Abstract
Nevirapine (NVP) is a nonnucleoside reverse transcriptase inhibitor (NNRTI) used worldwide as part of combination antiretroviral therapy in infants and children to treat HIV infection. Dosing based on either weight or body surface area has been approved by the U.S. Food and Drug Administration (FDA) but can be difficult to implement in resource-limited settings. The World Health Organization (WHO) has developed simplified weight band dosing for NVP, but it has not been critically evaluated. NVP pharmacokinetic data were combined from eight pediatric clinical trials (Pediatric AIDS Clinical Trials Group [PACTG] studies 245, 356, 366, 377, 403, 1056, and 1069 and Children with HIV in Africa Pharmacokinetics and Adherence of Simple Antiretroviral Regimens [CHAPAS]) representing subjects from multiple continents and across the pediatric age continuum. A population pharmacokinetic model was developed to characterize developmental changes in NVP disposition, identify potential sources of NVP pharmacokinetic variability, and assess various pediatric dosing strategies and their impact on NVP exposure. Age, CYP2B6 genotype, and ritonavir were independent predictors of oral NVP clearance. The Triomune fixed-dose tablet was an independent predictor of bioavailability compared to the liquid and other tablet formulations. Monte Carlo simulations of the final model were used to assess WHO weight band dosing recommendations. The final pharmacokinetic model indicated that WHO weight band dosing is likely to result in a percentage of children with NVP exposure within the target range similar to that obtained with FDA dosing. Weight band dosing of NVP proposed by the WHO has the potential to provide a simple and effective dosing strategy for resource limited settings.
INTRODUCTION
Nevirapine (NVP) is a nonnucleoside reverse transcriptase inhibitor (NNRTI) used worldwide as part of antiretroviral therapy. NVP in combination with two nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) is recommended by the World Health Organization (WHO) for first-line therapy in infants >24 months of age and in infants <24 months of age who were not exposed to maternal or infant NVP or other NNRTIs used for maternal treatment or prevention of mother-to-child transmission of HIV (7). The chemical and pharmacokinetic properties of NVP are advantageous in these settings, particularly in sub-Saharan Africa, as it can be formulated as a heat-stable liquid preparation and has fewer drug interactions than protease inhibitors, and its bioavailability is not affected by food intake (20).
NVP is metabolized by a variety of cytochrome P450 (CYP) enzymes but predominantly by CYP2B6 and CYP3A. Prior studies have found an association between the CYP2B6 516 GT single nucleotide polymorphism and NVP pharmacokinetics in adults and children. Lower clearance and higher trough concentrations have previously been observed in patients with the CYP2B6 516 TT genotype (22, 24).
The weight-adjusted oral clearance of NVP is higher in younger children than in older children or adults. The initial FDA pediatric dosing of 7 mg/kg twice daily in children less than 8 years of age was thus reduced to 4 mg/kg for children 8 years of age or older. Subsequently, an alternative dosing recommendation of 150 mg/m2 twice daily was approved by the FDA (25). In resource-limited settings, dosing based on body surface area (BSA) is not ideal, as it requires obtaining an accurate height measurement and utilizing a mathematical calculation to obtain the appropriate dose. An alternative approach is to dose NVP based on weight bands, which requires no calculations and is easy to implement in the developing world. The WHO selected weight bands rather than age for dosing in resource-poor countries, as obtaining an accurate age can be difficult due to poor record-keeping.
NVP has been studied extensively in adults, and multiple population pharmacokinetics (PK) studies have been published (1, 4, 6, 8, 11, 13, 21, 27). While many studies have looked at NVP in HIV-infected infants, children, and adolescents (12, 15, 17, 23, 26), no comprehensive population analyses have been performed to assess NVP across the pediatric age continuum. The current evaluation combines PK data from eight studies of NVP in infants, children, and adolescents, generating a robust NVP PK data set of pediatric patients from three continents. A population pharmacokinetic approach was used to determine the factors affecting NVP disposition and to compare the impact of different dosing approaches on achieving therapeutic NVP concentrations.
MATERIALS AND METHODS
NVP pharmacokinetic data were pooled from eight prior pediatric studies. Seven of the studies were protocols of the Pediatric AIDS Clinical Trials Group/International Maternal Pediatric and Adolescent AIDS Clinical Trials Network (PACTG/IMPAACT), namely, studies 245, 356, 366, 377, 403, P1056, and P1069. The final study was from the Children with HIV in Africa Pharmacokinetics and Adherence of Simple Antiretroviral Regimens (CHAPAS). Individual study characteristics are described below and presented in Table 1.
Table 1.
Summary of study and subject demographicsa
| Study | No. of NVP subjects | Study location | NVP maintenance dose (mg/m2) | Median no. of samples per subject (range) | Median age (yr) at PK visits (range) | No. of doses in formulationb | Median wt (kg) at PK visits (range) | Total no. of samples |
|---|---|---|---|---|---|---|---|---|
| 245, phase I | 238 | U.S. | 120 | 3 (1–6) | 7.3 (1.2–19.5) | 521 liquid | 21.8 (7.0–61.9) | 693 |
| 356, phase I/II | 54 | U.S. | 200 | 6.5 (1–16) | 0.3 (0.1–2.1) | 144 liquid | 5.9 (2.8–16.2) | 376 |
| 366, phase I/II | 85 | U.S. | 120 | 3 (1–9) | 5.9 (0.6–17.2) | 168 liquid, 13 tablet | 19.5 (8.4–64.6) | 305 |
| 377, phase I/II | 102 | U.S. | 120 | 6 (1–20) | 6.0 (0.5–14.7) | 248 liquid, 38 tablet | 20.9 (5.5–73.8) | 698 |
| 403, phase II | 16 | U.S. | 120 | 6 (1–12) | 6.3 (0.5–19.3) | 17 liquid, 7 tablet | 22.7 (9.2–56.5) | 91 |
| CHAPAS, phase I/II | 59 | Zambia | 150–200 | 7 (7–7) | 6.9 (0.6–13.6) | 59 Triomune | 16.0 (3.4–29.0) | 413 |
| P1056, phase I/II | 43 | Thailand | 120–200 | 14 (12–16) | 8.3 (0.7–12.0) | 43 liquid, 43 GPO-VIR | 21.0 (6.4–28.9) | 602 |
| P1069, phase I/II | 42 | Thailand | 150–200 | 14 (7–14) | 6.1 (0.5–12.3) | 41 liquid, 42 GPO-VIR | 19.1 (5.9–28.6) | 581 |
| Overall | 639 | 4 (1–20) | 6.5 (0.1–19.5) | 1,182 liquid, 58 tablet, 85 GPO-VIR, 59 Triomune | 19.7 (2.8–73.8) | 3,759 |
Age and weight are representative of the first PK visit.
“Tablet” refers to the Boehringer Ingelheim tablet; GPO-VIR and Triomune are fixed-dose combination tablets. The maximum dose was 200 mg.
PACTG 245 studied NVP in HIV-1-positive children from 6 months to 20 years of age. Children received combination therapy with NVP and NRTIs. NVP measurements included intensive PK studies, with up to three plasma samples collected throughout the dosing interval, and population pharmacokinetics, with a single sample from the dosing interval.
PACTG 356 studied NVP in HIV-1-positive children from 15 days through 2 years of age. Children received combination therapy with NVP, the protease inhibitor (PI) nelfinavir (NFV), and nucleoside reverse transcriptase inhibitors (NRTIs). NVP measurement included intensive PK studies, with up to five plasma samples collected throughout the dosing interval, and population pharmacokinetics, with up to two samples collected in the dosing interval (2, 18).
PACTG 366 studied NVP in nelfinavir and ritonavir (RTV) treatment-experienced HIV-1-infected children from 6 months to 21 years of age. Children received combination therapy with NVP, PIs (NFV and RTV), and NRTIs. NVP measurement included intensive PK studies in a subset of patients (27 total subjects) at week 4, with up to six plasma samples collected throughout the dosing interval, and population pharmacokinetics at weeks 4, 12, and 48, with one sample collected in the dosing interval (16).
PACTG 377 studied NVP in HIV-1 positive children from 4 months to 17 years of age. Children received combination therapy with NVP, PIs (NFV and RTV), and NRTIs. NVP measurements included intensive PK studies, with up to seven plasma samples collected over the dosing interval, and population pharmacokinetics, with up to two samples collected from the dosing interval (12).
PACTG 403 studied NVP in HIV-1-positive children between 4 months and 21 years of age. Children received combination therapy with NVP, PIs (NFV and RTV), and NRTIs. NVP measurement included intensive PK studies, with up to six plasma samples collected over the dosing interval, and population pharmacokinetics, with up to two samples collected from the dosing interval (15).
IMPAACT P1056 and P1069 studied HIV-1 infected children in Thailand from 6 months to 13 years of age designed to assess NVP-containing fixed-dose combination (FDC) tablets (manufactured by the Thai Government Pharmaceutical Organization) versus brand-name liquid formulations. In both studies, children received combination therapy with the appropriate FDC tablet (referred to as GPO-VIR), which contained NVP and two NRTIs, and an equivalent innovator liquid formulation. Following intensive PK sampling with seven plasma samples obtained over the dosing interval, patients were crossed over to the alternate formulation, and a repeat intensive PK profile was obtained (3, 26).
CHAPAS studied HIV-1-infected children in Zambia from 3 months to 14 years of age to assess the Triomune tablet (Cipla Pharmaceuticals, India). Children received combination therapy with Triomune, which contained NVP and two NRTIs. Intensive PK profiles were determined, with seven plasma samples collected throughout the dosing interval (17).
NVP concentrations were determined by validated high-performance liquid chromatography methods with UV detection in the following laboratories: PACTG 245, Boehringer Ingelheim Laboratory; PACTG 356 and 366, University of California San Diego Clinical Pharmacology and Assay Laboratory, San Diego, CA; PACTG 377, University of California San Francisco Clinical Pharmacology Laboratory, San Francisco, CA; PACTG 403, University of Alabama at Birmingham Antiviral Pharmacology Laboratory, Birmingham, AL; PACTG 1056 and 1069, PHPT-IRD Laboratory, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand; and CHAPAS, Radboud University Nijmegen Medical Centre, Laboratory of Pharmacy, Nijmegen, Netherlands. All labs participated in external quality assurance testing, including laboratory cross-validation, with the exception of Boehringer Ingelheim. CYP2B6 genotyping was performed using real-time PCR for PACTG 366, 377, and 1069 as previously described (24).
Pharmacokinetic analysis.
Using the computer program NONMEM (version VI) with a GNU Fortran G77 compiler, concentration-time data were fitted using a first-order conditional estimation method (FOCE) with interaction. A one-compartment pharmacokinetic structural model (ADVAN2, TRANS2 subroutine) with first-order absorption was used to describe the data. NVP concentration data were log transformed (ln) prior to analysis. An exponential-normal distribution error model was used for intersubject variability, and a proportional residual error model was used to describe the residual error not explained by the model or intersubject variability.
Pharmacokinetic parameters were scaled by subject size before evaluation of other potential covariates. An allometric approach was used, with clearance (CL) being scaled by allometric weight (WT0.75) and volume of distribution (V) being scaled by weight (WT1.0). Population (Thai or African as compared to U.S.), gender, nelfinavir (NFV), ritonavir (RTV), CYP2B6 genotype, and age were evaluated as potential covariates for clearance, while various solid formulations (tablet [Boehringer-Ingelheim], GPO-VIR, and Triomune) were evaluated for bioavailability to assess differences from the liquid formulation. Potential covariates were added to the model one at a time as either a linear or nonlinear function, with covariates that improved the model fitting by a change in the objective function of at least 4.0 (P < ∼0.05) being retained in the initial covariate screen. A backward elimination approach was utilized in the multivariate assessment. Covariates found to improve the objective function by 8.0 (P < ∼0.005) or more were retained in the final model.
Age was assessed as a potential covariate for clearance using both linear and nonlinear maturation functions. CYP2B6 516 genotype was available in a selected number of subjects and was modeled as poor metabolizer (516 TT) versus all others, as well as in three groups (poor metabolizers with the 516 GG genotype, extensive metabolizers with the 516 GT genotype, and unknown genotype).
To account for intrasubject variability between intensive PK visits, interoccasion variability was modeled on clearance. Separate residual errors were considered for U.S., Thai, and Zambian PK data. Empirical Bayesian estimates of individual infant pharmacokinetic parameters were generated from the final model using the POSTHOC subroutine. A 1,000-sample bootstrap assessment of the final model was performed using Wings for NONMEM.
Monte Carlo simulations were performed using the final population PK model to assess different dosing recommendations. Concentration profiles for 1,000 virtual children from 0.25 to 18 years of age were simulated. Weight distributions for simulations were derived from Centers for Disease Control and Prevention (CDC) 50th-percentile weights and a CYP2B6 poor-metabolizer frequency identical to that in the study population. NVP concentration profiles using the WHO weight band dosing [3 to 5.9 kg (50 mg), 6 to 9.9 kg (75 mg), 10 to 13.9 kg (100 mg), 14 to 19.9 kg (125 mg), 20 to 24.9 kg (150 mg), 25 to 34.9 kg (200 mg)] were compared to the FDA dosing (NVP 150 mg/m2 with a maximum of 200 mg twice daily). The target range for NVP trough concentrations was defined as a minimum of 3.0 μg/ml (5) with a maximum of 7.63 μg/ml (95th percentile of adult troughs) which corresponded to an area-under-the-curve (AUC) target range of 42 to 108 μg*h/ml, respectively (5). An additional simulation was performed based on a population of virtual children who were exclusively poor metabolizers (CYP2B6 516 TT genotype). RTV was not included as a covariate for simulations because this combination at therapeutic doses is no longer used in clinical practice.
RESULTS
Intensive and sparse pharmacokinetic data were available for 639 infants, children, and adolescents (3,759 NVP concentrations). Table 1 summarizes patient characteristics at first PK visit. CYP2B6 genotype information was available from 26% of the study subjects. Among the patients with CYP2B6 genotypes, the distribution was 11% homozygous poor metabolizers (CYP2B6 516 TT), 46% heterozygous individuals (CYP2B6 516 GT), and 42% homozygous extensive metabolizers (CYP2B6 516 GG).
Thai population, age, formulation, NFV, RTV, and CYP2B6 genotype were identified as potential covariates in the univariate screen. Triomune formulation, age, CYP2B6 genotype, and RTV were significant covariates retained in the final model. Separate residual errors were assigned to U.S., Thai, and Zambian data with an improvement of objective function.
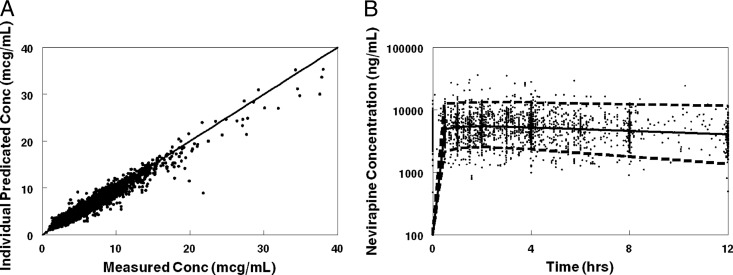
The final population model described the data without significant bias, as shown in Fig. 1. Final model parameter and variance estimates are shown in Table 2. All parameter estimates were within 95% confidence intervals of the bootstrap data sets. The residual error estimates for the Zambian and Thai data were significantly less than that for the U.S. data, likely due to the exclusive use of intensive PK sampling in the Zambian and Thai studies.
Fig 1.
(A) Goodness-of-fit plot. NVP concentrations from the study were compared to NONMEM individual predicted concentrations. (B) Visual predictive plots for an NVP population PK model for patients not taking RTV or Triomune and with active copies of the CYP2B6 gene (extensive metabolizers). Solid lines represent median concentrations, and dashed lines represent 97.5 and 2.5 percentiles from a Monte Carlo simulation of the final model.
Table 2.
Population PK final parameter and standard error estimatesa
| Parameter | Final value | SE | Median bootstrap estimate (95% CI) |
|---|---|---|---|
| Θ1 (V) | 3.02 | 0.14 | 2.91 (2.69–3.18) |
| Θ2 (CL) | 0.20 | 0.0046 | 0.20 (0.19–0.21) |
| Θ3 (Ka) | 1.30 | 0.13 | 1.41 (1.18–1.68) |
| Θ4 (RTV) | 0.75 | 0.04 | 0.78 (0.71–0.87) |
| Θ5 (CYP2B6) | 0.64 | 0.07 | 0.61 (0.50–0.73) |
| Θ6 (immature) | 0.39 | 0.17 | 0.42 (0.10–0.99) |
| Θ7 (KMAT) | 3.79 | 0.82 | 4.49 (2.43–9.91) |
| Θ8 (Triomune formulation) | 0.58 | 0.03 | 0.56 (0.51–0.62) |
| Variability | |||
| Intersubject, V | 28.2% | 3.52% | 19.11% (11.57%–26.78%) |
| Intersubject, CL | 47.6% | 1.63% | 40.50% (37.42%–43.47%) |
| Intersubject interaction (CL-V) | 18.6% | 5.11% | |
| Interoccasion (CL) | 50.7% | 2.69% | |
| Error | |||
| Proportional (US data) | 21.6% | 1.12% | 16.7% (−28.74%–68.38%) |
| Proportional (Thai data) | 11.4% | 0.57% | 39.62% (37.01%–42.07%) |
| Proportional (African data) | 13.3% | 0.76% | 15.13% (13.60% - 16.50%) |
Equations for clearance (CL), bioavailability (F), absorption constant (Ka), and volume of distribution (V) used in the final model were as follows: CL (liters/h) = WT0.75 × Θ2 × Θ4 × Θ5CYP2B6 × {Θ6 + [(1 − Θ6) × (1 − e−(age × Θ7)]}; F (%) = Θ8Triomune; Vd (liters) = WT × Θ1; Ka (1/h) = Θ3. CYP2B6 represents the effect of the TT genotype (poor metabolizers). Triomune = 1 for the Triomune FDC formulation, while Triomune = 0 for other formulations. “Immature” represents the activity level NVP CL at birth relative to full maturation, and KMAT is the rate constant for acquisition of NVP CL activity.
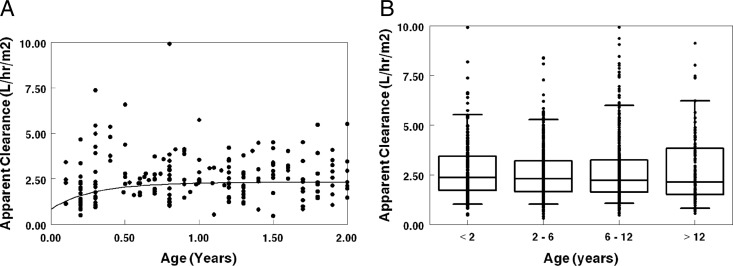
Age modeled as a nonlinear maturation function for clearance (see the equations in the footnote to Table 2) provided the greatest improvement in objective function and was utilized in the final model. This function estimated NVP clearance at birth to be 39% of the “mature” clearance with a “half-life” for NVP clearance gain of 3.2 months. The maturation function represented the effects of age accurately, as there was no relationship between age and the deviation from predicted (eta) clearance in the final model. As suggested based on the maturation function parameters, the age effect on clearance was modest and predominantly occurred in the first year of life (Fig. 2A). Grouping subjects by age (<2, 2 to <6, 6 to <12, and 12 years of age) did not demonstrate apparent age effects on clearance normalized to BSA (Fig. 2B).
Fig 2.
Population PK model results. (A) Estimated NVP apparent clearance (clearance/bioavailability) versus age for patients not taking RTV or Triomune and with active copies of the CYP2B6 gene (extensive metabolizers). The solid line represents the population-based apparent clearance profile, which was derived using the allometrically scaled median weight for each age group (CDC 50th-percentile weight). (B) Estimated NVP apparent clearance versus age.
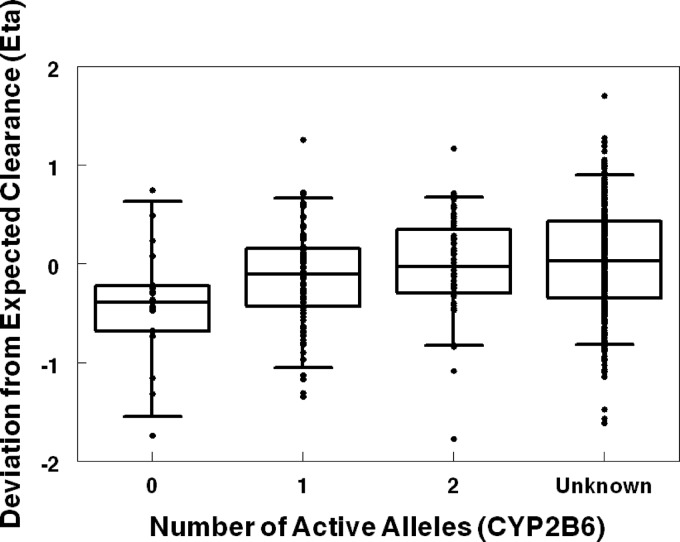
Despite the fact that CYP2B6 genotype information was available for only a limited number of subjects, CYP2B6 poor metabolizer status was identified as a significant covariate for NVP clearance. Figure 3 demonstrates the impact of CYP2B6 for the base model. Apparent clearance for the homozygous CYP2B6 516 TT poor metabolizer genotype was 64% of the clearance for other genotypes or the unknown genotype group. The overall proportion of poor metabolizers in the population is small, and thus the unknown group is predominately representative of patients with the extensive metabolizer genotype (CYP2B6 516 GG and GT).
Fig 3.
CYP2B6 active alleles compared to deviation from expected clearance (eta for clearance) in a population PK base model (allometric weight was the only covariate).
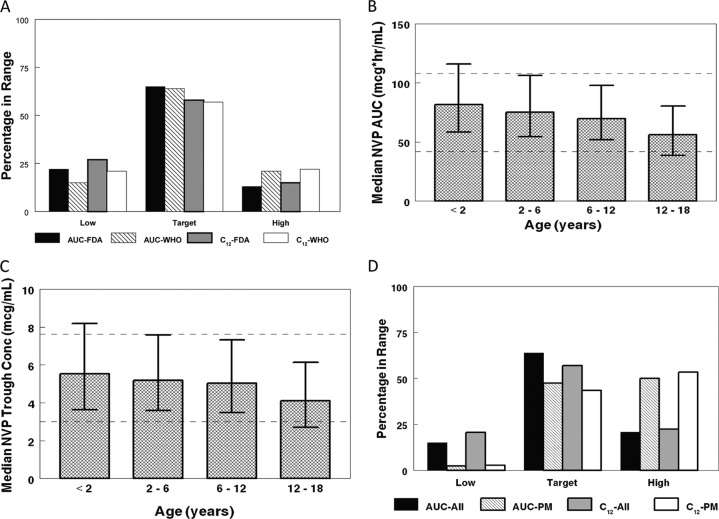
The Monte Carlo simulation comparison of AUCs and 12-h trough concentrations obtained with the WHO weight band and FDA dosing are shown in Fig. 4A. Although WHO weight band dosing represents a simplified dosing structure, it performs as well as FDA dosing, providing a similar percentage of AUC and trough concentrations in the target range. The WHO weight band dosing achieved similar NVP AUCs and trough concentrations across the pediatric age spectrum (Fig. 4B and C). The impact of CYP2B6 genotype status on AUC and trough concentrations was also assessed for the WHO weight band dosing. In a population of exclusively CYP2B6 poor metabolizers, the frequency of low AUC and trough concentrations was greatly reduced, while the frequency of above-target AUC and trough concentrations increased (Fig. 4D).
Fig 4.
Monte Carlo simulations with WHO weight band dosing recommendations. (A) FDA dose (150 mg/m2 with a maximum of 200 mg, twice daily) compared to WHO weight band dosing for NVP exposure (area under the curve) and 12-h trough concentrations (C12). (B) Median drug exposure (area under the curve) for WHO weight banding dosing. Data are medians and interquartile ranges of NVP AUC (μg · h/ml). Target AUC levels are represented by dashed lines (42 to 108 μg · h/ml). (C) Median 12-h concentrations (trough levels) for WHO weight band dosing. Data are medians and interquartile ranges of NVP concentration (μg/ml). Goal trough concentrations are represented by dashed lines (3 to 7.6 μg/ml). (D) WHO weight band dosing for the overall study distribution of CYP2B6 genotype compared to a population of poor metabolizers (PM) (CYP2B6 516 TT). Area under the curve (AUC) and C12 (trough concentrations) are given.
DISCUSSION
NVP remains a primary antiretroviral agent for the treatment and prevention of HIV and AIDS in resource-limited settings. NVP has many properties which make it useful for treating infants and children with HIV infection. It has high and consistent bioavailability, existing as both liquid and solid formulations, and is available in several fixed-dose combinations. Its relatively long half-life makes it tolerant to minor variability in dose administration time. However, low NVP concentrations in the presence of replicating virus can select high-level resistance from a single mutation; therefore, accurate dosing of NVP is critical (20).
The present study represents by far the largest NVP pharmacokinetic analysis in infants, children, and adolescents which includes data from three continents. The overall population pharmacokinetic parameters are similar to those obtained in prior noncompartmental analyses that have been performed on subsets of the data used in the current analysis (15, 17, 19, 23, 26). However, the comprehensive population PK model developed in the current analysis is better able to assess potential differences between the separate studies than comparing noncompartmental median exposures and trough concentrations. The median (interquartile range [IQR]) clearance in the present study was 2.3 (1.6 to 3.3) liters/m2/h, similar to the clearance values observed by Luzuriaga et al. (19), which ranged from 1.4 to 2.5 liters/m2/h in the multiple-oral-dose component of the study. However, Luzuriaga et al. found that clearance adjusted for body surface area decreased with increasing age, in contrast to the present study, which found that clearance increased with age in the first year of life. Whereas the study by Luzuriaga et al. had few subjects, the present study represents a continuum of ages, which allowed a better assessment of the age effect.
Prior studies have shown a potential effect of CYP2B6 gene activity on NVP concentrations with lower clearance (24) and higher trough concentrations (22) in homozygous poor-metabolizer patients, which is supported by the present study, where poor metabolizers had a lower NVP clearance. The Triomune tablet was a significant covariate in the model, providing a lower bioavailability and yielding significantly higher clearance levels, but was completely confounded by population. Triomune has been shown to be bioequivalent to the innovator product in adults. So while this difference may be due to formulation, it could also be related to race, study design, diet, or other factors. In contrast, the GPO-VIR tablet, which was evaluated with a crossover study design, was not found to alter NVP bioavailability. RTV decreases the clearance of NVP, consistent with its inhibition of CYP3A4 metabolism. However, RTV was used in only a subset of patients in two studies, where it was included at higher doses than when it is used as a pharmacologic enhancer to boost antiviral drug concentrations. Gender did not affect clearance in the current analysis, despite a prior study by Zhou et al. which found gender to affect NVP clearance in adults; however, their study did not include body size in the model, which may account for the difference (27). Race was not found to affect clearance in the current analysis, consistent with the work of de Maat et al., who found no significant effect of race (Asian, Caucasian, or African) in adults on NVP therapy (9).
Despite many years of NVP use for the treatment of pediatric HIV infection, the labeled pediatric dose is not practical for use in resource-poor settings. While the initial pediatric trials set NVP doses at 120 to 200 mg/m2, the initial FDA label dose was 7 mg/kg for young children, with a dose reduction to 4 mg/kg at 8 years of age despite no major change in NVP metabolism at that age. Many clinicians have opted to administer NVP at 120 to 200 mg/m2 (14), and more recently, the FDA approved an alternative pediatric dosage of 150 mg/m2 twice daily (25). Thus, there is a lack of a unified dosing methodology, and the more recent BSA-based dosing requires an accurate measurement of height and a calculation to estimate BSA. WHO weight band dosing has been proposed as a solution to the numerous problems associated with current NVP BSA- and prior mg/kg-based dosing, offering the potential for a simpler and more logical dosing regimen. However, since weight band dosing represents a simplification in dosing, it recommends the same dose for a wide range of subject weights. Thus, within a particular weight band, smaller subjects will have higher drug concentration levels and be at risk for toxicity, while larger subjects will have lower levels and be at risk for underdosing. One might expect fewer pediatric patients to achieve the therapeutic range with WHO weight band dosing than with FDA dosing.
The development of a population pharmacokinetic model for NVP allowed us to compare the expected frequency of therapeutic concentrations with different dosing approaches using Monte Carlo simulations. Prior studies indicated that an NVP trough concentration of 3.0 μg/ml, which corresponds to an AUC0–12 of 42 μg · h/ml, is likely sufficient to ensure a sustained virologic response (10). For the purposes of the present study, the upper limit of the target range was defined as a trough of 7.63 μg/ml (95th percentile of adult troughs), which corresponds to an AUC of 108 μg · h/ml, although there is no consensus on a maximum acceptable NVP concentration (5). Given the lack of evidence relating a high NVP concentration to increased serious drug toxicity, particularly hepatic toxicity, the current clinical emphasis is on avoiding underdosing of NVP to prevent development of viral drug resistance. Thus, although Monte Carlo simulation indicates that CYP2B6 poor metabolizers will have higher drug levels than extensive metabolizers, this is unlikely to result in a meaningful increase in toxicity. It is therefore important to target dosing toward CYP2B6 extensive metabolizers, who had lower trough levels in the Monte Carlo simulations, to avoid underdosing and the rapid development of high-level resistance in this group. The Monte Carlo simulations also indicated that WHO dosing maintained the majority of patients in the target range for drug exposure and trough level, with a similar percentage of patients in the goal range for therapy with WHO and FDA BSA dosing. While the WHO dosing produced higher overall mean AUCs (21% higher) and trough concentrations (13% higher) than FDA dosing, these modest differences are unlikely to have a clinical impact. Thus, despite the simplification of the WHO dosing guidelines, which use identical dosing for a range of subject weights, WHO dosing provides appropriate NVP exposure, similar to the more complicated FDA dosing.
The present study developed a comprehensive population PK model which can be updated and adjusted to specific populations. CYP2B6 gene frequency varies greatly between different populations. The frequency of poor metabolizers varies from as little as 0.14 in Korean and Japanese populations to as much as 0.62 in Papua New Guinea (4); thus, significant differences in NVP exposure can result. CYP2B6 differences between populations can be addressed through population pharmacokinetic modeling and Monte Carlo simulation, with drug dosing tailored to specific populations. The model additionally allows targeted dosing strategies based on age, race, genotype, other medications, and alternative formulations and thereby has the potential to improve dosing recommendations for pediatric subpopulations.
The present study is limited in that NVP levels were determined at six different laboratories. However, five of the laboratories participated in the same quality assurance testing. Additionally, the Triomune tablet was the only formulation assessed in the CHAPAS study. Thus, it could not be determined if changes in apparent clearance were due to formulation, race, or other potential confounders. The CYP2B6 genotype information was also limited, as it was unavailable for 74% of the combined study population; however, it still affected NVP clearance. Finally, due to the long half-life of NVP and limited early sampling, we were unable to characterize Ka and V as accurately as CL. Thus, the model has better precision in predicting AUC than minimum concentration.
In conclusion, integrating data across pediatric trials into a population pharmacokinetic analysis for NVP resulted in a robust model that allowed characterization of age and genotype effects on NVP clearance. The comprehensive population PK model developed can be used in combination with Monte Carlo simulation to predict drug exposure and adjust dosing in specific populations, thereby allowing more individualized dosing regimens. The model also demonstrated that WHO weight band dosing recommendations result in NVP troughs and AUCs comparable to those achieved with FDA dosing. Thus, the proposed WHO weight band dosing represents a simplified dosing approach for NVP in resource-limited countries.
ACKNOWLEDGMENTS
We thank all the children and families who participated in the PACTG/IMPAACT and CHAPAS studies involved in this analysis. E. V. Capparelli was funded by an NICHD RPDP grant (U54 HD071600-01).
We acknowledge the PACTG/IMPPACT P1056/P1069 investigators: Virat Sirisanthana and Linda Aurpibul (Research Institute for Health Sciences, Chiang Mai University), Kulkanya Chokephaibulkit and Nirun Vanprapar (Siriraj Hospital, Bangkok, Thailand), Suchat Hongsiriwon (Chonburi Hospital), Chaiwat Ngampiyaskul (Prapokklao Hospital), Pra-ornsuda Sukrakanchana and Yardpiron Taworn (PHPT-IRD174, Chiang Mai, Thailand). We also acknowledge the National Institute of Allergy and Infectious Diseases (award U01 AI 068632 IMPAACT Network Pharmacology and Host-Genetics Specialty Laboratories).
There were no conflicts of interest for any of the authors involved in the study.
Footnotes
Published ahead of print 6 August 2012
REFERENCES
- 1. Capparelli EV, et al. 2008. Chronic administration of nevirapine during pregnancy: impact of pregnancy on pharmacokinetics. HIV Med. 9:214–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Capparelli EV, et al. 2001. Pharmacokinetics of nelfinavir in human immunodeficiency virus-infected infants. Pediatr. Infect. Dis. J. 20:746–751 [DOI] [PubMed] [Google Scholar]
- 3. Chokephaibulkit K, et al. 2011. Pharmacokinetics and safety of a new paediatric fixed-dose combination of zidovudine/lamivudine/nevirapine in HIV-infected children. Antivir. Ther. 16:1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chou M, et al. 2010. Population pharmacokinetic-pharmacogenetic study of nevirapine in HIV-infected Cambodian patients. Antimicrob. Agents Chemother. 54:4432–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooper CL, van Heeswijk RP. 2007. Once-daily nevirapine dosing: a pharmacokinetics, efficacy and safety review. HIV Med. 8:1–7 [DOI] [PubMed] [Google Scholar]
- 6. Dailly E, et al. 2009. Influence of darunavir coadministration on nevirapine pharmacokinetics in HIV-infected patients: a population approach. HIV Med. 10:586–589 [DOI] [PubMed] [Google Scholar]
- 7. de Bethune MP. 2010. Non-nucleoside reverse transcriptase inhibitors (NNRTIs), their discovery, development, and use in the treatment of HIV-1 infection: a review of the last 20 years (1989–2009). Antiviral Res. 85:75–90 [DOI] [PubMed] [Google Scholar]
- 8. de Maat MM, et al. 2002. Population pharmacokinetics of nevirapine in an unselected cohort of HIV-1-infected individuals. Br. J. Clin. Pharmacol. 54:378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Maat MM, et al. 2004. Race is not associated with nevirapine pharmacokinetics. Ther. Drug Monit. 26:456–458 [DOI] [PubMed] [Google Scholar]
- 10. Duong M, et al. 2005. Low trough plasma concentrations of nevirapine associated with virologic rebounds in HIV-infected patients who switched from protease inhibitors. Ann. Pharmacother. 39:603–609 [DOI] [PubMed] [Google Scholar]
- 11. Elsherbiny D, et al. 2009. Population pharmacokinetics of nevirapine in combination with rifampicin-based short course chemotherapy in HIV- and tuberculosis-infected South African patients. Eur. J. Clin. Pharmacol. 65:71–80 [DOI] [PubMed] [Google Scholar]
- 12. Floren LC, et al. 2003. Nelfinavir pharmacokinetics in stable human immunodeficiency virus-positive children: Pediatric AIDS Clinical Trials Group Protocol 377. Pediatrics 112:e220–e227 [DOI] [PubMed] [Google Scholar]
- 13. Kappelhoff BS, et al. 2005. Pharmacokinetics of nevirapine: once-daily versus twice-daily dosing in the 2NN study. HIV Clin. Trials 6:254–261 [DOI] [PubMed] [Google Scholar]
- 14. King JR, Kimberlin DW, Aldrovandi GM, Acosta EP. 2002. Antiretroviral pharmacokinetics in the paediatric population: a review. Clin. Pharmacokinet. 41:1115–1133 [DOI] [PubMed] [Google Scholar]
- 15. King JR, et al. 2005. Efficacy, tolerability and pharmacokinetics of two nelfinavir-based regimens in human immunodeficiency virus-infected children and adolescents: pediatric AIDS clinical trials group protocol 403. Pediatr. Infect. Dis. J. 24:880–885 [DOI] [PubMed] [Google Scholar]
- 16. Kovacs A, et al. 2005. Immune reconstitution after receipt of highly active antiretroviral therapy in children with advanced or progressive HIV disease and complete or partial viral load response. J. Infect. Dis. 192:296–302 [DOI] [PubMed] [Google Scholar]
- 17. L'Homme RF, et al. 2008. Nevirapine, stavudine and lamivudine pharmacokinetics in African children on paediatric fixed-dose combination tablets. AIDS 22:557–565 [DOI] [PubMed] [Google Scholar]
- 18. Luzuriaga K, et al. 1997. Combination treatment with zidovudine, didanosine, and nevirapine in infants with human immunodeficiency virus type 1 infection. N. Engl. J. Med. 336:1343–1349 [DOI] [PubMed] [Google Scholar]
- 19. Luzuriaga K, et al. 1996. Pharmacokinetics, safety, and activity of nevirapine in human immunodeficiency virus type 1-infected children. J. Infect. Dis. 174:713–721 [DOI] [PubMed] [Google Scholar]
- 20. Milinkovic A, Martinez E. 2004. Nevirapine in the treatment of HIV. Expert Rev. Anti Infect. Ther. 2:367–373 [DOI] [PubMed] [Google Scholar]
- 21. Molto J, et al. 2008. Once- or twice-daily dosing of nevirapine in HIV-infected adults: a population pharmacokinetics approach. J. Antimicrob. Chemother. 62:784–792 [DOI] [PubMed] [Google Scholar]
- 22. Penzak SR, et al. 2007. Cytochrome P450 2B6 (CYP2B6) G516T influences nevirapine plasma concentrations in HIV-infected patients in Uganda. HIV Med. 8:86–91 [DOI] [PubMed] [Google Scholar]
- 23. Pollock L, et al. 2009. Pharmacokinetics of nevirapine in HIV-infected children with and without malnutrition receiving divided adult fixed-dose combination tablets. J. Antimicrob. Chemother. 64:1251–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saitoh A, et al. 2007. CYP2B6 genetic variants are associated with nevirapine pharmacokinetics and clinical response in HIV-1-infected children. AIDS 21:2191–2199 [DOI] [PubMed] [Google Scholar]
- 25. U. S. Food and Drug Administration 2010. Viramune (nevirapine) January prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/ 2010/020933s022, 020636s032lbl.pdf
- 26. Vanprapar N, et al. 2010. A chewable pediatric fixed-dose combination tablet of stavudine, lamivudine, and nevirapine: pharmacokinetics and safety compared with the individual liquid formulations in human immunodeficiency virus-infected children in Thailand. Pediatr. Infect. Dis. J. 29:940–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou XJ, et al. 1999. Population pharmacokinetics of nevirapine, zidovudine, and didanosine in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 43:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]