Abstract
Multidrug-resistant (MDR) Klebsiella pneumoniae may require combination therapy. We systematically investigated bacterial killing with colistin and doripenem mono- and combination therapy against MDR K. pneumoniae and emergence of colistin resistance. A one-compartment in vitro pharmacokinetic/pharmacodynamic model was employed over a 72-h period with two inocula (∼106 and ∼108 CFU/ml); a colistin-heteroresistant reference strain (ATCC 13883) and three clinical isolates (colistin-susceptible FADDI-KP032 [doripenem resistant], colistin-heteroresistant FADDI-KP033, and colistin-resistant FADDI-KP035) were included. Four combinations utilizing clinically achievable concentrations were investigated. Microbiological responses were examined by determining log changes and population analysis profiles (for emergence of colistin resistance) over 72 h. Against colistin-susceptible and -heteroresistant isolates, combinations of colistin (constant concentration regimens of 0.5 or 2 mg/liter) plus doripenem (steady-state peak concentration [Cmax] of 2.5 or 25 mg/liter over 8 h; half-life, 1.5 h) generally resulted in substantial improvements in bacterial killing at both inocula. Combinations were additive or synergistic against ATCC 13883, FADDI-KP032, and FADDI-KP033 in 9, 9, and 14 of 16 cases (4 combinations at 6, 24, 48, and 72 h) at the 106-CFU/ml inoculum and 14, 11, and 12 of 16 cases at the 108-CFU/ml inoculum, respectively. Combinations at the highest dosage regimens resulted in undetectable bacterial counts at 72 h in 5 of 8 cases (4 isolates at 2 inocula). Emergence of colistin-resistant subpopulations in colistin-susceptible and -heteroresistant isolates was virtually eliminated with combination therapy. Against the colistin-resistant isolate, colistin at 2 mg/liter plus doripenem (Cmax, 25 mg/liter) at the low inoculum improved bacterial killing. This investigation provides important information for optimization of colistin-doripenem combinations.
INTRODUCTION
The emergence of nosocomial Klebsiella pneumoniae resistant to almost all available antibiotics, including carbapenems, is an increasing problem worldwide (7, 18, 21, 22, 27, 38). Increasing resistance combined with a lack of novel antibiotics in the drug discovery pipeline for Gram-negative pathogens (7) has forced a reevaluation of “old” antibiotics, in particular colistin (polymyxin E), which retains activity against many of these multidrug-resistant (MDR) Gram-negative organisms (24, 30, 31, 33). As a consequence, the use of colistin has increased substantially over the past few years, particularly for critically ill patients. However, colistin-resistant K. pneumoniae has rapidly emerged (1, 6, 26, 35, 48).
Colistin is administered parenterally in the form of its sulfomethyl derivative, sodium colistin methanesulfonate (CMS). The inactive prodrug CMS undergoes conversion in vivo to the active species, colistin (4, 17, 43). Although CMS has been available in the clinic for more than 50 years, only recently has reliable information on the pharmacokinetics (PK) and pharmacodynamics (PD) of CMS and formed colistin emerged. It is evident from these investigations that the plasma colistin concentrations achieved in critically ill patients with currently recommended CMS dosage regimens are low and in many cases suboptimal (17, 43). Unfortunately, colistin-induced nephrotoxicity is a dose-limiting adverse effect in up to ∼50% of patients (11, 17, 19, 28), and thus increasing the daily dose may not be an acceptable option (17). Further complicating the use of CMS as monotherapy is the phenomenon of colistin heteroresistance, the presence of colistin-resistant subpopulations within an isolate that is susceptible based upon its MIC. Colistin heteroresistance, which has been identified in K. pneumoniae (37, 44), Acinetobacter baumannii (20, 32, 51), and Pseudomonas aeruginosa (3), is thought to contribute to the rapid emergence of colistin resistance observed with monotherapy. Collectively, these observations suggest caution with the use of colistin monotherapy and have led some to propose that colistin may be best used as part of combination therapy (17, 40). The aim of the present study was to systematically investigate using a range of isolates the extent of in vitro bacterial killing and the emergence of colistin resistance of K. pneumoniae, at both low and high inocula, using clinically relevant dosage regimens of colistin alone and in combination with doripenem.
(Parts of this study were presented at the 51st Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Chicago, IL, 17 to 20 September 2011, and the 22nd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), London, United Kingdom, 31 March to 3 April 2012.)
MATERIALS AND METHODS
Bacterial isolates.
K. pneumoniae ATCC 13883 (American Type Culture Collection, Rockville, MD) and three clinical isolates (FADDI-KP032, FADDI-KP033, and FADDI-KP035) were selected based upon differing patterns of susceptibility to colistin and doripenem and included colistin-heteroresistant strains (Table 1); the method of determining colistin heteroresistance is described below. FADDI-KP033 and FADDI-KP032 were kindly donated by Ben Howden (Austin Hospital, Melbourne, Australia) and were taken from the same patient before and after carbapenem therapy, whereas FADDI-KP035 was from Diagnostic Services of Manitoba (Health Sciences Centre, Winnipeg, Canada). All clinical strains in this study fulfilled the definition of MDR of the Centers for Disease Control and Prevention (45).
Table 1.
MICs for K. pneumoniae isolates used in this studya
| Isolate | MIC (mg/liter)b |
β-Lactamase typingc | Colistin heteroresistantd | |
|---|---|---|---|---|
| Colistin | Doripenem | |||
| ATCC 13883 | 1 | <0.125 | SHV | Yes |
| FADDI-KP033e | 1 | <0.125 | SHV and DHA | Yes |
| FADDI-KP032e | 1 | 8 | SHV and DHA | No |
| FADDI-KP035 | >128 | <0.125 | SHV | NA |
All clinical isolates were MDR according to the definition of the Centers for Disease Control and Prevention (45).
EUCAST breakpoints for colistin are ≤2 mg/liter for susceptibility and >2 mg/liter for resistance. For doripenem, the breakpoints are ≤1 mg/liter for susceptibility and >4 mg/liter for resistance (14).
The strain contains genes encoding SHV class A and/or DHA AmpC type β-lactamase.
Heteroresistance to colistin was defined as the existence, in an isolate for which the colistin MIC was ≤2 mg/liter, of subpopulations able to grow in the presence of >2 mg/liter colistin (44). NA, not applicable due to colistin resistance.
FADDI-KP033 and FADDI-KP032 were taken from the same patient before and after carbapenem therapy (isolates 16 and 17 from a previous study [44]).
MICs of colistin and doripenem were determined for each isolate in two replicates on separate days in cation-adjusted Mueller-Hinton broth (CAMHB) (containing 23.0 mg/liter Ca2+ and 11.5 mg/liter Mg2+ [Oxoid, Hampshire, England]) via broth microdilution (15); European Committee on Antimicrobial Susceptibility Testing (EUCAST) (Växjö, Sweden) breakpoints against the Enterobacteriaceae were employed (Table 1) (14). All isolates produced β-lactamases, although only FADDI-KP032 was resistant to doripenem based upon MICs (Table 1). Isolates were stored in tryptone soy broth (Oxoid, Basingstoke, Hampshire, England) with 20% glycerol (Ajax Finechem, Seven Hills, NSW, Australia) at −80°C.
Antibiotics.
For MIC determinations and in vitro PK/PD studies, colistin sulfate (lot 070M1499V; 23,690 U/mg) was purchased from Sigma-Aldrich (St. Louis, MO), while doripenem (lot 9GBSE00) was from Janssen-Cilag (Australia). Colistin sulfate was employed in the current study because colistin is the active antibacterial agent formed in vivo following administration of CMS (4). Colistin was prepared using Milli-Q water (Millipore Australia, North Ryde, New South Wales, Australia) at the beginning of each experiment and spiked into the sterile broth that was pumped into the central reservoir (see below) to achieve the desired concentration; we have previously shown that colistin is stable under these conditions for the duration of the experiment (5). Stock solutions of doripenem were similarly prepared using 0.9% sodium chloride immediately prior to each administration to the PK/PD model, protected from light to minimize loss from degradation, and sterilized by filtration with a 0.22-μm Millex-GP filter (Millipore, Bedford, MA). Concentrations of colistin and doripenem in the reservoirs were measured using high-performance liquid chromatography as described previously (5, 29).
In vitro pharmacokinetic/pharmacodynamic model.
A one-compartment in vitro PK/PD model was used to examine the microbiological response and emergence of resistance to various dosage regimens of colistin and doripenem alone and in combination over 72 h at two different starting inocula (∼106 and ∼108 CFU/ml) (5). All drug-containing regimens (whether mono- or combination therapy) mimicked the PK profiles of each drug achieved in critically ill patients (17, 39, 43). Since we have previously demonstrated that both colistin (2) and doripenem (5) are almost entirely unbound in CAMHB, the specified concentrations represent unbound (free) concentrations. Colistin (when included in the dosage regimen) was delivered at a clinically relevant concentration (Table 2) to mimic the flat colistin concentration-versus-time profiles observed across a dosage interval at steady state in patients receiving short-term infusions of CMS intravenously (i.v.) every 8 to 24 h (17, 43); since colistin was added to all media prior to each experiment, steady-state concentrations were present from the beginning of each experiment. For doripenem-containing regimens, doripenem was injected as a bolus dose into each treatment compartment following bacterial inoculation to achieve the desired steady-state peak concentration (Cmax), with intermittent dosing every 8 h thereafter (Table 2); no loading dose of doripenem was required to achieve steady-state concentrations, since doripenem does not accumulate with intermittent i.v. administration. The flow rate of sterile media through the system simulated a doripenem elimination half-life (t1/2) of 1.5 h, which approximates that in critically ill patients (36). Three monotherapy dosage regimens (each) were simulated for colistin and doripenem (for the colistin-resistant isolate, only colistin at 5 mg/liter was used), with four colistin-doripenem regimens used for combination therapy (Table 2).
Table 2.
Colistin and doripenem dosage regimens, PK/PD index values, and sampling times in the in vitro PK/PD modela
| Treatment regimen | PK/PD index valueb |
Sampling times (h) for microbiological measurementsƒ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ƒAUC/MIC |
ƒCmax/MIC |
ƒT>MIC |
|||||||||||
| ATCC 13883 | FADDI-KP033 | FADDI-KP032 | FADDI-KP035 | ATCC 13883 | FADDI-KP033 | FADDI-KP032 | FADDI-KP035 | ATCC 13883 | FADDI-KP033 | FADDI-KP032 | FADDI-KP035 | ||
| Col monotherapy,c target concn (mg/liter) | 0, 1, 2, 3, 4, 6, 8, 24, 26, 28, 48, 50, 52, 72 | ||||||||||||
| 0.5 | 12.0 | 12.0 | 12.0 | <0.09 | 0.50 | 0.50 | 0.50 | <0.004 | 0 | 0 | 0 | 0 | |
| 2.0 | 48.0 | 48.0 | 48.0 | <0.38 | 2.0 | 2.0 | 2.0 | <0.016 | 100 | 100 | 100 | 0 | |
| 5.0 | 120.0 | 120.0 | 120.0 | <0.94 | 5.0 | 5.0 | 5.0 | <0.039 | 100 | 100 | 100 | 0 | |
| Dor monotherapy,d target Cmax/Cmin (mg/liter) | 0, 1, 2, 3, 4, 6, 8, 24, 26, 28, 48, 50, 52, 72 | ||||||||||||
| 2.5/0.062 | >127 | >127 | 1.98 | >127 | >20 | >20 | 0.32 | >20 | 81.0 | 81.0 | 0 | 81.0 | |
| 25/0.62 | >1,266 | >1,266 | 19.8 | >1,266 | >200 | >200 | 3.13 | >200 | 100 | 100 | 30.8 | 100 | |
| 50/1.24 | >2,532 | >2,532 | 39.6 | >2,532 | >400 | >400 | 6.25 | >400 | 100 | 100 | 49.6 | 100 | |
| Combination therapye | 0, 1, 2, 3, 4, 6, 8, 24, 26, 28, 48, 50, 52, 72 | ||||||||||||
Dosage regimens were tested with ∼106- and ∼108-CFU/ml starting inocula.
Values shown are target values for PK/PD indices. For combination therapy, the PK/PD indices for each drug were the same as those for equivalent monotherapy. ƒAUC/MIC, area under the unbound drug concentration-time curve over 24 h in the steady state divided by the MIC; ƒCmax/MIC, unbound drug maximal concentration divided by the MIC; ƒT>MIC, cumulative percentage of a 24-h period that the unbound drug concentration exceeds the MIC under steady-state PK conditions.
Colistin (Col) dosage regimens involved a constant concentration of colistin simulating the flat colistin concentration-versus-time profiles observed across a dosing interval at steady state in patients receiving short-term infusions of CMS i.v. every 8 to 24 h. For the colistin-resistant isolate (FADDI-KP035), only colistin at 5 mg/liter was used as monotherapy. Values shown for isolate FADDI-KP035 at other dosages of colistin are those for combination therapy with the indicated dosage regimen of colistin.
Doripenem (Dor) dosage regimens involved intermittent administration (every 8 h) to achieve the targeted Cmax and Cmin.
Combination therapy was carried out with the following dosage regimens: colistin at 0.5 mg/liter plus a doripenem Cmax of 2.5 mg/liter, colistin at 0.5 mg/liter plus a doripenem Cmax of 25 mg/liter, colistin at 2 mg/liter plus a doripenem Cmax of 2.5 mg/liter, and colistin at 2 mg/liter plus a doripenem Cmax of 25 mg/liter.
The number of CFU/ml was determined at all time points. Full PAPs were generated at 0 and 72 h; mini-PAPs were generated at 6, 24, and 48 h.
Microbiological response and emergence of resistance to colistin.
Serial samples (1 ml) were collected aseptically from the central reservoir of the PK/PD model at the times shown in Table 2 for determination of colistin and doripenem concentrations, as well as viable cell counting and real-time population analysis profiles (PAPs) (5). Viable counting and PAPs were conducted immediately after sampling by spiral plating (WASP2 spiral plater; Don Whitley Scientific Ltd., United Kingdom) 50 μl of sample (appropriately diluted with 0.9% saline) on either nutrient agar (viable counting) or Mueller-Hinton agar (PAPs), followed by incubation for 24 h (48 h for plates with small colonies) at 35°C. The limit of detection was 20 CFU/ml (equivalent to 1 colony per plate), whereas the limit of quantification was 400 CFU/ml (equivalent to 20 colonies per plate). Colistin heteroresistance was defined as a colistin-susceptible isolate (i.e., MIC ≤ 2 mg/liter) in which subpopulations were able to grow in the presence of >2 mg/liter colistin in the PAPs (44).
PD analysis.
The log change method comparing the change in log10 (CFU/ml) from 0 h (CFU0) to time t (6, 24, 48, or 72 h; CFUt), calculated as log change = log10 CFUt − log10 CFU0, was used to examine microbiological responses to monotherapy and combination therapy. Single-antibiotic or combination regimens causing a reduction of ≥1 log10 CFU/ml below the initial inoculum at the specified time were considered active. Synergy was defined as ≥2-log10 killing for the combination relative to that for its most active component at the specified time; additivity was defined as a 1- to <2-log10 superior killing for the combination (42).
RESULTS
PK validation.
The colistin concentrations achieved (mean ± standard deviation [SD]) were 0.48 ± 0.13 mg/liter (n = 40), 1.92 ± 0.35 mg/liter (n = 46), and 5.11 ± 0.83 mg/liter (n = 19) for the targeted concentrations of 0.50, 2.00, and 5.00 mg/liter, respectively. The measured doripenem Cmax was 2.84 ± 0.41 mg/liter (n = 52) for the targeted value of 2.50 mg/liter, 24.5 ± 2.78 mg/liter (n = 56) for the targeted value of 25.0 mg/liter, and 49.0 ± 4.06 mg/liter (n = 17) mg/liter for the targeted value of 50.0 mg/liter.
Microbiological response.
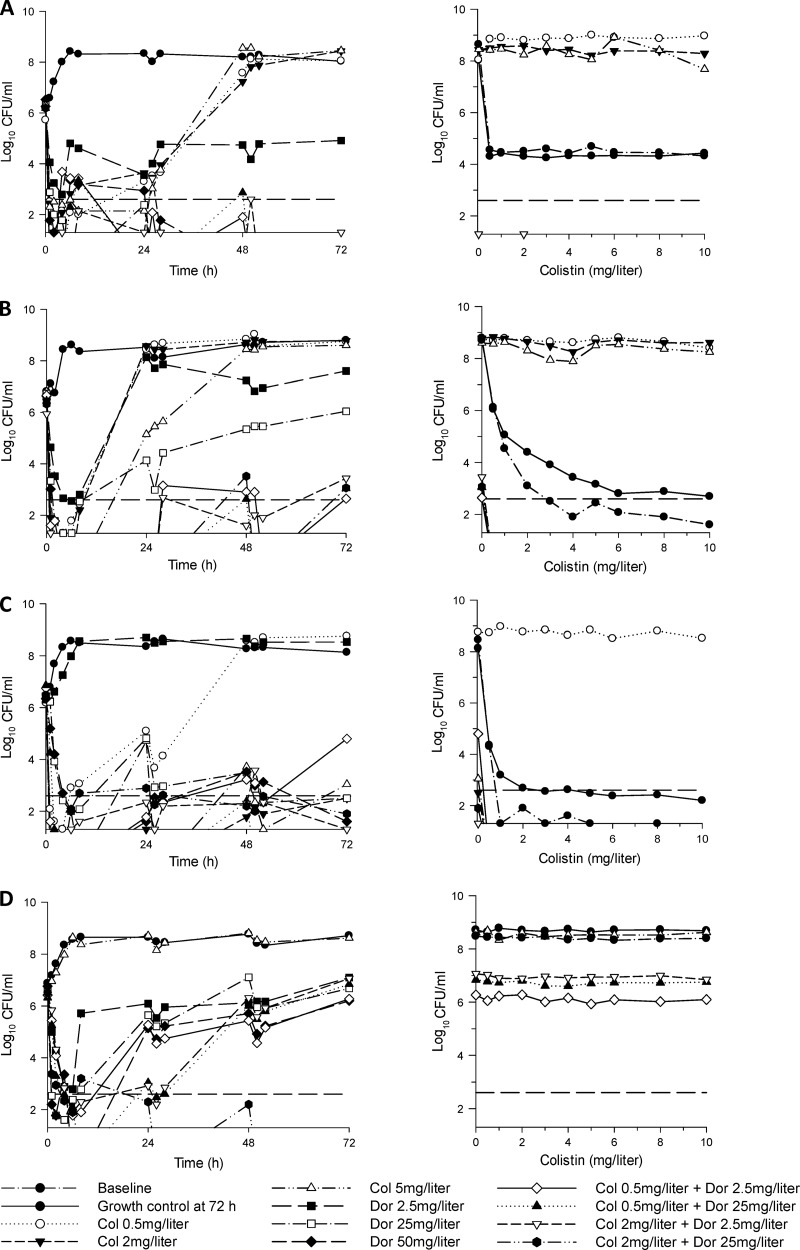
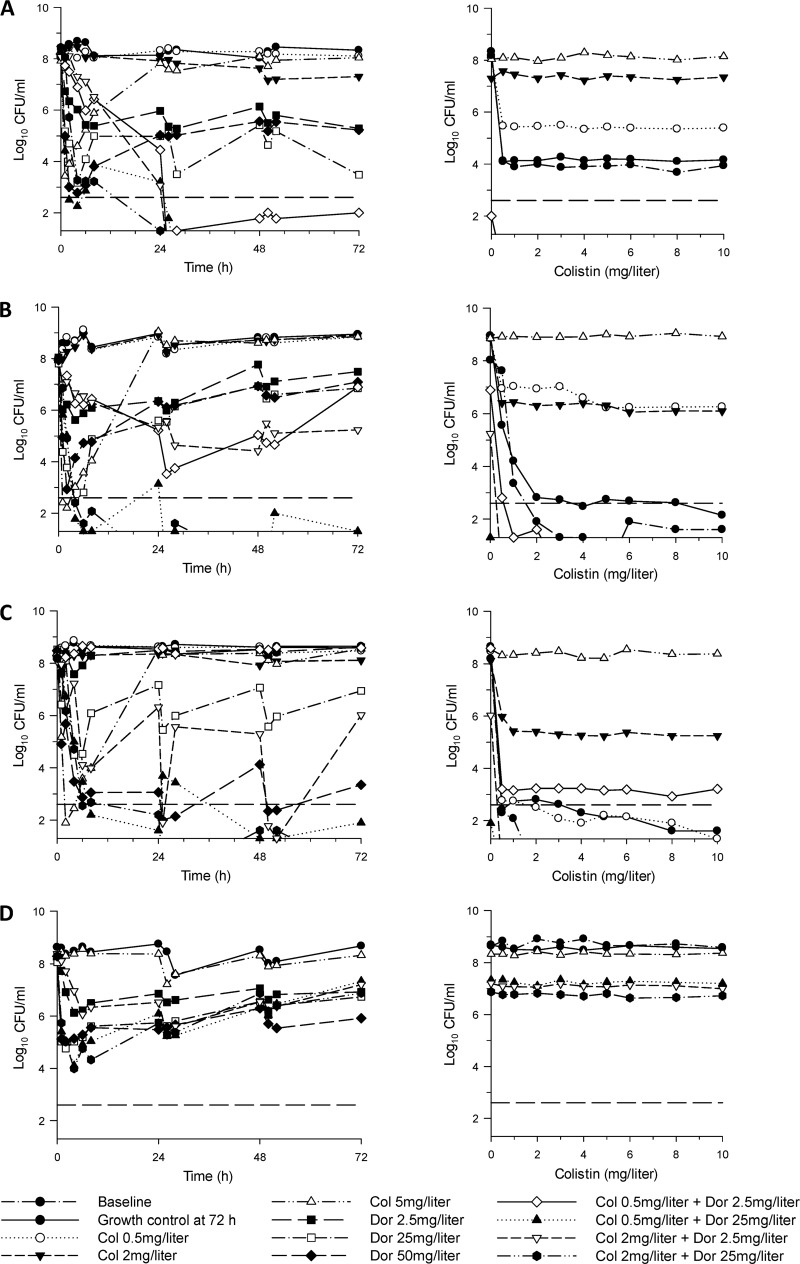
For the targeted inoculum of ∼106 CFU/ml, initial log10 CFU/ml (mean ± SD) were 6.21 ± 0.19 (n = 11), 6.54 ± 0.25 (n = 11), 6.48 ± 0.19 (n = 11), and 6.56 ± 0.18 (n = 9) for ATCC 13883, FADDI-KP033, FADDI-KP032, and FADDI-KP035, respectively; the corresponding values for the targeted inoculum of ∼108 CFU/ml were 8.17 ± 0.15 (n = 11), 7.94 ± 0.08 (n = 11), 8.28 ± 0.13 (n = 11), and 8.31 ± 0.16 (n = 8) log10 CFU/ml. The colistin PAPs conducted at baseline at an inoculum of ∼108 CFU/ml revealed the presence of preexisting colistin-resistant subpopulations (i.e., colistin heteroresistance) in some isolates (Fig. 1, baseline). The time course profiles of bacterial numbers achieved with all dosage regimens for each of the four isolates at both inocula are shown in Fig. 1 and 2. Log changes in viable cell counts at each inoculum with mono- and combination therapy are presented in Table 3.
Fig 1.
(Left) Time-kill curves with various clinically relevant dosage regimens of colistin (Col) and doripenem (Dor) alone and in combination at an inoculum of ∼106 CFU/ml. (Right) PAPs at baseline and after 72 h of exposure to colistin monotherapy, colistin-doripenem combination therapy, or neither antibiotic (growth control). Doripenem monotherapy regimens were not included in colistin-PAP examination. (A) ATCC 13883 (colistin heteroresistant, doripenem susceptible, MDR); (B) FADDI-KP033 (colistin heteroresistant, doripenem susceptible, MDR); (C) FADDI-KP032 (colistin susceptible, doripenem resistant, MDR); (D) FADDI-KP035 (colistin resistant, doripenem susceptible, MDR). The y axis starts at the limit of detection, and the limit of quantification is indicated by the dashed horizontal line.
Fig 2.
(Left) Time-kill curves with various clinically relevant dosage regimens of colistin (Col) and doripenem (Dor) alone and in combination at an inoculum of ∼108 CFU/ml. (Right) PAPs at baseline and after 72 h of exposure to colistin monotherapy, colistin-doripenem combination therapy, or neither antibiotic (growth control). Doripenem monotherapy regimens were not included in colistin-PAPs examination. (A) ATCC 13883 (colistin heteroresistant, doripenem susceptible, MDR); (B) FADDI-KP033 (colistin heteroresistant, doripenem susceptible, MDR); (C) FADDI-KP032 (colistin susceptible, doripenem resistant, MDR); (D) FADDI-KP035 (colistin resistant, doripenem susceptible, MDR). The y axis starts at the limit of detection, and the limit of quantification is indicated by the dashed horizontal line.
Table 3.
Log changes in numbers of CFUa

Log changes at 6, 24, 48, and 72 h at an inoculum of 106 or 108 CFU/ml with colistin (Col) and/or doripenem (Dor) against four K. pneumoniae isolates. The gray background indicates activity (a reduction of ≥1-log10 CFU/ml below the initial inoculum); the green background indicates synergy (a ≥2-log10 decrease in the number of CFU/ml between the combination and its most active component); the red background indicates additivity (a 1.0- to <2-log10 decrease in the number of CFU/ml between the combination and its most active component). NA, not available.
bColistin-resistant isolate; colistin monotherapy performed with 5 mg/liter only. The calculations of synergy/additivity were based on colistin at 5 mg/liter.
Colistin monotherapy.
At the 106-CFU/ml inoculum, colistin monotherapy regimens (a constant concentration of 0.5, 2, or 5 mg/liter) against the colistin-susceptible and -heteroresistant isolates produced rapid and extensive initial killing to ∼2-log10 CFU/ml or below (Fig. 1). For the colistin-susceptible isolate KP032 and the heteroresistant clinical isolate KP033, no viable bacteria were detected by 1 to 2 h with colistin at 2 and 5 mg/liter (and colistin at 0.5 mg/liter for KP033). For KP032, regrowth equivalent to that of the control occurred by 48 h with colistin at 0.5 mg/liter, whereas regrowth with the higher colistin concentration regimens was substantially reduced across the 72 h. For both heteroresistant strains, regrowth approaching that of the control occurred by 24 to 48 h with all colistin regimens (0.5, 2, and 5 mg/liter). An inoculum effect with colistin monotherapy was evident. At the 108-CFU/ml inoculum, colistin at 0.5 and 2 mg/liter produced virtually no bacterial killing at any time against the colistin-susceptible or -heteroresistant strains. Although colistin at 5 mg/liter produced rapid and extensive initial killing (∼5- to 6-log10 CFU/ml) against these isolates, regrowth was evident by 4 to 6 h, and bacterial counts had returned close to control values by 24 h in all cases (Fig. 2). Against the colistin-resistant isolate KP035, bacterial growth in the presence of colistin at 5 mg/liter was essentially no different from that of the growth control at either inoculum (Fig. 2).
Doripenem monotherapy.
For doripenem-susceptible isolates at the 106-CFU/ml inoculum, all doripenem regimens (Cmax of 2.5, 25, or 50 mg/liter) produced rapid initial bacterial killing by ≥∼3-log10 CFU/ml. For ATCC 13883, with a doripenem Cmax of 2.5 mg/liter, regrowth had begun by 6 h, and bacterial numbers remained ∼3-log10 CFU/ml below control values across the 72 h (Fig. 1); regrowth with doripenem Cmax values of 25 and 50 mg/liter was greatly suppressed for this strain, with no viable bacteria detected from 48 h onwards. For the remaining two doripenem-susceptible isolates, regrowth with doripenem Cmax values of 2.5 and 25 mg/liter was evident by 8 h and was within ∼1- to 3-log10 CFU/ml of control values by 72 h. With the highest doripenem dosage regimen (Cmax of 50 mg/liter), no viable bacteria were detected by 2 to 6 h, although substantial regrowth beginning at 24 h occurred for KP035; for KP033, no viable bacteria were detected after 2 h. For the doripenem-resistant isolate (KP032), doripenem Cmax values of 25 and 50 mg/liter each produced substantial early bacterial killing despite a doripenem MIC of 8 mg/liter, with regrowth to ∼2-log10 CFU/ml by 72 h; bacterial growth in the presence of a doripenem Cmax of 2.5 mg/liter was essentially no different from that of the growth control. An inoculum effect was generally observed with monotherapy against doripenem-susceptible isolates.
Combination therapy.
For the colistin-susceptible isolate KP032, the addition of doripenem at a Cmax of 2.5 or 25 mg/liter to colistin at 0.5 mg/liter at the low inoculum produced ∼1.5-log10-greater initial killing than monotherapy, with no viable bacteria detected from 2 to 4 h; both combinations resulted in synergy at virtually all time points across 72 h. Improved activity was particularly noticeable with the combination of colistin at 0.5 mg/liter and doripenem at 2.5 mg/liter, which produced ∼4- to 5-log10-greater killing than monotherapy at 48 and 72 h. There was no enhancement of bacterial activity at this inoculum with combinations containing colistin at 2 mg/liter, with similarly large activity compared to that in the presence of equivalent colistin monotherapy (Fig. 1C and Table 3). At the 108-CFU/ml inoculum, bacterial killing was not improved when colistin and doripenem were combined in the lowest-dosage regimens (constant 0.5 mg/liter of colistin and a doripenem Cmax of 2.5 mg/liter); however, all other combinations resulted in substantially greater activity with synergistic (predominantly) or additive effects at nearly all time points (Fig. 2C and Table 3). Combinations with colistin at 0.5 or 2 mg/liter plus a doripenem Cmax of 25 mg/liter were particularly active and produced ∼5- to 7-log10-greater killing than the most active monotherapy (doripenem) at 48 and 72 h, with no viable colonies detected across the 72-h period on at least one occasion.
Against both colistin-heteroresistant strains, bacterial killing was substantially enhanced at most time points across 72 h with most combinations at both inocula (Fig. 1 and 2 and Table 3). For clinical isolate KP033 at the low inoculum, combinations containing a doripenem Cmax of 2.5 mg/liter with colistin (0.5 or 2 mg/liter) generally resulted in ∼4- to 5-log10 greater killing across 24 to 72 h compared to results with monotherapy, whereas both combinations containing a doripenem Cmax of 25 mg/liter generally resulted in ∼2- to 3-log10 greater killing across the same period; all combinations resulted in sustained periods where no viable bacteria were detected. Of the 12 cases from 24 to 72 h (i.e., 4 combinations across 3 time points), 11 were synergistic, with the remaining case being additive (Fig. 1B and Table 3). Though activity was reduced somewhat at the higher inoculum with combinations containing a doripenem Cmax of 2.5 mg/liter, both combinations containing a doripenem Cmax of 25 mg/liter substantially increased activity and resulted in ∼5- to 7-log10-greater killing at most time points across 24 to 72 h (Fig. 2B and Table 3). The latter combinations resulted in undetectable bacterial counts on at least two occasions and, for the combination of colistin at 2 mg/liter plus a doripenem Cmax of 25 mg/liter, no viable bacteria from 48 h onwards. Bacterial killing was similarly enhanced for reference strain ATCC 13883. Although the combinations of colistin at 0.5 or 2 mg/liter plus a doripenem Cmax of 25 mg/liter failed to substantially improve activity at the low inoculum (owing to the potent bacterial killing of equivalent doripenem monotherapy), the remaining two combinations at the low inoculum and all combinations at the high inoculum substantially increased bacterial killing over the corresponding monotherapy. Notably, at the high inoculum, all combinations produced bacterial killing to below the limit of detection by 26 to 28 h (∼3- to 4-log10-greater killing than with doripenem monotherapy with a Cmax of 25 mg/liter), with no viable bacteria detected after this time for all combinations except that of colistin at 0.5 mg/liter plus a doripenem Cmax of 2.5 mg/liter; with the latter combination, regrowth to ∼2-log10 CFU/ml occurred by 72 h, which was >3-log10 CFU/ml lower than that with the most active equivalent monotherapy (doripenem).
For colistin-resistant isolate KP035 at the 106-CFU/ml inoculum, only the combination of colistin at 2 mg/liter plus a doripenem Cmax of 25 mg/liter resulted in enhanced bacterial killing. This combination provided additional killing of ∼3- and 6.5-log10 CFU/ml at 24 and 72 h, respectively, over that achieved with doripenem monotherapy; from 50 h onwards, no viable bacteria were detected with this combination (Fig. 1D and Table 3). At the high inoculum, bacterial killing and regrowth with combination therapy were very similar to those produced with equivalent doripenem monotherapy (Fig. 2D and Table 3).
Emergence of colistin resistance.
Apart from a small shift to the right at the 106-CFU/ml inoculum, PAPs for the growth controls at 72 h were generally similar to those observed at baseline. For the colistin-susceptible isolate (KP032), monotherapy with colistin at 0.5 mg/liter at the 106 CFU/ml inoculum resulted in an increase in colistin-resistant subpopulations by 24 h, growing in the presence of colistin at 10 mg/liter at 72 h; with higher colistin regimens (2 and 5 mg/liter), the low regrowth observed across 72 h at this inoculum precludes meaningful interpretation of the PAPs (Fig. 1C). At the high inoculum, a small number of resistant colonies emerged by 24 h with the colistin 0.5-mg/liter regimen, with the proportion of resistant colonies remaining approximately the same up to 72 h. At this inoculum, the colistin 5-mg/liter regimen resulted in a rapid and substantial increase in colistin-resistant colonies such that by 24 h, virtually all colonies grew in the presence of colistin at 10 mg/liter; the colistin 2-mg/liter regimen led to a slower but substantial emergence of colistin-resistant subpopulations across 72 h (Fig. 2C). For the two heteroresistant strains, all colistin monotherapy regimens (0.5, 2, and 5 mg/liter) resulted in substantial increases in colistin-resistant subpopulations by 24 to 48 h. At the 106-CFU/ml inoculum, all colistin regimens led to emergence of resistance, with growth in the presence of 8 or 10 mg/liter colistin by 24 to 48 h (data not shown); at the high inoculum, all regimens resulted in a substantial increase in resistant subpopulations with colistin at 5 mg/liter at 72 h, resulting in nearly all subpopulations growing in the presence of colistin at 10 mg/liter on the PAP plates (Fig. 2A and B). For the colistin-resistant isolate KP035, the PAPs at baseline and across the 72-h incubation period did not change irrespective of inoculum, with virtually all colonies in the control and treatment groups growing in the presence of colistin at 10 mg/liter (Fig. 1D and 2D).
Combination therapy against the colistin-susceptible isolate KP032 resulted in the emergence of colistin-resistant subpopulations only with the combination of colistin at 0.5 mg/liter plus a doripenem Cmax of 2.5 mg/liter and only at the high inoculum (Fig. 2C). Although many combinations at both inocula resulted in substantial bacterial killing, for KP032 no colistin-resistant colonies were detected at any time with the combination of colistin at 2 mg/liter and a doripenem Cmax of 2.5 mg/liter at the 108-CFU/ml inoculum despite substantial regrowth (to ∼6-log10 CFU/ml) at 72 h (Fig. 2C). For the colistin-heteroresistant strains, no colistin-resistant colonies were detected following the initiation of combination therapy at any time at either inoculum, even on the few occasions where regrowth was extensive, e.g., with isolate KP033 at the high inoculum, where regrowth to ∼7-log10 CFU/ml occurred at 72 h with the combinations of colistin at 0.5 or 2 mg/liter plus a doripenem Cmax of 2.5 mg/liter (Fig. 2B). Combination therapy had no effect on colistin resistance of the MDR colistin-resistant isolate.
DISCUSSION
With colistin (and polymyxin B) monotherapy, regrowth of K. pneumoniae as well as other bacterial species considered susceptible to colistin based upon MIC measurement is well documented both in vitro (3, 32, 44) and in vivo (25), even with concentrations well in excess of those which can be safely achieved clinically. Amplification of colistin-resistant subpopulations, which has been shown to contribute to the observed regrowth, may be particularly important for K. pneumoniae, which has a high frequency of colistin heteroresistance (75% [37] and > 90% [44] of isolates in previous studies). With currently recommended CMS dosage regimens achieving low colistin concentrations in plasma (17, 43) and toxicity limiting dose escalation in many patients (11, 17), CMS monotherapy may be suboptimal. Consequently, the investigation of rational colistin combinations is essential. We systematically investigated the effectiveness of colistin alone and in combination with doripenem against a diverse array of K. pneumoniae isolates which included colistin-susceptible, -heteroresistant, and -resistant strains.
The dosage regimens of colistin and doripenem employed in the present study were carefully designed to reflect the plasma concentration-time profiles achieved in critically ill patients (17, 39, 43). Previously we have shown that both colistin and doripenem are almost entirely unbound in CAMHB (2, 5). Currently, there is no information on unbound plasma concentrations of colistin in humans. However, assuming plasma binding of colistin in patients is similar to that in animals (i.e., ∼50% bound) (29, 52), the colistin dosage regimens of 0.5 and 2 mg/liter used in our combinations are indeed clinically achievable (17, 43). Colistin was administered as a constant infusion to mimic the flat concentration-versus-time profiles observed across a dosage interval at steady state (17, 43). All doripenem dosage regimens employed in the combinations are readily achieved in plasma after consideration of protein binding (5). Considering the inoculum effect of colistin and carbapenems (9, 10), experiments were conducted at both ∼106 and ∼108 CFU/ml; the latter inoculum mimics the high bacterial densities found in some infections (34, 47). In general, the colistin-and-doripenem combination maintained its excellent activity at both inocula (Table 3) and was less subject to an inoculum effect compared to monotherapy with either agent.
The PK/PD index that best predicts colistin activity against K. pneumoniae is currently unknown. Against P. aeruginosa (2, 12) and A. baumannii (13), area under the unbound drug concentration-time curve over 24 h in the steady state divided by the MIC (fAUC/MIC) is the index for colistin most closely correlated with bacterial killing. For P. aeruginosa, an fAUC/MIC of >∼20 was required to achieve a 1-log reduction at 24 h against most isolates (2, 12); this value was somewhat lower (∼10) for equivalent killing of A. baumannii (13). If an fAUC/MIC target of >20 is applied to the present study, only the colistin 0.5-mg/liter regimen failed to reach this value against colistin-susceptible and -heteroresistant isolates (fAUC/MIC of 12), whereas all regimens were considerably below a fAUC/MIC of 20 for the colistin-resistant isolate (Table 2). However, even with a fAUC/MIC of 48 (colistin 2-mg/liter regimen) or 120 (colistin 5-mg/liter regimen), monotherapy with colistin reduced bacterial growth by >1 log at 24 h only with the 106-CFU/ml inoculum (in five of six cases; Table 3). The PK/PD index most closely associated with doripenem activity against Gram-negative bacilli is the cumulative percentage of a 24-h period that the unbound drug concentration exceeds the MIC under steady-state PK conditions (ƒT>MIC) (50). For doripenem, an ƒT>MIC of 35 to 45% is required for a >1-log reduction in bacterial growth (50). Against the doripenem-susceptible isolates, this target was achieved with all doripenem regimens; against the doripenem-resistant isolate, only the doripenem Cmax 50-mg/liter regimen achieved this target (Table 2). While a >1-log bacterial kill at 24 h was achieved against the reference strain and all isolates at both inocula with doripenem Cmax 25- and 50-mg/liter regimens, this was achieved in only half the cases across both inocula with the Cmax 2.5-mg/liter regimen (Table 3). Combining colistin with doripenem produced substantially enhanced bacterial killing at 24 h in 20 out of 24 cases against colistin-susceptible and -heteroresistant isolates, even with a low index value for each drug. For example, with the colistin 0.5-mg/liter (fAUC/MIC of 12) plus doripenem Cmax 2.5- or 25-mg/liter (ƒT>MIC < 35%) regimen against doripenem-resistant KP032, >1-log killing at 24 h occurred in 3 of 4 cases with the combination compared to 3 of 6 cases with equivalent monotherapy (Table 3).
Despite all isolates containing β-lactamases, improvements in bacterial killing over that of equivalent monotherapy were observed across the 72-h duration at both inocula against colistin-susceptible and -heteroresistant strains. Although enhanced bacterial killing was generally present with all combinations, the killing effect was particularly strong for combinations containing a doripenem Cmax of 25 mg/liter with colistin dosage regimens of either 0.5 or 2 mg/liter. That the addition of a doripenem Cmax of 25 mg/liter to low-dosage regimens of colistin (e.g., 0.5 mg/liter) can substantially improve bacterial killing is an important observation given that many patients will achieve only low plasma colistin concentrations with currently recommended CMS dosage regimens (17, 43). Encouragingly, improvements in activity with combination therapy (colistin at 2 mg/liter and a doripenem Cmax of 25 mg/liter) were also observed against the colistin-resistant isolate, albeit only with the 106-CFU/ml inoculum.
Previous studies have utilized static time-kill methods to investigate the combination of a polymyxin (colistin or polymyxin B) and a carbapenem against K. pneumoniae (8, 16, 23, 41, 46, 49). While these studies generally reported enhanced activity (usually reported as synergy) with the combination, only a single, generally low inoculum (∼106 CFU/ml) was employed, and experiments were conducted for no longer than 24 h. Only one study employed more than one dosage regimen of polymyxin B (41), and importantly, the emergence of polymyxin resistance (e.g., by using real-time PAPs) was not reported. To the best of our knowledge, the present study is the first to investigate polymyxin and carbapenem combination therapy against K. pneumoniae using an in vitro PK/PD model and to investigate the emergence of colistin resistance with combination therapy. On only one occasion were colistin-resistant colonies detected following combination therapy for the colistin-susceptible or -heteroresistant isolates, and this occurred at the high inoculum with the lowest-dosage regimens of colistin and doripenem (0.5 mg/liter and Cmax at 2.5 mg/liter, respectively). Although the extensive killing generally observed against these isolates with the combination regimens complicates the interpretation of PAPs, on the few occasions where extensive regrowth (e.g., up to ∼7-log10 CFU/ml) occurred with combination therapy, no colistin-resistant colonies were detected; the reason for the observed regrowth despite an apparent lack of colistin resistance is unknown and is under investigation. This important finding clearly suggests that combining doripenem with colistin against K. pneumoniae may reduce the emergence of colistin-resistant subpopulations.
As multidrug resistance increases and we await active new agents, rationally designed combinations of existing antibiotics are of paramount importance. We have shown for the first time that clinically relevant dosage regimens of colistin and doripenem in combination substantially increase bacterial killing against colistin-susceptible, -heteroresistant (and doripenem-resistant), and colistin-resistant MDR K. pneumoniae and limit the emergence of colistin resistance. Further investigations of colistin combinations in animal models and patients are warranted.
ACKNOWLEDGMENTS
The study was supported by R01AI079330 and R01AI070896 from the National Institute of Allergy and Infectious Diseases (NIAID). T.V., R.L.N., and J.L. are supported by the Australian National Health and Medical Research Council (NHMRC). J.L. is an Australian NHMRC senior research fellow. T.V. is an Australian NHMRC Industry Career Development research fellow. J.B.B. is supported by an Australian Research Council (ARC) DECRA fellowship. Z.Z.D. is a Ph.D. candidate cosponsored by the Malaysian government and Universiti Sains Malaysia.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Published ahead of print 16 July 2012
REFERENCES
- 1. Antoniadou A, et al. 2007. Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: first report of a multiclonal cluster. J. Antimicrob. Chemother. 59:786–790 [DOI] [PubMed] [Google Scholar]
- 2. Bergen PJ, et al. 2010. Pharmacokinetic/pharmacodynamic investigation of colistin against Pseudomonas aeruginosa using an in vitro model. Antimicrob. Agents Chemother. 54:3783–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergen PJ, et al. 2011. Clinically relevant plasma concentrations of colistin in combination with imipenem enhance pharmacodynamic activity against multidrug-resistant Pseudomonas aeruginosa at multiple inocula. Antimicrob. Agents Chemother. 55:5134–5142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergen PJ, Li J, Rayner CR, Nation RL. 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:1953–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bergen PJ, et al. 2011. Synergistic killing of multidrug-resistant Pseudomonas aeruginosa at multiple inocula by colistin combined with doripenem in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 55:5685–5695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bogdanovich T, et al. 2011. Colistin-resistant, Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae belonging to the international epidemic clone ST258. Clin. Infect. Dis. 53:373–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boucher HW, et al. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1–12 [DOI] [PubMed] [Google Scholar]
- 8. Bratu S, et al. 2005. Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J. Antimicrob. Chemother. 56:128–132 [DOI] [PubMed] [Google Scholar]
- 9. Bulitta JB, et al. 2010. Attenuation of colistin bactericidal activity by high inoculum of Pseudomonas aeruginosa characterized by a new mechanism-based population pharmacodynamic model. Antimicrob. Agents Chemother. 54:2051–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conejo MC, Rodríguez-Martínez JM, Serrano L, Pascual Á. 2011. Comparative activity of doripenem, ertapenem and imipenem against clinical isolates of Klebsiella pneumoniae producing extended-spectrum beta-lactamases, plasmid-mediated AmpC-type beta-lactamases or both, associated or not with porin deficiency, abstr. P1182. Twenty-first Eur. Congr. Clin. Microbiol. Infect. Dis. (ECCMID)-27th Int. Congr. Chemother. (ICC), Milan, Italy, 7 to 10 May 2011 European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland [Google Scholar]
- 11. DeRyke CA, Crawford AJ, Uddin N, Wallace MR. 2010. Colistin dosing and nephrotoxicity in a large community teaching hospital. Antimicrob. Agents Chemother. 54:4503–4505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dudhani RV, et al. 2010. Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models. Antimicrob. Agents Chemother. 54:1117–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dudhani RV, Turnidge JD, Nation RL, Li J. 2010. fAUC/MIC is the most predictive pharmacokinetic/pharmacodynamic index of colistin against Acinetobacter baumannii in murine thigh and lung infection models. J. Antimicrob. Chemother. 65:1984–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. European Committee on Antimicrobial Susceptibility Testing 5 January 2011. Breakpoint tables for interpretation of MICs and zone diameters, version 1.3 European Committee on Antimicrobial Susceptibility Testing; http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/EUCAST_breakpoints_v1.3_pdf.pdf [Google Scholar]
- 15. European Committee on Antimicrobial Susceptibility Testing 2003. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 9:ix–xv [Google Scholar]
- 16. Farrell A, Clancy C, Press E, Shields RK. 2011. A combination of colistin+doripenem+ertapenem is effective against Klebsiella pneumoniae producing carbapenemase (KPC) isolates with high-level colistin (C) and carbapenem (CBM) resistance, abstr. P-252. Infect. Dis. Soc. Am. (IDSA) Annu. Meet., Boston, MA, 20 to 23 October 2011 [Google Scholar]
- 17. Garonzik SM, et al. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 55:3284–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gootz TD. 2006. The forgotten Gram-negative bacilli: what genetic determinants are telling us about the spread of antibiotic resistance. Biochem. Pharmacol. 71:1073–1084 [DOI] [PubMed] [Google Scholar]
- 19. Hartzell JD, et al. 2009. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin. Infect. Dis. 48:1724–1728 [DOI] [PubMed] [Google Scholar]
- 20. Hawley JS, Murray CK, Jorgensen JH. 2008. Colistin heteroresistance in Acinetobacter and its association with previous colistin therapy. Antimicrob. Agents Chemother. 52:351–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ho J, Tambyah PA, Paterson DL. 2010. Multiresistant Gram-negative infections: a global perspective. Curr. Opin. Infect. Dis. 23:546–553 [DOI] [PubMed] [Google Scholar]
- 22. Infectious Diseases Society of America 2011. Combating antimicrobial resistance: policy recommendations to save lives. Clin. Infect. Dis. 52:S397–S428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jernigan MG, Press EG, Nguyen MH, Clancy CJ, Shields RK. 19 March 2012. The combination of doripenem and colistin is bactericidal and synergistic against colistin-resistant, carbapenemase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. doi:10.1128/AAC.06364-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karabinis A, et al. 2004. Colistin for Klebsiella pneumoniae-associated sepsis. Clin. Infect. Dis. 38:e7–e9 doi:10.1086/380461 [DOI] [PubMed] [Google Scholar]
- 25. Kethireddy S, et al. 2007. In vivo pharmacodynamics of colistin against Pseudomonas aeruginosa in thighs of neutropenic mice, abstr. A-4, p 1. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 26. Kontopoulou K, et al. 2010. Hospital outbreak caused by Klebsiella pneumoniae producing KPC-2 β-lactamase resistant to colistin. J. Hosp. Infect. 76:70–73 [DOI] [PubMed] [Google Scholar]
- 27. Kumarasamy KK, et al. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 10:597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kwon J-A, et al. 2010. Predictors of acute kidney injury associated with intravenous colistin treatment. Int. J. Antimicrob. Agents 35:473–477 [DOI] [PubMed] [Google Scholar]
- 29. Li J, et al. 2003. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob. Agents Chemother. 47:1766–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li J, Nation RL. 2006. Old polymyxins are back: is resistance close? Clin. Infect. Dis. 43:663–664 [DOI] [PubMed] [Google Scholar]
- 31. Li J, et al. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 6:589–601 [DOI] [PubMed] [Google Scholar]
- 32. Li J, et al. 2006. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 50:2946–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lim LM, et al. 2010. Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy 30:1279–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mah T-FC, O'Toole GA. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34–39 [DOI] [PubMed] [Google Scholar]
- 35. Marchaim D, et al. 2011. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob. Agents Chemother. 55:593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matthews SJ, Lancaster JW. 2009. Doripenem monohydrate, a broad-spectrum carbapenem antibiotic. Clin. Ther. 31:42–63 [DOI] [PubMed] [Google Scholar]
- 37. Meletis G, Tzampaz E, Sianou E, Tzavaras I, Sofianou D. 2011. Colistin heteroresistance in carbapenemase-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 66:946–947 [DOI] [PubMed] [Google Scholar]
- 38. Moellering RC. 2010. NDM-1—a cause for worldwide concern. N. Engl. J. Med. 363:2377–2379 [DOI] [PubMed] [Google Scholar]
- 39. Nandy P, Samtani MN, Lin R. 2010. Population pharmacokinetics of doripenem based on data from phase 1 studies with healthy volunteers and phase 2 and 3 studies with critically ill patients. Antimicrob. Agents Chemother. 54:2354–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nation RL, Li J. 2009. Colistin in the 21st century. Curr. Opin. Infect. Dis. 22:535–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pankey GA, Ashcraft DS. 2011. Detection of synergy using the combination of polymyxin B with either meropenem or rifampin against carbapenemase-producing Klebsiella pneumoniae. Diagn. Microbiol. Infect. Dis. 70:561–564 [DOI] [PubMed] [Google Scholar]
- 42. Pillai SK, Moellering RC, Jr, Eliopoulos GM. 2005. Antimicrobial combinations, p 365–424 In Lorian V. (ed), Antibiotics in laboratory medicine, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 43. Plachouras D, et al. 2009. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by Gram-negative bacteria. Antimicrob. Agents Chemother. 53:3430–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Poudyal A, et al. 2008. In vitro pharmacodynamics of colistin against multidrug-resistant Klebsiella pneumoniae. J. Antimicrob. Chemother. 62:1311–1318 [DOI] [PubMed] [Google Scholar]
- 45. Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee 2006. Management of multidrug-resistant organisms in healthcare settings, 2006, p 5 Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/hicpac/pdf/MDRO/MDROGuideline2006.pdf [DOI] [PubMed] [Google Scholar]
- 46. Souli M, et al. 2009. Does the activity of the combination of imipenem and colistin in vitro exceed the problem of resistance in metallo-β-lactamase-producing Klebsiella pneumoniae isolates? Antimicrob. Agents Chemother. 53:2133–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stressmann FA, et al. 2011. Does bacterial density in cystic fibrosis sputum increase prior to pulmonary exacerbation? J. Cyst. Fibros. 10:357–365 [DOI] [PubMed] [Google Scholar]
- 48. Tóth Á, et al. 2010. Emergence of a colistin-resistant KPC-2-producing Klebsiella pneumoniae ST258 clone in Hungary. Eur. J. Clin. Microbiol. Infect. Dis. 29:765–769 [DOI] [PubMed] [Google Scholar]
- 49. Urban C, Mariano N, Rahal JJ. 2010. In vitro double and triple bactericidal activities of doripenem, polymyxin B, and rifampin against multidrug-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli. Antimicrob. Agents Chemother. 54:2732–2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van Wart SA, Andes DR, Ambrose PG, Bhavnani SM. 2009. Pharmacokinetic-pharmacodynamic modeling to support doripenem dose regimen optimization for critically ill patients. Diagn. Microbiol. Infect. Dis. 63:409–414 [DOI] [PubMed] [Google Scholar]
- 51. Yau W, et al. 2009. Colistin hetero-resistance in multidrug-resistant Acinetobacter baumannii clinical isolates from the Western Pacific region in the SENTRY antimicrobial surveillance programme. J. Infect. 58:138–144 [DOI] [PubMed] [Google Scholar]
- 52. Ziv G, Wanner M, Nicolet J. 1980. Clinical pharmacology of polymyxin B, colistin and colistimethate in young dairy calves. J. Vet. Pharmacol. Ther. 3:87–94 [Google Scholar]