Abstract
From June to September 2011, a total of 305 ertapenem-nonsusceptible Enterobacteriaceae isolates (MICs of ertapenem ≥ 1 μg/ml) were collected from 11 hospitals in different parts of Taiwan. The MICs of 12 antimicrobial agents against these isolates were determined using the broth microdilution method, and genes for carbapenemases were detected using PCR. Genotypes of isolates possessing carbapenemase genes were identified by pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing. The ertapenem-nonsusceptible Enterobacteriaceae isolates included Klebsiella pneumoniae (n = 219), Escherichia coli (n = 64), Enterobacter cloacae (n = 15), and other species (n = 7). Seven (2.3%) of the ertapenem-nonsusceptible Enterobacteriaceae isolates exhibited colistin MICs of >4 μg/ml, and 24 (7.9%) were not susceptible to tigecycline (MICs > 2 μg/ml). A total of 29 (9.5%) isolates carried genes encoding carbapenemases, namely, K. pneumoniae carbapenemase-2 (KPC-2) in 16 (7.3%) isolates of K. pneumoniae (KPC-2-KP) and IMP-8 in 5 (2.3%) isolates of K. pneumoniae, 5 (33.3%) isolates of E. cloacae, 1 isolate of E. coli, 1 isolate of Klebsiella oxytoca, and one isolate of Citrobacter freundii. The 16 KPC-2-KP isolates were isolated from patients at four different hospitals in northern Taiwan. All 16 of the KPC-2-KP isolates were susceptible to amikacin and colistin and had a similar pulsotype (pulsotype 1) and the same sequence type (sequence type 11). Infections due to KPC-2-KP mainly occurred in severely ill patients in the intensive care unit (n = 14, 88%). Four patients with infections due to KPC-2-KP died within 14 days of hospitalization. The findings are the first to demonstrate intrahospital and interhospital dissemination of KPC-2-KP in northern Taiwan.
INTRODUCTION
Carbapenems remain the first-line therapy for severe infections caused by extended-spectrum β-lactamase (ESBL)-producing and multidrug-resistant Enterobacteriaceae (1, 19, 24, 30). Global surveillance studies have revealed that all carbapenems (ertapenem, imipenem, meropenem, and doripenem) are highly active against these resistant Enterobacteriaceae isolates (7, 8, 18). Recently, carbapenem-resistant Enterobacteriaceae isolates, particularly Klebsiella pneumoniae carbapenemase-producing K. pneumoniae (KPC-KP) and New Delhi metallo-β-lactamase-1 (NDM-1)-producing Enterobacteriaceae have emerged in many countries as a result of intracontinental and intercontinental spreading (3–6, 20, 23, 25, 27, 31, 32, 34, 35, 38). Carbapenemases, however, are not the only mechanisms associated with resistance to carbapenems. For example, studies have shown that the combination of ESBL-AmpC hyperproduction with porin loss contributes to carbapenem nonsusceptibility (13, 14, 20, 36, 37).
In Taiwan, ESBL production, AmpC β-lactamase overproduction, and decreased outer membrane protein expression combined with an active efflux pump have also been reported to contribute to resistance of Enterobacteriaceae to ertapenem (36, 37). KPC-KP- and NDM-1-producing organisms were not reported in Taiwan until 2011, and all of them were imported from China (10, 22).
The purpose of this multicenter surveillance study comprising 11 teaching hospitals located in different parts of Taiwan was to delineate the current status of carbapenem resistance and the molecular basis for the increase in carbapenem resistance among Enterobacteriaceae species in Taiwan during the period from June to September 2011.
MATERIALS AND METHODS
Bacterial isolates and hospital settings.
From June 2011 to September 2011, a total of 305 nonduplicate isolates of ertapenem-nonsusceptible Enterobacteriaceae (MIC values ≥ 1 μg/ml) were collected from 11 hospitals (bed number range, 1,000 to 3,000) located in northern Taiwan (Taipei City and New Taipei City; n = 7), central Taiwan (Taichung; n = 2), and southern Taiwan (Tainan and Kaohsiung; n = 2). The ertapenem-nonsusceptible Enterobacteriaceae isolates were obtained from various clinical specimens, namely, sputum (n = 116, 38.0%), urine (n = 95, 31.1%), wound or pus (n = 36, 11.8%), and blood (n = 35, 11.5%) and included Klebsiella pneumoniae (n = 219), Escherichia coli (n = 64), Enterobacter cloacae (n = 15), Enterobacter aerogenes (n = 2), Serratia marcescens (n = 2), and 1 isolate each of Klebsiella oxytoca, Citrobacter freundii, and C. diversus (see Table S1 in the supplemental material). The isolates were initially identified using a Phoenix PMIC/ID-30 identification system (Becton Dickinson Diagnostic Systems, Sparks, MD) (Table 1).
Table 1.
MICs of 17 KPC-2-KP isolates to 12 antimicrobial agents and microbiological characteristics of these isolates
| Isolate | MIC (μg/ml)a |
Microbiological characteristics |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FEP | ATM | ERT | DOR | IMI | MEM | GM | AN | FOS | SXT | CL | TGC | MHT result | Pulsosubtypeb | ST | |
| A1 | >128 | >128 | >128 | 32 | 32 | 64 | 0.5 | 2 | >256 | 0.25 | 1 | 0.5 | + | PST-2 | 11 |
| A2 | >128 | >128 | >128 | 128 | 64 | 128 | 0.5 | 1 | >256 | 0.25 | 0.5 | 0.5 | + | PST-5 | 11 |
| B1 | >128 | >128 | >128 | >128 | >128 | >128 | 0.5 | 1 | >256 | 0.25 | 0.5 | 0.5 | + | PST-2 | 11 |
| B2 | >128 | >128 | >128 | 64 | 32 | 64 | 0.5 | 2 | >256 | 0.25 | 0.5 | 1 | + | PST-2 | 11 |
| B3 | 64 | >128 | >128 | 64 | 64 | 128 | 0.5 | 1 | >256 | 2 | 0.5 | 1 | + | PST-1 | 11 |
| B4 | 128 | >128 | >128 | 64 | 64 | 128 | 64 | 1 | 128 | 16 | 1 | 1 | + | PST-4 | 11 |
| B5 | 128 | >128 | >128 | 64 | 32 | 128 | 0.5 | 2 | >256 | >32 | 0.5 | 1 | + | PST-2 | 11 |
| B6 | >128 | >128 | >128 | 128 | 32 | 128 | 1 | 2 | >256 | 2 | 0.5 | 1 | + | PST-2 | 11 |
| C1 | 128 | >128 | >128 | 32 | 32 | 64 | 0.5 | 1 | 64 | 2 | 0.5 | 1 | + | PST-1 | 11 |
| C2 | 128 | >128 | >128 | 64 | 64 | 128 | 0.5 | 1 | 256 | 2 | 0.5 | 1 | + | PST-1 | 11 |
| C3 | 128 | >128 | >128 | 64 | 32 | 128 | 0.5 | 1 | 128 | 2 | 0.5 | 1 | + | PST-1 | 11 |
| C4 | >128 | >128 | >128 | 128 | 64 | 128 | 0.25 | 0.5 | 64 | 2 | 0.5 | 1 | + | PST-6 | 11 |
| C5 | 128 | >128 | >128 | 64 | 32 | 64 | 0.5 | 0.5 | 128 | 2 | 0.5 | 1 | + | PST-1 | 11 |
| C6 | 128 | >128 | >128 | 64 | 32 | 64 | 0.5 | 0.5 | 256 | 2 | 0.5 | 1 | + | PST-1 | 11 |
| C7 | 64 | >128 | >128 | 32 | 16 | 64 | 0.5 | 2 | 64 | 2 | 0.5 | 2 | + | PST-3 | 11 |
| D1 | 128 | >128 | >128 | 32 | 32 | 64 | 0.5 | 1 | >256 | 2 | 1 | 1 | + | PST-1 | 11 |
| NTUH- 9047 (9) | >128 | >128 | >128 | 32 | 64 | 64 | >128 | >128 | >256 | 2 | 0.5 | 1 | + | PST-1 | 11 |
AN, amikacin; ATM, aztreonam; CL, colistin; DOR, doripenem; ERT, ertapenem; ESBL, extended-spectrum β-lactamase production detected by the disk diffusion method (10); FEP, cefepime; FOS, fosfomycin; GM, gentamicin; IMI, imipenem; MEM, meropenem; MHT, modified Hodge test (11); PST, pulsosubtype; ST, sequence type; SXT, trimethoprim-sulfamethoxazole; TGC, tigecycline.
All 17 KPC-KP isolates belonged to PT-1.
Antimicrobial susceptibility testing.
MIC values were initially determined by the broth microdilution method using the Phoenix PMIC/ID-30 system (Becton Dickinson Diagnostic Systems, Sparks, MD), and the MICs of 12 antimicrobial agents against all isolates that harbored any carbapenemase gene were determined using the agar dilution method according to the guidelines recommended by the Clinical and Laboratory Standards Institute (CLSI) (12). The antimicrobial agents used for susceptibility testing were obtained from their corresponding manufacturers. The concentrations of antimicrobial agents ranged from 0.03 μg/ml to 128 μg/ml. The MIC of each antimicrobial agent was defined as the lowest concentration that inhibited visible growth of the organism. Susceptibility to tigecycline was defined on the basis of the criteria proposed by the U.S. Food and Drug Administration (FDA) (MICs ≤ 2 μg/ml) (16) and the European Committee on Antimicrobial Susceptibility Testing-2011 (EUCAST-2011) (susceptible, MICs ≤ 1 μg/ml) (15).
ESBL-producing isolates were identified using the disk diffusion method as recommended by the CLSI (11). The modified Hodge test was also performed for all isolates harboring carbapenemase genes (11).
Determination of carbapenemase genes.
Genes encoding different classes of carbapenemases, including those of class A (KPC, NMC, SME, IMI, and GES), class B (NDM, IMP, VIM, SPM, GIM, and SIM), and class D (OXA-48 and 51-like, 23-like, 24-like, and 58-like enzymes), were identified as previously described (13, 29).
Molecular typing.
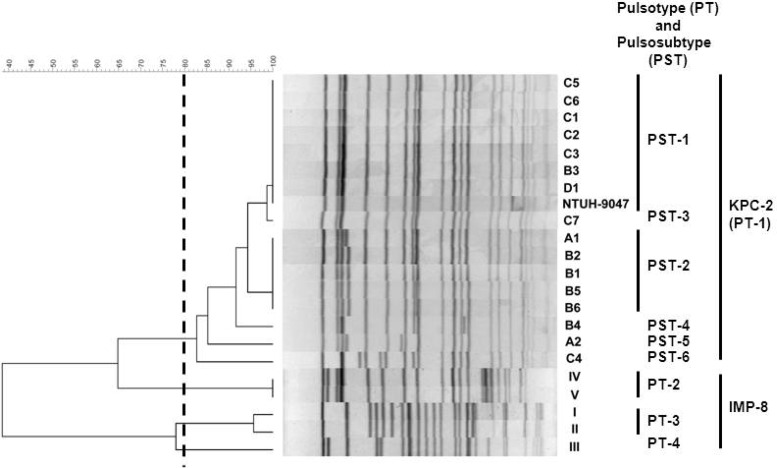
Genotypes of isolates harboring carbapenemase genes were determined using pulsed-field gel electrophoresis (PFGE) with the restriction enzyme XbaI and multilocus sequence typing (MLST) (http://www.pasteur.fr/recherche/genopole/PF8/mlst/Kpneumoniae.html) (28). Isolates exhibiting PFGE profiles with more than 80% similarity were considered to be closely related strains and defined as being of the same pulsotype (PT). Isolates with the same pulsosubtype (PST) were defined as being closely related strains possessing identical (100% similarity) PFGE profiles. Isolates with the same PST and sequence type (ST) were considered to belong to the same clone. NTUH-9047, the first isolate of KPC-2-producing K. pneumoniae (KPC-2-KP) recovered in Taiwan, was also included in this study (9).
Plasmid identification.
Plasmid DNA was extracted from isolates of KPC-2 K. pneumoniae with a Qiagen midikit (Qiagen, Germantown, MD). HindIII-digested plasmids were separated on a 0.8% agarose gel and transferred to nylon membranes for Southern hybridization. KPC-containing fragments were identified by hybridization with a digoxigenin (Dig)-labeled blaKPC-specific probe (Roche Diagnostics, GmbH, Germany) (13, 29).
RESULTS
Antimicrobial susceptibilities of all ertapenem-nonsusceptible Enterobacteriaceae isolates.
The MIC ranges, MIC50 values, and MIC90 values of 20 antimicrobial agents against the 305 ertapenem-nonsusceptible Enterobacteriaceae isolates, as well as the rates of susceptibility to said agents, are shown in Table S2 in the supplemental material. Among all ertapenem-nonsusceptible Enterobacteriaceae isolates, 69% were susceptible to imipenem, 82% were susceptible to meropenem, and 25% were susceptible to cefepime. The rate of susceptibility to cefepime was higher among E. coli (47%) and E. cloacae (60%) isolates than among K. pneumoniae isolates (16%). The rates of susceptibility to amikacin were 66% for K. pneumoniae, 97% for E. coli, and 100% for E. cloacae. The overall rate of susceptibility to amikacin was 75%. The range of tigecycline MICs was 0.06 μg/ml to 8 μg/ml (MIC90 value, 2 μg/ml), and that of colistin was ≤1 μg/ml to >4 μg/ml (MIC90 value, ≤1 μg/ml). Seven (2.3%) of the ertapenem-nonsusceptible Enterobacteriaceae isolates, including six (2.7%) isolates of K. pneumoniae and one (6.7%) isolate of E. cloacae, exhibited colistin MIC values of >4 μg/ml. Fifty-five (18.0%) isolates, including 43 (19.6%) K. pneumonia isolates, 8 (53.3%) E. cloacae isolates, 2 S. marcescens isolates, and 1 isolate each of K. oxytoca and E. aerogenes, were not susceptible to tigecycline on the basis of the criteria (MICs > 1 μg/ml) proposed by EUCAST (15). Twenty-eight isolates (9.2%), including 22 (10.0%) of K. pneumonia, 4 (26.7%) of E. cloacae, and 2 of S. marcescens, were not susceptible to tigecycline (MICs > 2 μg/ml) on the basis of FDA criteria (16). Rates of susceptibility to other agents tested were low.
Genes encoding carbapenemases.
Among the 305 ertapenem-nonsusceptible Enterobacteriaceae isolates, genes for carbapenemases were detected in 29 (9.5%) isolates, namely, 16 (7.3%) isolates of KPC-2-KP and 13 isolates of IMP-8-producing organisms, including 5 (2.3%) isolates of K. pneumoniae, 5 (33.3%) isolates of E. cloacae, 1 isolate of E. coli, 1 isolate of K. oxytoca, and 1 isolate of C. freundii (Tables 1 and 2).
Table 2.
MICs of 13 isolates possessing genes encoding IMP-8 to 12 antimicrobial agentsa and their pulsotypes
| Isolate no. (hospital) | Bacterium (designation) | ESBL production/MHT result | MIC (μg/ml) |
Pulsotype | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FEP | ATM | ERT | DOR | IMI | MEM | GM | AN | FOS | SXT | CL | TGC | ||||
| 1 (A) | K. pneumoniae (I) | +/+ | 32 | 0.5 | 8 | 2 | 2 | 1 | 1 | 2 | 256 | >32 | 0.5 | 4 | PT-3 |
| 2 (A) | K. pneumoniae (II) | +/+ | 16 | 0.25 | 4 | 1 | 1 | 0.5 | 1 | 2 | 256 | >32 | 0.5 | 4 | PT-3 |
| 3 (A) | K. pneumoniae (III) | +/+ | 16 | 0.25 | 4 | 1 | 0.5 | 0.5 | 1 | 2 | >256 | >32 | 0.5 | 1 | PT-4 |
| 4 (H) | K. pneumoniae (IV) | +/+ | 32 | 16 | 4 | 0.5 | 0.5 | 0.5 | 32 | 1 | 32 | >32 | 0.5 | 2 | PT-2 |
| 5 (H) | K. pneumoniae (V) | +/+ | 32 | 16 | 2 | 0.5 | 0.5 | 0.5 | 32 | 1 | 64 | >32 | 0.5 | 2 | PT-2 |
| 6 (A) | E. coli | +/+ | 32 | 64 | 1 | 0.12 | 0.5 | 0.12 | 128 | 2 | 1 | >32 | 0.5 | 0.5 | NP |
| 7 (K) | E. cloacae | NA/+ | 8 | 128 | 1 | 0.25 | 0.5 | 0.25 | 128 | 8 | 8 | >32 | 0.5 | 1 | PT-5 |
| 8 (K) | E. cloacae | NA/+ | 8 | 128 | 1 | 0.06 | 0.25 | 0.12 | 128 | 8 | 16 | >32 | 0.25 | 4 | PT-6 |
| 9 (K) | E. cloacae | NA/+ | 16 | >128 | 4 | 0.25 | 0.5 | 0.25 | 1 | 1 | 32 | >32 | 0.5 | 4 | PT-7 |
| 10 (K) | E. cloacae | NA/+ | 2 | 128 | 2 | 0.12 | 0.25 | 0.25 | 1 | 1 | 32 | >32 | 0.5 | 4 | PT-7 |
| 11 (E) | E. cloacae | NA/− | 2 | 128 | 1 | 0.06 | 0.12 | 0.06 | 128 | 8 | 32 | >32 | 0.25 | 2 | PT-8 |
| 12 (A) | K. oxytoca | +/− | 16 | 0.25 | 2 | 0.5 | 1 | 0.5 | 1 | 1 | 16 | 0.25 | 0.5 | 1 | NP |
| 13 (E) | C. freundii | NA/+ | 64 | 128 | 4 | 1 | 2 | 0.5 | 128 | 2 | 0.5 | 0.12 | 0.5 | 0.5 | NP |
AN, amikacin; ATM, aztreonam; CL, colistin; DOR, doripenem; ERT, ertapenem; ESBL, extended-spectrum β-lactamase production detected by the disk diffusion method (10); FEP, cefepime; FOS, fosfomycin; GM, gentamicin; IMI, imipenem; MEM, meropenem; MHT, modified Hodge test (11); NA, not available; NP, not performed; SXT, trimethoprim-sulfamethoxazole; TGC, tigecycline.
MICs of 11 antimicrobial agents against carbapenemase-producing ertapenem-nonsusceptible Enterobacteriaceae.
The MIC ranges of 11 antimicrobial agents against the 16 KPC-2-KP isolates and NTUH-9047, the first isolate of KPC-2 K. pneumoniae isolated in Taiwan, were as follows: cefepime, 64 to >128 μg/ml; ertapenem, >128 μg/ml; imipenem, 16 to >128 μg/ml; meropenem, 64 to >128 μg/ml; doripenem, 32 to >128 μg/ml; amikacin, 0.5 to 2 μg/ml; gentamicin, 0.5 to 64 μg/ml (1 isolate with an MIC of 64 mg/liter); sulfamethoxazole-trimethoprim, 0.25 to >32 μg/ml (1 isolate with an MIC of 16 mg/liter and 1 with an MIC of >32 μg/ml); and fosfomycin, 64 to >256 μg/ml (Table 1). The 16 KPC-2-KP isolates were susceptible to colistin (range, 0.5 to 1 μg/ml) and tigecycline (range, 0.5 to 2 μg/ml) (Table 1), and all exhibited positive reactions in the modified Hodge test.
Of the 13 IMP-8-producing ertapenem-nonsusceptible Enterobacteriaceae isolates, 2 (1 each of K. pneumoniae and C. freundii) demonstrated intermediate susceptibility to imipenem, 1 (K. pneumoniae) showed intermediate susceptibility to doripenem, and all were susceptible to meropenem (Table 2). Eleven (84.6%) of the isolates were resistant to trimethoprim-sulfamethoxazole (MICs, >32 μg/ml). Tigecycline MICs of 4 μg/ml were found in five (5/14, 38.5%) isolates (two K. pneumoniae and three E. cloacae isolates). All the five isolates were susceptible to colistin, with MICs of ≤0.5 μg/ml.
Genotypes of KPC-2-KP and IMP-8-producing isolates.
The 16 KPC-2-KP and NTUH-9047 isolates were closely related (all belonged to PT-1), had the same sequence type (ST11) (Fig. 1), and were isolated from patients hospitalized at four hospitals in northern Taiwan: hospitals A (n = 2), B (n = 6), C (n = 7), and D (n = 1). The distance between hospitals B and C is less than 10 kilometers. Among the 16 KPC-2-KP isolates, six PSTs were identified. Two main PSTs were identified: PST-1 (n = 8) in four hospitals (hospital A [NTUH-9047], B [n = 1], C [n = 5], and D [n = 1]) and PST-2 (n = 5) in two hospitals (hospitals A [n = 1] and B [n = 4]). The pulsosubtype (PST-1) of the NTUH-9047 isolate differed from the PSTs (PST-2 and PST-5, respectively) of two subsequently identified KPC-2-KP isolates in hospital A. Similar PTs were also found in four IMP-8-producing K. pneumoniae isolates (PT-2 in two isolates from hospital H and PT-3 in two isolates from hospital A) and in two IMP-8-producing E. cloacae isolates (PT-6 in two isolates from hospital K) (Fig. 1 and Table 2). A plasmid location of the blaKPC-2 gene was found among the six main PSTs of 16 KPC-2-producing K. pneumoniae isolates (Fig. 2).
Fig 1.
Pulsed-field gel electrophoresis profiles and dendrogram of the 16 KPC-2-KP isolates, 1 KPC-2-KP isolate previously reported (NTUH-9024) (10), and 5 IMP-8-producing K. pneumoniae isolates in Taiwan in 2011. See Tables 2 and 3 for isolate designations and the sources of the isolates.
Fig 2.
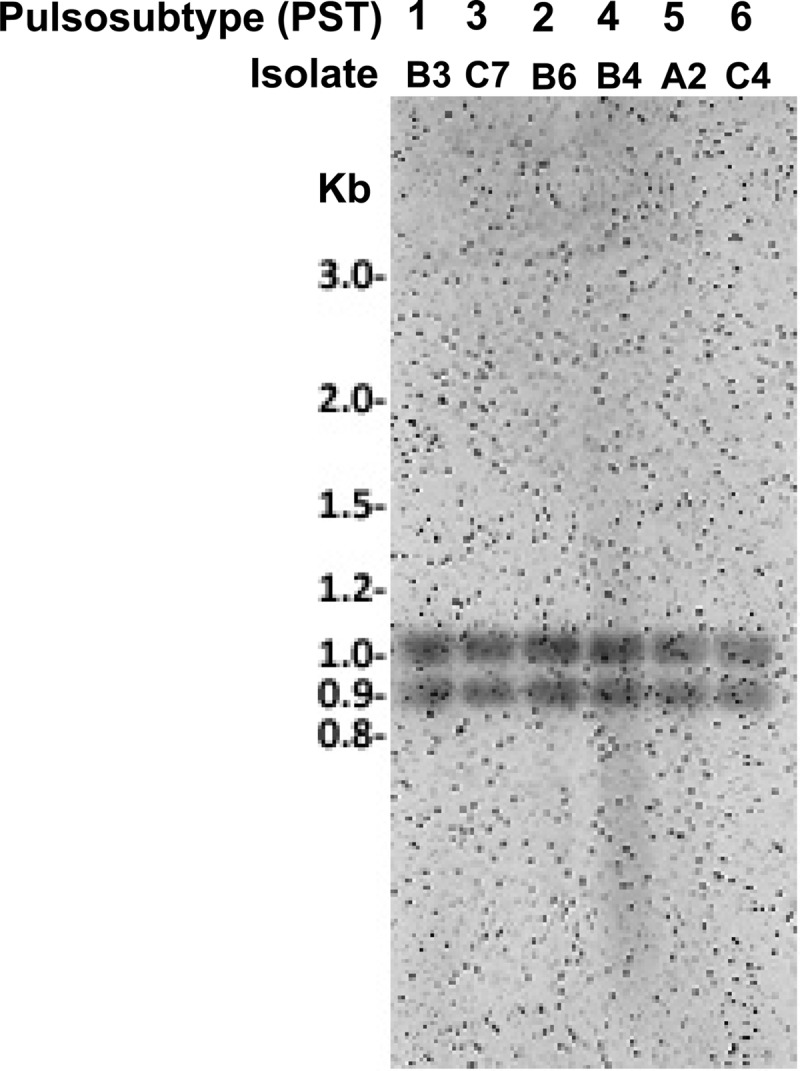
Southern hybridization of plasmids containing KPC fragments obtained from six main PSTs of the 16 KPC-2-producing K. pneumoniae isolates.
Clinical characteristics of patients with infections due to KPC-2-KP.
Demographic and clinical information for the patients with isolations of KPC-2-KP is summarized in Table 3. Patients with KPC-2-KP were found in four different hospitals, and all of them are located in northern Taiwan. The distance between these four hospitals is about 10 kilometers. None of the 16 patients had traveled to China prior to hospitalization, but all had previously received treatment at hospital A, the hospital in which the first case of KPC-2-KP had been isolated (10). Most (14 of 16, 87.5%) of the patients from whom KPC-2-KP was isolated were in intensive care units and were receiving aggressive medical interventions and treatment with multiple antibiotics. Among these 16 patients, 6 had received carbapenems within 2 weeks prior to the isolation of KPC-2-KP. The airway was the most common site of isolation. The duration from admission to KPC-2-KP isolation varied.
Table 3.
Demographic and clinical information for patients infected/colonized with KPC-2-KPa
| Patient (age [yr]/sex) | Hospital/isolate designation | Date (yr/mo/day) of: |
Underlying medical condition(s) | ICU related | Site of isolation/type of infection or colonization | Antibiotic regimen within 2 wk: |
Mortality |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Admission | KPC-KP isolation | Prior to KPC-KP isolation | After KPC-2-KP isolation | 14 days | In hospital | |||||
| 1 (22/M) | A/A1 | 2011/6/17 | 2011/6/20 | Becker muscular dystrophy, cardiac arrest after CPR, and ECMO | Yes | Wound/SSI (ECMO related) | Vancomycin, ceftazidime, piperacillin-tazobactam | Piperacillin-tazobactam, tigecycline, colistin | No | No |
| 2 (31/M) | A/A2 | 2011/6/18 | 2011/8/21 | DM, CKD, ARDS after ECMO | Yes | Sputum/colonization | Vancomycin, cefepime, colistin | Imipenem, colistin, moxifloxacin, vancomycin | No | Yes |
| 3 (81/F) | B/B1 | 2011/6/14 | 2011/7/2 | Colon cancer stage IV postoperation and chemotherapy, recurrence with bowel obstruction postoperation | Yes | Retroperitoneal abscess | Piperacillin-tazobactam | Doripenem, amikacin | No | Yes |
| 4 (83/F) | B/B2 | 2011/6/26 | 2011/7/25 | Colon cancer with liver and lung metastasis | Yes | Sputum/colonization | Piperacillin-tazobactam | Imipenem, gentamicin | No | Yes |
| 5 (67/M) | B/B3 | 2011/7/22 | 2011/8/9 | Subdural hemorrhage postoperation, dementia | Yes | Sputum/colonization | Piperacillin-tazobactam | Piperacillin-tazobactam | No | Np |
| 6 (45/M) | B/B4 | 2011/8/10 | 2011/9/5 | Cellulitis, acute myeloid leukemia postchemotherapy, neutropenic fever | No | Blood/bacteremia | Vancomycin, cefepime, metronidazole | Colistin, imipenem, amikacin | Yes | Yes |
| 7 (74/F) | B/B5 | 2011/7/9 | 2011/9/25 | COPD, stroke | Yes | Sputum/colonization | Piperacillin-tazobactam | Piperacillin-tazobactam | No | No |
| 8 (86/F) | B/B6 | 2011/9/23 | 2011/10/12 | DM, seizure, sick sinus syndrome post-pacemaker installation | Yes | Sputum/colonization | Piperacillin-tazobactam | Levofloxacin, trimethoprim- sulfamethoxazole | No | No |
| 9 (81/M) | C/C1 | 2011/5/17 | 2011/6/8 | DM, CAD, post-coronary artery bypass graft | Yes | Sputum | Piperacillin-tazobactam, amikacin | Amikacin | Yes | Yes |
| 10 (84/M) | C/C2 | 2011/6/17 | 2011/7/2 | DM, dissection of aorta | Yes | Sputum/colonization | Cefmetazole, cefepime, metronidazole | No | Yes | Yes |
| 11 (57/F) | C/C3 | 2011/5/1 | 2011/6/22 | DM, CAD, post-coronary artery bypass graft | Yes | Wound/SSI | Piperacillin-tazobactam, meropenem | Meropenem | No | Yes |
| 12 (84/F) | C/C4 | 2011/6/21 | 2011/6/24 | Pneumonia with respiratory failure | Yes | Urine/colonization | Ceftriaxone, sulbactam | Cefmetazole, amikacin | No | Yes |
| 13 (78/M) | C/C5 | 2011/6/28 | 2011/7/6 | Parkinson's disease, pneumonia with respiratory failure | Yes | Sputum/VAP | Piperacillin-tazobactam | Piperacillin-tazobactam, amikacin | No | No |
| 14 (89/M) | C/C6 | 2011/8/1 | 2011/8/1 | Pneumonia with septic shock | No | Sputum/VAP | Cefazolin | Imipenem | Yes | Yes |
| 15 (77/M) | C/C7 | 2011/7/28 | 2011/8/29 | Pressure sore, septic shock, UTI | Yes | Urine/UTI | Piperacillin-tazobactam, daptomycin | Doripenem | No | No |
| 16 (90/M) | D/D1 | 2011/8/2 | 2011/8/15 | CKD, urothelial carcinoma with lung and brain metastasis | Yes | Sputum/colonization | Cefazolin, cefoperazone-sulbactam | No | No | Yes |
ARDS, acute respiratory distress syndrome; CAD, coronary arterial disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CPR, cardiopulmonary resuscitation; DM, diabetes mellitus; ECMO, extracorporeal membrane oxygenation; F, female; ICU, intensive care unit; M, male; SSI, surgical site infection; UTI, urinary tract infection; VAP, ventilator-associated pneumonia.
DISCUSSION
Our findings in this multicenter, prospective study clearly indicate that there was intrahospital and interhospital dissemination of KPC-2-KP in northern Taiwan and that intrahospital spread of IMP-8-possessing ertapenem-nonsusceptible Enterobacteriaceae occurred in several Taiwanese hospitals. We also found that all of the isolates of KPC-2-KP (n = 16) had the same pulsotype (PT-1) and the same sequence type (ST11) as the first isolate of KPC-2-KP (NTUH-9047) found in hospital A (10). In addition, the majority of IMP-8 producers were found in ertapenem-nonsusceptible K. pneumoniae and E. cloacae isolates. The presence of IMP-8 along with ESBL production, AmpC β-lactamase overproduction, and decreased outer membrane protein expression combined with an active efflux pump has been reported to contribute to ertapenem resistance in E. cloacae in Taiwan and in other countries (14, 26, 37).
Previous reports demonstrated that the ST11 KPC-KP clone dominated in China and in other Asian countries (2, 29). Interestingly, the endemic clone found in this study shared the same sequence type (29). None of the 16 patients had recently traveled to China; however, because of the close geographic relationship between China and Taiwan and the frequency with which individuals travel between the two countries, the strain associated with this outbreak could have spread from China to Taiwan and could have resided in the community and hospitals without being noticed. In this study, KPC-2-KP was isolated within 3 days of admission in three patients. None of these three patients had recent hospitalization or contact with KPC-2 carriers, suggesting that KPC-2-KP existed not only in the hospitals but also in the community, although the isolation of KPC-2-carrying isolates was not documented in their families or nearby individuals or pets.
Inadequate infection control and prior antimicrobial exposure in hospitalized patients, particularly severely ill patients, are the main forces driving the spread of carbapenem-resistant organisms (5, 6, 21, 28). The antimicrobial agents causing selective pressure for KPC-KP include carbapenems, fluoroquinolones, extended-spectrum cephalosporins, β-lactam–β-lactamase inhibitor combinations, and agents with antianaerobe activity (17, 21, 39). Among the 16 patients with infections due to KPC-2-KP, the most common antibiotic administered prior to KPC-KP isolation was piperacillin-tazobactam. In addition, 14 of those patients had received agents with antianaerobe activities, and only 1 had received a carbapenem within 2 weeks prior to the isolation of KPC-2-KP. We speculate that the antianaerobe agents used in these patients might have created a survival niche for K. pneumoniae (KPC-KP), a common intestinal bacterium, thereby allowing it to spread from patient to patient and hospital to hospital.
In this study, the 14-day mortality rate among the 16 patients with isolations of KPC-2-KP was 25%, and the in-hospital mortality rate was 62.5%. These findings are similar to those previously reported (17, 21, 28, 33). Gasink et al. reported that KPC-KP infection/colonization was independently associated with in-hospital mortality (17). The effect of KPC-2-KP isolation on the outcomes for these 16 patients is difficult to evaluate because the majority of these patients were severely ill and had multiple comorbidities. The inability to distinguish between colonization and infection (especially the isolates from the airway specimens), the number of coisolates with KPC-2-KP, and the limited activities of antimicrobial agents administered all contributed to the outcomes for these patients (17, 28). Furthermore, based on the MIC values of antibiotics against this epidemic strain, tigecycline, colistin, and aminoglycosides should have been effective for the treatment of infections caused by this organism (39). However, among the eight patients who received the above-mentioned agents, the in-hospital mortality rate was 75%. The one patient with KPC-2-KP bacteremia died in spite of concomitant colistin and amikacin treatment.
In conclusion, in this multicenter surveillance study, we identified the first outbreak of intrahospital and interhospital dissemination of KPC-2-KP in northern Taiwan. Although only four hospitals from central and southern Taiwan were included in this study, the fact that no KPC-bearing isolates were identified in these regions does not mean that these regions must be clear of KPC-bearing isolates. Further nationwide surveillance of KPC-KP is necessary, and strict infection control measures should be enforced.
Supplementary Material
ACKNOWLEDGMENT
This study was partly supported by the Centers for Disease Control, Taiwan (DOH100-DC-1030).
Footnotes
Published ahead of print 16 July 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Alhambra A, Cuadros JA, Cacho J, Gómez-Garcés JL, Alós JI. 2004. In vitro susceptibility of recent antibiotic-resistant urinary pathogens to ertapenem and 12 other antibiotics. J. Antimicrob. Chemother. 53:1090–1094 [DOI] [PubMed] [Google Scholar]
- 2. Balm MN, Ngan G, Jureen R, Lin RT, Teo J. 2012. Molecular characterization of newly emerged blaKPC-2-producing Klebsiella pneumoniae in Singapore. J. Clin. Microbiol. 50:475–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bratu S, et al. 2005. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch. Intern. Med. 165:1430–1435 [DOI] [PubMed] [Google Scholar]
- 4. Bratu S, et al. 2005. Emergence of KPC-possessing Klebsiella pneumoniae in Brooklyn, New York: epidemiology and recommendations for detection. Antimicrob. Agents Chemother. 49:3018–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention 2009. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR Morb. Mortal. Wkly. Rep. 58:256–260 [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention 2011. Carbapenem-resistant Klebsiella pneumoniae associated with a long-term-care facility—West Virginia, 2009-2011. MMWR Morb. Mortal. Wkly. Rep. 60:1418–1420 [PubMed] [Google Scholar]
- 7. Chen M, Nafziger AN, Drusano GL, Ma L, Bertino JS., Jr 2006. Comparative pharmacokinetics and pharmacodynamic target attainment of ertapenem in normal-weight, obese, and extremely obese adults. Antimicrob. Agents Chemother. 50:1222–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen YH, et al. 2011. Antimicrobial susceptibility profiles of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections in the Asia-Pacific region according to currently established susceptibility interpretive criteria. J. Infect. 62:280–291 [DOI] [PubMed] [Google Scholar]
- 9. Chen YH, et al. 2012. Trends in the susceptibility of clinically important resistant bacteria to tigecycline: results from the Tigecycline In Vitro Surveillance in Taiwan study, 2006 to 2010. Antimicrob. Agents Chemother. 56:1452–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung KP, et al. 2011. Arrival of Klebsiella pneumoniae carbapenemase (KPC)-2 in Taiwan. J. Antimicrob. Chemother. 66:1182–1184 [DOI] [PubMed] [Google Scholar]
- 11. Clinical and Laboratory Standard Institute 2011. Performance standards for antimicrobial susceptibility testing (M100-S21). Clinical and Laboratory Standards Institute, Wayne, PA: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clinical and Laboratory Standards Institute 2012. Performance standards for antimicrobial susceptibility testing (M100-S22). Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 13. Dallenne C, Da Costa A, Decré D, Favier C, Arlet G. 2010. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 65:490–495 [DOI] [PubMed] [Google Scholar]
- 14. Doumith M, Ellington MJ, Livermore DM, Woodford N. 2009. Molecular mechanisms disrupting porin expression in ertapenem-resistant Klebsiella and Enterobacter spp. clinical isolates from the UK. J. Antimicrob. Chemother. 63:659–667 [DOI] [PubMed] [Google Scholar]
- 15. European Society of Clinical Microbiology and Infectious Diseases 2011. Clinical breakpoints. In European Committee on Antimicrobial Susceptibility Testing, London, United Kingdom: http://www.eucast.org/ [Google Scholar]
- 16. Food and Drug Administration 2011. Class II special controls guidance document: antimicrobial susceptibility test (AST) systems. U.S. Department of Health and Human Services, Food and Drug Administration, Washington, DC: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm080564.htm Accessed 22 March 2012 [Google Scholar]
- 17. Gasink LB, Edelstein PH, Lautenbach E, Synnestvedt M, Fishman NO. 2009. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect. Control Hosp. Epidemiol. 30:1180–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hawser SP, et al. 2011. Susceptibility of Klebsiella pneumoniae isolates from intra-abdominal infections and molecular characterization of ertapenem-resistant isolates. Antimicrob. Agents Chemother. 55:3917–3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsu MS, et al. 2011. In vitro susceptibilities of clinical isolates of ertapenem-non-susceptible Enterobacteriaceae to nemonoxacin, tigecycline, fosfomycin and other antimicrobial agents. Int. J. Antimicrob. Agents 37:276–278 [DOI] [PubMed] [Google Scholar]
- 20. Hu F, et al. 2012. Emergence of carbapenem-resistant clinical Enterobacteriaceae isolates from a teaching hospital in Shanghai, China. J. Med. Microbiol. 61:132–136 [DOI] [PubMed] [Google Scholar]
- 21. Hussein K, et al. 2009. Carbapenem resistance among Klebsiella pneumoniae isolates: risk factors, molecular characteristics, and susceptibility patterns. Infect. Control Hosp. Epidemiol. 30:666–671 [DOI] [PubMed] [Google Scholar]
- 22. Lai CC, et al. 2011. Pelvic abscess caused by New Delhi metallo-β-lactamase-1-producing Klebsiella oxytoca in Taiwan in a patient who underwent renal transplantation in China. Diagn. Microbiol. Infect. Dis. 71:474–475 [DOI] [PubMed] [Google Scholar]
- 23. Lascols C, et al. 2011. Increasing prevalence and dissemination of NDM-1 metallo-β-lactamase in India: data from the SMART study (2009). J. Antimicrob. Chemother. 66:1992–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leavitt A, et al. 2009. Ertapenem resistance among extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae isolates. J. Clin. Microbiol. 47:969–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lomaestro BM, Tobin EH, Shang W, Gootz T. 2006. The spread of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae to upstate New York. Clin. Infect. Dis. 43:e26–e28 [DOI] [PubMed] [Google Scholar]
- 26. Marchaim D, Navon-Venezia S, Schwaber MJ, Carmeli Y. 2008. Isolation of imipenem-resistant Enterobacter species: emergence of KPC-2 carbapenemase, molecular characterization, epidemiology, and outcomes. Antimicrob. Agents Chemother. 52:1413–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 17:1791–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. 2008. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect. Control Hosp. Epidemiol. 29:1099–1106 [DOI] [PubMed] [Google Scholar]
- 29. Qi Y, et al. 2011. ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. J. Antimicrob. Chemother. 66:307–312 [DOI] [PubMed] [Google Scholar]
- 30. Queenan AM, Bush K. 2007. Carbapenemases: the versatile beta-lactamases. Clin. Microbiol. Rev. 20:440–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steinmann J, et al. 2011. Outbreak due to a Klebsiella pneumoniae strain harbouring KPC-2 and VIM-1 in a German university hospital, July 2010 to January 2011. Euro Surveill. 16(33):pii=19944 [PubMed] [Google Scholar]
- 32. Tseng SH, Lee CM, Lin TY, Chang SC, Chang FY. 2011. Emergence and spread of multi-drug resistant organisms: think globally and act locally. J. Microbiol. Immunol. Infect. 44:157–165 [DOI] [PubMed] [Google Scholar]
- 33. Weisenberg SA, Morgan DJ, Espinal-Witter R, Larone DH. 2009. Clinical outcomes of patients with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae after treatment with imipenem or meropenem. Diagn. Microbiol. Infect. Dis. 64:233–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Won SY, et al. 2011. Emergence and rapid regional spread of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin. Infect. Dis. 53:532–540 [DOI] [PubMed] [Google Scholar]
- 35. Woodford N, et al. 2004. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York medical center. Antimicrob. Agents Chemother. 48:4793–4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yan JJ, Wu JJ, Lee CC, Ko WC, Yang FC. 2010. Prevalence and characteristics of ertapenem-nonsusceptible Escherichia coli in a Taiwanese university hospital, 1999 to 2007. Eur. J. Clin. Microbiol. Infect. Dis. 29:1417–1425 [DOI] [PubMed] [Google Scholar]
- 37. Yang FC, Yan JJ, Hung KH, Wu JJ. 2012. Characterization of ertapenem-resistant Enterobacter cloacae in a Taiwanese university hospital. J. Clin. Microbiol. 50:223–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zarfel G, et al. 2011. Emergence of carbapenem-resistant Enterobacteriaceae in Austria, 2001–2010. Clin. Microbiol. Infect. 17:E5–E8 [DOI] [PubMed] [Google Scholar]
- 39. Zarkotou O, et al. 2010. Risk factors and outcomes associated with acquisition of colistin-resistant KPC-producing Klebsiella pneumoniae: a matched case-control study. J. Clin. Microbiol. 48:2271–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.