Abstract
The challenges of developing new drugs to treat tuberculosis (TB) are indicated by the relatively small number of candidates entering clinical trials in the past decade. To overcome these issues, we reexamined two FDA-approved antibacterial drugs, sulfamethoxazole (SMX) and trimethoprim (TMP), for use in TB treatment. SMX and TMP inhibit folic acid biosynthesis and are used in combination to treat infections of the respiratory, urinary, and gastrointestinal tracts. The MICs of SMX and TMP, alone and in combination, were determined for drug-susceptible, multidrug-resistant (MDR), and extensively drug-resistant Mycobacterium tuberculosis strains. While TMP alone was not effective against M. tuberculosis, the combination of TMP and SMX was bacteriostatic against M. tuberculosis. Surprisingly, the combination of SMX and TMP was also active against a subset of MDR M. tuberculosis strains. Treatment of M. tuberculosis with TMP-SMX and a first-line anti-TB drug, either isoniazid or rifampin, was bactericidal, demonstrating that the combination of TMP and SMX with isoniazid or rifampin was not antagonistic. Moreover, the addition of SMX-TMP in combination with either isoniazid or rifampin also prevented the emergence of drug resistance in vitro. In conclusion, this study further illustrates the opportunity to reevaluate the activity of TMP-SMX in vivo to prevent the emergence of drug-resistant M. tuberculosis.
INTRODUCTION
Tuberculosis (TB), a disease caused by the bacillus Mycobacterium tuberculosis, has resurfaced as one of the most deadly bacterial infections worldwide. Between coinfections with HIV and the emergence of Mycobacterium tuberculosis strains that are resistant to most, if not all, TB drugs, the tools available to fight the disease are becoming obsolete. Developing new drugs to fight a disease is a lengthy process with a very low success rate. The last TB drug that was approved by the FDA, i.e., rifampin (RIF), is 40 years old. There is a very rich pipeline of new TB drugs being developed, but they still must pass clinical trials and obtain FDA approval. A possible path to circumvent this process is to test already approved FDA drugs against M. tuberculosis.
Trimethoprim (TMP) and sulfamethoxazole (SMX) have been used in combination in clinical settings for more than 40 years (7). The combination of TMP and SMX is commercialized under various names (including Bactrim and Septra) and is used to treat a variety of bacterial infections of the respiratory, urinary, and gastrointestinal tracts. TMP and SMX target successive steps of the folate biosynthesis pathway. SMX inhibits the dihydropteroate synthase FolP, which catalyzes the addition of dihydropterin diphosphate to p-aminobenzoic acid, a structural analog of SMX. The product of FolP, 7,8-dihydropteroate, reacts with glutamate to form dihydrofolate, which is reduced to tetrahydrofolate by the dihydrofolate reductase Dhfr, the target of TMP. Bacteria, fungi, and plants synthesize folate de novo, but mammals lack the dihydropteroate synthase and therefore cannot produce folate. Tetrahydrofolate is an essential cofactor involved in the transfer of a one-carbon unit and is implicated in the biosynthesis of purine and pyrimidine and in the biosynthesis and catabolism of some amino acids. The combination of TMP and SMX prevents the emergence of drug resistance and was demonstrated to be synergistic in many bacteria (2).
Although TMP and SMX are used to treat infections caused by organisms such as Nocardia, Salmonella, Shigella, staphylococci, and streptococci, few studies have been conducted on the effects of TMP and/or SMX on M. tuberculosis. Initially, M. tuberculosis was reported to be resistant to TMP-SMX (2, 18). The MIC of TMP against M. tuberculosis H37Ra was found to be >128 μg/ml (19). A study by Forgacs et al. showed that drug-susceptible and drug-resistant M. tuberculosis strains were susceptible to the TMP-SMX combination, with bactericidal activity observed with 2 and 38 μg/ml of TMP and SMX, respectively (4). Subsequently, an analysis of 12 drug-susceptible clinical isolates of M. tuberculosis from Australia showed that M. tuberculosis was susceptible to SMX on its own at a concentration below 38 μg/ml (15). A recent clinical study of a patient infected with an extensively drug-resistant (XDR) strain of M. tuberculosis reported that this XDR-TB strain was sensitive to TMP-SMX at ≥1.6 μg/ml TMP and 8.3 μg/ml SMX (3). Finally, 117 clinical isolates of M. tuberculosis from Taiwan with various drug resistance patterns were analyzed for susceptibility to TMP and SMX and found to be susceptible to SMX, with an MIC90 of 9.5 μg/ml (6). The authors concluded that SMX was active against M. tuberculosis and that the addition of TMP to SMX did not provide any additional benefits.
The present study aimed at testing (i) the susceptibility of various drug-susceptible and drug-resistant M. tuberculosis strains to TMP and SMX; (ii) the synergy between TMP and SMX against M. tuberculosis; and (iii) the compatibility of TMP and SMX with isoniazid (INH) and RIF, the two major first-line TB drugs (FLDs).
MATERIALS AND METHODS
Bacterial strains and media.
M. tuberculosis strains were obtained from laboratory stocks. The strains were grown in Middlebrook 7H9 medium (Difco, Sparks, MD) supplemented with 10% (vol/vol) OADC enrichment (0.85% NaCl, 5% bovine serum albumin [BSA] fraction V [Roche Applied Science], 2% d-dextrose, 0.004% catalase, and 0.05% oleic acid), 0.2% (vol/vol) glycerol, and 0.05% (vol/vol) tyloxapol. The solid medium used was Middlebrook 7H10 medium (Difco) supplemented with 10% (vol/vol) OADC enrichment and 0.2% (vol/vol) glycerol. All other chemicals used in this study were obtained from Sigma (St. Louis, MO).
MIC determination.
The compounds were dissolved in dimethyl sulfoxide (DMSO). The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was used to determine the MICs (11). Twofold serial dilutions in 96-well plates were done for each compound tested. The strains were added to each well (104 to 105 CFU), and the plates were incubated at 37°C for 7 days. A solution of MTT (0.015 ml; 5 mg/ml MTT in phosphate-buffered saline [PBS]) was added to each well, and the plate was further incubated at 37°C for 24 h. The reaction was stopped by adding 0.05 ml of 20% sodium dodecyl aqueous solution-50% aqueous dimethylformamide (1/1) to each well and incubating the plate for 24 h. The MIC was set as the lowest concentration of compound that prevented the conversion of MTT (yellow solution) to formazan (blue-violet solution).
MBC determination.
Twofold serial dilutions of the compounds were added to 2 ml of 7H9 medium. The strains tested were diluted to 105 to 106 CFU/ml, and 100-μl aliquots of the dilutions were added to the tubes containing the compounds. The tubes were incubated with shaking at 37°C for 2 weeks, and serial dilutions were plated onto 7H10 plates. The plates were incubated at 37°C for 3 weeks. The minimum bactericidal concentration (MBC) was determined as the concentration of compound that reduced the initial bacterial load by 99%.
MIC determination for drug combinations.
Determination of the MICs of drug combinations was done in 96-well plates (12 columns by 8 rows). The outside wells were filled with 0.2 ml of PBS. The inside wells received 0.1 ml of Middlebrook 7H9 medium. Drug A, dissolved in 0.1 ml of Middlebrook 7H9 medium, was added to the wells of column 2 (rows B to G). The top well (row B, column 2) received twice the amount of drug A than the other wells in column 2. Twofold serial dilutions were then performed from column 2 to column 9. Drug B, dissolved in 0.1 ml of Middlebrook 7H9 medium, was added to the wells of row B (columns 2 to 10). Twofold serial dilutions were then performed from row B to row F. M. tuberculosis H37Rv cultures were grown to mid-log phase in Middlebrook 7H9 medium and then diluted 1/1,000. Diluted cultures (0.1 ml) were added to the inside wells. The plates were incubated at 37°C for 7 days. Growth of M. tuberculosis was determined using the MTT assay (see above). The fractional inhibitory concentration index (FICI) was calculated as follows: FICI = (MIC of drug A in combination/MIC of drug A alone) + (MIC of drug B in combination/MIC of drug B alone). A FICI value of ≤0.5 indicates synergy between drugs A and B, and a FICI value of >4 indicates antagonism.
Growth of M. tuberculosis under anaerobic conditions.
M. tuberculosis mc26230 (H37Rv ΔRD1 ΔpanCD) was grown under an aerobic atmosphere in 7H9 medium supplemented as described above, with the addition of 50 μg/ml pantothenic acid, to an optical density at 600 nm (OD600) of 0.5 to 1.0. The strain was then shifted to a hypoxic glove box (Coy Laboratory Products, Grass Lake, MI) containing an anaerobic atmosphere (<0.0001% O2, 5% CO2, 10% H2, and 85% N2). After 24 h of anaerobic incubation, bacilli were inoculated at a 1:50 ratio into anaerobic 7H9 medium (supplemented as described above), and the compounds to be tested were added. At each time point, a sample of each culture was taken, and serial dilutions were plated on Middlebrook 7H10 plates containing 50 μg/ml of pantothenic acid. Plates were incubated under a normal aerobic atmosphere at 37°C for 3 to 4 weeks, and CFU were counted.
RESULTS
M. tuberculosis is resistant to TMP and susceptible to SMX.
The MICs of SMX and TMP were first determined against the drug-susceptible M. tuberculosis strain H37Rv (Table 1). TMP had no activity against M. tuberculosis (MIC = 512 μg/ml), while SMX had an MIC of 8.5 μg/ml. The MBCs of TMP and SMX against M. tuberculosis H37Rv were 768 μg/ml and 17 μg/ml, respectively. TMP and SMX were also tested against 5 other drug-susceptible M. tuberculosis strains. The strains were resistant to TMP and had different levels of susceptibility to SMX (Table 1).
Table 1.
MICs of TMP and SMX against drug-susceptible and drug-resistant M. tuberculosis strains
| Strain | Drug resistance(s)a | Genotypeb | MIC (μg/ml) |
Source and/or reference | |
|---|---|---|---|---|---|
| TMP | SMX | ||||
| H37Rv | 512 | 8.5 | Trudeau Institute | ||
| CDC1551 | 512 | 2.1 | CSU | ||
| Beijing | 512 | 34 | Laboratory stock | ||
| Erdman | 1,024 | 8.5 | Trudeau Institute | ||
| V4207 | 256 | ≤2.1 | 8; clinical isolate from KwaZulu-Natal, South Africa | ||
| V9124 | 512 | 8.5–17 | 8; clinical isolate from KwaZulu-Natal, South Africa | ||
| H37Rv/pMV261::dhfr | 1,024 | 17 | 22 | ||
| mc24977 | INH | katG del371g | 128 | 8.5 | Laboratory strain (H37Rv background) |
| CI5447 | INH | ND | 512 | 17 | Clinical isolate from Mexico |
| CI5297 | INH | ND | 256 | ≤2.1 | 20; clinical isolate from Mexico |
| mc24986 | RIF | rpoB H445R | 512 | 4.25 | Laboratory strain (H37Rv background) |
| CVOF1 | OF | ND | 1,024 | 8.5 | Laboratory strain (H37Rv background) |
| mc24914 | INH, ETH | PmabAinhA(c-15t) | 512 | 8.5 | 21; laboratory strain (H37Rv background) |
| mc24997 | INH, RIF | katG del, rpoB H451W | 512 | 8.5 | Laboratory strain (H37Rv background) |
| mc25854 | INH, RIF | katG L546P, rpoB S450L | 512 | 8.5 | Laboratory strain (H37Rv background) |
| mc25857 | INH, RIF | katG S315I, rpoB S450L | 512 | 8.5 | Laboratory strain (H37Rv background) |
| mc25858 | INH, RIF | katG insg317, rpoB H445D | 512 | 8.5 | Laboratory strain (H37Rv background) |
| CI5058 | INH, RIF | ND | 512 | 8.5 | Clinical isolate from Mexico |
| CI5324 | INH, RIF | 512 | 8.5 | 20; clinical isolate from Mexico | |
| CI5400 | INH, RIF | 512 | 8.5 | 20; clinical isolate from Mexico | |
| V2475 | INH, RIF | 512–1,024 | 17 | 8; clinical isolate from KwaZulu-Natal, South Africa | |
| mc25855 | INH, RIF, OF | katG del, rpoB H451W, gyrA A90V | 512 | 4.25 | Laboratory strain (H37Rv background) |
| mc25856 | INH, RIF, SM | katG del, rpoB H451W, rrs c516t | 512 | 4.25 | Laboratory strain (H37Rv background) |
| CI5072 | INH, RIF, SM | ND | 1,024 | 8.5 | 20; clinical isolate from Mexico |
| CI5071 | INH, SM | ND | 512 | 4.25 | 20; clinical isolate from Mexico |
| CI5358 | INH, EMB, ETH | ND | 512 | 8.5 | 20; clinical isolate from Mexico |
| CI5459 | INH, EMB, ETH | ND | 256 | 8.5 | Clinical isolate from Mexico |
| CI5483 | EMB, SM | ND | 512 | 8.5 | Clinical isolate from Mexico |
| CI12081 | INH, RIF, SM, EMB, ETH | ND | 128 | 2.1 | Clinical isolate from Mexico |
| TF275 | INH, RIF, SM, EMB, OF, KM | 640 | 8.5 | 8; clinical isolate from KwaZulu-Natal, South Africa | |
OF, ofloxacin; ETH, ethionamide; SM, streptomycin; EMB, ethambutol; KM, kanamycin.
ND, not determined.
Since TMP and SMX have been shown to be synergistic against a variety of bacteria (2), we tested whether TMP and SMX had a synergistic effect against M. tuberculosis. A checkerboard method (5) was used, with concentrations of SMX varying from 1.06 μg/ml to 17 μg/ml and concentrations of TMP varying from 4 μg/ml to 1,024 μg/ml. The FICI was below 1 for TMP concentrations ranging from 32 to 256 μg/ml. One combination (TMP at 128 μg/ml and SMX at 2.125 μg/ml) gave a FICI value of 0.5, indicating that a synergistic effect could occur between TMP and SMX at specific concentrations (Table 2).
Table 2.
MICs of TMP-SMX combination against M. tuberculosis H37Rv
| TMP MIC (μg/ml) | SMX MIC (μg/ml) | FICI value for combination |
|---|---|---|
| 0 | 8.5 | |
| 32 | 4.25 | 0.56 |
| 64 | 4.25 | 0.625 |
| 128 | 2.125 | 0.5 |
| 256 | 1.06 | 0.625 |
| 512 | 0 |
Twenty-three drug-resistant laboratory and clinical M. tuberculosis strains were then tested for susceptibility to TMP and SMX. The strains were either monoresistant to INH, RIF, or ofloxacin (OF), resistant to 2 or more first-line or second-line TB drugs, or XDR (TF275) (Table 1). The drug-resistant M. tuberculosis strains were also highly resistant to TMP, but the majority were as susceptible to SMX as M. tuberculosis H37Rv.
Time course analysis shows that a one-time dose of TMP and SMX stops the growth of drug-susceptible and drug-resistant M. tuberculosis strains.
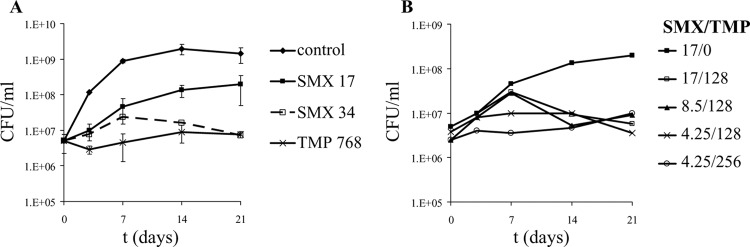
Cultures of M. tuberculosis H37Rv were treated with TMP and SMX at their MBCs (768 and 17 μg/ml, respectively). At these concentrations, we did not observe any killing of M. tuberculosis. TMP prevented the growth of M. tuberculosis, while SMX showed a dose-dependent effect on the growth of M. tuberculosis (Fig. 1A). To test whether the combination of TMP and SMX would result in better killing of M. tuberculosis than that with either drug alone, M. tuberculosis H37Rv was treated with a combination of TMP and SMX (Fig. 1B). The addition of a subinhibitory concentration of TMP (128 or 256 μg/ml) to M. tuberculosis cultures had no effect on the growth of M. tuberculosis (data not shown). When subinhibitory concentrations of TMP were added to SMX at the MIC or MBC, a viability curve with similar kinetics to that for M. tuberculosis treated with SMX alone was obtained for the first 7 days of treatment, but after that point, a decrease in CFU was observed, leading to a bacteriostatic phenotype after 21 days. Interestingly, the addition of subinhibitory concentrations of TMP to M. tuberculosis treated with a subinhibitory concentration of SMX followed a different kinetics, with a reduced increase in CFU at the beginning of the treatment, also leading to a bacteriostatic effect on the growth of M. tuberculosis. Growth inhibition by subinhibitory concentrations of SMX and TMP was stable over time and continued for over 2 months of incubation (data not shown).
Fig 1.
Activity of SMX and TMP against M. tuberculosis H37Rv. (A) M. tuberculosis H37Rv cultures were treated with SMX or TMP and incubated at 37°C for 3 weeks. CFU were determined by plating 10-fold serial dilutions of each culture onto Middlebrook 7H10 agar plates. Each growth curve was done at least in triplicate, and the average with standard deviation was plotted. (B) M. tuberculosis H37Rv was treated with a combination of SMX and TMP. Conditions for the experiment were the same as those for panel A. Concentrations are given in μg/ml.
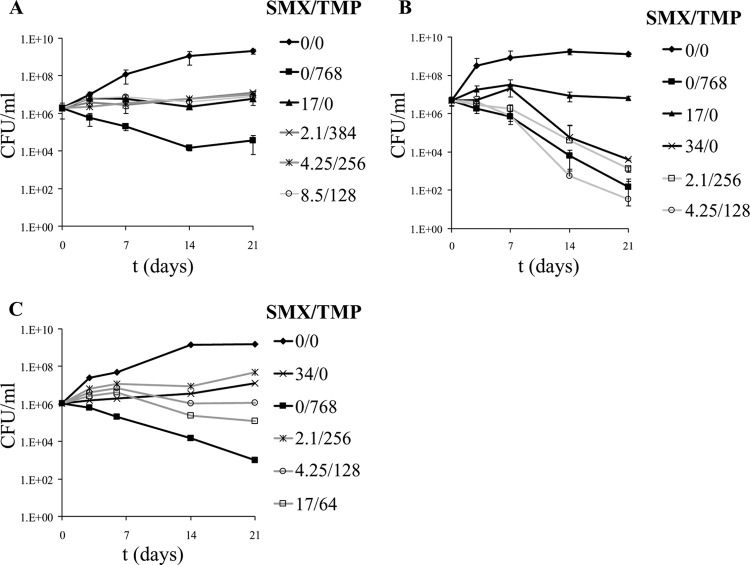
Three multidrug-resistant TB (MDR-TB) strains were treated with TMP and SMX, alone or in combination, for 3 weeks (Fig. 2). SMX alone showed more activity against the MDR-TB strains than against the drug-susceptible M. tuberculosis strain H37Rv, preventing the growth of all strains but mc25856. All of the MDR-TB strains were also more susceptible to a high concentration of TMP (768 μg/ml). A lower concentration of TMP (128 μg/ml) had no effect on the growth of the drug-resistant strains, while 256 μg/ml of TMP alone slowed down the growth of M. tuberculosis but did not induce any bacterial killing (data not shown). The combination of subinhibitory or inhibitory concentrations of TMP and SMX was bacteriostatic for the MDR-TB strains. One strain, mc25855, was particularly sensitive to SMX and its combination with TMP (Fig. 2B), resulting in a bactericidal effect (2- to 4-log decrease in CFU) after 3 weeks of treatment.
Fig 2.
Growth of multidrug-resistant M. tuberculosis strains in the presence of TMP and SMX. Three MDR-TB strains were treated with TMP and SMX for 3 weeks. (A) mc25854 (INHr RIFr); (B) mc25855 (INHr RIFr OFr); (C) mc25856 (INHr RIFr SMr). At each time point, samples were taken, and serial dilutions were plated onto Middlebrook 7H10 agar plates. Concentrations are given in μg/ml. OF, ofloxacin; SM, streptomycin.
TMP and SMX have limited activity against M. tuberculosis grown under anaerobic conditions.
One of the challenges facing TB drug development is the ability of M. tuberculosis to stay dormant for many years. Drugs are required to treat active as well as latent TB infections. To mimic in vitro latent or dormant TB, the Wayne model was developed, in which mycobacterial cells are subjected to a slow decrease in oxygen content to reproduce the hypoxic conditions in granulomas in the lungs (23). In order to test the activity of TMP and SMX against nongrowing hypoxic M. tuberculosis cells, an anaerobic chamber was used to incubate M. tuberculosis, with the level of oxygen kept at <0.0001%. For safety reasons, these experiments were done with mc26230 (M. tuberculosis H37Rv ΔRD1 ΔpanCD), an M. tuberculosis strain that can be used in a biosafety level 2 laboratory. The strain and medium were preincubated in the chamber for 24 h prior to the addition of TMP (768 μg/ml), SMX (17 μg/ml), or a combination of TMP and SMX (256 and 2.1 μg/ml, respectively, or 128 and 4.25 μg/ml, respectively). After 4 weeks of treatment, the difference in CFU between untreated M. tuberculosis and M. tuberculosis treated with TMP and SMX, either alone or in combination, was on the order of 1 log (data not shown), suggesting that TMP and SMX have limited activity in the killing of hypoxic M. tuberculosis cells.
The combination of TMP and/or SMX with INH or RIF results in bactericidal killing of M. tuberculosis.
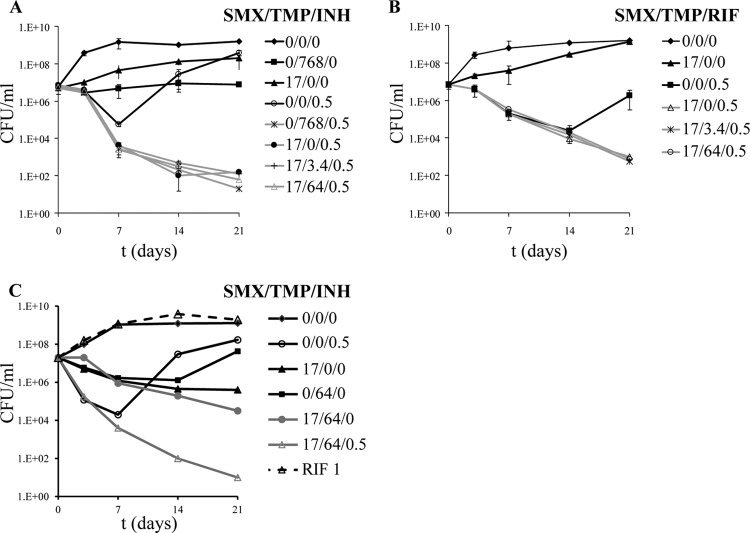
The current drug regimen for treating patients infected with a drug-susceptible M. tuberculosis strain is INH, RIF, pyrazinamide, and ethambutol for 2 months followed by 4 months of INH and RIF. To prevent the emergence of drug resistance, monotherapy is not an option in the treatment of TB. Therefore, any drug developed for treatment of TB should work in combination with FLDs and/or second-line TB drugs. To test if TMP-SMX could be added to the current drug regimen without any antagonistic side effects, M. tuberculosis H37Rv was treated with INH or RIF in the presence of TMP and SMX, alone or in combination (Fig. 3). In vitro, INH and RIF are bactericidal for the first 7 to 14 days of treatment, followed by the emergence of drug-resistant mutants. When TMP and/or SMX was added to INH-treated M. tuberculosis cultures, an increased killing of M. tuberculosis (about 1 log) compared to that of M. tuberculosis treated with INH alone was observed after 7 days (Fig. 3A). Furthermore, the emergence of INH-resistant mutants observed in the INH-treated culture did not occur in the cultures treated with INH and TMP-SMX. After 3 weeks of cotreatment with INH, TMP, and SMX at various concentrations, a 4- to 5-log decrease in CFU was achieved.
Fig 3.
Combination of TMP and SMX with FLDs. M. tuberculosis H37Rv was treated with TMP-SMX in combination with INH (A) or RIF (B). (C) A RIF-resistant M. tuberculosis strain, mc24986, was treated with RIF, INH, TMP, and SMX, alone or in combination. The cultures were incubated with shaking at 37°C. CFU were determined by plating 10-fold serial dilutions onto Middlebrook 7H10 plates. Concentrations are given in μg/ml.
The effect of cotreatment with RIF, TMP, and/or SMX was slightly different from that with INH. The killing of M. tuberculosis with RIF, SMX, and TMP was undistinguishable from that with RIF alone until RIF-resistant mutants started to emerge in the M. tuberculosis culture treated with RIF alone (Fig. 3B). After 3 weeks of treatment, the titers of the M. tuberculosis cultures treated with RIF-SMX and with or without TMP had been reduced by 4 log, while the RIF-treated M. tuberculosis culture was growing and contained mostly RIF-resistant mutants. Müller-Brundaler et al. showed previously that RIF resistance could not arise in M. tuberculosis cultures treated with RIF-SMX-TMP, while M. tuberculosis cultures treated with RIF alone generated RIF-resistant mutants (13). To confirm that SMX-TMP also prevented the emergence of INH-resistant mutants, nonmutagenized M. tuberculosis cultures were plated on Middlebrook 7H10 plates containing INH (0.5 μg/ml), INH (0.5 μg/ml)-SMX (17 μg/ml), or INH (0.5 μg/ml)-SMX (17 μg/ml)-TMP (3.4 or 64 μg/ml). The plates were incubated at 37°C for 4 weeks, and colonies were counted. INH-resistant mutants were isolated at a frequency of 2 × 10−5 mutant/inoculum from the INH plates. No colonies were isolated from the plates containing INH-SMX with or without TMP (frequency of mutation, <10−9 mutant/inoculum).
To test whether the combination of SMX-TMP with FLDs would also work on monoresistant M. tuberculosis strains, mc24986, a RIF-resistant M. tuberculosis strain, was treated with RIF, INH, SMX, TMP, and a combination of SMX-TMP and INH (Fig. 3C). Treatment of the RIF-resistant M. tuberculosis strain with INH alone followed the same pattern as that for wild-type M. tuberculosis, with rapid killing of the strain for the first 7 days followed by the emergence of INH-resistant mutants. SMX or TMP alone had bacteriostatic activity against mc24986, while the SMX-TMP combination was bactericidal. When INH was added to the SMX-TMP combination, a 6-log reduction in CFU was obtained after 3 weeks, and no emergence of INH-resistant mutants was observed.
Overexpression of dhfr results in increased resistance to TMP and SMX in M. tuberculosis.
Since Koser et al. questioned the role of overexpression of dhfr in increased resistance to TMP-SMX in M. tuberculosis (9), the minimum concentrations of TMP and SMX that inhibited the growth of an M. tuberculosis laboratory strain overexpressing dhfr, i.e., H37Rv/pMV261::dhfr (22), were measured and found to be 1,024 μg/ml and 17 μg/ml, respectively. These concentrations were 2-fold higher than the MICs for M. tuberculosis H37Rv (Table 1), suggesting that overexpression of dhfr in M. tuberculosis does slightly increase resistance to TMP, which targets dhfr, but also to SMX, which inhibits FolP, a different enzyme in the folate biosynthesis pathway. Measuring the MICs of a combination of TMP and SMX against M. tuberculosis H37Rv/pMV261::dhfr revealed that TMP and SMX had mostly an additive effect, with FICI values falling between 0.5 and 1.
The mammalian Dhfr inhibitor MTX is more active than TMP against M. tuberculosis.
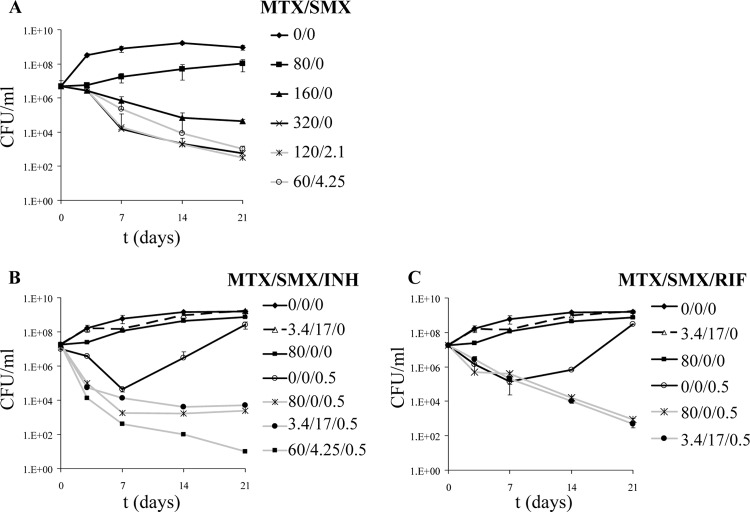
TMP has no activity against M. tuberculosis on its own, although it increases the activity of SMX against M. tuberculosis. TMP is in fact a poor inhibitor of M. tuberculosis Dhfr, with a 50% inhibitory concentration (IC50) of 16.5 μM (24). To examine whether Dhfr is indeed a good drug target for M. tuberculosis drug development, we tested the mammalian Dhfr inhibitor methotrexate (MTX) against M. tuberculosis. While TMP is a specific inhibitor of bacterial Dhfr, MTX inhibits the DHFR enzymes of Plasmodium berghei, Escherichia coli, and humans (17). MTX is actually a better inhibitor of the M. tuberculosis Dhfr enzyme (IC50 = 8.3 nM) than TMP is (24). MTX has a chemical structure very similar to that of folic acid and binds to Dhfr 1,000 times more tightly than folate does. The MIC of MTX against M. tuberculosis is 150 μg/ml. When MTX was tested in our culture model, a high concentration of MTX (160 μg/ml) was found to be bactericidal against M. tuberculosis H37Rv, which we had not observed with TMP alone (Fig. 4A). Furthermore, the combination of MTX and SMX was also bactericidal. Subinhibitory concentrations of SMX and MTX resulted in a 3- to 4-log decrease in CFU after 3 weeks of treatment.
Fig 4.
Activity of MTX against M. tuberculosis H37Rv. M. tuberculosis H37Rv was treated with MTX and SMX (A), MTX, SMX, and INH (B), or MTX, SMX, and RIF (C). The cultures were incubated at 37°C for 3 weeks. CFU were determined by plating 10-fold serial dilutions of each culture onto Middlebrook 7H10 agar plates. Concentrations are given in μg/ml.
MTX was also tested in combination with INH (Fig. 4B) or RIF (Fig. 4C). The combination of MTX-SMX and INH or RIF mimicked the results obtained with TMP, SMX, and INH or RIF. MTX and SMX were not antagonistic to INH and RIF, and in both cases, the addition of MTX to INH- or RIF-treated M. tuberculosis cultures prevented the growth of INH- or RIF-resistant mutants. The combined addition of MTX, SMX, and INH or RIF resulted in a 4- to 6-log decrease in CFU compared to the original inoculum.
DISCUSSION
The combination of TMP and SMX has been used clinically for over 40 years, with great success, to treat infections from Gram-positive and Gram-negative bacteria (18), yet few studies have investigated the activity of TMP and SMX against M. tuberculosis. The findings of such studies are highly divergent, from TMP and SMX being totally inactive against M. tuberculosis (2) to SMX alone having good activity (15) or the combination being efficient at treating patients infected with drug-susceptible or XDR-TB strains (3, 4). This study was aimed at determining the activity of TMP and SMX, alone and in combination, against drug-susceptible and drug-resistant M. tuberculosis strains. Our conclusion is that M. tuberculosis is susceptible to SMX and to the combination of SMX and TMP.
The synergy between TMP and SMX was established in different bacteria both in vitro and in vivo (1). Our data suggest that there is indeed a synergy between TMP and SMX in M. tuberculosis. Increased activities were observed in establishing the MICs for both compounds as well as in studying the effects of TMP and SMX on the viability of drug-susceptible and drug-resistant M. tuberculosis strains in cultures. On the other hand, the addition of TMP to INH-SMX- or RIF-SMX-treated M. tuberculosis cultures did not improve the early bactericidal killing of M. tuberculosis, but neither was it antagonistic. Nonetheless, since the commercial formulation of SMX-TMP contains 5 times more SMX than TMP, this could still be used to test the in vivo efficiency of the combination of TMP and SMX against drug-susceptible and MDR-TB isolates. Cohen-Bacrie et al. showed that patients receiving TMP-SMX (960 mg-4.8 g) daily had serum levels of 4.4 μg/ml of TMP and 81.5 μg/ml of SMX (3). This SMX concentration is almost 10 times higher than the measured MIC and several times higher than the SMX concentrations used in our in vitro studies.
In in vitro cultures of M. tuberculosis treated with INH or RIF, drug-resistant mutants appeared consistently after 7 or 14 days of treatment. When TMP and SMX were added to INH- or RIF-treated M. tuberculosis cultures, a 4- to 5-log decrease in M. tuberculosis CFU was observed after 3 weeks, with no emergence of drug-resistant mutants. Surprisingly, although TMP and SMX either alone or in combination were bacteriostatic at best against M. tuberculosis, an extra 1- to 2-log decrease in CFU was observed in the M. tuberculosis cultures treated with SMX-TMP-INH or SMX-TMP-RIF after the drug-resistant mutants had started to grow in the M. tuberculosis cultures treated with INH or RIF alone. This suggests either that SMX (with or without TMP) was more efficient at killing emerging drug-resistant mutants or that SMX-TMP prevented their growth. An INH-resistant mutant isolation experiment showed that when M. tuberculosis was exposed to INH alone, the frequency at which such mutants arose was 2 × 10−5 mutant/inoculum. In contrast, no mutant could be isolated on INH-SMX plates, with or without TMP. In this case, the frequency of mutation was below 10−9 mutant/inoculum. This implies that the addition of TMP-SMX to TB treatment regimens could be useful for preventing the emergence of INH- or RIF-resistant mutants.
This study also suggests that the folate biosynthesis pathway is a good M. tuberculosis target for drug development. TMP is a poor inhibitor of M. tuberculosis Dhfr. As a proof-of-principle experiment to test whether better Dhfr inhibitors would show enhanced activity against M. tuberculosis, we treated M. tuberculosis with the mammalian Dhfr inhibitor MTX. MTX had a bactericidal effect against M. tuberculosis, while TMP was only bacteriostatic at a concentration that is irrelevant to drug testing (768 μg/ml). Furthermore, the combination of subinhibitory concentrations of MTX and SMX, with and without FLDs, was also bactericidal. It is interesting that TMP is usually 20 to 100 times more active than SMX in most bacteria (2), further supporting the fact that the inhibition of M. tuberculosis Dhfr by TMP is weak. In a study that described an analysis of TMP analogs against M. tuberculosis and Mycobacterium avium, the IC50 of TMP against M. avium Dhfr was 400 nM (19). Two of the deazapteridine derivatives identified in that study had useful MICs (6 to 12.5 μg/ml) against M. tuberculosis H37Rv, with low cytotoxicity, and their IC50s against Mycobacterium avium Dhfr were 2 and 8 nM (19). Better M. tuberculosis Dhfr inhibitors could therefore be synthesized that might have good bactericidal activities against M. tuberculosis. Furthermore, since no first-line or second-line TB drugs target this pathway, folate biosynthesis inhibitors would be a good addition to the TB pharmacopeia. PAS (p-amino salicylic acid), which was used in the treatment of TB in the late 1940s (10) and is now used mostly to treat MDR- and XDR-TB cases (3, 12), was thought to be involved in the folate pathway (16, 25) but was later shown to inhibit the conversion of salicylic acid to mycobactin (14).
A more in-depth study of the effect of adding TMP and SMX to the current TB drug regimen is warranted. The short-course chemotherapy of 6 months for treating a drug-susceptible TB infection becomes a 2-year treatment minimum when the patient is infected with a RIF-resistant M. tuberculosis strain. Our data show that a combination of INH, SMX, and TMP was extremely bactericidal to a RIF-resistant M. tuberculosis strain. Therefore, it would be interesting to test whether treating mice infected with a RIF-resistant M. tuberculosis strain with a combination of TMP-SMX and INH would shorten chemotherapy. We believe that the addition of TMP-SMX to the regular TB drug therapy could lead to quicker sterilization and prevent the emergence of drug resistance. Furthermore, since the commercial formulation of TMP and SMX is widely available and inexpensive, clinical trials including TMP and SMX in a set of TB drugs would be extremely useful for validating the published clinical cases that have reported a potential benefit of adding TMP and SMX to TB treatment. In light of the fact that all effective M. tuberculosis sterilization regimens require multiple drugs, we think that the addition of TMP-SMX to clinical trials is well worth pursuing. Finally, with the challenges that drug discovery poses in terms of length, cost, and success rate, testing FDA-approved drugs against M. tuberculosis could be a solution to quickly uncover safe and effective drug regimens to cure TB.
ACKNOWLEDGMENTS
We thank David Alland and Michelle Larsen for their generous gifts of clinical isolates and John Blanchard for helpful discussions.
W.R.J. acknowledges generous support from NIH Centers for AIDS Research (CFAR) grant AI-051519 to the Albert Einstein College of Medicine. This work was supported by National Institutes of Health grant AI26170 to W.R.J.
Footnotes
Published ahead of print 23 July 2012
REFERENCES
- 1. Bushby SR. 1975. Synergy of trimethoprim-sulfamethoxazole. Can. Med. Assoc. J. 112:63–66 [PMC free article] [PubMed] [Google Scholar]
- 2. Bushby SR. 1973. Trimethoprim-sulfamethoxazole: in vitro microbiological aspects. J. Infect. Dis. 128(Suppl):442–462 [DOI] [PubMed] [Google Scholar]
- 3. Cohen-Bacrie S, et al. 2011. Imported extensively drug-resistant Mycobacterium tuberculosis Beijing genotype, Marseilles, France, 2011. Euro Surveill. 16:19846 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19846 [PubMed] [Google Scholar]
- 4. Forgacs P, et al. 2009. Tuberculosis and trimethoprim-sulfamethoxazole. Antimicrob. Agents Chemother. 53:4789–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsieh MH, Yu CM, Yu VL, Chow JW. 1993. Synergy assessed by checkerboard. A critical analysis. Diagn. Microbiol. Infect. Dis. 16:343–349 [DOI] [PubMed] [Google Scholar]
- 6. Huang TS, et al. 2012. Susceptibility of Mycobacterium tuberculosis to sulfamethoxazole, trimethoprim and their combination over a 12 year period in Taiwan. J. Antimicrob. Chemother. 67:633–637 [DOI] [PubMed] [Google Scholar]
- 7. Huovinen P, Sundstrom L, Swedberg G, Skold O. 1995. Trimethoprim and sulfonamide resistance. Antimicrob. Agents Chemother. 39:279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ioerger TR, et al. 2009. Genome analysis of multi- and extensively-drug-resistant tuberculosis from KwaZulu-Natal, South Africa. PLoS One 4:e7778 doi:10.1371/journal.pone.0007778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koser CU, Summers DK, Archer JA. 2010. Role of the dihydrofolate reductase DfrA (Rv2763c) in trimethoprim-sulfamethoxazole (cotrimoxazole) resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 54:4951–4952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lehmann J. 1946. Para-aminosalicylic acid in the treatment of tuberculosis. Lancet i:15. [DOI] [PubMed] [Google Scholar]
- 11. Martin A, et al. 2005. Multicenter study of MTT and resazurin assays for testing susceptibility to first-line anti-tuberculosis drugs. Int. J. Tuberc. Lung Dis. 9:901–906 [PubMed] [Google Scholar]
- 12. Mitnick C, et al. 2003. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N. Engl. J. Med. 348:119–128 [DOI] [PubMed] [Google Scholar]
- 13. Müller-Brundaler U, Dingfelder G, Urbanczik R. 1997. Improbability of selection for RIF resistance in Mycobacterium tuberculosis by accidental exposure during short-course therapy with cotrifazid. Chemotherapy 43:451–452 [DOI] [PubMed] [Google Scholar]
- 14. Nagachar N, Ratledge C. 2010. Knocking out salicylate biosynthesis genes in Mycobacterium smegmatis induces hypersensitivity to p-aminosalicylate (PAS). FEMS Microbiol. Lett. 311:193–199 [DOI] [PubMed] [Google Scholar]
- 15. Ong W, Sievers A, Leslie DE. 2010. Mycobacterium tuberculosis and sulfamethoxazole susceptibility. Antimicrob. Agents Chemother. 54:2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rengarajan J, et al. 2004. The folate pathway is a target for resistance to the drug para-aminosalicylic acid (PAS) in mycobacteria. Mol. Microbiol. 53:275–282 [DOI] [PubMed] [Google Scholar]
- 17. Schweitzer BI, Dicker AP, Bertino JR. 1990. Dihydrofolate reductase as a therapeutic target. FASEB J. 4:2441–2452 [DOI] [PubMed] [Google Scholar]
- 18. Smilack JD. 1999. Trimethoprim-sulfamethoxazole. Mayo Clin. Proc. 74:730–734 [DOI] [PubMed] [Google Scholar]
- 19. Suling WJ, et al. 1998. Susceptibilities of Mycobacterium tuberculosis and Mycobacterium avium complex to lipophilic deazapteridine derivatives, inhibitors of dihydrofolate reductase. J. Antimicrob. Chemother. 42:811–815 [DOI] [PubMed] [Google Scholar]
- 20. Vilchèze C, et al. 2011. Novel inhibitors of InhA efficiently kill Mycobacterium tuberculosis under aerobic and anaerobic conditions. Antimicrob. Agents Chemother. 55:3889–3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vilchèze C, et al. 2006. Transfer of a point mutation in Mycobacterium tuberculosis inhA resolves the target of isoniazid. Nat. Med. 12:1027–1029 [DOI] [PubMed] [Google Scholar]
- 22. Wang F, et al. 2010. Mycobacterium tuberculosis dihydrofolate reductase is not a target relevant to the antitubercular activity of isoniazid. Antimicrob. Agents Chemother. 54:3776–3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wayne LG, Hayes LG. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. White EL, Ross LJ, Cunningham A, Escuyer V. 2004. Cloning, expression, and characterization of Mycobacterium tuberculosis dihydrofolate reductase. FEMS Microbiol. Lett. 232:101–105 [DOI] [PubMed] [Google Scholar]
- 25. Winder FG. 1964. The antimicrobial action of streptomycin, isoniazid and PAS. Butterworth, London, United Kingdom [Google Scholar]