Abstract
A nutritional characteristic of trypanosomatid protozoa is that they need a heme compound as a growth factor. Because of the cytotoxic activity of heme and its structural similarity to cobalamins, we have investigated the in vitro and in vivo effect of vitamin B12 (or cyanocobalamin) on the different forms of Trypanosoma cruzi. Cyanocobalamin showed a marked antiparasitic activity against epimastigotes (50% inhibitory concentration [IC50], 2.42 μM), amastigotes (IC50, 10.69 μM), and trypomastigotes (IC50, 9.46 μM). Anti-epimastigote and -trypomastigote values were 1.7 to 4 times lower than those obtained with the reference drug benznidazole (Bnz). We also found that B12 and hemin do not interact with each other in their modes of action. Our results show that B12 increases intracellular oxidative activity and stimulates both superoxide dismutase (50%) and ascorbate peroxidase (20%) activities, while the activity of trypanothione reductase was not modified. In addition, we found that the antioxidants dithiothreitol and ascorbic acid increase the susceptibility of the parasite to the cytotoxic action of B12. We propose that vitamin B12 exerts its growth-inhibitory effect through the generation of reactive oxygen species. In an in vivo assay, a significant reduction in the number of circulating parasites was found in T. cruzi-infected mice treated with cyanocobalamin and ascorbic acid. The reduction of parasitemia in benznidazole-treated mice was improved by the addition of these vitamins. According to our results, a combination of B12 and Bnz should be further investigated due to its potential as a new therapeutic modality for the treatment of Chagas' disease.
INTRODUCTION
Trypanosoma cruzi is the causative agent of Chagas' disease, which is a major disease that is endemic to Latin America. Current treatments employ benznidazole (Bnz) or nifurtimox, two drugs that have been in use for more than 40 years. The chemotherapy for this disease is not satisfactory due to the limited efficacy and the toxicity associated with long-term treatments (15, 16). Research and development of new drugs effective in the treatment of this disease, which affects 16 to 18 million people in the Americas (25), is a real need and requires new strategies for drug development (2, 22).
Heme compounds are necessary as growth factors for T. cruzi (7). However, hemin and related porphyrins have an important cytotoxic action through the generation of reactive oxygen species (ROS), such as superoxide anion (O2−), hydrogen peroxide (H2O2), and the highly reactive hydroxyl radical (OH ·) (6). We have previously studied the effect of hemin on growth and the antioxidant defense system in T. cruzi epimastigotes (4), demonstrating the correlation between higher hemin concentrations in the culture medium and oxidative damage in the cells. Concentrations above 15 μg/ml hemin produced a clear decrease in growth rate, inducing the transformation of epimastigotes into amastigotes accompanied by a marked injury to the antioxidant enzymatic machinery of the parasite (4). Similar results have been reported for Leishmania donovani promastigotes cultured in vitro (14).
Since the structures and uptake systems of heme and cobalamin are alike (5), and the known cytotoxic and antitumor activity of cobalamins administered either alone or combined with antioxidants are also similar (10, 18, 19, 23), it was of great interest to investigate the effect of cyanocobalamin (vitamin B12) on T. cruzi.
Here, we have evaluated the in vitro antiparasitic activity of vitamin B12 against different forms of T. cruzi, and we have explored its possible mode of action. Additionally, we have analyzed the interaction of B12 with hemin and the improvement in activity by the addition of antioxidants. More importantly, we have investigated the capacity of B12 to control parasitemia in the murine model, showing its effect combined with Bnz treatment as a novel therapeutic modality.
MATERIALS AND METHODS
Chemicals.
Hemin, NADPH, EDTA, NADH, chlorophenol red-β-d-galactopyranoside (CPRG), RPMI 1640 medium, hydrogen peroxide, ascorbic acid, and 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) were obtained from Sigma Chemical Co. (St. Louis, MO). Yeast extract, tryptose, powdered beef liver, and brain heart infusion were from Difco Laboratories (Sparks, MD). Trypanothione was purchased from Bachem Bioscience Inc. Bnz was kindly provided by Roche (Argentina). All other chemicals were of the highest purity commercially available.
Parasites.
Trypanosoma cruzi epimastigotes (Tulahuen strain) were grown at 28°C in a liquid medium containing 0.3% yeast extract, 0.9% tryptose, 0.4% dextrose, 1% disodium phosphate 2-hydrate, 0.36% sodium chloride, 0.04% potassium chloride, 0.15% powdered beef liver, 0.5% brain heart infusion, and 0.5 to 1.0 mg/100 ml hemin. T. cruzi bloodstream trypomastigotes were obtained from infected CF1 mice by cardiac puncture at the peak of parasitemia on day 15 postinfection. Trypomastigotes were routinely maintained by infecting 21-day-old CF1 mice. T. cruzi parasites from the Tulahuen strain stably expressing the β-galactosidase (β-gal) gene were kindly provided by F. S. Buckner (3).
Animals.
Outbred CF1 male and inbred C3H/HeN female mice were nursed at the Departamento de Microbiología, Facultad de Medicina, Universidad de Buenos Aires. Animals were treated in accordance with guidelines established by the Animal Care and Use Committee of the Argentine Association of Specialists in Laboratory Animals (AADEALC).
In vitro assays for anti-T. cruzi activity.
To evaluate the growth inhibition of T. cruzi epimastigotes, parasites from a 3-day-old culture were inoculated into fresh culture medium to reach an initial concentration of 1.5 × 107 to 2.5 × 107 cells/ml. Cells were cultured in the presence of 0.125 to 15 μM B12 or 0.75 to 25 μM Bnz (used as a positive control) for 3 days (or the time indicated in the experiment). The compound's ability to inhibit the growth of the parasite was evaluated in triplicate and compared to the control without drug. Cell growth was monitored by counting the number of cells per ml of culture using a Neubauer chamber and was expressed as cellular density (CD). The percent inhibition (%I) was calculated as %I = {1 − [(CD3t − CD0)/(CD3c − CD0)]} × 100, where CD3t is the cellular density of treated parasites at day 3, CD0 is the cellular density of parasites at day 0, and CD3c is the cellular density of untreated parasites (control) at day 3. To evaluate the combinatory effect of B12 and Bnz, the 50% inhibitory concentrations (IC50s) for Bnz in the presence of different B12 concentrations (0.8 to 2.4 μM) were calculated. The inhibitory activity of B12 (0.25 μM) was also evaluated in the presence of antioxidant agents such as dithiothreitol (DTT; 0.5 to 1 μM) and vitamin C or ascorbic acid (0.5 to 1.5 μM).
The trypanocidal effects were also tested on bloodstream trypomastigotes according to a standard WHO protocol with minor modifications (20, 21). Briefly, parasites were counted in a Neubauer chamber and the blood was diluted in RPMI culture medium to a final concentration of 1.5 × 106 trypomastigotes/ml. Parasites were seeded (150 μl/well) in duplicate in a 96-well microplate in the presence of B12 (0.37 to 72 μM) or Bnz (0.38 to 38 μM). Plates were incubated for 24 h, and the remaining live parasites were counted in a Neubauer chamber. The results were expressed as the percentage of lysed parasites (%L) relative to the number of parasites in the control (without adding the drug).
An amastigote growth inhibition assay was performed on 96-well tissue culture plates seeded with a murine macrophage cell line, J774, at 5 × 103 per well in 100 μl complete RPMI medium without phenol red and incubated for 2 h at 37°C and 5% CO2. Cells were infected with transfected trypomastigotes expressing β-galactosidase at a parasite/cell ratio of 10:1 as previously described (12). After 24 h of coculture, plates were washed to remove unbound parasites and drug compounds were added in duplicate. Controls included uninfected J774 cells (0% infection control) and cell monolayers infected with trypomastigotes (100% infection control). On day 7, the assays were developed by addition of CPRG (100 μM final concentration) and Nonidet P-40 (1% final concentration). Plates were incubated for 4 to 6 h at 37°C. Wells with galactosidase activity turned substrate from yellow to red, and this was quantified using a microplate reader (A570; Bio-Rad Laboratories). Percent inhibition (%I) was calculated as 100 − {[(absorbance of treated infected cells − absorbance of treated uninfected cells)/(absorbance of untreated infected cells)] × 100}, and the IC50 was estimated. Because B12 is colored and shows significant absorbance at 570 nm, blanks of uninfected cells with the different doses of B12 were done and their absorbances were subtracted.
Cytotoxicity assay.
Cytotoxic activity was evaluated in vitro using Vero cells cultured under standard conditions. Cells (9 × 105 cell/ml) were seeded in a 24-well plate, and after 48 h different concentrations of B12 (6 to 2,400 μM) or Bnz (3 to 3000 μM) were added. After 24 h of incubation, cells were washed twice with phosphate-buffered saline (PBS), and 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) was added at a final concentration of 0.5 mg/ml. Plates were incubated for 1 h at 37°C. Finally, blue precipitates were dissolved in 0.5 ml of dimethyl sulfoxide (DMSO) and were read on a plate reader (Spectra Count BS 10001) at a wavelength of 570 nm. Values from blank wells containing only medium and reagents were subtracted from the values of the samples. The values of absorbance showed a good correlation with viable cell counts using trypan blue. All MTT assays were repeated at least three times by using four samples per assay. The selectivity index (SI) was calculated as the 50% cytotoxic concentration on Vero cells divided by the IC50 of the compound for T. cruzi cells.
Assay of intracellular oxidative activity.
The intracellular oxidative activity was assessed using the oxidant-sensitive fluorescent probe H2DCFDA. After 3, 7, or 24 h of treatment with 15, 30, and 60 μM B12 (these conditions are herein referred to as short-time treatment), the parasites were harvested, suspended in PBS at a concentration of 1 × 106 cells/ml, and stained for 30 min in the dark at 37°C with 10 μM H2DCFDA taken from a 0.2 mM stock solution in DMSO. The fluorescence of dichlorofluorescein (DCF) in cells was analyzed by a Becton, Dickinson FACScalibur flow cytometer with an excitation wavelength of 480 nm and an emission wavelength of 530 nm. The flow cytometry results were expressed by the ratio Gmt/Gmc, where Gmt and Gmc correspond to the geometric means of histograms obtained for treated and untreated (control) cells, respectively.
Enzymatic determinations.
All steps were performed at 2 to 4°C. Parasites (200 ml of culture) coming from short-time treatment were harvested by centrifugation at 12,000 × g for 10 min, washed once, and resuspended in 5 ml of (i) Tris-HCl buffer for ascorbate peroxidase (APx) activity, (ii) sodium phosphate buffer for superoxide dismutase (SOD) activity, and (iii) potassium phosphate buffer for trypanothione reductase (TryR) activity. Cells in suspension were disrupted by sonication in an MSE Soniprep 150 ultrasonic disintegrator for 45 s. The resulting homogenate was centrifuged at 5,000 × g for 15 min, the precipitate was discarded, and the supernatant was employed as the source of enzymes. Protein concentration was determined according to the method described by Lowry et al. (8), and these values were considered to express the enzymatic activities as specific activities (enzyme unit [EU]/mg of protein).
SOD activity has been assayed by a spectrophotometric method based on the inhibition of superoxide-driven NADH oxidation as previously described (4). One EU is defined as the amount of protein required to inhibit 50% NADH oxidation.
APx activity was measured following the change in absorbance at 265 nm due to ascorbate oxidation at 25°C (4). Enzyme activity was calculated by using an ε value of 16.00 × 103 M−1 cm−1. The EU is defined as the amount of enzyme forming 1 nmol of product per s under standard incubation conditions.
TryR activity was determined following NADPH oxidation at 340 nm at 25°C (1). The activity was calculated using an extinction coefficient of 6.22 × 103 M−1 cm−1. One EU is defined as the amount of enzyme forming 1 nmol of product per min under standard incubation conditions.
In vivo trypanocidal activity assay.
Groups of five C3H/HeN mice (6 to 8 weeks old) maintained under standard conditions were infected with 5 × 103 bloodstream T. cruzi trypomastigotes by the intraperitoneal route. Five days after infection, the presence of circulating parasites was confirmed by a microhematocrit method. Mice were treated with B12 (1.5 mg/kg of body weight/day), B12 (1.5 mg/kg/day) plus ascorbic acid (1.5 mg/kg/day), Bnz (0.75 mg/kg/day), or a combination of the treatments. The administration of the drugs was performed from Monday through Friday during 2 weeks (days 5 to 9 and 12 to 16 postinfection) by the intraperitoneal route. Drugs were resuspended in 0.1 M PBS (pH 7.2), and this vehicle also was employed as a negative control. In addition, control groups receiving ascorbic acid (1.5 mg/kg/day) or a combination of both at half doses (0.75 mg/kg/day each) were included. Levels of parasitemia were monitored every 2 days in 5 μl of blood diluted 1:5 in lysis buffer (0.75% NH4Cl, 0.2% Tris, pH 7.2) by counting parasites in a Neubauer chamber. The number of deaths was recorded daily.
Statistical analysis.
All data are expressed as means ± standard errors of the means (SEM), and parasitemia is also expressed as the area under the curve. To calculate the IC50s, the %I or %L values were plotted against the log of drug concentration (μM) and fitted with a straight line determined by a linear regression (Sigma Plot 10 software). The fractional inhibitory concentrations (FICs) were calculated as the ratio of the IC50 of the drug in combination and the IC50 of the drug alone. The FIC index (FICI) for two drugs was the FIC of B12 plus the FIC of Bnz. The significance of differences was evaluated using Student's t test, taking P < 0.05 as significant. The results presented are representative of three to four independent experiments. Survival curves were compared using a log-rank test.
RESULTS
In vitro antiparasitic activity.
T. cruzi epimastigotes were grown for 7 days in culture medium containing 10 μg/ml hemin plus different concentrations of B12 (Fig. 1), and cells were counted daily. All of the tested B12 concentrations produced a decrease in the growth rate compared to the control in a dose-dependent manner, as observed on the third day. A concentration of B12 as high as 45 μM presented trypanocidal activity showing a negative slope until day 3. For longer times, growth rates were similar, showing that the B12 antiparasitic effect only lasts for short periods of time, which may be explained by the instability of B12 in the culture.
Fig 1.
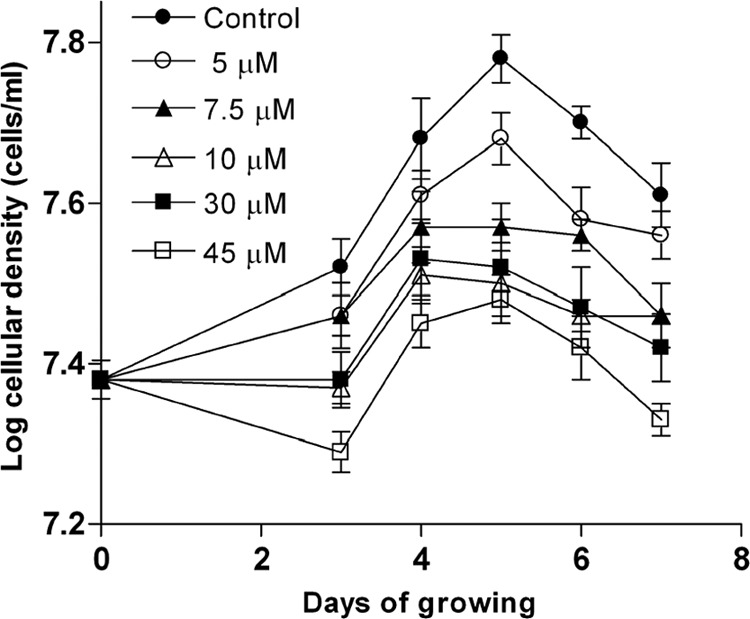
Effect of vitamin B12 on the proliferation of T. cruzi epimastigotes. The parasites were cultured at 28°C for 7 days with different B12 concentrations (0 to 45 μM) and were counted daily. All other experimental conditions were as indicated in Materials and Methods.
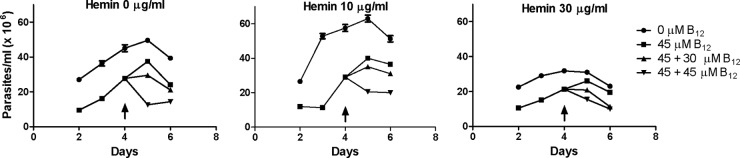
Independently of the doses and exposure time, B12 did not produce morphological changes, as observed by optical microscopy, but motility was markedly diminished (data not shown). The inhibitory effect of B12 was manifested independently of the hemin concentration added to the cultures (Fig. 2). To corroborate if the parasites were still B12 susceptible after 4 days of treatment, a new addition was made showing a dose-dependent response. The results obtained in the absence of B12 corroborate the inhibitory effect of high concentrations of hemin (30 μg/ml) on the growth of T. cruzi epimastigotes already reported (4) (Fig. 2).
Fig 2.
Effect of vitamin B12 on epimastigotes cultured with different hemin concentrations. T. cruzi epimastigotes were grown in the presence (45 μM) or absence of B12 in culture medium containing different hemin concentrations (0, 10, and 30 μg/ml). The cells were counted daily between days 2 and 6. On the fourth day, vitamin B12 was added again at a concentration of 30 or 45 μM (arrow). All other experimental conditions were those described in Materials and Methods.
We next analyzed the inhibitory activity of B12 and Bnz, the drug currently used for treatment of Chagas' disease, against the three stages of T. cruzi (Table 1). Similar IC50s for B12 on trypo- and amastigote forms were found. B12 was found to be 1.7 to 3.6 and 2.6 to 4 times more active than Bnz on the epimastigote and trypomastigote forms, respectively.
Table 1.
Activity of vitamin B12 and Bnz on epi-, trypo-, and amastigote forms of T. cruzia
| Compound | Epimastigote IC50 (μM) after 3 days | Trypomastigote IC50 (μM) after day | Amastigote IC50 (μM) after 7 days |
|---|---|---|---|
| B12 | 2.42 ± 0.54 | 9.46 ± 1.20 | 10.69 ± 1.50 |
| Bnz | 5.86 ± 0.93 | 30.26 ± 2.85 | 4.10 ± 0.55 |
IC50s were calculated as indicated in Materials and Methods.
In epimastigotes the combined effect of B12 and Bnz was also investigated. The isobologram depicted in Fig. 3 shows an additive effect for the combination of both drugs.
Fig 3.
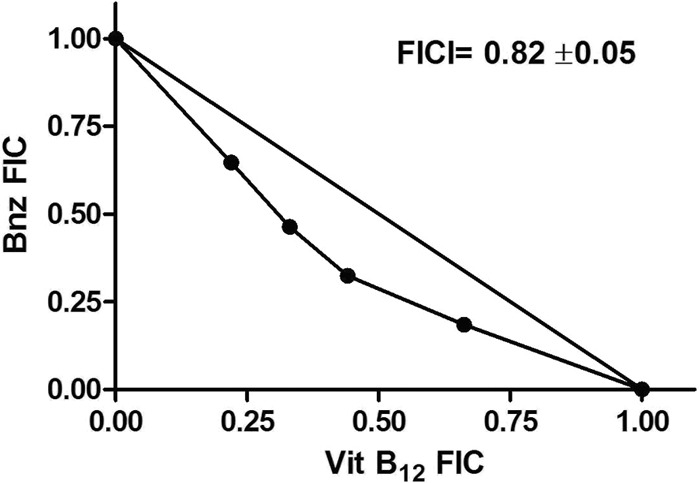
Isobologram describing the interaction between vitamin B12 and Bnz against epimastigotes of T. cruzi. The fractional inhibitory concentrations (FICs) and the FIC index (FICI) were determined as described in Materials and Methods. An FICI of >0.5 to 4.0 indicates no interaction between vitamin B12 and Bnz.
Cytotoxicity assay.
The results of the cytotoxic activity of B12 or Bnz on Vero cells are shown in Fig. 4. For B12 concentrations as high as 2,400 μM, no cytotoxic effect was found, while for Bnz the 50% cytotoxic concentration was 82.79 ± 2.75 μM. The SI was employed to compare the toxicity for mammalian cells and the activity against the parasites. The SIs for the epimastigote form of T. cruzi were >991.7 for B12 and 14.1 for Bnz, while for the trypomastigote forms, values of >253.7 for B12 and 2.7 for Bnz were found. For the amastigote form, the SI value for B12 was 224.5.
Fig 4.
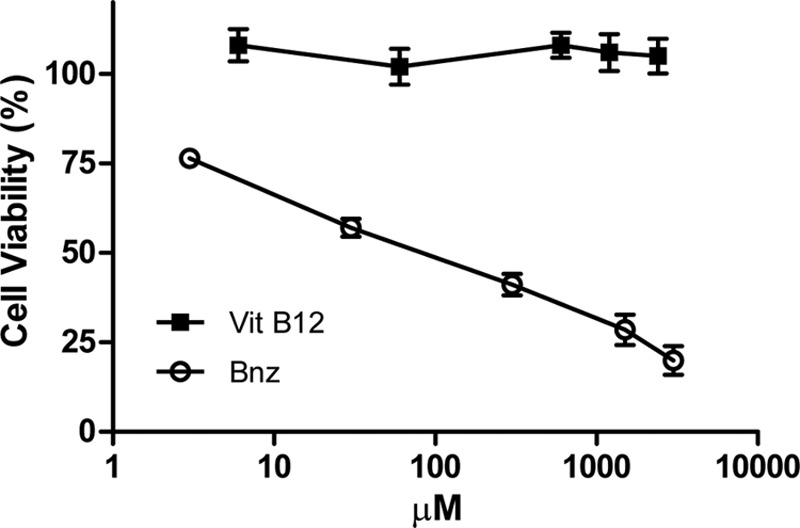
Effect of vitamin B12 and Bnz on Vero cell viability. Cells were cultured for 24 h in the presence of different concentrations of B12 (6 to 2,400 μM) and Bnz (3 to 3,000 μM). Cell viability was determined by the MTT assay as described in Materials and Methods.
Short-time treatment and action mode.
To elucidate the mechanism of action, high concentrations of B12 (15 to 60 μM) and short-time treatment (3 to 24 h) on epimastigotes of T. cruzi were employed. We expected that B12, by analogy to hemin, would act by inducing the generation of ROS. Results obtained by flow cytometry to evaluate intracellular oxidative stress are shown in Fig. 5. Independently of B12 concentration, the fluorescence of H2DCFDA-loaded epimastigotes increased ∼12 to 14 times after only 3 h of treatment and was kept markedly high (∼16 to 18 times) until the end of treatment (24 h). The addition of 0.2 mM H2O2 as a positive control caused a similar increase of around 15 times in cell fluorescence intensity (data not shown).
Fig 5.
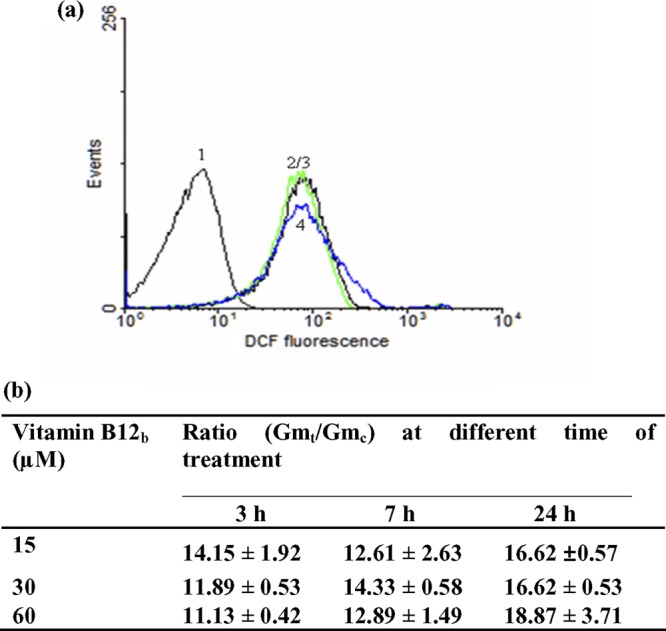
Intracellular oxidative stress during the short-time treatment with vitamin B12. Epimastigotes were treated with B12 (15, 30, or 60 μM) for 3, 7, or 24 h. Intracellular oxidative stress then was evaluated by flow cytometry (n = 20,000 cells/analysis). (a) Histograms corresponding to untreated cells (curve 1, control) and treated with 30 μM B12 for 3, 7, or 24 h (curves 2, 3, and 4, respectively). (b) Time course of the Gmt/Gmc ratio (see Materials and Methods) for parasites treated with the different concentrations of B12.
Because of evidence for oxidative stress on treated parasites, we studied the antioxidant enzyme activities in short-time treatments with 30 μM B12 (Fig. 6). Similar behaviors of SOD and APx activities were observed. During the first 3 h of treatment, the oxidative damage of the enzymes is evident, presenting an equally reduced activity (around 25% below control). The antioxidant activity of these enzymes was manifested after 7 and 24 h with an increased activity for SOD (50 to 55% above control) and APx (20 to 25% above control). During the evaluated period of time, the activity of TryR was not significantly different from that of the control. Taking these results together, it can be considered that treatment with B12 induces the generation of superoxide anion and hydrogen peroxide, and that despite the increased activity of SOD and APx, the intracellular oxidative stage persists due to an incomplete metabolism of those reactive species and/or the lack of an increase in TryR activity.
Fig 6.
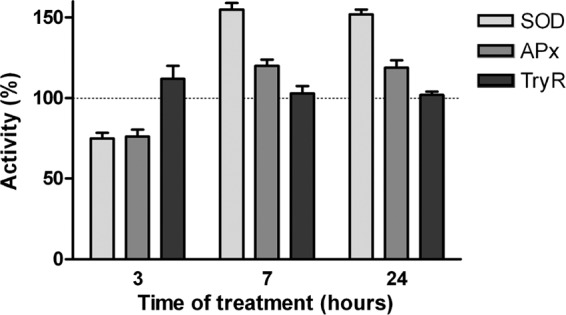
Effect of vitamin B12 on activities of SOD, Apx, and TryR. Experimental conditions were as described in Materials and Methods. The concentration of B12 used was 30 μM. For each enzyme, the activity value obtained in the absence of B12 was considered the control value (100%).
Effect of antioxidant agents.
To obtain further information regarding the mode of action of B12, the effect of antioxidant agents such as DTT and ascorbic acid (vitamin C) was evaluated (Table 2). Employing a low concentration of B12 (0.25 μM), both antioxidants enhanced considerably the antiparasitic activity in vitro. As expected (9, 17), the antioxidants showed a dose-dependent antiparasitic activity, reaching 8-fold inhibition when DTT and ascorbic acid were used at 1.0 and 1.5 μM, respectively (Table 2).
Table 2.
Effect of DTT and ascorbic acid on the anti-T. cruzi activity of vitamin B12a
| Antioxidant concn (μM) | Vitamin B12 activity (μM) | Inhibition (%) |
|---|---|---|
| None | 0.25 | 5.4 ± 1.5 |
| DTT | ||
| 0.5 | 0 | 5.9 ± 1.9 |
| 1.0 | 0 | 21.3 ± 2.4 |
| 0.5 | 0.25 | 23.9 ± 1.8 |
| 1.0 | 0.25 | 41.4 ± 2.2 |
| Vitamin C | ||
| 0.5 | 0 | 3.7 ± 1.7 |
| 1.0 | 0 | 2.4 ± 1.2 |
| 1.5 | 0 | 10.3 ± 1.4 |
| 0.5 | 0.25 | 18.4 ± 1.2 |
| 1.0 | 0.25 | 22.9 ± 1.6 |
| 1.5 | 0.25 | 47.7 ± 1.9 |
Zero percent inhibition corresponds to parasites cultured in the absence of either B12 or antioxidant compound.
In vivo antiparasitic activity.
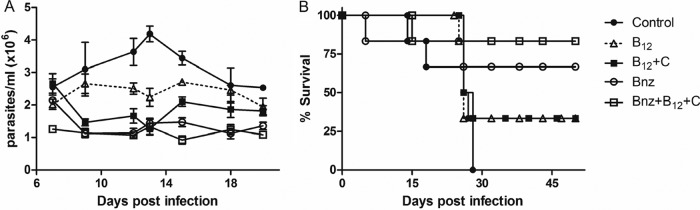
To determine whether treatments could be useful for in vivo therapies, an acute murine model was employed. Thus, 7 groups of mice were infected with T. cruzi trypomastigotes and injected with B12 alone, B12 plus ascorbic acid, Bnz alone, and Bnz plus ascorbic acid and B12. Individual parasitemia levels were assessed every other day. At the peak of parasitemia, around day 13, all treated mice presented an important decrease in the number of circulating parasites compared to the control group (P < 0.01) (Fig. 7A). When B12 was administered alone, a reduction in parasitemia could be observed ([2.23 ± 0.28] × 106; the control level was [4.18 ± 0.02] × 106 parasites/ml), thus this reduction was improved by the administration of B12 simultaneously with ascorbic acid ([1.26 ± 0.17] × 106 parasites/ml). Moreover, when infected animals were treated with half of the doses of the vitamins, we still observed a significant reduction in parasitemia ([1.62 ± 0.23] × 106; the control level was [4.18 ± 0.02] × 106; P < 0.01) (data not shown).
Fig 7.
Effect of vitamin B12 and Bnz on the treatment of infected mice. Parasitemia levels (A) and survival curve (B) during the acute infection period in C3H mice infected with 5 × 103 bloodstream trypomastigotes of T. cruzi. Mice were treated from days 5 to 10 and 12 to 17 of infection. Parasitemia was determined by counting the number of trypomastigotes in 5 μl of fresh blood collected from the tail every other day. Mortality was recorded every day.
At the peak of parasitemia, mice treated with Bnz alone or combined with vitamins presented levels of circulating parasites ([1.43 ± 0.12] × 106 and [1.33 ± 0.25] × 106 parasites/ml, respectively) similar to those after mice were treated with the vitamins. Important differences in the kinetics of parasitemia could be observed throughout the acute phase of infection. Thus, calculating the area under the curve, decreases in the number of circulating parasites of 43.9, 58.6, and 64.6% were observed for the mice treated only with vitamins, only with Bnz, or the combination of both, respectively (Fig. 7A). The reduced number of parasites was crucial for animal survival, as shown in Fig. 7B. Control mice presented high levels of parasitemia, leading to death between days 14 and 28 postinfection. In contrast, 83.3% of animals treated with Bnz plus vitamins survived until the end of the experiment (100 days postinfection). Only animals receiving Bnz combined with vitamins presented significant survival rates compared to control animals (P < 0.05).
DISCUSSION
The effect of B12 on the parasite T. cruzi was investigated for the first time. B12 produced a marked decrease in epimastigote growth rate (Fig. 1), together with significant changes in motility. Unlike hemin, which at 25 μg/ml produces the epimastigote transformation to amastigotes (4), no morphological changes were observed for B12 concentrations up to 45 μM. The inhibitory effect of B12 on epimastigote growth was increased in a dose-dependent manner by a second addition of B12 on the fifth day. Previously, we have demonstrated that high concentrations of hemin produce an antiproliferative effect on T. cruzi epimastigotes (4). Although there are structural similarities between B12 and hemin, the antiproliferative effect of cyanocobalamin occurs regardless of the hemin concentration present in the culture medium (Fig. 2). Even in the presence of B12, the trypanocidal effect of high concentrations of hemin was manifested, demonstrating that B12 does not influence its effect. These results corroborate that B12 and hemin do not interact with each other in their modes of action. At the concentrations assayed, apparently there was not a competitive effect between both compounds.
Cyanocobalamin showed a marked in vitro anti-T. cruzi activity, with IC50s of 2.42 ± 0.54, 10.69 ± 1.50, and 9.46 ± 1.20 μM for epimastigote, amastigote, and trypomastigote forms, respectively (Table 1). B12 showed activity levels between 1.7 and 4 times higher than those of Bnz in epimastigote and trypomastigote stages. Moreover, the effect produced by a combination of B12 and Bnz was the sum of the effects produced by the components alone (Fig. 3).
Due to the high trypanocidal activity and low cytotoxicity, cyanocobalamin presented high SI values (>200) in all parasite stages. This is particularly significant since an SI of >50 is considered adequate for trypanocidal drugs under development (13).
The short-time treatment of T. cruzi epimastigotes with high doses of B12 (15 to 60 μM) showed a significant increase in the cellular oxidative state (Fig. 5). Superoxide anion generation may cause the increased activity of SOD (50% above the control value; Fig. 6), which would transform the superoxide anion into hydrogen peroxide. Under these conditions, it was expected that hydrogen peroxide-metabolizing enzymes would increase their activity. Although catalase and selenocysteine-containing glutathione peroxidases are absent from T. cruzi (24), the parasite has an efficient, redundant, and ubiquitously distributed antioxidant defense system. The enzymes APx and tryparedoxin peroxidase would be responsible for degrading the hydrogen peroxide (24). In this work, we have evaluated the TryR activity as an indirect way to measure APx and tryparedoxin peroxidase activities, finding that there were not significant differences from the untreated control (Fig. 6). TryR is an enzyme that is likely to be pivotal to peroxide metabolism in all trypanosomatids, because reduced Try is the molecule reducing dehydroascorbate to ascorbate and oxidized tryparedoxin to reduced tryparedoxin (11). The lack of increased activity of TryR above control levels and the slight increase of APx activity could be responsible for the accumulation of hydrogen peroxide within the cell. This excess of hydrogen peroxide and the rest of the superoxide anions that may not have been metabolized by SOD would be the species responsible for intracellular oxidative stress induced by B12. A deleterious action of B12 treatment only became evident for SOD and APx (with activity values significantly lower than the control) at 3 h of treatment with B12 (Fig. 5). At later times the antioxidant action of these enzymes was manifested, but it was not enough to reconstitute the cellular equilibrium.
As was observed with hemin (4), vitamin B12 appears to express its cytotoxic action on the parasite through the generation of ROS. By adding antioxidants such as DTT or vitamin C, the oxidative damage increases instead of decreases, probably due to the well-known prooxidant effect of these compounds combined with transition metal ions (Fe, Cu, and Co), which makes itself evident through the generation of ROS (18, 19). Concerning the possible mechanism of action of B12, additional studies should carried out. The oxygen-reduction products have been implicated in the mechanism of action of several trypanocidal agents active in vitro and in vivo (11), and they make the parasite very vulnerable because of its partially deficient antioxidant defense system.
It is relevant to highlight that, in the presence of ascorbic acid for the epimastigote stage, a decrease of 10 times the IC50 of B12 can be achieved (data not shown). In our in vivo model, the addition of ascorbic acid to B12 treatment produced an important increase in the antiparasitic activity, since mice treated with both presented a reduction 2 times higher than those which received B12 alone at the peak of parasitemia. The fact that an antioxidant enhances the effect of B12 would ensure that concentrations of B12 required to express antiparasitic activity do not exceed the maximum concentration that can be achieved in circulation.
Benznidazol is the reference drug used currently; however, it is unsatisfactory because of its limited efficacy and its toxic side effects, such as anorexia, vomiting, peripheral polyneuropathy, and allergic dermopathy. Instead, B12 rarely presents side effects and is an over-the-counter drug. As shown in Fig. 7, the administration of vitamins together with Bnz was able to improve the antiparasitic effect of the Bnz treatment, showing its potential as a candidate for novel therapeutic modalities for the treatment of Chagas' disease.
ACKNOWLEDGMENTS
F.M.F., E.L.M., A.B., and M.E.L. hold the post of Scientific Researchers at the Argentine National Research Council (CONICET). A.B.C., F.M.F., E.L.M., and M.E.L. are members of the University of Buenos Aires. A.B.C. is a Postdoctoral Fellow of CONICET.
This work was supported by the University of Buenos Aires (UBACYT X083 and 20020090200478) and the Argentine National Research Council, CONICET (PIP 5263).
Footnotes
Published ahead of print 6 August 2012
REFERENCES
- 1. Ariyanayagam M, Fairlamb A. 2001. Ovothiol and trypanothione as antioxidants in trypanosomatids. Mol. Biochem. Parasitol. 115:189–198 [DOI] [PubMed] [Google Scholar]
- 2. Buckner FS, Navabi N. 2010. Advances in Chagas disease drug development: 2009–2010. Curr. Opin. Infect. Dis. 23:609–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buckner FS, Verlinde CL, La Flamme AC, Van Voorhis WC. 1996. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 40:2592–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ciccarelli A, Araujo L, Batlle A, Lombardo E. 2007. Effect of hemin on growth, protein content and the antioxidant defense system in Trypanosoma cruzi. Parasitology 134:959–965 [DOI] [PubMed] [Google Scholar]
- 5. Eakanunkul S, et al. 2005. Characterization of the periplasmic heme-binding protein ShuT from the heme uptake system of Shigella dysenteriae. Biochemistry 44:13179–13191 [DOI] [PubMed] [Google Scholar]
- 6. Kumar S, Bandyopadhyay U. 2005. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 157:175–188 [DOI] [PubMed] [Google Scholar]
- 7. Lombardo ME, Araujo LS, Batlle A. 2003. 5-Aminolevulinic acid synthesis in epimastigotes of Trypanosoma cruzi. Int. J. Biochem. Cell Biol. 35:1263–1271 [DOI] [PubMed] [Google Scholar]
- 8. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275 [PubMed] [Google Scholar]
- 9. Macao LB, et al. 2007. Antioxidant therapy attenuates oxidative stress in chronic cardiopathy associated with Chagas' disease. Int. J. Cardiol. 123:43–49 [DOI] [PubMed] [Google Scholar]
- 10. Marguerite V, Beri-Dexheimer M, Ortiou S, Guéant JL, Merten M. 2007. Cobalamin potentiates vinblastine cytotoxicity through downregulation of mdr-1 gene expression in HepG2 cells. Cell. Physiol. Biochem. 20:967–976 [DOI] [PubMed] [Google Scholar]
- 11. Maya JD, et al. 2007. Mode of action of natural and synthetic drugs against Trypanosoma cruzi and their interaction with the mammalian host. Comp. Biochem. Physiol. A 146:601–620 [DOI] [PubMed] [Google Scholar]
- 12. Muscia GC, et al. 2011. Synthesis, trypanocidal activity and molecular modeling studies of 2-alkylaminomethyl-quinoline derivatives. Eur. J. Med. Chem. 46:3696–4703 [DOI] [PubMed] [Google Scholar]
- 13. Nwaka S, Hudson A. 2006. Innovative lead discovery strategies for tropical diseases. Nat. Rev. Drug Discov. 5:941–955 [DOI] [PubMed] [Google Scholar]
- 14. Pal J, Joshi-Purandare M. 2001. Dose-dependent differential effect of hemin on protein synthesis and cell proliferation in Leishmania donovani promastigotes in vitro. J. Biosci. 26:225–231 [DOI] [PubMed] [Google Scholar]
- 15. Pinazo MJ, et al. 2010. Tolerance of benznidazole in treatment of Chagas' disease in adults. Antimicrob. Agents Chemother. 54:4896–4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pinazo MJ, et al. 2010. Successful treatment with posaconazole of a patient with chronic Chagas disease and systemic lupus erythematosus. Am. J. Trop. Med. Hyg. 82:583–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ribeiro CM, et al. 2010. Antioxidant therapy attenuates oxidative insult caused by benzonidazole in chronic Chagas' heart disease. Int. J. Cardiol. 145:27–33 [DOI] [PubMed] [Google Scholar]
- 18. Solovieva ME, Soloviev VV, Akatov VS. 2007. Vitamin B12b increases the cytotoxicity of short-time exposure to ascorbic acid, inducing oxidative burst and iron-dependent DNA damage. Eur. J. Pharmacol. 566:206–214 [DOI] [PubMed] [Google Scholar]
- 19. Solovieva ME, Solovyev VV, Kudryavtsev AA, Trizna YA, Akatov VS. 2008. Vitamin B12b enhances the cytotoxicity of dithiothreitol. Free Radic. Biol. Med. 44:1846–1856 [DOI] [PubMed] [Google Scholar]
- 20. Sülsen VP, et al. 2008. Trypanocidal and leishmanicidal activities of sesquiterpene lactones from Ambrosia tenuifolia Sprengel (Asteraceae). Antimicrob. Agents Chemother. 52:2415–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sülsen VP, et al. 2011. Psilostachyin C: a natural compound with trypanocidal activity. Int. J. Antimicrob. Agents 37:536–543 [DOI] [PubMed] [Google Scholar]
- 22. Trouiller P, et al. 2002. Drug development for neglected diseases: a deficient market and a public health policy failure. Lancet 359:2188–2194 [DOI] [PubMed] [Google Scholar]
- 23. Truong DH, Mihajlovic A, Gunness P, Hindmarsh W, O'Brien PJ. 2007. Prevention of hydrogen sulfide (H2S)-induced mouse lethality and cytotoxicity by hydrocobalamin (vitamin B12a). Toxicology 242:16–22 [DOI] [PubMed] [Google Scholar]
- 24. Turrens JF. 2004. Oxidative stress and antioxidant defense: a target for the treatment of diseases caused by parasitic protozoa. Mol. Aspects Med. 25:211–220 [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization 2002. Control of Chagas' disease. World Health Organ. Tech. Rep. Ser. 905:1–109 [PubMed] [Google Scholar]