Abstract
There is increased interest in intermittent regimen of liposomal amphotericin B, which may facilitate use in ambulatory settings. Little is known, however, about the most appropriate dosage and schedule of administration. Plasma pharmacokinetic data were acquired from 30 patients receiving liposomal amphotericin B for empirical treatment of suspected invasive fungal infection. Two cohorts were studied. The first cohort received 3 mg of liposomal amphotericin B/kg of body weight/day; the second cohort received 10 mg of liposomal amphotericin B/kg at time zero, followed by 5 mg/kg at 48 and 120 h. The levels of liposomal amphotericin B were measured by high-pressure liquid chromatography (HPLC). The pharmacokinetics were estimated by using a population methodology. Monte Carlo simulations were performed. D-optimal design was used to identify maximally informative sampling times for both conventional and intermittent regimens for future studies. A three-compartment pharmacokinetic model best described the data. The pharmacokinetics for both conventional and intermittent dosing were linear. The estimates for the mean (standard deviation) for clearance and the volume of the central compartment were 1.60 (0.85) liter/h and 20.61 (15.27) liters, respectively. Monte Carlo simulations demonstrated considerable variability in drug exposure. Bayesian estimates for clearance and volume increased in a linear manner with weight, but only the former was statistically significant (P = 0.039). D-optimal design provided maximally informative sampling times for future pharmacokinetic studies. The pharmacokinetics of a conventional and an intermittently administered high-dose regimen liposomal amphotericin B are linear. Further pharmacokinetic-pharmacodynamic preclinical and clinical studies are required to identify safe and effective intermittent regimens.
INTRODUCTION
Liposomal amphotericin B (AmBisome; Gilead Sciences Europe Ltd.) is a widely used antifungal compound for the treatment of invasive fungal infections (9). This lipid formulation consists of amphotericin B embedded in the wall of a unilamellar liposome of approximately 80 nm in diameter. A regimen of 3 mg/kg of body weight/day is effective for the treatment of invasive infections caused by Candida spp. and Aspergillus spp. (7, 18). Liposomal amphotericin B is also used for the treatment of cryptococcal meningitis and infections caused by the Mucorales order (16, 19).
Despite extensive clinical trial data that support a regimen of 3 mg/kg/day, there is increased interest in the use of alternative regimens. A longer dosing interval extends the utility of liposomal amphotericin B to ambulatory settings, which may be especially useful in the setting of prophylaxis (primary and secondary) and for consolidation therapy for infections where the use of orally bioavailable compounds is precluded. At this time, there is considerable uncertainty regarding safe and effective intermittent regimens. The clinical studies that have been performed are relatively small and have not been designed to detect differences in clinical outcomes (see, for example, references 6 and 10). Further clinical studies are currently being planned and conducted.
Here, we describe the population pharmacokinetics of liposomal amphotericin B. These data were acquired as a component of a clinical study that compared a conventional (i.e., 3 mg/kg/day) with an intermittent regimen (10 mg/kg on day 1, followed by 5 mg/kg at 48 and 120 h) of liposomal amphotericin B for patients with profound and prolonged neutropenia with fever that was refractory to broad-spectrum antimicrobial agents. The clinical aspects of this study have been previously reported (12). The current study reports the population pharmacokinetics of liposomal amphotericin B in these patients and represents a first critical step for the future rational design of intermittent regimens.
MATERIALS AND METHODS
Patients, dosage, and schedule of administration of liposomal amphotericin B.
The primary aim of this study was to assess the safety of an intermittent regimen of liposomal amphotericin B. The results of this study have been reported elsewhere (12). Briefly, the study was approved by the Al Ain Medical District Human Research Ethics Committee. All patients were treated at Tawam-Johns Hopkins Hospital, Al-Ain, United Arab Emirates. All recruited patients provided informed, written consent. The patients had hematological malignancy and were undergoing induction chemotherapy. All patients had profound and prolonged neutropenia (<0.5 × 109 neutrophils/liter) with fever (≥38.3°C as a single measurement or ≥38°C for ≥1 h) that was refractory to broad-spectrum antibacterial therapy with piperacillin-tazobactam. The underlying conditions and other demographics are summarized in Table 1.
Table 1.
Demographics of the 30 patients enrolled in this studya
| Demographic characteristicb | Value for group |
|
|---|---|---|
| Intermittent regimen | Conventional regimen | |
| Age, yr (range) | 36 (17–55) | 38 (18–55) |
| No. of males/no. of females | 13:2 | 9:6 |
| No. of patients with AML | 9 | 11 |
| No. of patients with ALL | 4 | 3 |
| No. of patients with lymphoma | 2 | 1 |
Data summarized in this table have been previously reported (12).
AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia.
Liposomal amphotericin was administered until the resolution of fever and neutropenia and/or a minimum of 14 days had elapsed. The patients were randomized to receive conventional regimen of 3 mg/kg of body weight/day (n = 15) or an intermittent regimen of 10 mg/kg of body weight at time zero, followed by 5 mg/kg at 48 and 120 h (n = 15). The study size was selected to demonstrate a difference in nephrotoxicity between the two regimens of liposomal amphotericin B. Liposomal amphotericin B was reconstituted according to the manufacturer's instructions in 5% dextrose and infused over a 2-hour period. All patients had serial blood samples drawn throughout the study period to enable estimation of their plasma pharmacokinetics.
The patients were monitored daily for evidence of drug toxicity, intolerance to study medication, and the development of an invasive fungal infection (clinical, radiological, or microbiological evidence of a mold infection or a positive fungal blood culture). The patients that developed an invasive fungal infection were withdrawn from the study and treated with voriconazole or caspofungin.
Sampling for measurement of amphotericin B concentrations in plasma.
Blood samples were taken to estimate the concentrations of liposomal amphotericin B in plasma. For the intermittent arm of the study, blood samples were taken at 0.5, 2, 4, 9, and 24 h after infusion on days 1, 3, and 5, and at the time of bone marrow aspiration if performed. For the cohort receiving drug on a daily basis, blood samples were taken on days 1, 3, 6, 10, and 14. The total number of samples available for this analysis was 538. The mean numbers of samples per patient in the intermittent versus daily arm were 16.14 and 23, respectively.
Measurement of liposomal amphotericin B HPLC.
Liposomal amphotericin B was measured in serum by high-pressure liquid chromatography (HPLC). A C18 5-μm 50- by 20-mm column (Agilent, Cheshire, United Kingdom) was used. Active drug was extracted with 300 μl methanol (Fisher, United Kingdom). The mobile phase was 0.1% aqueous formic acid (Fisher, United Kingdom) (solution A) and 0.1% formic acid in acetonitrile (Fisher, United Kingdom) (solution B). A gradient was used. The gradient began with a 80:20 ratio of solution A-solution B, and the ratio increased to 30:70 over 7 min. A flow rate of 0.8 ml/min was used. Amphotericin B was measured using UV detection at a wavelength of 385 nm. The internal standard was piroxicam in methanol (2 μg/ml). Serum concentrations were estimated using a standard curve in the concentration range from 0 to 100 mg/liter. The injection volume was 50 μl. The retention times for amphotericin B and the internal standard were 4.0 and 2.3 min, respectively. The limit of detection was 0.05 mg/liter. The intra- and interday variation in the assay were both <10%.
Population pharmacokinetic modeling.
All data were modeled using a population methodology and with the pharmacokinetic program Nonparametric Adaptive Grid (NPAG) (20). The weighting function for each patient was obtained from the maximum likelihood estimator in the pharmacokinetic program ADAPT 5 (8), as previously utilized by us and others (see, for example, reference 17). Data from one patient were not available, and for another patient, there was significant uncertainty regarding the validity of the measured values. This left a total of 28 patients, with 14 patients in each study arm. Our approach provided estimates for the extent of interindividual variability in pharmacokinetics, but we did not model interoccasion variability or use an interacting multiple model approach that would enable the patient's pharmacokinetic parameter values to change through the study period (23).
A number of structural models were (empirically) fitted to the data. Initially, a standard two-compartment model with intravenous (i.v.) infusion into the central compartment and first-order elimination was used. Subsequently, a three-compartment model was also fitted. This model enabled the relatively high peak concentrations and persistently detectable concentrations after cessation of dosing to be better described. These two- and three-compartment models were compared and distinguished on the basis of the observed-predicted values both before and after the Bayesian step, the coefficient of determination of the linear regression of these data, the log likelihood value, a measure of bias (mean weighted error), and precision (bias-adjusted weighted mean squared error). Statistically significant differences in the log likelihood values were assessed by calculating twice the difference and comparing this value against a chi-squared distribution with the appropriate number of degrees of freedom (i.e., differences in the number of parameters between the respective models).
The ordinary differential equations that describe this three-compartment model were as follows:
| (1) |
| (2) |
| (3) |
where X1, X2, and X3 represent the amount of drug (in milligrams) in compartments 1, 2, and 3, respectively. R1 represents the infusion of drug. SCL is the clearance from the central compartment. Vc is the volume of the central compartment. K12, K21, K23, and K32 are the first-order rate constants that connect the respective compartments (e.g., K12 is the first-order rate constant for compartment 1 to compartment 2). Equations 1, 2, and 3 described the rate of change of liposomal amphotericin B in compartments 1, 2, and 3, respectively. The mean and median parameter values were compared by assessing the observed and predicted values both before and after the Bayesian step.
Monte Carlo simulation.
Monte Carlo simulations were performed to provide a further insight into the extent of pharmacokinetic variability of both the intermittent and conventional dosing regimens—this variability could have an impact on the pharmacodynamics, especially for intermittent regimens. All simulations were performed using ADAPT 5 (8). The parameter means and the full covariance matrix were inserted into subroutine PRIOR of ADAPT 5 (8). Both normal and log normal parameter distributions were evaluated and distinguished on the basis of their ability to recapitulate the original parameter values and their dispersions. The 5th and 95th percentiles of the simulated population were plotted (note that this is not the same as confidence intervals, which cannot be accurately determined using a nonparametric approach).
Statistical methods.
The Bayesian estimates for volume and clearance for patients receiving intermittent versus conventional daily regimen were plotted on a histogram and compared by using the Mann-Whitney U test and the statistical program SYSTAT 11. A linear regression of the Bayesian estimates for both volume and clearance against weight was performed. An assessment was made as to whether the slope of the regression lines deviated in a statistically significant manner from zero was performed by using the statistical package SYSTAT 11.
Optimal sampling times.
Optimal sampling times for both a conventional and intermittent regimen were estimated using the SAMPLE module of ADAPT 5. An estimate of these times for the population was obtained using the method originally described by Tam et al. (25). Briefly, the multiple model file from the output of NPAG was obtained; this file contains the support points for the entire population and their associated probabilities. Liposomal amphotericin B at 3 mg/kg or 10 mg/kg was administered to each of these support points (infused over 2 h). Optimal sampling times were obtained throughout the subsequent 24 and 96 h for the conventional and intermittent regimen, respectively. As the sampling times from each support point were obtained, they were weighted by multiplying by the probability of each support point and then transferred to a histogram. For the 3 mg/kg/day group, 15-min increments were used. For the group given 10 mg/kg every 96 h, hourly increments were used.
RESULTS
Patients and demographics.
Patient demographics are summarized in Table 1. There were no significant differences in weight and age between the patients receiving a conventional versus intermittent regimen.
Population pharmacokinetic modeling.
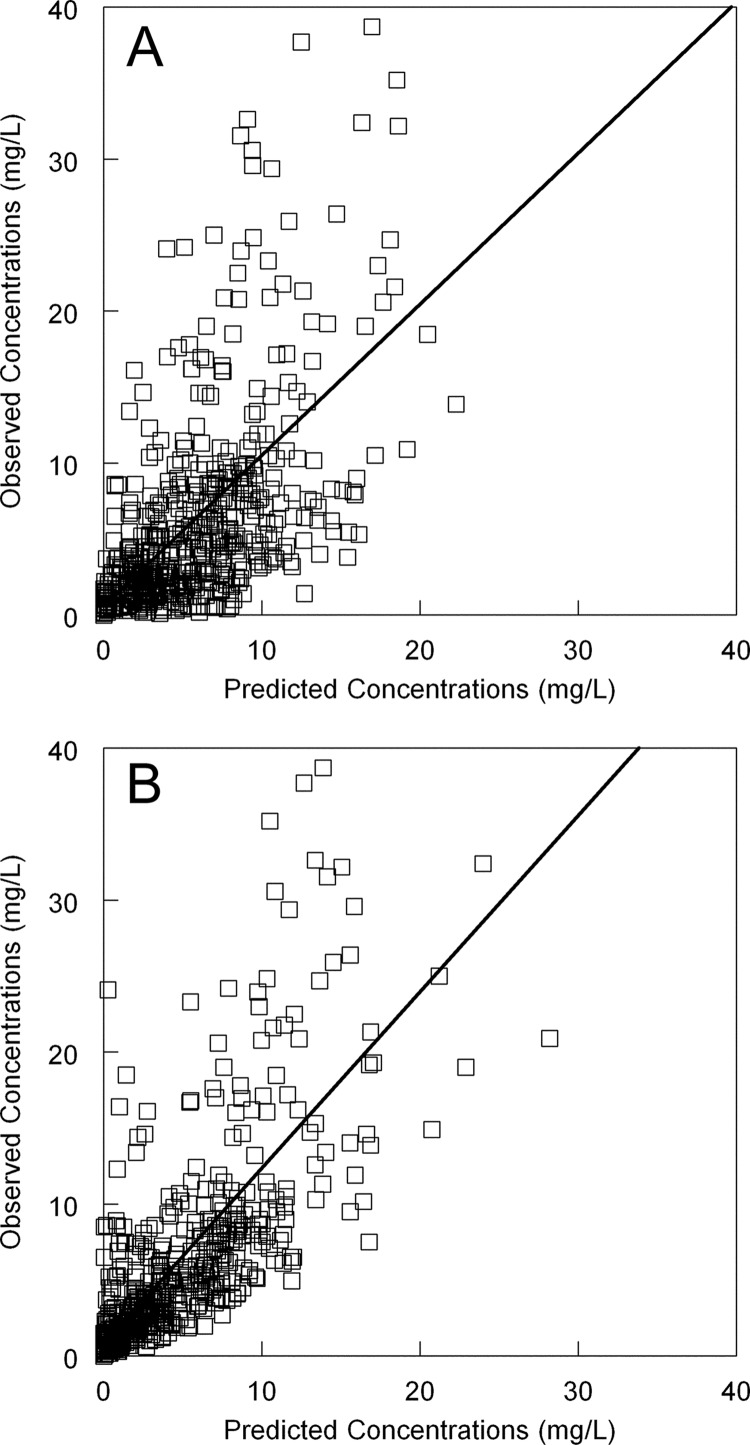
A three-compartment model resulted in a higher log likelihood value than the two-compartment model (twice difference in log likelihood value [9.026] compared to a chi-squared distribution with two degrees of freedom [P = 0.01]). The estimates for the population parameter means, medians, and standard deviations are shown in Table 2. Both the mean and median parameter values could have reasonably been used with comparable measures of precision and bias and with similar regression lines before and after the Bayesian step. Ultimately, the means were chosen for the subsequent simulations. The fit of the model to the population (i.e., prior to the Bayesian step) was acceptable (Fig. 1A). The mean weighted error (a measure of bias) and bias-adjusted mean weighted squared error (a measure of precision) were 1.15 and 15.30, respectively. After the Bayesian step, these measures were −0.17 and 1.43, respectively (Fig. 1B).
Table 2.
Population parameter valuesa
| Parameter (unit) | Mean | Median | Standard deviation |
|---|---|---|---|
| Volume (liter) | 20.61 | 16.82 | 15.27 |
| K12 (h−1) | 1.94 | 1.12 | 3.00 |
| K21 (h−1) | 15.34 | 15.31 | 12.71 |
| Clearance (liter/h) | 1.60 | 1.36 | 0.85 |
| K23 (h−1) | 11.35 | 3.59 | 10.31 |
| K32 (h−1) | 5.69 | 0.24 | 9.90 |
These estimates are from the overall model fitted to patients receiving an intermittent and conventional regimen.
Fig 1.
(A) Fit of the three-compartment pharmacokinetic model to the serum data from the population. Predicted concentration = 0.59 + (0.99 × observed concentration); r2 = 0.35. (B) Observed-predicted values for fit of Bayesian estimates to the data from each individual patient. Predicted concentration = 0.77 + (1.16 × observed concentration); r2 = 0.56.
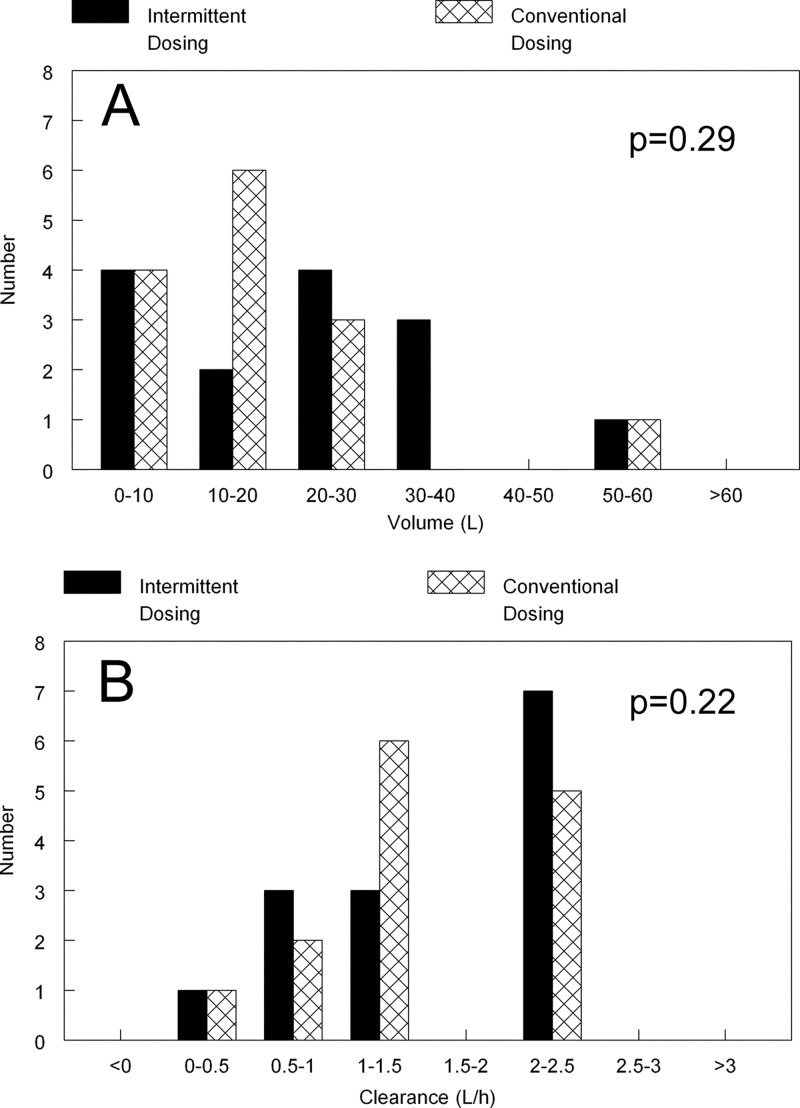
There were no differences in the Bayesian estimates for volume and clearance for patients receiving an intermittent versus conventional regimen (Fig. 2). The P values using the Mann-Whitney U test for the two groups of patients for volume and clearance were 0.22 and 0.29, respectively.
Fig 2.
Bayesian estimates for volume (A) and clearance (B) for patients receiving an intermittent regimen and a daily regimen (conventional dosing). There were no statistical differences in the estimates using the Mann-Whitney U test.
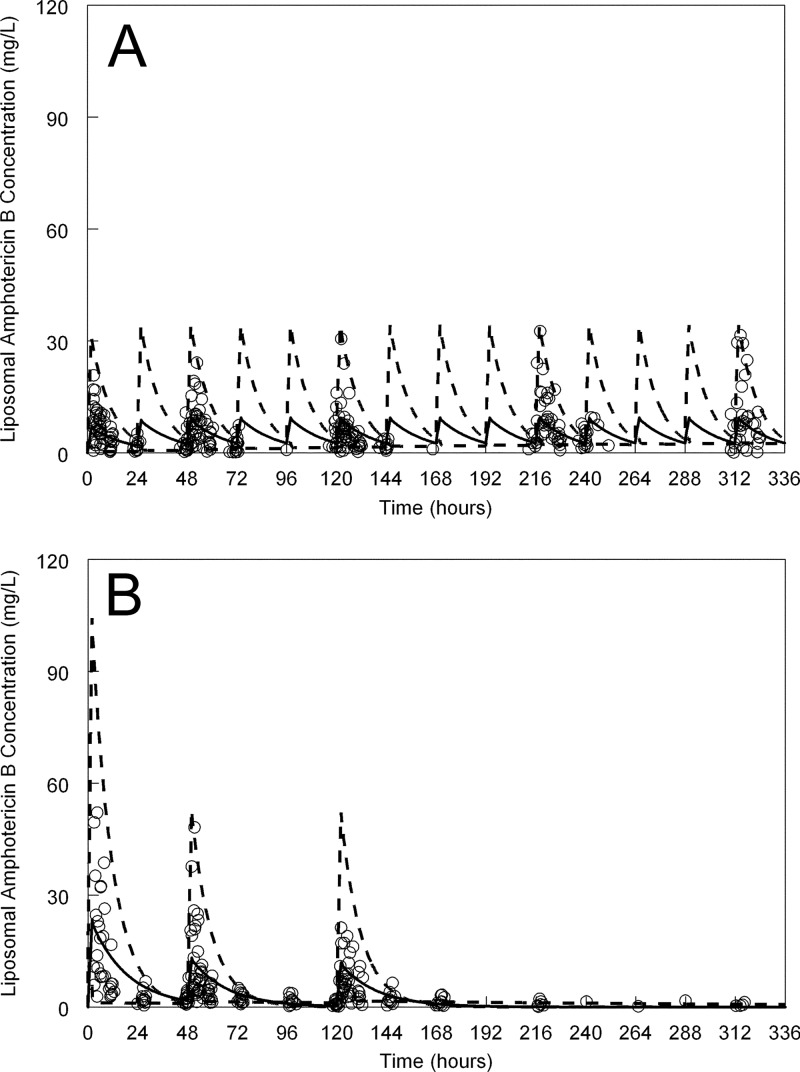
The mean concentration-time profiles for a patient weighing 68 kg (the mean weight of the study population) and receiving a conventional and intermittent regimen are shown in Fig. 3A and B, respectively. As is evident from Fig. 3, there was considerable variability in the concentrations of liposomal amphotericin B, especially in terms of peak concentrations. The extent of this pharmacokinetic variability is further reflected in the Monte Carlo simulations that are shown in Fig. 3. Approximately 10% of the observations fell outside the concentration-time trajectories delineated by the 5th and 95th percentiles, although these were all low concentrations (i.e., all <5th percentile).
Fig 3.
Pharmacokinetics of liposomal amphotericin B administered at different doses. Liposomal amphotericin B was given at a dose of 3 mg/kg/day (A) and in doses of 10 mg/kg, 5 mg/kg, and 5 mg/kg at 0, 48, and 120 h, respectively (B). The open circles are the raw data points from patients (n = 14 in each group; 28 patients total). The solid black line is the mean concentration-time profile of liposomal amphotericin B for a patient weighing 68 kg. The broken lines in both panels represents the 5th and 95th percentile for the simulated population.
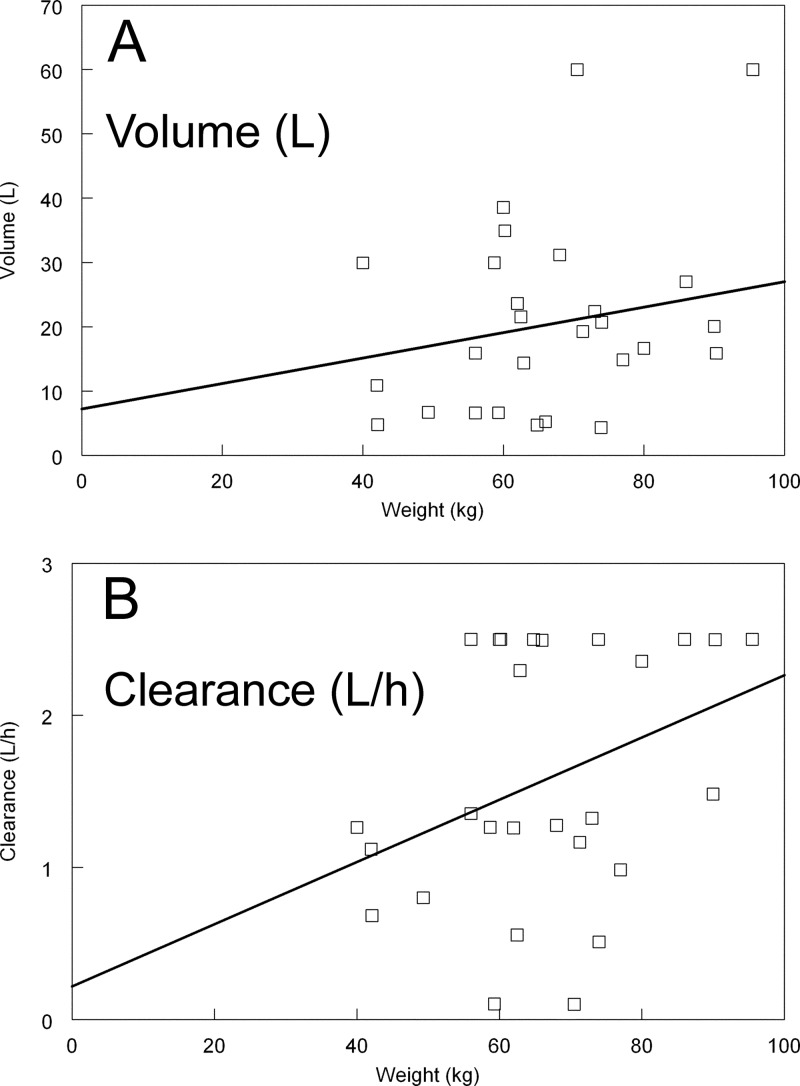
The linear regressions of the Bayesian estimates for volume and clearance versus weight are shown in Fig. 4. Heavier patients tended to have higher estimates for volume. The regression line was given by the following equation: volume (in liters) = 7.20 + (0.20 × weight). The slope of the regression line for volume versus weight was not statistically significant (i.e., the estimate did not deviate in a statistically significant manner from zero; P = 0.27). Conversely, the regression line for the relationship between weight and clearance was statistically significant (Fig. 4B). The regression line was given by the following equation: clearance (liter/h) = 0.22 + (0.02 × weight). The P value of the slope was 0.039.
Fig 4.
Relationship between weight and volume (A) and weight and clearance (B). The regression of weight versus clearance was statistically significant (P = 0.039).
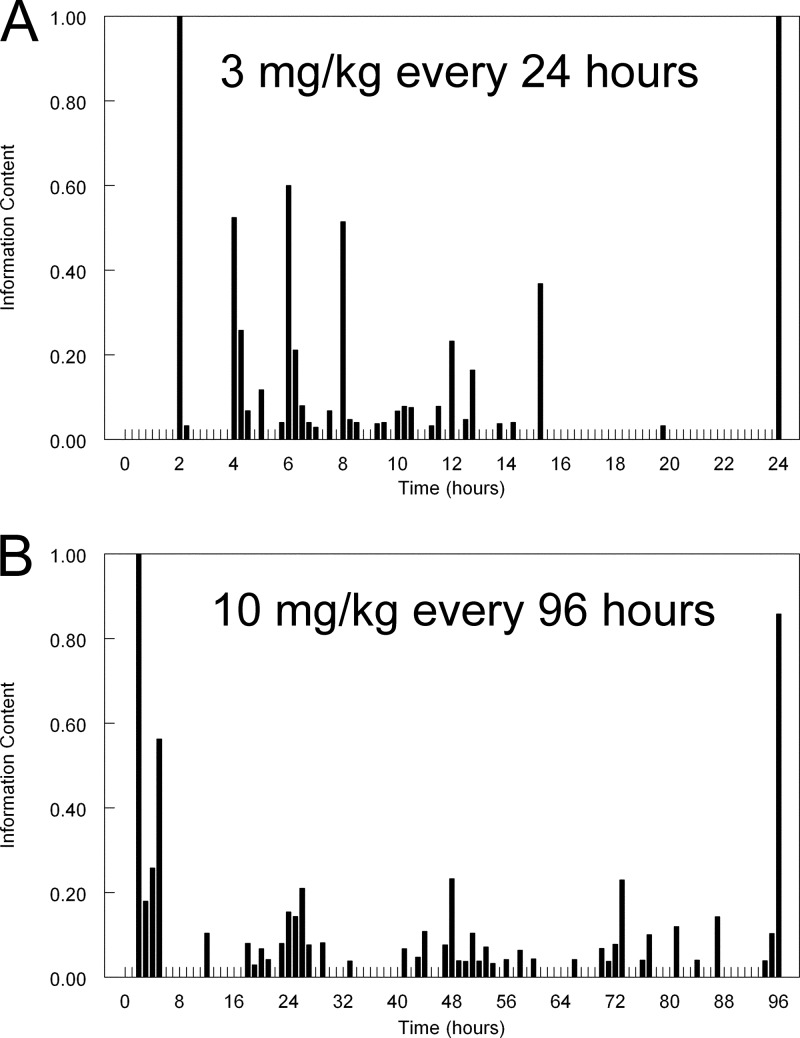
The histograms containing the optimal sampling times for 3 mg/kg administered daily and 10 mg/kg administered every 96 h are shown in Fig. 5. The most informative sampling times for 3 mg/kg administered every day were 2, 4, 6, 8, 15.25, and 24 h. The most informative sampling times for 10 mg/kg administered every 96 h were 2, 5, 26, 48, 73, and 96 h. As seen in Fig. 5B, information-rich sampling times clustered around the end of the initial infusion and then at the end of days 1, 2, 3, and 4.
Fig 5.
Optimal sampling times for two liposomal amphotericin B regimens. The two liposomal amphotericin B regimens were 3 mg/kg/day (A) and 10 mg/kg administered every 96 h (B).
DISCUSSION
Liposomal amphotericin B (AmBisome; Gilead Sciences Europe Ltd.) is used extensively for the treatment of suspected or established invasive fungal infections. There have been considerable investigations to identify safe and effective regimens for humans (7, 18, 28–30). Most authorities recommend a regimen of 3 mg/kg/day for the treatment if the patient has invasive candidiasis or invasive aspergillosis and empirical treatment of patients with profound and prolonged neutropenia that is refractory to broad-spectrum antibacterial agents (24, 27, 28). While the pharmacokinetics are relatively poorly understood, there is increasing interest in the administration of liposomal amphotericin B for intervals exceeding 24 h (11), and limited clinical data suggest that this may be an effective strategy for the prevention of invasive fungal infections (6, 10). Nevertheless, there is considerable uncertainty regarding the optimal dosage and schedule of administration. While amphotericin B appears to exhibit concentration-dependent antifungal effects in preclinical models (potentially supporting the use of less fractionated regimens) (2, 3), the use of progressively longer dosing intervals means that drug concentrations at the site of infection must ultimately decline to levels that enable fungal regrowth. The length of this interval and the minimum effective concentration at the relevant site of infection are not known.
There is an increased understanding of the serum pharmacokinetics of liposomal amphotericin B. In this regard, the findings of this study are largely consistent with previous studies (4, 5, 15, 33). Importantly, the conclusions of this study cannot be extrapolated to other lipid formulations of amphotericin B, including compounds otherwise referred to as “liposomal amphotericin.” Liposomal amphotericin B has a relatively small volume of distribution (circa 20 liters) and a clearance of approximately 1 to 2 liters/h. The pharmacokinetics of conventional dosages (3 mg/kg) appear linear, relatively predictable, and readily described by two- or three-compartment pharmacokinetic models. Importantly, however, there is less certainty about the pharmacokinetics of higher dosages of liposomal amphotericin B. While the pharmacokinetics of the higher dosages in this study remained linear, other investigators have described progressively lower estimates for the maximum concentration of drug in serum (Cmax) and area under the concentration-time curve (AUC) for patients receiving 7.5 to 15 mg/kg (29). This observation has led to the hypothesis that there may be a concentration-dependent clearance pathway(s). Perhaps this was not evident in our study, because the dosages that were used were not high enough to trigger these alternative clearance mechanisms. The relationship between weight and clearance identified in this study, while weak, is statistically significant and does support the use of weight-based dosing. Such a relationship between weight and clearance was not evident in a recent population pharmacokinetic study (33). The likely explanation for this is that inherent pharmacokinetic variability swamps any signal related to weight.
The relatively dependable plasma pharmacokinetics belie the complicated cellular and molecular pharmacology of liposomal amphotericin B. Much has been written about the potential importance of tissue pharmacokinetics in governing the shape of the plasma concentration-time curve and potentially providing improved efficacy compared with other formulations. While liposomal amphotericin B may achieve higher concentrations in some tissue subcompartments such as reticuloendothelial tissues (21), the precise relationship between amphotericin B and the liposome carrying drug to these tissue sites is unclear. The majority of active drug in tissues probably remains preferentially bound to the liposome rather than existing as free drug. Some have proposed that active compound is released under the effect of phagocytic degradation of the liposome (31). A further possibility is that the liposome becomes embedded within host cellular membranes, and active drug is able to engage with the fungus when the cell is breached in the course of invasion (22). These considerations are further complicated by the extensive and relatively complicated binding properties of amphotericin B itself, where the active drug is bound to lipoproteins such as high-density lipoprotein (HDL).
The use of an intermittent dosing regimen of liposomal amphotericin B may be facilitated by favorable tissue pharmacokinetics. Drug concentrations at the site of infection may be significantly higher than plasma drug concentrations, or drug may persist in tissue long after serum concentrations have become undetectable. Following systemic administration, liposomal amphotericin B achieves quantifiable concentrations in the liver, spleen, lung tissue, epithelial lining fluid, and buccal mucosa in both laboratory animals and humans (14, 15, 21, 26, 32). The clinical relevance of these concentrations remains poorly understood. The MIC is frequently used to provide an insight into potentially effective concentrations, but the biological relevance of this measure of antifungal potency in tissues is completely unknown. Persistence of drug at the site of infection may also be clinically relevant. In this circumstance, there may be relatively high tissue concentrations despite undetectable plasma concentrations, and this may account for ongoing antifungal activity that appears disconnected with drug administration. This concept is supported by the resistance of mice to infection with Histoplasma 7 days after receiving a single dose of liposomal amphotericin B (13).
Well-conducted pharmacokinetic studies describing both plasma and tissue drug concentrations are a necessary but insufficient step to support a strategy of intermittent dosing; the drug may be in the wrong tissue compartment or cellular subcompartment or may not achieve effective concentrations at the site of infection. Rather, a combination of pharmacokinetic and pharmacodynamic data are required to demonstrate that intermittent dosing is potentially effective. Some of these studies have already been performed in laboratory animal models. For example, the effect of liposomal amphotericin B in doses of 1 mg/kg/day for 4 days is comparable to the effect produced by 4 mg/kg administered once (1). Comprehensive pharmacokinetic-pharmacodynamic studies are now required where concentrations of liposomal amphotericin B in plasma and tissues are directly linked with the observed antifungal effect. Mathematical modeling and bridging studies can then be employed to identify potentially effective regimens for humans with a range of invasive fungal infections.
ACKNOWLEDGMENTS
William Hope is supported by a National Institute of Health (NIHR) Clinician Scientist Fellowship. Timothy Felton is supported by a Medical Research Council (MRC) Fellowship in Clinical Pharmacology. William Hope has given talks, acted as a consultant, and received research funding from Gilead Inc.
Footnotes
Published ahead of print 6 August 2012
REFERENCES
- 1. Adler-Moore JP, Olson JA, Proffitt RT. 2004. Alternative dosing regimens of liposomal amphotericin B (AmBisome) effective in treating murine systemic candidiasis. J. Antimicrob. Chemother. 54:1096–1102 [DOI] [PubMed] [Google Scholar]
- 2. Andes D, Safdar N, Marchillo K, Conklin R. 2006. Pharmacokinetic-pharmacodynamic comparison of amphotericin B (AMB) and two lipid-associated AMB preparations, liposomal AMB and AMB lipid complex, in murine candidiasis models. Antimicrob. Agents Chemother. 50:674–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andes D, Stamsted T, Conklin R. 2001. Pharmacodynamics of amphotericin B in a neutropenic-mouse disseminated-candidiasis model. Antimicrob. Agents Chemother. 45:922–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bekersky I, et al. 2002. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob. Agents Chemother. 46:828–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bekersky I, et al. 2002. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob. Agents Chemother. 46:834–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cordonnier C, et al. 2008. Safety of a weekly high dose of liposomal amphotericin B for prophylaxis of invasive fungal infection in immunocompromised patients: PROPHYSOME Study. Int. J. Antimicrob. Agents 31:135–141 [DOI] [PubMed] [Google Scholar]
- 7. Cornely OA, et al. 2007. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin. Infect. Dis. 44:1289–1297 [DOI] [PubMed] [Google Scholar]
- 8. D'Argenio DZ, Schumitzky A, Wang X. 2009. ADAPT 5 user's guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles, CA [Google Scholar]
- 9. Denning DW, Hope WW. 2010. Therapy for fungal diseases: opportunities and priorities. Trends Microbiol. 18:195–204 [DOI] [PubMed] [Google Scholar]
- 10. El-Cheikh J, et al. 2007. High-dose weekly liposomal amphotericin B antifungal prophylaxis following reduced-intensity conditioning allogeneic stem cell transplantation. Bone Marrow Transplant. 39:301–306 [DOI] [PubMed] [Google Scholar]
- 11. Ellis M. 2008. New dosing strategies for liposomal amphotericin B in high-risk patients. Clin. Microbiol. Infect. 14(Suppl 4):55–64 [DOI] [PubMed] [Google Scholar]
- 12. Ellis M, et al. 2009. A safety and feasibility study comparing an intermittent high dose with a daily standard dose of liposomal amphotericin B for persistent neutropenic fever. J. Med. Microbiol. 58:1474–1485 [DOI] [PubMed] [Google Scholar]
- 13. Garcia A, Adler-Moore JP, Proffitt RT. 2000. Single-dose AmBisome (liposomal amphotericin B) as prophylaxis for murine systemic candidiasis and histoplasmosis. Antimicrob. Agents Chemother. 44:2327–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Groll AH, et al. 2006. Compartmentalized intrapulmonary pharmacokinetics of amphotericin B and its lipid formulations. Antimicrob. Agents Chemother. 50:3418–3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gubbins PO, Amsden JR, McConnell SA, Anaissie EJ. 2009. Pharmacokinetics and buccal mucosal concentrations of a 15 milligram per kilogram of body weight total dose of liposomal amphotericin B administered as a single dose (15 mg/kg), weekly dose (7.5 mg/kg), or daily dose (1 mg/kg) in peripheral stem cell transplant patients. Antimicrob. Agents Chemother. 53:3664–3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamill RJ, et al. 2010. Comparison of 2 doses of liposomal amphotericin B and conventional amphotericin B deoxycholate for treatment of AIDS-associated acute cryptococcal meningitis: a randomized, double-blind clinical trial of efficacy and safety. Clin. Infect. Dis. 51:225–232 [DOI] [PubMed] [Google Scholar]
- 17. Howard SJ, et al. 2011. Pharmacodynamics of echinocandins against Candida glabrata: requirement for dosage escalation to achieve maximal antifungal activity in neutropenic hosts. Antimicrob. Agents Chemother. 55:4880–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuse ER, et al. 2007. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 369:1519–1527 [DOI] [PubMed] [Google Scholar]
- 19. Lanternier F, et al. 2012. A global analysis of mucormycosis in France: the RetroZygo Study (2005–2007). Clin. Infect. Dis. 54(Suppl 1):S35–S43 [DOI] [PubMed] [Google Scholar]
- 20. Leary R, Jelliffe R, Schumitzky A, van Guilder M. 2001. An adaptive grid, non-parametric approach to pharmacokinetic and dynamic (PK/PD) models, p 389–394 In Proceedings of the Fourteenth IEEE Symposium on Computer-Based Medical Systems IEEE Computer Society, Washington, DC [Google Scholar]
- 21. Lee JW, et al. 1994. Pharmacokinetics and safety of a unilamellar liposomal formulation of amphotericin B (AmBisome) in rabbits. Antimicrob. Agents Chemother. 38:713–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lestner JM, et al. 2010. Pharmacokinetics and pharmacodynamics of amphotericin B deoxycholate, liposomal amphotericin B, and amphotericin B lipid complex in an in vitro model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 54:3432–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Macdonald I, Staatz CE, Jelliffe RW, Thomson AH. 2008. Evaluation and comparison of simple multiple model, richer data multiple model, and sequential interacting multiple model (IMM) Bayesian analyses of gentamicin and vancomycin data collected from patients undergoing cardiothoracic surgery. Ther. Drug Monit. 30:67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pappas PG, et al. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tam VH, Preston SL, Drusano GL. 2003. Optimal sampling schedule design for populations of patients. Antimicrob. Agents Chemother. 47:2888–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vogelsinger H, et al. 2006. Amphotericin B tissue distribution in autopsy material after treatment with liposomal amphotericin B and amphotericin B colloidal dispersion. J. Antimicrob. Chemother. 57:1153–1160 [DOI] [PubMed] [Google Scholar]
- 27. Walsh TJ, et al. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327–360 [DOI] [PubMed] [Google Scholar]
- 28. Walsh TJ, et al. 1999. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N. Engl. J. Med. 340:764–771 [DOI] [PubMed] [Google Scholar]
- 29. Walsh TJ, et al. 2001. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob. Agents Chemother. 45:3487–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walsh TJ, et al. 1998. Safety, tolerance, and pharmacokinetics of a small unilamellar liposomal formulation of amphotericin B (AmBisome) in neutropenic patients. Antimicrob. Agents Chemother. 42:2391–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wasan KM, Lopez-Berestein G. 1996. Characteristics of lipid-based formulations that influence their biological behavior in the plasma of patients. Clin. Infect. Dis. 23:1126–1138 [DOI] [PubMed] [Google Scholar]
- 32. Weiler S, et al. 2009. Pulmonary epithelial lining fluid concentrations after use of systemic amphotericin B lipid formulations. Antimicrob. Agents Chemother. 53:4934–4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wurthwein G, et al. 2012. Population pharmacokinetics of liposomal amphotericin B and caspofungin in allogeneic hematopoietic stem cell recipients. Antimicrob. Agents Chemother. 56:536–543 [DOI] [PMC free article] [PubMed] [Google Scholar]