral-naive patients infected with non-B HIV-1 subtypes. Antimicrob. Agents Chemother. 54:728–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manosuthi W, et al. 2010. Patients infected with HIV type 1 subtype CRF01_AE and failing first-line nevirapine- and efavirenz-based regimens demonstrate considerable cross-resistance to etravirine. AIDS Res. Hum. Retroviruses 26:609–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marcelin A-G. 2006. Resistance to nucleoside reverse transcriptase inhibitors. In Geretti AM, Antiretroviral resistance in clinical practice. Mediscript Ltd, London, United Kingdom: http://www.ncbi.nlm.nih.gov/books/NBK2239/ [Google Scholar]
- 49. Marcelin AG, et al. 2010. Mutations selected in patients displaying treatment failure under an etravirine-containing regimen. Antivir. Ther. 15(Suppl 2):A64. [DOI] [PubMed] [Google Scholar]
- 50. McCormick AL, et al. 2011. Impact of the N348I mutation in HIV-1 reverse transcriptase on nonnucleoside reverse transcriptase inhibitor resistance in non-subtype B HIV-1. Antimicrob. Agents Chemother. 55:1806–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mewshaw JP, et al. 2002. Dioxolane guanosine, the active form of the prodrug diaminopurine dioxolane, is a potent inhibitor of drug-resistant HIV-1 isolates from patients for whom standard nucleoside therapy fails. J. Acquir. Immune Defic. Syndr. 29:11–20 [DOI] [PubMed] [Google Scholar]
- 52. Michailidis E, et al. 2009. Mechanism of inhibition of HIV-1 reverse transcriptase by 4′-ethynyl-2-fluoro-2′-deoxyadenosine triphosphate, a translocation-defective reverse transcriptase inhibitor. J. Biol. Chem. 284:35681–35691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Miller V, et al. 2000. HIV-1 reverse transcriptase (RT) genotype and susceptibility to RT inhibitors during abacavir monotherapy and combination therapy. AIDS 14:163–171 [DOI] [PubMed] [Google Scholar]
- 54. Haddad M, Stawiski E, Benhamida J, Coakley E. 2010. Improved genotypic algorithm for predicting etravirine susceptibility: comprehensive list of mutations identified through correlation with matched phenotype, abstr 574. Abstr. 17th Conf. Retrovir. Oppor. Infect., San Francisco, CA [Google Scholar]
- 55. Molina JM, et al. 2011. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet 378:238–246 [DOI] [PubMed] [Google Scholar]
- 56. Moyle G, et al. 2010. Phase 2a randomized controlled trial of short-term activity, safety, and pharmacokinetics of a novel nonnucleoside reverse transcriptase inhibitor, RDEA806, in HIV-1-positive, antiretroviral-naive subjects. Antimicrob. Agents Chemother. 54:3170–3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Murphy RL, et al. 2010. Antiviral activity and tolerability of amdoxovir with zidovudine in a randomized double-blind placebo-controlled study in HIV-1-infected individuals. Antivir. Ther. 15:185–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nadler JP, et al. 2007. Efficacy and safety of etravirine (TMC125) in patients with highly resistant HIV-1: primary 24-week analysis. AIDS 21:F1–F10 [DOI] [PubMed] [Google Scholar]
- 59. Nel AM, et al. 2010. Pharmacokinetic assessment of dapivirine vaginal microbicide gel in healthy, HIV-negative women. AIDS Res. Hum. Retroviruses 26:1181–1190 [DOI] [PubMed] [Google Scholar]
- 60. Nel AM, et al. 2009. Safety, tolerability, and systemic absorption of dapivirine vaginal microbicide gel in healthy, HIV-negative women. AIDS 23:1531–1538 [DOI] [PubMed] [Google Scholar]
- 61. Neogi U, Shet A, Shamsundar R, Ekstrand ML. 2011. Selection of nonnucleoside reverse transcriptase inhibitor-associated mutations in HIV-1 subtype C: evidence of etravirine cross-resistance. AIDS 25:1123–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Oliveira M, et al. 2009. Apricitabine does not select additional drug resistance mutations in tissue culture in human immunodeficiency virus type 1 variants containing K65R, M184V, or M184V plus thymidine analogue mutations. Antimicrob. Agents Chemother. 53:1683–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Painter GR, et al. 2007. Evaluation of hexadecyloxypropyl-9-R-[2-(phosphonomethoxy)propyl]-adenine, CMX157, as a potential treatment for human immunodeficiency virus type 1 and hepatitis B virus infections. Antimicrob. Agents Chemother. 51:3505–3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Palella FJ, Jr, et al. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853–860 [DOI] [PubMed] [Google Scholar]
- 65. Panel on Antiretroviral Guidelines for Adults and Adolescents 2011. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents, p 1–167 Department of Health and Human Services, Washington, DC: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf [Google Scholar]
- 66. Paredes R, et al. 2010. Pre-existing minority drug-resistant HIV-1 variants, adherence, and risk of antiretroviral treatment failure. J. Infect. Dis. 201:662–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Parikh UM, Bacheler L, Koontz D, Mellors JW. 2006. The K65R mutation in human immunodeficiency virus type 1 reverse transcriptase exhibits bidirectional phenotypic antagonism with thymidine analog mutations. J. Virol. 80:4971–4977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Parikh UM, Zelina S, Sluis-Cremer N, Mellors JW. 2007. Molecular mechanisms of bidirectional antagonism between K65R and thymidine analog mutations in HIV-1 reverse transcriptase. AIDS 21:1405–1414 [DOI] [PubMed] [Google Scholar]
- 69. Poveda E, et al. 2010. Etravirine resistance associated mutations in HIV-infected patients failing efavirenz or nevirapine in the Spanish antiretroviral resistance database. AIDS 24:469–471 [DOI] [PubMed] [Google Scholar]
- 70. Poveda E, et al. 2007. Prevalence of etravirine (TMC-125) resistance mutations in HIV-infected patients with prior experience of non-nucleoside reverse transcriptase inhibitors. J. Antimicrob. Chemother. 60:1409–1410 [DOI] [PubMed] [Google Scholar]
- 71. Ray AS, et al. 2006. Mechanism of active renal tubular efflux of tenofovir. Antimicrob. Agents Chemother. 50:3297–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ray AS, et al. 2008. Intracellular metabolism of the nucleotide prodrug GS-9131, a potent anti-human immunodeficiency virus agent. Antimicrob. Agents Chemother. 52:648–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ren J, et al. 1995. High resolution structures of HIV-1 RT from four RT-inhibitor complexes. Nat. Struct. Biol. 2:293–302 [DOI] [PubMed] [Google Scholar]
- 74. Ribera Pascuet E, Curran A. 2008. Current role of tenofovir in clinical medicine. Enferm. Infecc. Microbiol. Clin. 26(Suppl 8):45–54 (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 75. Rimsky L, et al. 2012. Genotypic and phenotypic characterization of HIV-1 isolates obtained from patients on rilpivirine therapy experiencing virologic failure in the phase 3 ECHO and THRIVE studies: 48-week analysis. J. Acquir. Immune Defic. Syndr. 59:39–46 [DOI] [PubMed] [Google Scholar]
- 76. Roberts JD, Bebenek K, Kunkel TA. 1988. The accuracy of reverse transcriptase from HIV-1. Science 242:1171–1173 [DOI] [PubMed] [Google Scholar]
- 77. Ruane P, et al. 2012. GS-7340 25 mg and 40 mg demonstrate superior efficacy to tenofovir 300 mg in a 10-day monotherapy study of HIV-1+ patients, abstr 103. Abstr. 19th Conf. Retrovir. Oppor. Infect., Seattle, WA [Google Scholar]
- 78. Ruxrungtham K, et al. 2008. Impact of reverse transcriptase resistance on the efficacy of TMC125 (etravirine) with two nucleoside reverse transcriptase inhibitors in protease inhibitor-naive, nonnucleoside reverse transcriptase inhibitor-experienced patients: study TMC125-C227. HIV Med. 9:883–896 [DOI] [PubMed] [Google Scholar]
- 79. Schader SM, Colby-Germinario SP, Schachter JR, Xu H, Wainberg MA. 2011. Synergy against drug-resistant HIV-1 with the microbicide antiretrovirals, dapivirine and tenofovir, in combination. AIDS 25:1585–1594 [DOI] [PubMed] [Google Scholar]
- 80. Schader SM, et al. 2012. In vitro resistance profile of the candidate HIV-1 microbicide drug dapivirine. Antimicrob. Agents Chemother. 56:751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Selhorst P, et al. 2011. Human immunodeficiency virus type 1 resistance or cross-resistance to nonnucleoside reverse transcriptase inhibitors currently under development as microbicides. Antimicrob. Agents Chemother. 55:1403–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sohl CD, et al. 2012. Mechanism of interaction of human mitochondrial DNA polymerase γwith the novel nucleoside reverse transcriptase inhibitor 4′-ethynyl-2-fluoro-2′-deoxyadenosine indicates a low potential for host toxicity. Antimicrob. Agents Chemother. 56:1630–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tambuyzer L, Nijs S, Daems B, Picchio G, Vingerhoets J. 2011. Effect of mutations at position E138 in HIV-1 reverse transcriptase on phenotypic susceptibility and virologic response to etravirine. J. Acquir. Immune Defic. Syndr. 58:18–22 [DOI] [PubMed] [Google Scholar]
- 84. Tambuyzer L, et al. 2010. Characterization of genotypic and phenotypic changes in HIV-1-infected patients with virologic failure on an etravirine-containing regimen in the DUET-1 and DUET-2 clinical studies. AIDS Res. Hum. Retroviruses 26:1197–1205 [DOI] [PubMed] [Google Scholar]
- 85. Trottier B, et al. 2010. Impact of the background regimen on virologic response to etravirine: pooled 48-week analysis of DUET-1 and -2. HIV Clin. Trials 11:175–185 [DOI] [PubMed] [Google Scholar]
- 86. Varghese V, et al. 2009. Minority variants associated with transmitted and acquired HIV-1 nonnucleoside reverse transcriptase inhibitor resistance: implications for the use of second-generation nonnucleoside reverse transcriptase inhibitors. J. Acquir. Immune Defic. Syndr. 52:309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vernazza P, et al. 2011. Efficacy and safety of lersivirine (UK-453, 061) vs. efavirenz in antiretroviral treatment-naive HIV-1-infected patients: week 48 primary analysis results from an ongoing, randomised, double-blind, phase IIb trial (study A5271015), abstr TUAB0101. Abstr. 6th IAS Conf. HIV Pathog. Treat. Prev., Rome, Italy [DOI] [PubMed] [Google Scholar]
- 88. Vingerhoets J, et al. 2005. TMC125 displays a high genetic barrier to the development of resistance: evidence from in vitro selection experiments. J. Virol. 79:12773–12782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vingerhoets J, et al. 2010. Resistance profile of etravirine: combined analysis of baseline genotypic and phenotypic data from the randomized, controlled phase III clinical studies. AIDS 24:503–514 [DOI] [PubMed] [Google Scholar]
- 90. Weber J, et al. 2008. Drug susceptibility profile of OBP-601, a novel NRTI, using a comprehensive panel of NRTI and/or NNRTI resistant viruses, abstr 726b. Abstr. 15th Conf. Retrovir. Oppor. Infect., Boston, MA [Google Scholar]
- 91. Xu W, et al. 2008. Resistance to RDEA806 requires multiple mutations which have limited cross-resistance to other NNRTIs, poster H-1222. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 92. Yeni PG, et al. 2002. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society-USA panel. JAMA 288:222–235 [DOI] [PubMed] [Google Scholar]